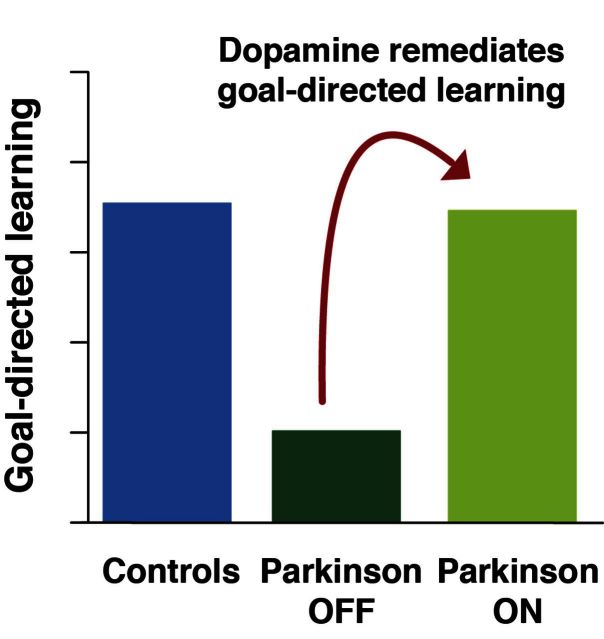
Patients with Parkinson’s disease show impaired reinforcement learning, but the exact nature of this deficit and the role of dopamine remain unclear. Using computational methods, Sharp et al. show that patients with Parkinson’s disease have a specific model-based, i.e. goal-directed, learning deficit that can be completely remediated by dopamine replacement.
Keywords: reinforcement learning, Parkinson’s disease, dopamine, model-free, model-based
Patients with Parkinson’s disease show impaired reinforcement learning, but the exact nature of this deficit and the role of dopamine remain unclear. Using computational methods, Sharp et al. show that patients with Parkinson’s disease have a specific model-based, i.e. goal-directed, learning deficit that can be completely remediated by dopamine replacement.
Abstract
Patients with loss of dopamine due to Parkinson’s disease are impaired at learning from reward. However, it remains unknown precisely which aspect of learning is impaired. In particular, learning from reward, or reinforcement learning, can be driven by two distinct computational processes. One involves habitual stamping-in of stimulus-response associations, hypothesized to arise computationally from ‘model-free’ learning. The other, ‘model-based’ learning, involves learning a model of the world that is believed to support goal-directed behaviour. Much work has pointed to a role for dopamine in model-free learning. But recent work suggests model-based learning may also involve dopamine modulation, raising the possibility that model-based learning may contribute to the learning impairment in Parkinson’s disease. To directly test this, we used a two-step reward-learning task which dissociates model-free versus model-based learning. We evaluated learning in patients with Parkinson’s disease tested ON versus OFF their dopamine replacement medication and in healthy controls. Surprisingly, we found no effect of disease or medication on model-free learning. Instead, we found that patients tested OFF medication showed a marked impairment in model-based learning, and that this impairment was remediated by dopaminergic medication. Moreover, model-based learning was positively correlated with a separate measure of working memory performance, raising the possibility of common neural substrates. Our results suggest that some learning deficits in Parkinson’s disease may be related to an inability to pursue reward based on complete representations of the environment.
Introduction
It is widely accepted that phasic dopamine signals in the striatum play a critical role in learning to update actions based on outcomes, often referred to as reward or reinforcement learning (Houk et al., 1995; Schultz et al., 1997). Patients with Parkinson’s disease have a severe striatal dopamine deficit, offering a test of the necessary role of striatal dopamine in learning in humans. Consistent with the role of striatal dopamine in reinforcement learning, patients with Parkinson’s disease have been shown to be impaired at reinforcement learning tasks (Knowlton et al., 1996; Frank et al., 2004; Shohamy et al., 2004). However, it is becoming increasingly apparent that learning from reward is not a unitary process, and in particular that some facets of it appear to be heavily dependent on prefrontal, executive processes such as working memory (Dickinson and Balleine, 2002; Balleine and O'Doherty, 2010; Collins and Frank, 2012; Otto et al., 2013a, b, 2015). This raises the question: what specific mechanisms of reinforcement learning are impaired in Parkinson’s disease? Moreover, do the learning impairments specifically relate to the striatal dopaminergic deficit (Braak et al., 2003; Kordower et al., 2013) or do extra-striatal effects of the disease (Braak et al., 2003; O'Callaghan et al., 2014; Pereira et al., 2014) also (or instead) contribute? Addressing these questions has important implications both for understanding the effects of dopamine treatment, as well as for understanding to what extent early cognitive deficits of Parkinson’s disease, such as planning and multi-tasking, may share a common substrate with reward learning.
