Abstract
Introduction:
Many studies on prenatal tobacco exposure (PTE) effects have relied on single item retrospective measures of PTE. However, it is unclear how these single item measures may relate to more intensive maternal self-reports and to biological markers of maternal use and/or fetal exposure. It is also unclear whether these measures may be more valid predictors of fetal growth (gestational age, birthweight, head circumference, and birth length).
Methods:
Data were obtained from 258 women during their pregnancy. PTE was assessed by four methods: a single item question, a calendar-based self-report measure from each trimester of pregnancy, maternal salivary cotinine assays, and nicotine and metabolites in infant meconium. We hypothesized that the more intensive measures and biological assays would account for additional variance in birth outcomes, above and beyond the single item measure.
Results:
The single item self-report measure was not related to fetal growth. However, the more intensive calendar based self-report measure and the biological assays of PTE (ie, maternal salivary assays and infant meconium) were significant predictors of poor fetal growth, even with the single item measure in the model.
Conclusions:
The negative effects of PTE on important child outcomes may be greatly underestimated in the literature as many studies use single item self-report measures to ascertain PTE. Whereas more intensive self-report measures or biological assays may be cost prohibitive in large scale epidemiological studies, using a combination of measures when possible should be considered given their superiority both identifying prenatal smokers and predicting poor fetal growth.
Implications:
The present work underscores the importance of measurement issues when assessing associations between PTE and fetal growth. Results suggest that we may be greatly underestimating the negative effects of prenatal smoking on fetal growth and other important child outcomes if we rely solely on restricted single item self-report measures of prenatal smoking. Researchers should consider more intensive prospective self-report measures and biological assays as viable and superior alternatives to single item self-report measures.
Introduction
Despite the known risks to mother and fetus, prenatal tobacco use remains a major public health problem as approximately 15.4% of pregnant women in the United States acknowledge smoking during pregnancy.1 At that rate, over 400 000 infants affected by prenatal tobacco exposure (PTE) are born each year. This makes PTE one of the largest preventable causes of maternal, fetal, and childhood morbidity and mortality in the United States.2 Extant studies of PTE have often relied on retrospective measures of prenatal tobacco using a single question regarding amount smoked to determine exposure status. However, there are often major changes in the number of cigarettes smoked per day during pregnancy, with the vast majority of pregnant smokers having significant reductions upon pregnancy recognition, with continuing changes reflected in patterns of reduction and relapse.3,4 Few studies have examined the association between single item measures of smoking status or amount smoked and more intensive, prospective self-reported smoking during pregnancy as well as biological assays. In one of the few studies examining measurement issues, Pickett et al.5 noted that there was less congruence among retrospective recall of amount smoked and prospective measures and biological assays, especially among heavier smokers. Similarly, a comparison of brief self-reports to maternal serum cotinine in the 1999–2006 National Health and Nutrition Examination Survey data indicated that approximately 23% of pregnant smokers did not disclose their smoking status.2 However, these few studies did not examine how these measures may be differentially predictive of neonatal birth outcomes.
One of the most consistent effects of PTE is decreased fetal growth, resulting in poor birth outcomes. Birth weight deficits in infants prenatally exposed to tobacco range from 200 to 327 grams, depending on the nicotine dose,2 and it is estimated that 20% of low birthweight and small for gestational age infants are attributable to PTE.6 Women who smoke during pregnancy are also more likely to have a preterm delivery.2 Nicotine interacts with receptors in placental vasculature resulting in decreased placental blood flow and fetal vasoconstriction, which leads to a disruption of the delivery of oxygen and nutrients to the fetus. This reduced blood flow leads to fetal malnutrition and is thought to be a causal mechanism for the effects of PTE on poor fetal growth.2
One of the most commonly used assessments of maternal smoking during pregnancy is a self-report measure, which asks mothers to recall how many cigarettes they smoked while pregnant.2 For practical and financial reasons, these assessments are typically no more than a few questions, and are often asked retrospectively months, or perhaps even years after their pregnancy. This typically only allows for a dichotomous classification of smoking versus not, and, consequently, does not allow researchers to examine potential dose–response effects. In fact, a recent meta-analysis reported that 70 of the 71 studies they examined utilized self-report measures to assess prenatal smoking.7 Another recent meta-analysis reported that half of their included studies used a dichotomous measure of maternal pregnancy smoking, which was collected after delivery in 56% of the studies. None of the studies utilized biochemical measures of maternal pregnancy smoking.8 However, even when data on average number of cigarettes during pregnancy is collected, there is only moderate congruence between retrospective and prospective reports.5 This may be because these prospective reports often happen after pregnancy recognition, upon which many women quit or substantially cut down on their smoking. Thus, even prospective measures may miss the vital time before pregnancy recognition when smoking is typically heavier, necessitating the use of more intensive self-report measures which include the time before pregnancy recognition.
