Abstract
Essentials.
There is no data on the use of idarucizumab in children with venous thromboembolism (VTE).
We present the design of a trial that will assess the safety of idarucizumab in children with VTE.
Patients will be recruited from two ongoing trials in children treated with dabigatran for VTE.
Idarucizumab provides additional re‐assurance when rapid reversal of dabigatran effects is needed.
Background
The incidence of venous thromboembolism (VTE) in children has been increasing. Anticoagulants are the mainstay of treatment but are associated with bleeding events that may be life‐threatening. Idarucizumab is a fragment antigen‐binding (fab) that provides immediate, complete, and sustained reversal of dabigatran's anticoagulant effects in adults.
Objective and Methods
This phase III, open‐label, single‐arm, multicenter, multinational trial will assess the safety of idarucizumab in children participating in two ongoing trials investigating dabigatran etexilate. Eligible patients will be children with VTE (aged 0–≤18 years; n = ~5) with life‐threatening or uncontrolled bleeding (group A), and children who require emergency surgery/urgent procedures for a condition other than bleeding (group B). Patients will receive idarucizumab up to 5 g as two consecutive intravenous infusions over 5‐10 minutes each, as two 10‐15‐minute drips or as two bolus injections (15 minutes apart) and will be monitored for 30 days. The primary endpoint will be the safety of idarucizumab assessed by the occurrence of drug‐related adverse events (including immune reactions) and all‐cause mortality. Secondary endpoints will be the reversal of dabigatran anticoagulant effects assessed by changes in diluted thrombin time and ecarin clotting time, time to achieve complete reversal and the duration of the reversal and bleeding severity (group A). The formation of antidrug antibodies at 30 days post‐dose and cessation of bleeding will also be assessed.
Conclusion
This study will report the safety of idarucizumab in children with VTE who require rapid reversal of the anticoagulant effects of dabigatran. Clinical trial registration: NCT02815670.
Keywords: children, dabigatran etexilate, idarucizumab, oral anticoagulants, venous thromboembolism
1. INTRODUCTION
The incidence of venous thromboembolism (VTE) in children has been increasing over time.1 Anticoagulants such as unfractionated heparin, low‐molecular‐weight heparin (LMWH), and vitamin K antagonists (VKAs) are widely used therapeutic options for the treatment and prevention of VTE, albeit with some disadvantages. LMWH, for example, is administered by subcutaneous injection, VKAs require intensive anticoagulation monitoring, and are associated with multiple food and drug interactions.2 The direct oral anticoagulants (DOACs), such as dabigatran etexilate, overcome many of the shortcomings associated with the use of the current standard of care for VTE, namely LMWHs and VKAs. Dabigatran etexilate, a direct thrombin inhibitor, which is administered orally, showed a predictable pharmacokinetic profile, no food interactions and few drug–drug interactions, and no requirement for regular anticoagulation monitoring in adults.2 However, bleeding is a potential adverse event (AE) associated with the use of dabigatran etexilate, as with all anticoagulants, and in rare cases, bleeding might be life‐threatening or uncontrolled.3, 4, 5 To date, dabigatran etexilate is not yet approved in children but is undergoing pediatric clinical trials.
With the increasing use of anticoagulants, there is an unmet need for agents that can reverse anticoagulant effects in patients who require emergency surgery or urgent procedures, or who have life‐threatening or uncontrolled bleeding. Current standards of care for reversal of anticoagulant effects are suboptimal and have shown undesired effects.6, 7, 8, 9, 10, 11
Moreover, although non‐specific reversal agents (eg, prothrombin complex concentrates [PCCs], activated PCCs, and recombinant factor VIIa) are considered for the reversal of the effects of DOACs, there is a lack of evidence supporting their use, especially in children. Overall, the limitations associated with the current standard of care and non‐specific reversal agents for anticoagulant reversal highlight the necessity of specific reversal agents.12
Currently, dabigatran is the only DOAC to have a compound‐specific reversal agent (idarucizumab) approved for use in adults. Idarucizumab is a monoclonal antibody fragment that binds specifically and with high affinity to dabigatran, resulting in immediate, complete, and sustained reversal of the anticoagulant effects of dabigatran.13, 14 If clinically necessary, a second dose of idarucizumab may be required.15 The safety and efficacy of idarucizumab have been confirmed in adults receiving dabigatran etexilate.16, 17 However, these are yet to be established in children.