A classic dichotomy exists between two dissociable sorts of instrumental behaviours, known as habits and goal-directed actions (Dickinson and Balleine, 2002). These are often distinguished using post-training reward-devaluation procedures, with habits (but not goal-directed actions) being characteristically insensitive to devaluation. Recent advances in computational neuroscience have led to more specific hypotheses about how these two classes of behaviours are acquired, connecting these psychological categories to the computational neuroscience of learning and permitting researchers to link these computational mechanisms (such as the reinforcing influence of reward) to measurable neural (e.g. dopamine neuron firing) or behavioural (e.g. animal and human choices) outcomes during learning (Dolan and Dayan, 2013). Altogether, it is now widely recognized that reinforcement learning can be driven by two separate but concurrent processes with distinct neural substrates, with each relying on different parts of frontal cortex and striatum (Balleine and O'Doherty, 2010; Daw et al., 2011).
The first process relies on reward prediction errors carried by phasic dopamine responses, which stamp in stimulus-response associations in the striatum, consistent with the predominant hypothesis for a dopaminergic role in reinforcement learning (Schultz et al., 1997). Computationally, this account is known as ‘model-free’ learning because preferences are formed through direct experience rather than through an understanding of a model of the environment. This form of learning is hypothesized to support the learning of habits (Sutton and Barto, 1998; Daw et al., 2005). Model-free learning is accompanied by a second, dissociable mechanism for ‘model-based’ learning, which integrates feedback with knowledge of a model of the environment. The ‘model’ of the environment is assumed to comprise multiple action-outcome (or, in computational terms, state-action-state) associations that represent the often-complex map of cues and actions that ultimately lead to reward. In contrast to the reflexively reinforced stimulus-response associations of model-free learning, in model-based learning, the value of candidate actions is computed more constructively from the combination of these associations and the values of the resulting outcomes.
Model-based learning has been proposed to be the computational implementation for learning goal-directed actions. Its neural substrates are less well-defined than those of model-free learning but are believed to include parts of the prefrontal cortex as well as the striatum (Daw et al., 2011; Smittenaar et al., 2013; Deserno et al., 2015). Although model-based learning is mechanistically quite distinct from model-free learning—in particular, it does not in its standard form make use of a dopaminergic reward prediction error—it may also be sensitive to dopaminergic function. For instance, dopamine is known to support working memory through innervation of the prefrontal cortex (Sawaguchi and Goldman-Rakic, 1991), which could in turn support the manipulation of action-outcome and outcome-value representations in computing action values. Indeed, model-based learning has been shown to be sensitive to core components of executive function, such as working memory and cognitive control (Otto et al., 2013a, b, 2015).
It has generally been assumed that patients with Parkinson’s disease are specifically impaired at habitual learning of stimulus-response associations (Knowlton et al., 1996; Shohamy et al., 2004), related to impaired reward signalling in the striatum. However, although this interpretation of reinforcement learning deficits in patients with Parkinson’s disease is often appealed to as key evidence supporting the hypothesis that dopaminergic reward prediction errors drive (model-free) reinforcement learning (Shohamy and Daw, 2014), most existing data are actually ambiguous on this point. This is in large part because previous work has not effectively dissociated the contributions from model-free versus model-based learning. Only one recent study has addressed the (related) habit versus goal-directed learning dichotomy in Parkinson’s disease. Using an instrumental conflict task with a devaluation procedure (de Wit et al., 2011), they found that, contrary to predictions, patients had preserved habitual stimulus-response learning. Based on these surprising findings, and the fact that Parkinson’s disease also affects extra-striatal executive functions, which may contribute to model-based learning, such as working memory (Lange et al., 1992; Owen et al., 1997; Lewis et al., 2005; Beato et al., 2008), we applied computational methods to more closely examine whether patients with Parkinson’s disease have a model-based deficit and, critically, whether this deficit is dopamine-mediated.