The purpose of this study was twofold. First, to examine the utility of a single item self-report measure of PTE as a predictor of fetal growth outcomes such as birthweight, gestational age, birth length, and head circumference; and second, to determine the utility of alternate measures of PTE in predicting these outcomes above and beyond the single item measure. We focused on fetal growth as our primary outcome of interest mainly because of the consistent and well established effects of PTE on fetal growth. If it is true that single item measures of PTE are good predictors of fetal growth, then it may not be necessary to employ more expensive and time consuming methods to determine PTE status or amount of exposure. If this is not the case, using these measures may be substantially underestimating the relationship not just between PTE and fetal growth, but other consequential infant and child outcomes of interest.
Methods
Participants
This study included 258 mother/infant dyads, with 181 infants prenatally exposed to tobacco (99 boys and 82 girls), and 77 not exposed (35 boys and 42 girls). In this case, children were considered exposed if either maternal self-report, maternal saliva, infant meconium, or any combinations of these indices were positive for tobacco use. Pregnant women were recruited at their first prenatal appointment in a local area hospital and screened for eligibility using a self-reported health screening measure, followed by HIPAA consent, and medical record review, with all procedures and consents approved by the Institutional Review Board. Eligibility criteria included: less than 20 weeks gestation, no multiple birth, 18 years or older, no illicit drug use except cannabis, no heavy alcohol use (4 or more standard drinks in one occasion or more than 1 drink/day upon pregnancy recognition), no heavy cannabis use (an average of more than 1 joint/day or 4 or more on one occasion), and were English speakers. At the conclusion of each recruitment month, participating smokers were matched on maternal age and highest educational attainment with the closest eligible nonsmoking woman, who was then invited to participate. The smoking group was oversampled such that one nonsmoker was recruited for every two smokers. This allowed for a full range of light to heavy smokers to be represented, as well as for the possibility of higher attrition in the smoking group across time.
Procedures and Instruments
Prenatal Tobacco Use
Maternal prenatal tobacco use was measured via both self-report and biological verification methods. First, the eligibility screener included a single item asking how many cigarettes they were smoking per day in their current pregnancy (single item self-report). The Timeline Follow-Back Interview (TLFB)9 was used once at the end of each trimester to gather daily tobacco use for the previous 3 months. The TLFB has good test–retest reliability, and is highly correlated with other intensive self-report measures.10 The TLFB yielded daily data on the number of cigarettes smoked per day. This was averaged to number of cigarettes per day across the entire pregnancy for these analyses.
Maternal saliva specimens were collected once at the end of each trimester and assayed for cotinine, the primary nicotine biomarker, with enzyme-linked immunosorbent assay (ELISA for the first 42 participants at the first trimester only) or liquid chromatography–tandem mass spectrometry (LC–MSMS) at 10 ng/ml cutoff. Maternal salivary cotinine ranged from 0 to 569 ng/ml of saliva. Results were dummy coded across pregnancy for these analyses such that a negative result in each of the 3 trimesters was a 0, and a positive saliva result in any of the 3 trimesters was a 1. If maternal saliva was collected in less than 3 trimesters, we used only the trimesters for which saliva was available to determine smoking status. For example, six women did not have saliva results in trimester 1, but of those, five had saliva results in the next two trimesters, and 1 woman had results in trimester 2 only.