1.1. Study objective
We present here the design of a trial that will evaluate the safety and efficacy of idarucizumab in children aged ≤18 years who are being treated with dabigatran etexilate within the ongoing pediatric clinical trials for treatment (NCT01895777) or secondary prevention (NCT02197416) of VTE, and who require emergency surgery/urgent procedures or who have life‐threatening or uncontrolled bleeding.
2. METHODS
2.1. Study design
This is a phase III, open‐label, single‐arm, multicenter, multinational safety study of idarucizumab in children aged from 0 to 18 years. The patients will be recruited from two ongoing pediatric trials of pediatric patients being treated with dabigatran etexilate for the treatment and secondary prevention of VTE in up to 100 sites in about 30 countries. Patients in whom a rapid reversal of dabigatran anticoagulant effect is required will receive idarucizumab and will be followed up for 30 days. Reversal of anticoagulant activity with idarucizumab will be recorded in all patients. The assessments performed at each time‐point are described in Table 1.
Table 1.
Specific assessments carried out at each visit during the study
| Trial period/procedure | Screening | Treatment period | Follow‐up period | |||
|---|---|---|---|---|---|---|
| Visit number | 1.0 | 2.1 | 2.2 | 3 | 4 | 5 |
| Visit description | Patient screening (baseline)a | Dosing (vial 1) | Dosing (vial 2) | 24‐hour post‐dosing | ||
| Study day (visit window) | 1 | 1 | 1 (no later than 15 minutes after vial 1) | 2 (± 2 hours) | 7 (± 3 days) | 30 (+ 7 days) |
| Evaluation of bleeding events | X | X | X | X | X | X |
| Surgery/procedure assessment | X | X | X | X | X | X |
| Drug administration | – | X | X | – | – | – |
| PK blood sampleb | – | X | X | X | – | – |
| PD blood sample (coagulation biomarkers)b | – | X | X | X | – | – |
| ADA blood samplec | – | X | – | – | – | X |
| Adverse events | X | X | X | X | X | X |
ADA, anti‐idarucizumab antibodies; aPTT, activated partial thromboplastin time; dTT, diluted thrombin time; ECT, ecarin clotting time; PD, pharmacodynamic; PK, pharmacokinetic; TT, thrombin time.
Due to the need for urgent care in these patients, it is expected that the majority of screening procedures will already have been performed as part of the hospitalization and diagnosis procedures.
Blood will be collected prior to drug administration of each vial of idarucizumab. Post‐dose sampling will be collected at 30 minutes (±10 minutes), 4 hours (±30 minutes), 12 hours (±1 hour), and 24 hours (±2 hours) for PD coagulation biomarkers (dTT, ECT, aPTT, and TT) and for PK samples (dabigatran and idarucizumab concentration).
Pre‐dose sampling will occur only prior to vial 1 administration. Post‐dose sampling will be collected at day 30. “X” indicates “present”; “‐ ” indicates “not present.”
2.2. Administrative structure of the trial
The trial is sponsored by Boehringer Ingelheim. A coordinating investigator is responsible for managing investigators at different centers. The independent data monitoring committee that was established for the ongoing dabigatran pediatric trials will assess safety, tolerability and efficacy during the conduct of the trial, and will provide recommendations to the sponsor regarding continuation, modification or termination of the study (Figure 1).
Figure 1.