To address these questions we used a task previously shown to successfully distinguish model-free from model-based learning in both healthy and patient populations (Daw et al., 2011; Eppinger et al., 2013; Voon et al., 2015) and tested patients with Parkinson’s disease in a within-subject design, ON versus OFF dopaminergic medication.
Materials and methods
Participants
Twenty-two patients with idiopathic Parkinson’s disease (13 males, mean age 61 ± 7 years) (diagnosed as per UK Brain Bank criteria) were recruited either from the Center for Parkinson’s Disease and other Movement Disorders at the Columbia University Medical Center or from the Michael J Fox Foundation Trial Finder website. Twenty-one healthy control participants (11 males, mean age 63 ± 7 years) were recruited from the local community. All participants provided informed consent and were paid $12/h for their participation. The study was approved by the Institutional Review Board of Columbia University.
Patients were in the mild-to-moderate stage of disease [mean Unified Parkinson’s Disease Rating Scale (UPDRS) OFF 19 ± 6, as examined by a movement disorders neurologist, disease duration: 2–14 years; Table 1]. All patients had been receiving levodopa treatment for at least 6 months (mean total daily levodopa dose 522 ± 235 mg, Supplementary Table 2). Nine patients were additionally taking dopamine agonists.
Table 1.
Demographic and clinical characteristics of participants
| Parkinson’s patients | Healthy controls | P-value | |
|---|---|---|---|
| Age | 61.1 (6.5) | 62.8 (6.8) | 0.4 |
| Sex (male) | 13/22 | 11/21 | 0.7 |
| Education | 17 (2) | 16 (3) | 0.3 |
| MoCA | 28.5 (1.3) | 28.9 (0.5) | 0.1 |
| F-A-S fluency | 49 (17) | 52 (13) | 0.5 |
| Trails B | 86 (43) | 66 (24) | 0.08 |
| Stroopa | 61 (19) | 64 (17) | 0.6 |
| Digit Span totalb | 12.9 (2.4) | 13.5 (2.0) | 0.4 |
| Geriatric Depression Scale | 3.0 (2.5) | 1.1 (1.4) | 0.01 |
| Starkstein Apathy Scale | 24 (6) | 22 (5) | 0.2 |
| BIS-11 | 61 (9) | 54 (7) | 0.001 |
| UPDRS OFF | 18.6 (6.0) | – | – |
| UPDRS ONc | 13.3 (6.0) | – | – |
| Disease duration | 6.8 (2.9) | – | – |
| Daily levodopa dose (mg) | 522 (235) | – | – |
| LEED (mg)d | 715 (273) | – | – |
Table shows mean (SD). MoCA = Montreal Cognitive Assessment; BIS-11 = Barratt Impulsiveness Scale; UPDRS = Unified Parkinson’s Disease Rating Scale–Part III; LEED = Levodopa equivalent dose. P-values are based on t-tests.
aStroop score calculated as difference between colour and interference stages.
bDigit span total = sum of forward and backward span.
cUPDRS ON was significantly lower than UPDRS OFF (P < 0.001).
dLEED includes levodopa, dopamine agonists, amantadine, monoamine oxidase inhibitors and catechol-O-methyl transferase inhibitors.
Participants completed a battery of neuropsychological tests focusing on executive function [Montreal Cognitive Assessment (MoCA), Trails A and B, Stroop, Digit Span and Phonemic word fluency] and psychiatric domains [Geriatric Depression Scale, Starkstein Apathy Scale, Barratt Impulsiveness Scale-11 (BIS-11)] (Table 1). Participants had no history of other major neurological or psychiatric disease. Participants with dementia (based on MoCA < 26) were excluded.
The patients with Parkinson’s disease and controls did not differ in age, sex distribution, education, and general measures of cognition (Table 1). As expected (Weintraub et al., 2006), patients scored higher on the Geriatric Depression Scale (P = 0.01) and on the BIS-11 (P = 0.001).