Infant meconium, the first neonatal feces, was collected after birth twice daily until the appearance of milk stools. Meconium was transferred to storage containers and frozen at −80°C until transport to the National Institute on Drug Abuse. Meconium was assayed with a validated quantitative LS–MSMS method11 for nicotine, cotinine, and trans-3’–hydroxycotinine (OHCOT; see Gray et al.12 for further details). Limits of quantification were 2.5 ng/g for nicotine, 1 ng/g for cotinine, and 5 ng/g for OHCOT. As the gold standard for measuring fetal exposure, meconium is a reliable measure of fetal tobacco exposure in the third trimester specifically.12 Meconium results were also dummy coded such that a negative result was 0 and a positive result was 1.
Fetal Growth
Three measures of fetal growth were used: birth weight (g), birth length (cm), and head circumference (cm) taken by trained obstetrical nurses in the delivery room. Gestational age was calculated using dates by trained study staff. Confirmatory factor analyses indicated that these four indicators of fetal growth and gestational age loaded on one latent factor termed Fetal Growth. Goodness of fit indices indicated that the measurement model fit the data well (ie, χ2(2) = 5.73, p = .06, CFI = 0.99; RMSEA = 0.085).
Data Analytic Strategy
We examined associations between the four PTE measures and birth outcomes using Pearson correlations (Table 1). These included the single item eligibility screener question regarding number of cigarettes smoked per day (single item self-report; n = 254, 129 smokers), the dose–response variable of the average number of cigarettes per day across pregnancy (TLFB; n = 258, 168 smokers), the biological verification indicator of whether maternal saliva was positive for nicotine at any time during pregnancy (n = 258, 141 smokers), and the biological indicator of whether infant meconium was positive for nicotine and metabolites or not (fetal exposure; n = 203, 105 smokers). Analyses to guide selection of potential covariates were conducted using bivariate correlations. Demographic or perinatal risk variables that were associated with maternal prenatal smoking and fetal growth at p < .10 were included in subsequent models as covariates (Table 1). Maternal age and parity were considered as potential covariates, but did not meet this criterion and were thus excluded from the models. Structural Equation Modeling (SEM, using AMOS version 2313) was used to examine: (1) the role of the single item self-report measure as a predictor of fetal growth, and (2) whether alternative measures of PTE had incremental predictive utility after including the single item self-report measure in the model.
Table 1.
Bivariate Correlations
| Mean (SD)/n(%) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Single item self-report | 2.85 (3.94) | 1 | |||||||||||
| 2. Average number of cigarettes per day across pregnancy (TLFB) | 3.56 (4.63) | 0.76** | 1 | ||||||||||
| 3. Maternal salivary cotinine across pregnancy | n = 141 (54.7%) positive | 0.57** | 0.64** | 1 | |||||||||
| 4. Infant meconium | n = 105 (51.7%) positive | 0.53** | 0.61** | 0.75** | 1 | ||||||||
| 5. Birthweight (kg) | 3233 (575.7) | −0.03 | −0.08 | −0.16* | −0.19** | 1 | |||||||
| 6. Head circumference (cm) | 33.95 (1.86) | −0.02 | −0.08 | −0.20** | −0.29** | 0.63** | 1 | ||||||
| 7. GA (weeks) | 38.94 (1.81) | 0.02 | −0.04 | −0.12 | −0.19** | 0.56** | 0.47** | 1 | |||||
| 8. Birth length (cm) | 50.10 (2.79) | −0.02 | −0.07 | −0.16* | −0.13 | 0.67** | 0.64** | 0.44** | 1 | ||||
| 9. Maternal Edu. | 12.32 (1.87) | −0.13* | −0.14* | −0.18** | −0.15* | 0.15* | 0.16* | 0.11 | 0.07 | 1 | |||
| 10.TANF | n = 36 (14%) receiving | 0.09 | 0.14* | 0.10 | 0.18* | −0.12 | −0.15* | −0.10 | −0.13* | −0.29** | 1 | ||
| 11. Race | n = 178 (69%) minority | 0.17** | 0.25** | 0.09 | 0.06 | 0.26** | 0.30** | 0.10 | 0.21** | 0.14* | −0.17** | 1 | |
| 12. Child sex | n = 134 (51.9%) male | −0.10 | −0.11 | −0.06 | −0.06 | −0.10 | −0.10 | −0.06 | −0.12 | 0.04 | 0.02 | −0.06 | 1 |
Edu. = Education; GA = Gestational Age; SD = standard deviation; TANF = Temporary Assistance for Needy Families; TLFB = Timeline Follow Back. Bivariate n’s ranged from 199 to 258 due to missing data on meconium and head circumference as described in the methods section. Incidence of preterm birth in smoking mothers was 6% when smoking status was determined by single item self-report (n = 258), 7.7% when identified using the TLFB (n = 258) 7.8% when identified by maternal saliva (n = 258), 7.6% when identified by infant meconium (n = 203).