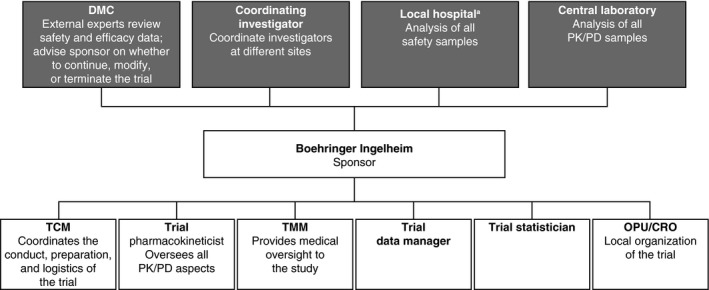
Trial administrative and oversight structure. aLocal laboratories may be employed, after consultation with the sponsor, to evaluate some assays, such as dTT, for the evaluation of dabigatran activity, international normalized ratio, serum creatinine, and hemoglobin. CRO, Contract Research Organization; DMC, Data Monitoring Committee; dTT, diluted thrombin time; OPU, local Boehringer Ingelheim operating unit; PD, pharmacodynamics; PK, pharmacokinetics; SOP, standard operating procedure; TCM, Trial Clinical Monitor; TMM, Team Member Medicine
The trial will be carried out in compliance with the approved protocol and the ethical principles laid down in the Declaration of Helsinki, in accordance with the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Tripartite Guideline for Good Clinical Practice, European Union regulation 536/2014, and other relevant regulations.18, 19 A written informed consent/assent will be obtained from each patient and/or the patient's legally authorized representative.
2.3. Participants
The following patients will be eligible to enter the present study. Patients receiving dabigatran etexilate for the treatment and secondary prevention of VTE in the ongoing pediatric trials NCT01895777 and NCT02197416: who develop life‐threatening/uncontrolled bleeding (group A) and who are without bleeding but need emergency surgery/urgent procedures (group B), therefore requiring a rapid reversal of anticoagulant effects of dabigatran. Key inclusion and exclusion criteria are listed in Table 2.
Table 2.
Key inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
2.4. Treatment
Idarucizumab will be administered intravenously as two consecutive intravenous infusions of over 5‐10 minutes each, as two 10‐15‐minute drips, or as two bolus injections (15 minutes apart); a dedicated intravenous line is not required. Idarucizumab may be given through an existing intravenous line infusing with 0.9% normal saline only, intermittent intravenous lock, peripheral intravenous, or a central venous catheter. A dose of 5 g of idarucizumab was chosen as a reference dose because it is sufficient to reverse the body loads of dabigatran measured in adults. Idarucizumab binds and inactivates dabigatran following a 1:1 stoichiometry. Assuming that dabigatran plasma concentrations in children are similar to those achieved in adults and that the dabigatran volume of distribution correlates with body weight, an approach to downscale idarucizumab pediatric dosing based only on body weight will be taken. The pediatric dose will therefore range from 300 to 5000 mg, where 5000 mg is the reference dose for a patient with a body weight of ≥71 kg and 300 mg is the dose for pediatric patients in the lowest weight category, 2.5 to 5 kg. The detailed methods of pediatric dose selection of idarucizumab are provided in Table S1.
2.5. Study outcomes
The full list of primary and secondary study endpoints is presented in Table 3. The primary endpoint is to assess the safety of idarucizumab in this population. The investigators will collect and maintain records of all AEs and deaths reported during the trial. Secondary endpoints include bleeding severity for the index bleed (group A) or any unusual bleed associated with surgery that cannot be delayed by at least 8 hours (group B). Bleeding assessments during the treatment period will be performed at or near the times of blood withdrawal (ie, the time‐points of the phlebotomy). The severity of bleeding will be classified according to recommendations made by the Perinatal and Paediatric Haemostasis Subcommittee during the 56th‐58th Scientific and Standardization Committee Meetings (SSC) of the International Society on Thrombosis and Haemostasis (ISTH).20
Table 3.
Study outcomes
| Primary endpoint | Secondary endpoints |
|---|---|
Safety of idarucizumab as assessed by the occurrence of:
|
The reversal of dabigatran anticoagulant activity as assessed by:
|
ADA, anti‐idarucizumab antibodies; AE, adverse event; dTT, diluted thrombin time; ECT, ecarin clotting time.