Procedure
Participants were tested in two sessions, in counterbalanced order: OFF, after an overnight withdrawal (>16 h, which is at least 10 half-lives for the carbidopa-levodopa and two half-lives for the dopamine agonists) of all their Parkinson’s medications; and ON, 1–1.5 h after their usual dose of levodopa (mean 151 ± 61 mg). Patients who were also on regular doses of dopamine agonists did not receive them for the ON session because we wanted to isolate the effects of levodopa, which most closely mimics normal synaptic release (Pothos et al., 1996). As expected, UPDRS-III scores were significantly lower when measured ON than OFF (P < 0.0001). Healthy control participants were also tested twice to control for practice effects. The interval between sessions ranged from 1 to 3 weeks.
Task
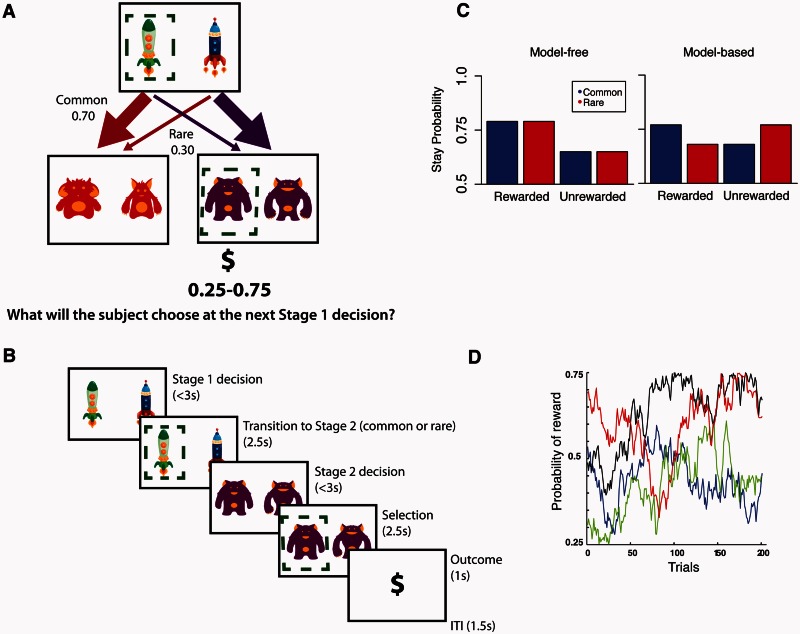
We used a reward learning task with a design feature that allowed for dissociation of model-free and model-based contributions to behaviour (Fig. 1; see also Daw et al., 2011). Specifically, each trial proceeded in two stages, each of which required a decision. The first stage decision was between two spaceships destined for two different planets; this choice determined which options were presented at the second stage. The second stage represented which planet the participant visited and required a decision between two aliens on the planet. The choice of alien at this second stage led to a possible ‘space gold’ reward, with each piece of gold worth $0.10. The winnings were dispensed at the end of the experiment. Because the transition from the first stage choice to the second stage planet was stochastic (Fig. 1), first stage choices could be dissociated from the second stage choices that determined their ultimate reward. This allowed dissociating two learning strategies, either model-free (in which second stage rewards are associated directly with the preceding first stage decision), or model-based (in which second stage rewards and knowledge of the probabilistic structure of the task are used to infer the value of the first stage outcomes in terms of the second stage planets to which they predominantly lead). Participants were pressed to learn continually because the reward probabilities associated with each of the four second stage options were determined by independently drifting Gaussian random walks [standard deviation (SD) = 0.025] with a lower boundary of 0.25 probability of reward and an upper boundary of 0.75, such that probability of reward from any particular second stage option changed very slowly from trial to trial (Fig. 1D).
Figure 1.