*p < .05. **p < .01.
Missing Data
As with any longitudinal study, there were incomplete data for some of the participants on one or more of the variables included in this study. Of the 258 mothers and infants who were enrolled in the study, 55 infants had missing results from meconium testing. Meconium was not collected if either (1) milk stool appeared before collection could occur (n = 28), or (2) the participant changed her prenatal care such that she delivered at another hospital (n = 23). For an additional three infants, meconium was collected and sent to the laboratory, but the quantity was not sufficient for analysis. Finally, one infant was excluded because the infant’s meconium was positive for prenatal methamphetamine exposure, leaving 203 infants with fetal exposure results. In addition, there were 23 infants missing information on head circumference. There were no significant differences between families with complete versus missing data on maternal age, maternal education, whether they received welfare, or on their prenatal smoking. Data were thus determined to fit criteria for missing at random, and were analyzed using the Full Information Maximum Likelihood Estimation feature in AMOS 23.
Results
Maternal age ranged from 18 to 39 years at the time of their first appointment (M = 24.01 years, SD = 4.96), with 52% African American, 30% Caucasian, 18% Hispanic, and 8% other or mixed race, with several identifying as more than one race. Forty-five percent of the expectant mothers were married or living with their partner, 33% were in a relationship but not living with their partner, 21% were single, and 1% were divorced. Twenty-nine percent of the women had less than a high school education, 29% completed high school, 29% completed some college, 9% had a vocational/technical or associates degree, and 4% had a bachelor’s degree.
Descriptive statistics for both demographic and substance use variables for participants in the smoking group and the nonsmoking group indicate that, in this predominantly low-income sample, mothers in the smoking group were not significantly different than nonsmoking mothers with respect to their age, education, welfare status, or whether they were married or living with their partner. Women in the smoking group were on average smoking about five cigarettes per day during pregnancy as measured by the TLFB.
When using the single item self-report exclusively, there were 125 women identified as nonsmokers. Of those 125 women, 34 were classified as smokers based on the more intensive self-report, 21 of the 125 reported nonsmokers were classified as smokers based on the salivary assay, and 19 of the 125 nonsmokers were classified as smokers based on infant meconium. Of those misidentified as nonsmokers by the single item self-report, 19 of the women were identified as smokers by more than one of the other more intensive assessments, and 11 were identified as smokers by all three. These numbers suggest that the use of the single item self-report alone misclassified 15% to 25% of women as nonsmokers when they were indeed smokers. Associations among the four different measures of smoking were examined using Pearson correlations and are presented in Table 1. There were significant differences on several infant outcomes based on maternal salivary cotinine (positive vs. negative) and tobacco metabolites in infant meconium (positive vs. negative). Infants in the maternal saliva positive group had lower birthweight, t(256) = 2.56, p < .01, lower head circumference t(233) = 2.50, p = .01, and lower birth length, t(249) = 2.5, p < .01 compared to the saliva negative group. Positive saliva was also associated with lower gestational age, but the difference was not statistically significant, t(256) = 1.76, p = .08. Infants who had tobacco metabolites in meconium had lower birthweight, t(201) = 2.8, p < .01, lower head circumference t(197) = 4.24, p < .01, and lower gestational age, t(201) = 2.71, p < .01 compared to the meconium negative group. Positive meconium was also associated with lower birth length, but the difference was not statistically significant, t(201) = 1.89, p = .06. Descriptive statistics are provided in Table 1.