Bleeding will be classified according to recommendations made by the Perinatal and Paediatric Haemostasis Subcommittee during the 56th‐58th Scientific and Standardization Committee (SSC) Meetings of the International Society on Thrombosis and Haemostasis (ISTH) into major, clinically relevant non‐major and minor bleeding.20
For group B patients who had unexpected abnormal bleeding during or post procedure, bleeding will be categorized by the treating clinician as: normal/expected haemostasis; mildly abnormal hemostasis (eg, unexpected slight oozing); moderate abnormality (eg, unexpected controllable bleeding); and severe abnormal hemostasis (eg, unexpected severe refractory hemorrhage).
As part of the secondary endpoints, the reversal of anticoagulant effects of dabigatran will be assessed by the reversal of diluted thrombin time (dTT) and ecarin clotting time (ECT), time to achieve complete reversal and the duration of the reversal. Additional anticoagulant efficacy measurements (as part of further endpoints) include assessments of activated partial thromboplastin time (aPTT) and thrombin time (TT).
Additionally, also as part of the secondary endpoints, an anti‐drug antibodies (ADAs) assay will be performed for idarucizumab.
Pharmacokinetic (PK) assays will be performed for idarucizumab and for dabigatran as part of further endpoints.
2.6. Statistical analysis
2.6.1. Sample size
The design of this trial (including the definition of endpoints and sample size) has been agreed with the Paediatric Committee of the European Medicines Agency. Because the recruitment of patients is expected to be slow, with an average rate of <1‐2 patients per year, the target number of total entered patients (treated and evaluable) in this trial is five. All patients previously or currently treated with dabigatran are eligible for the described study as soon as they develop uncontrolled major bleeding or emergency that requires urgent surgery.
2.6.2. Planned analysis
All statistical analyses are descriptive in nature. The two patient groups (groups A and B) will be summarized separately and together, with an overall conclusion, if possible.
Safety analyses will be based on all treated patients who have received any idarucizumab. Occurrence of drug‐related AEs (including immune reactions) and all‐cause mortality during the trial (from the start of dosing until 30 days after the end of dosing) will be reported using descriptive statistics. Potential specific risks, such as local injection site reactions, and systemic hypersensitivity, including anaphylaxis or immune complex disease, are considered immune reactions. The reversal of coagulation tests (dTT, ECT, aPTT, and TT) will be reported descriptively. Time to achieve complete reversal and duration of complete reversal of the effects of dabigatran sustained at 24 hours post‐dose based on dTT, ECT, aPTT, and TT will be reported by listing the reversal values at planned time points. The cessation of bleeding will be reported using a binary yes/no outcome (group A only). Bleeding status and other clinical conditions that may contribute to bleeding (group A only) during the trial will be reported descriptively. Detection of ADAs at 30 days post dose of idarucizumab will be reported. It is not planned to impute missing values of efficacy and safety evaluations.
3. DISCUSSION
This article describes the rationale and design of the trial that will evaluate the safety and efficacy of idarucizumab, a dabigatran‐specific reversal agent, in children from 0 to 18 years of age who are treated with dabigatran etexilate for managing VTE and in whom a reversal of dabigatran effects is required.