Four separate SEM models were tested; one assessing the association between each measure of PTE with fetal growth. Exogenous variables included the single item self-report measure, maternal race, years of education, child sex, receiving Temporary Assistance for Needy Families (TANF) or not. Model 1 only included the single item self-report measure. The TLFB measure was added in Model 2. Model 3 included maternal saliva results in addition to the single item self-report measure. Model 4 included infant meconium results in addition to the single item self-report measure.
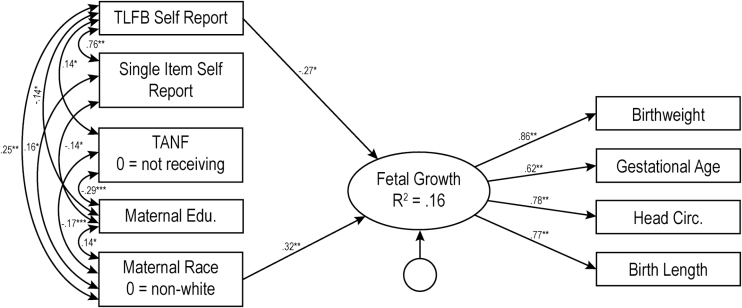
Model 1 (not pictured) indicated that the single item self-report measure of maternal smoking was not a significant predictor of fetal growth (b = −0.08, p = .32). Though the overall model explained 12.6% of the variance in fetal growth (χ2(17) = 18.53, p = .36, CFI = 0.99, RMSEA = 0.02, 90% CIs [00, 0.06]), this was driven primarily by the covariates included in the model. The more intensive TLFB self-report method, yielding number of cigarettes per day across pregnancy and reflecting dose–response associations between PTE and fetal growth (Model 2, Figure 1), accounted for significant variance in fetal growth (b = −0.293, p = .01) above and beyond the single item measure, with the overall model explaining 15.5% of the variance in fetal growth (χ2(20) = 20.93, p = .40, CFI = 0.99, RMSEA = 0.01, 90% CIs [0.000–0.056]).
Figure 1.
Model 2: Results of structural equations modeling for the intensive self-report measure of prenatal tobacco exposure. The numbers represent standardized path coefficients. Unstandardized coefficients are presented in text. For ease of presentation, nonsignificant paths are not depicted in the figure, nor is child sex, a covariate which was not related to any other variable. Covariances between all exogenous variables were included in model testing, but only significant covariances are depicted in figure. The error terms for the measured indicator are not depicted in figure. TANF = Temporary Assistance for Needy Families; Edu = Education; TLFB = Timeline Followback. Variance inflation factors for this model ranged from 1.15 to 2.62. Additional models were run using each of the 3 trimester averages separately rather than the combined average across pregnancy. Average cigarettes per day in each trimester significantly predicted fetal growth, and resulting R2’s ranged from 0.144 to 0.148. *p < .05; **p < .01.