Dabigatran etexilate is indicated for stroke risk reduction in patients with atrial fibrillation and in the prevention and treatment of VTE in adults.21 Dabigatran exhibited a similar efficacy when compared with VKA, with an improved bleeding profile in adult patients with VTE.22, 23, 24 The efficacy and safety of dabigatran was also demonstrated in trials for stroke prevention in patients with atrial fibrillation4 and in real‐world studies, such as in elderly Medicare patients.25
Nevertheless, bleeding events continue to be relevant AEs associated with dabigatran, as is the case with all anticoagulants.26 However, DOACs have demonstrated similar or lower major bleeding rates than warfarin. In phase 3 trials in adults, the major bleeding rates were found to be 2.71% vs 3.36% for dabigatran 110 mg bid4; 3.6% vs 3.4% for rivaroxaban27; 2.13% per year vs 3.09% per year for apixaban28 and 2.75% per year vs 3.43% per year for edoxaban 60 mg when compared with warfarin.29 In addition, patients with VTE treated with dabigatran etexilate or another anticoagulant and requiring emergency surgery and/or urgent procedures where anticoagulation treatment would unduly increase the risk of intra‐ and post‐operative bleeding may benefit from a reversal agent. In children, there are limited data available on bleeding rates with DOACs–no major bleeding was observed in a phase 1 study in children for rivaroxaban30 and in phase 2 studies in children and adolescents for dabigatran.31 The major bleeding rate for LMWH was 3% when used in children with venous thrombosis.32 However, a few observational studies have shown that the bleeding risks with LMWH in children ranged from 0.0‐10.8%.33 The bleeding risk in children on warfarin is estimated at 0.5%.33 However, bleeding rates of up to 24% were reported in critically ill children receiving unfractionated heparin and who were at increased risk of bleeding.34, 35 The current management of symptomatic bleeding associated with dabigatran has some similarities to that observed in patients taking VKA, such as the cessation of anticoagulant treatment and the use of supportive care guided towards hemodynamic stabilization. In addition, hemodialysis may represent an option to expedite the removal of systemic dabigatran.36 Specific reversal agents for DOACs may be advantageous in managing serious bleeding or when a rapid reversal of DOAC effect is required.37 With the approval of idarucizumab, a dabigatran‐specific reversal agent, an additional treatment option during emergency situations is available.38 A first‐in‐human study demonstrated that idarucizumab had a favorable PK profile, with no effects on pharmacodynamic parameters and no procoagulant effect in the absence of dabigatran, and that it was safe and well tolerated in healthy individuals.13, 39 Idarucizumab reversed dabigatran‐induced anticoagulation in healthy volunteers, elderly patients, and patients with renal impairment in a dose‐dependent manner.14, 40 A German case series assessed the clinical use of idarucizumab in antagonizing the effects of dabigatran in patients with symptoms of ischemic stroke or ICH. The administration of recombinant tissue plasminogen activator (rt‐PA) after idarucizumab use in the ischemic stroke patients was feasible and safe–79% of patients benefitted from rt‐PA thrombolysis following idarucizumab treatment. No bleeding complications were observed. Only two out of 12 patients with ICH showed hematoma growth and the mortality rate was low (6.5%).41 An interim analysis of an ongoing, phase III trial of idarucizumab in dabigatran‐treated adult patients with life‐threatening or uncontrolled bleeding, or in whom emergency surgery or an urgent procedure is required, demonstrated complete reversal of the anticoagulant effects of dabigatran as measured by dTT and ECT, and with no safety concerns.16, 17 Idarucizumab is specific for dabigatran reversal; antithrombotic therapy with unfractionated heparin or LMWH can therefore be started at any time after the surgery if the patient is stable and ongoing anticoagulation after the surgery is possible.
The use of anticoagulants, including dabigatran etexilate, to manage VTE in children is unlicensed or off‐label, suggesting a paucity of clinical data in children. Therapeutic guidelines for treatment of VTE in children are based on a limited number of small pediatric trials but, in many cases, the guidelines are extrapolated from adult studies, which may be inappropriate.37 In addition, guidelines recommend generic methods, such as use of gastric lavage and oral activated charcoal in case of anticoagulant overdose, use of fresh frozen plasma or activated prothrombin complex concentrate in case of serious bleeding, and performing dialysis to reverse the anticoagulant effects of dabigatran.36, 42 Nevertheless, although experience is very limited with the use of reversal agents in children receiving anticoagulants, anecdotal evidence from a case report has demonstrated the potential utility of idarucizumab in children with overdose of dabigatran. For example, a case report that involved a 15‐year‐old girl who presented with dabigatran overdose demonstrated that use of idarucizumab (5 g) completely and immediately reversed the anticoagulant effects of excess dabigatran (>1000 ng/mL).43 Moreover, the pediatric investigation plan for dabigatran etexilate and idarucizumab approved by the European Medicines Agency requires data generation in children. Therefore, there is a need to conduct trials of reversal agents in children taking anticoagulants who may develop uncontrolled or life‐threatening bleeding or in whom emergency surgery is required. The present planned study will provide evidence supporting the safety of idarucizumab in children in whom reversal of dabigatran effects is required.