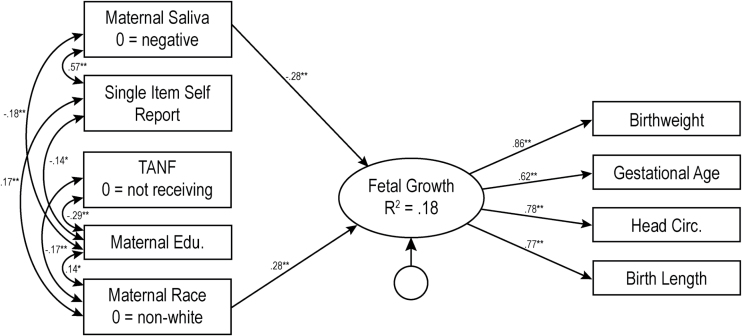
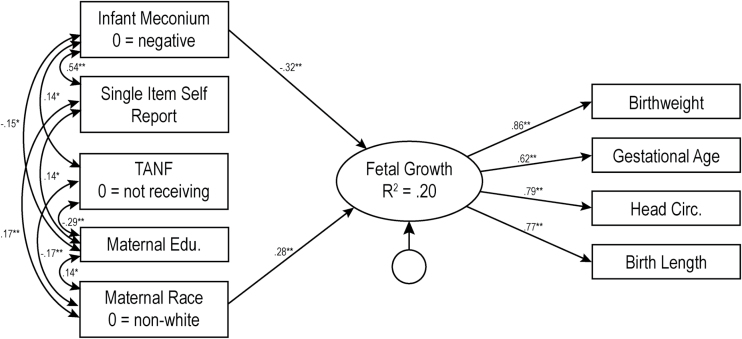
For the biological verification measures, nicotine metabolite (present vs. absent) in maternal saliva during pregnancy (Model 3, Figure 2) accounted for significant variance in fetal growth (b = −2.78, p < .001), again after consideration of the single item self-report measure. This overall model explained 17.8% of the variance in fetal growth (χ2(20) = 23.50, p = .27, CFI = 0.994, RMSEA = 0.026, 90% CIs [0.000–0.062]). Finally, infant meconium (positive vs. negative, Model 4, Figure 3) also accounted for significant variance in fetal growth (b = −3.08, p < .001) above and beyond the single item measure of PTE, with the overall model explaining 19.6% of the variance in fetal growth (χ2(20) = 30.94, p = 0.06, CFI = 0.98, RMSEA = 0.046, 90% CIs [0.000–0.077]).
Figure 2.
Model 3: Results of structural equations modeling for biologically validated maternal salivary analysis measure of prenatal tobacco exposure. The numbers represent standardized path coefficients. Unstandardized coefficients are presented in text. For ease of presentation, nonsignificant paths are not depicted in the figure, nor is child sex, a covariate which was not related to any other variable. Covariances between all exogenous variables were included in model testing but only significant covariances are depicted in figure. The error terms for the measured indicator are not depicted in figure. TANF = Temporary Assistance for Needy Families; Edu = Education; TLFB = Timeline Followback. Variance inflation factors for this model ranged from 1.09 to 1.51. *p < .05; **p < .01.
Figure 3.
Model 4: Results of structural equations modeling for biologically validated infant meconium measure of prenatal tobacco exposure. The numbers represent standardized path coefficients. Unstandardized coefficients are presented in text. For ease of presentation, nonsignificant paths are not depicted in the figure, nor is child sex, a covariate which was not related to any other variable. Covariances between all exogenous variables were included in model testing but only significant covariances are depicted in figure. The error terms for the measured indicator are not depicted in figure. TANF = Temporary Assistance for Needy Families, Edu = Education, TLFB = Timeline Followback. Variance inflation factors for this model ranged from 1.06 to 1.41. *p < .05; **p < .01.
Discussion
The negative impact of PTE on fetal growth is well-established and reduction in fetal growth is one of the most rigorously studied complications of smoking during pregnancy.14–17 Prenatal smoking, for instance, was linked with a reduction in head circumference, femur length, and abdominal circumference,18 with studies demonstrating that prenatal smoking reduces birthweight by approximately 10–12 g per cigarette per day.19 These results clearly illustrate the significant negative impact of PTE on fetal growth, providing further support to this large body of literature. However, the present work underscores the importance of carefully considering how PTE is measured and highlights the possibility that many studies of PTE based on single item self-report measures may be underestimating effects on child outcomes.
Results from this study indicate that assessing PTE via a single question regarding number of cigarettes smoked per day lead to inaccurate and erroneous findings concerning links between PTE and fetal growth. Despite the fact that all of the assessments of PTE were correlated, their validity for predicting fetal growth was not equal. As noted, 15%–25% of the women identified as nonsmokers by the single item self-report were misclassified based upon the other measures. Indeed, when assessing PTE using this single item prospective measure, results suggested that PTE was not predictive of fetal growth. Yet, when PTE was measured prospectively with the more intensive calendar-based self-report measure (TLFB) or with the biological measures (ie, maternal salivary assays and infant meconium), results clearly demonstrated that PTE is predictive of poorer fetal growth. Specifically, in the present work the strongest associations between PTE and fetal growth were observed when prenatal smoking was assessed via concentrations of nicotine markers in infant meconium. It may be that this association is strongest because infant meconium reflects the amount of nicotine that has actually crossed the placental barrier to the fetus.
Our current findings are startling considering that single item measures of PTE that ask mothers to report retrospectively how many cigarettes they smoked while they were pregnant generally dominate the field due to their practical and financial appeal. Moreover, these single item measures of PTE tend to be further reduced down to a dichotomous classification of smoking versus not, which does not permit researchers to examine potential dose–response effects. A meta-analysis, for example, reported that half of their included studies used a dichotomous measure of maternal pregnancy smoking, which was collected after delivery and none of the studies incorporated biochemical measures of maternal pregnancy smoking.8 Consequently, we may be greatly underestimating the negative effects of PTE on fetal growth and other important child outcomes with our reliance on restricted single item self-report measures of prenatal smoking. In our study, the use of the more intensive self-report method yielded the identification of 34 additional smokers. While these methods may be more time consuming, intensive self-report methods are not as costly as the biological assays. Use of more intensive self-report measures also addresses the issue raised by Pickett and colleagues5 regarding the timing of prospective measures of PTE, as these measures cover the time period between conception and pregnancy recognition, when smoking is often heavier. Whereas we looked at the predictive value of each trimester separately (see note in Figure 1), the average across the entire pregnancy explained more variance in fetal growth than any individual trimester, suggesting that the more intensive self-report measures should be collected across the entire prenatal period. Likewise, the use of biological verification methods also identified a larger number of smokers than the single item self-report measure, which aligns with the National Health and Nutrition Examination Survey finding that nearly 23% of pregnant smokers did not accurately disclose their smoking status based on comparisons of self-report and serum cotinine.2 While biological assays are more costly than the intensive self-report methods, they are less time consuming for research participants and staff. Both types of measures should be considered as viable and superior alternatives to single item self-report questions. Future research should examine the utility of using different combinations of measures of PTE for predicting fetal growth.
The present work must be understood in light of its limitations. First, our results may only be generalizable to primarily lower socioeconomic status smokers with high school or below high school education. Further, our study was restricted to pregnant smokers with low levels of alcohol use, low to moderate cannabis use during pregnancy, and no other illicit substance use during pregnancy. It may be possible that results will differ for women with different demographic characteristics. Yet, in the United States, prenatal smoking is more prevalent among younger, low income women with less education,20 which signifies the importance of examining prenatal smoking with the population under study.
In summary, precise measurement of prenatal smoking is complex, and our results emphasize the importance of more intensive measurement methods. Pregnant and postpartum women are often disinclined to reveal substance use information. Furthermore, retrospective assessments may be problematic due to considerable recall bias. Consequently, in light of these issues and our results demonstrating that single item self-report measures of prenatal smoking are poor predictors of fetal growth, future studies are encouraged to assess prenatal smoking via continuous dose–response associations between prenatal smoking and fetal growth using both intensive self-report measurements and biochemical verification methods moving forward to better advance the field. Similarly, if biochemical verification is not possible in clinical contexts, more intensive self-report measures may not only provide more accurate information regarding amount of smoking, but may also provide a context for clinical intervention.
Funding
This research was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number R01DA019632. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Interests
None declared.