The primary endpoint is the safety of idarucizumab, which will be assessed by the occurrence of drug‐related AEs (including immune reactions) and all‐cause mortality during the study. A single intravenous dose of idarucizumab, a monoclonal antibody fragment, has a low potential to stimulate ADA formation. Nevertheless, the use of a biologic agent may be a potential risk factor for immunogenicity in the form of ADA production.44 A pooled analysis of data from three phase 1 studies found that pre‐existing and treatment‐emergent antidrug antibodies were present at low levels and had no impact on the PK/PD of idarucizumab in adults.45 However, testing the immunogenic potential of a biologic agent is suggested in the regulatory guidance and is included in this study as a secondary endpoint.46
A secondary endpoint is coagulation‐time testing. These assays can give an objective measure of the reversal of anticoagulation. dTT and ECT are appropriate for dabigatran as the correlation between these parameters and dabigatran plasma concentration showed a high degree of linearity.36 Bleeding assessment is a more subjective metric, and is dependent upon the initial bleeding event or procedure, the presence of damaged blood vessels, timing, and patient characteristics.
A potential limitation of this trial is that the number of patients will be small because dabigatran etexilate is not yet approved for use in children. Hence, the target population for this trial is limited to those who are enrolled in the dabigatran etexilate trials and those who may then require reversal of dabigatran effects. Obtaining informed consent may be challenging in this emergency setting, particularly in children. An informed consent should be given by parent(s) or by the legal representative prior to trial participation. In addition, depending on the age of the child, an assent by the child should be given prior to trial participation. Where possible, permitted by the local regulatory body and approved by the local ethics committee, an adjusted informed consent procedure may be used due to the nature of the emergency medical condition of the patient (eg, verbal informed consent followed by written informed consent). The appropriate level of dosing may be challenging in children as the dose has to be adjusted in relation to the body weight of the patient.
Since DOACs are being considered as a potential replacement for the existing standard of care for managing children with VTE, the safety study of idarucizumab could further improve the benefit/risk profile of dabigatran etexilate use in this patient population.
AUTHOR CONTRIBUTIONS
M. Albisetti, M. Brueckmann, S. Glund, and A. Schlosser: made substantial contributions to the concept and study design. Each author critically reviewed and participated in revising the manuscript, and each author approved the final draft.
RELATIONSHIP DISCLOSURE
Dr. Albisetti is a co‐ordinating investigator for the present trial and is a member of the Pediatric Expert Working Group for Boehringer Ingelheim for which she received personal fees. Dr. Brandão has received honoraria from Boehringer Ingelheim as a consultant to Boehringer Ingelheim. Dr. Brueckmann, Dr. Gropper, and Dr. Tartakovsky are employees of Boehringer Ingelheim GmbH & Co. KG, Ingelheim, Germany. A. Schlosser is an employee of Boehringer Ingelheim bv, Alkmaar, the Netherlands. Dr. Glund is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany. Dr. Reilly is an employee of Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA.
Supporting information
Albisetti M, Schlosser A, Brueckmann M, et al. Rationale and design of a phase III safety trial of idarucizumab in children receiving dabigatran etexilate for venous thromboembolism. Res Pract Thromb Haemost. 2018;2:69–76. 10.1002/rth2.12053
Funding information
The study was funded by Boehringer Ingelheim Pharma GmbH & Co. KG. Medical writing assistance, supported by Boehringer Ingelheim Pharma GmbH & Co. KG, was provided by Anil Dandu, MPharm, PhD, of PAREXEL, during the preparation of this article
REFERENCES
- 1. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–8. [DOI] [PubMed] [Google Scholar]
- 2. Kerlin BA. Current and future management of pediatric venous thromboembolism. Am J Hematol. 2012;87(Suppl 1):S68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 4. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 5. Connolly SJ, Wallentin L, Ezekowitz MD, et al. The Long‐Term Multicenter Observational Study of Dabigatran Treatment in Patients With Atrial Fibrillation (RELY‐ABLE) Study. Circulation. 2013;128:237–43. [DOI] [PubMed] [Google Scholar]
- 6. Feuring M, van RJ. The discovery of dabigatran etexilate for the treatment of venous thrombosis. Expert Opin Drug Discov 2016;11:717–31. [DOI] [PubMed] [Google Scholar]
- 7. Franchini M, Lippi G. Prothrombin complex concentrates: an update. Blood Transfus. 2010;8:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee GM, Welsby IJ, Phillips‐Bute B, Ortel TL, Arepally GM. High incidence of antibodies to protamine and protamine/heparin complexes in patients undergoing cardiopulmonary bypass. Blood. 2013;121:2828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suarez CJ, Gayoso DP, Gude SF, Gomez Zincke JM, Rey AH, Fontanillo Fontanillo MM. Method to calculate the protamine dose necessary for reversal of heparin as a function of activated clotting time in patients undergoing cardiac surgery. J Extra Corpor Technol. 2013;45:235–41. [PMC free article] [PubMed] [Google Scholar]
- 10. Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban–an oral, direct factor Xa inhibitor. Thromb Haemost. 2012;108:876–86. [DOI] [PubMed] [Google Scholar]
- 11. Vigue B. Bench‐to‐bedside review: optimising emergency reversal of vitamin K antagonists in severe haemorrhage ‐ from theory to practice. Crit Care. 2009;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grottke O, Aisenberg J, Bernstein R, et al. Efficacy of prothrombin complex concentrates for the emergency reversal of dabigatran‐induced anticoagulation. Crit Care. 2016;20:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glund S, Moschetti V, Norris S, et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb Haemost. 2015;113:943–51. [DOI] [PubMed] [Google Scholar]
- 14. Glund S, Stangier J, Schmohl M, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo‐controlled, double‐blind phase 1 trial. Lancet. 2015;386:680–90. [DOI] [PubMed] [Google Scholar]
- 15. Summary of product characteristics, Praxbind. [cited 2017 July 3]. Available from http://www ema europa eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/003986/WC500197462 pdf. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003986/WC500197462.pdf.
- 16. Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–20. [DOI] [PubMed] [Google Scholar]
- 17. Pollack CV, Reilly PA, van Ryn J , et al. Idarucizumab for dabigatran reversal: updated results of the RE‐VERSE AD study. Circulation. 2016;134(Suppl 1): E714–E715. [Google Scholar]
- 18. ICH Harmonised Tripartite Guideline . Guideline for Good Clinical Practice E6. [cited 2017 February 20]. Available form http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf.
- 19. World Medical Association Declaration of Helsinki . Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 20. Mitchell LG, Goldenberg NA, Male C, Kenet G, Monagle P, Nowak‐Gottl U. Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thrombosis and pulmonary embolism in children. J Thromb Haemost. 2011;9:1856–8. [DOI] [PubMed] [Google Scholar]
- 21. Pradaxa, European Medicines Agency (EMA) . EMA Web site. [cited 2016 Aug 19]. Available form http://www.ema.europa.eu/docs/en_GB/document_library/EPAR-Product_Information/human/000829/WC500041059.pdf.