Acknowledgments
We would like to thank the mothers and infants who participated in the study as well as the research staff who were responsible for conducting and coding the assessments. A special thanks to Dr. Amol Lele and the Women and Children’s Hospital of Buffalo for their collaboration in the data collection process.
References
- 1. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Department of Health and Human Services; 2014. [Google Scholar]
- 2. U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014. [Google Scholar]
- 3. Eiden RD, Homish GG, Colder CR, Schuetze P, Gray TR, Huestis MA. Changes in smoking patterns during pregnancy. Subst Use Misuse. 2013;48(7):513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pickett KE, Wakschlag LS, Dai L, Leventhal BL. Fluctuations of maternal smoking during pregnancy. Obstet Gynecol. 2003;101(1):140–147. [DOI] [PubMed] [Google Scholar]
- 5. Pickett KE, Kasza K, Biesecker G, Wright RJ, Wakschlag LS. Women who remember, women who do not: a methodological study of maternal recall of smoking in pregnancy. Nicotine Tob Res. 2009;11(10):1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. U.S. Department of Health and Human Services. Women and Smoking: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. [Google Scholar]
- 7. Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735–744. [DOI] [PubMed] [Google Scholar]
- 8. Marufu TC, Ahankari A, Coleman T, Lewis S. Maternal smoking and the risk of still birth: systematic review and meta-analysis. BMC Public Health. 2015;15(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JPet al. , eds. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press, Inc; 1992:41–72. [Google Scholar]
- 10. Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol. Addict. Behav. 1998;12(2):101–112. [Google Scholar]
- 11. Gray TR, Shakleya DM, Huestis MA. A liquid chromatography tandem mass spectrometry method for the simultaneous quantification of 20 drugs of abuse and metabolites in human meconium. Anal Bioanal Chem. 2009;393(8):1977–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gray TR, Eiden RD, Leonard KE, Connors G, Shisler S, Huestis MA. Nicotine and metabolites in meconium as evidence of maternal cigarette smoking during pregnancy and predictors of neonatal growth deficits. Nicotine Tob Res. 2010;12(6):658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arbuckle JL. Amos (Version 23.0)[Computer Program]. Chicago, IL: IBM SPSS; 2014. [Google Scholar]
- 14. La Merrill M, Stein CR, Landrigan P, Engel SM, Savitz DA. Prepregnancy body mass index, smoking during pregnancy, and infant birth weight. Ann Epidemiol. 2011;21(6):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyer S, Raisig A, Gortner L, Ong MF, Bücheler M, Tutdibi E. In utero tobacco exposure: the effects of heavy and very heavy smoking on the rate of SGA infants in the Federal State of Saarland, Germany. Eur J Obstet Gynecol Reprod Biol. 2009;146(1):37–40. [DOI] [PubMed] [Google Scholar]
- 16. Olsen J. Cigarette smoking in pregnancy and fetal growth. Does the type of tobacco play a role? Int. J. Epidemiol. 1992;21(2):279–284. [DOI] [PubMed] [Google Scholar]
- 17. Vardavas CI, Chatzi L, Patelarou E, et al. Smoking and smoking cessation during early pregnancy and its effect on adverse pregnancy outcomes and fetal growth. Eur J Pediatr. 2010;169(6):741–748. [DOI] [PubMed] [Google Scholar]
- 18. Jaddoe VW, Verburg BO, de Ridder MA, et al. Maternal smoking and fetal growth characteristics in different periods of pregnancy: the generation R study. Am J Epidemiol. 2007;165(10):1207–1215. [DOI] [PubMed] [Google Scholar]
- 19. Papoz L, Eschwege E, Pequignot G, Barrat J, Schwartz D. Maternal smoking and birth weight in relation to dietary habits. Am J Obstet Gynecol. 1982;142(7):870–876. [DOI] [PubMed] [Google Scholar]
- 20. Gilman SE, Abrams DB, Buka SL. Socioeconomic status over the life course and stages of cigarette use: initiation, regular use, and cessation. J Epidemiol Commun Health. 2003;57(10):802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]