- 22. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. [DOI] [PubMed] [Google Scholar]
- 23. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–18. [DOI] [PubMed] [Google Scholar]
- 24. Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–72. [DOI] [PubMed] [Google Scholar]
- 25. Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–64. [DOI] [PubMed] [Google Scholar]
- 26. Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:623–7. [DOI] [PubMed] [Google Scholar]
- 27. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 28. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 29. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 30. Young G, Kubitza D, Kenet G, et al. Development of a rivaroxaban dosing regimen for treatment of VTE in children aged 12 to 18 years. J Thromb Haemost. 2017;13(Suppl. 2):1–997. [Google Scholar]
- 31. Boehringer Ingelheim . Safety and tolerability of dabigatran etexilate in adolescents. [cited 2017 June 16]. Available from ClinicalTrials.gov; Bethesda, MD:National Library of Medicine (US) 2000 NLMIdentifier: NCT00844415. https://clinicaltrials.gov/ct2/show/NCT00844415.
- 32. Nowak‐Gottl U, Bidlingmaier C, Krumpel A, Gottl L, Kenet G. Pharmacokinetics, efficacy, and safety of LMWHs in venous thrombosis and stroke in neonates, infants and children. Br J Pharmacol. 2008;153:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan AK, Monagle P. Updates in thrombosis in pediatrics: where are we after 20 years? Hematology Am Soc Hematol Educ Program. 2012;2012:439–43. [DOI] [PubMed] [Google Scholar]
- 34. Kuhle S, Eulmesekian P, Kavanagh B, Massicotte P, Vegh P, Mitchell LG. A clinically significant incidence of bleeding in critically ill children receiving therapeutic doses of unfractionated heparin: a prospective cohort study. Haematologica. 2007;92:244–7. [DOI] [PubMed] [Google Scholar]
- 35. Trucco M, Lehmann CU, Mollenkopf N, Streiff MB, Takemoto CM. Retrospective cohort study comparing activated partial thromboplastin time versus anti‐factor Xa activity nomograms for therapeutic unfractionated heparin monitoring in pediatrics. J Thromb Haemost. 2015;13:788–94. [DOI] [PubMed] [Google Scholar]
- 36. Rahmat NA, Lip GY. Monitoring the effects and antidotes of the non‐vitamin K oral anticoagulants. Arrhythm Electrophysiol Rev. 2015;4:90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e737S–801S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. PRAXBIND prescribing information . 2015.
- 39. Schmohl M, Glund S, Harada A, et al. Idarucizumab does not have procoagulant effects: assessment of thrombosis biomarkers in healthy volunteers. Thromb Haemost 2017;117:269–76. [DOI] [PubMed] [Google Scholar]
- 40. Glund S, Stangier J, van Ryn J, et al. Effect of age and renal function on idarucizumab pharmacokinetics and idarucizumab‐mediated reversal of dabigatran anticoagulant activity in a randomized, double‐blind, crossover phase Ib study. Clin Pharmacokinet. 2017;56:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kermer P, Eschenfelder CC, Diener HC, et al. Antagonizing dabigatran by idarucizumab in cases of ischemic stroke or intracranial hemorrhage in Germany–A national case collection. Int J Stroke. 2017;12:383–91. [DOI] [PubMed] [Google Scholar]
- 42. Peacock WF, Rafique Z, Singer AJ. Direct‐acting oral anticoagulants: practical considerations for emergency medicine physicians. Emerg Med Int. 2016;2016:1781684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shapiro S, Bhatnagar N, Khan A, Beavis J, Keeling D. Idarucizumab for dabigatran overdose in a child. Br J Haematol. 2016. [Epub ahead of print]. 10.1111/bjh.14371. [DOI] [PubMed] [Google Scholar]
- 44. Sethu S, Govindappa K, Alhaidari M, Pirmohamed M, Park K, Sathish J. Immunogenicity to biologics: mechanisms, prediction and reduction. Arch Immunol Ther Exp (Warsz). 2012;60:331–44. [DOI] [PubMed] [Google Scholar]
- 45. Norris S, Ramael S, Ikushima I, et al. Evaluation of the immunogenicity of the dabigatran reversal agent idarucizumab during Phase I studies. Br J Clin Pharmacol. 2017;83:1815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. US Department of Health and Human Services, Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) . Guidance for industry: immunogenicity assessment for therapeutic protein products. [cited 2015 June 23]. Available from http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm338856.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