Trial structure, logic and simulated data for the task. (A) Task design. At Stage 1, participants chose between two spaceships. This choice determined the transition to the next stage according to a fixed probability scheme: each spaceship was predominantly associated with one or the other Stage 2 states (i.e. planets) and led there 70% of the time. At Stage 2, participants chose one of two aliens to discover if they would be rewarded. Each alien was associated with a probabilistic reward (range: 0.25–0.75) that changed gradually over time as determined by a Gaussian random walk. (B) Timing of stages within a single trial. (C) Simulated data. Model-based and model-free strategies predict different patterns of behaviour. A pure model-free learner (left) shows only a main effect of reward, choosing to stay with the same Stage 1 choice after a reward irrespective of the transition type (common versus rare) associated with that reward on the last trial. Conversely, a pure model-based learner (right) is influenced by the interaction between reward and transition type on the last trial. For instance, after winning on the planet less commonly visited (rare transition) a participant will be less likely to stay with the same spaceship and will instead pick the alternate spaceship in order to maximize chances of returning to that same planet. Previous studies show that healthy participants use a mixture of both model-free and model-based processes (Daw et al., 2011; Eppinger et al., 2013; Otto et al., 2013b). (D) Example of one of the sets of Gaussian random walks that determined the probability of reward at each of the four Stage 2 options.
Analysis
Stay-switch behaviour
Model-free and model-based learning can be dissociated by examining how participants adjust their first stage choices in response to feedback. Consider what happens following a trial in which a first stage choice is followed by a rare transition (to the planet less likely to follow that choice) and then reward. On the subsequent trials, repeating the same first stage choice (i.e. ‘staying’ with the same spaceship) indicates model-free learning, guided only by reward, and insensitivity to the probability structure (rare versus common transition, i.e. the ‘model’). In contrast, making the alternate first stage choice (i.e. switching to the other spaceship commonly associated with the planet that produced the reward) indicates model-based learning because the effect of reward is mediated by the probability structure (Fig. 1B).
Accordingly, to quantify the contributions of model-free and model-based learning and the effects of disease and medication state on these contributions, we conducted a mixed effects logistic regression where the dependent variable was the probability of repeating the same first stage choice (staying = 1, switching = 0) on each trial. [Such a regression represents a simplified limiting case of fitting a more elaborate reinforcement learning model incorporating model-free and model-based components (Daw et al., 2011); and is more robustly estimated for the purpose of studying within- and between-subject individual differences.] The basic within-subject (random-effect) binary explanatory variables were reward at the preceding trial (reward = 1, no reward = −1: the model-free effect, MF), transition at preceding trial (common = 1, rare = −1), and the interaction of reward and transition (reward × transition: the model-based effect, MB). We also included two binary covariates, each fully interacted with all the preceding effects: the between-subject effect of disease [disease: Parkinson’s disease (PD) = 0, control = 1], and the within-subject effect of medication state (med: ON = 1, OFF = 0, where all controls were considered OFF).
This model defines the PD-OFF as the baseline group allowing comparison to PD-ON (such that the MB × med interaction reflects the effect of medications on model-based learning among disease, and the reward × med interaction reflects the effect of medication on model-free learning) and to Controls (where similarly, the MB × disease and MF × disease terms reflect the difference in model-based and model-free learning, respectively between controls and PD-OFF) (see Supplementary material for additional details). Statistical analyses were performed in R (R Development Core and Team, 2015), using the lme4 package (Bates et al., 2013).
Finally, we were also interested in the association between model-based contribution to learning and certain clinical variables. Because of previous findings indicating a relationship between model-based learning and executive function (Otto et al., 2013a)—working memory in particular (Eppinger et al., 2013; Otto et al., 2013b; Smittenaar et al., 2013)—we tested for this association among our participants using total digit span score as a measure of working memory. We tested working memory only once in this study, and in the case of the patients with Parkinson’s disease, it was tested during the ON session in 17/22 patients, and during the OFF session in the remaining 5/22. Because we were interested in memory capacity as a predictor of model-based learning, we included only the 17/22 patients whose memory was tested while ON in these models. We ran logistic regressions as detailed above but for each group separately (controls, patients with Parkinson’s disease ON, and patients OFF) and included mean-centred digit span score as a fully interacted covariate. Running all the previous analyses with this subgroup of 17 patients yielded the same results, with no new differences in demographic or cognitive measures (Supplementary Table 3). Similarly, because it has been suggested that motor symptom severity could reflect striatal dopamine deficiency (Broussolle et al., 1999), we tested for an association between UPDRS-III score and model-based contribution (we used the UPDRS-III score measured while OFF medications to model this association because it best reflects motor symptom severity). We ran a separate logistic regression in the patients with Parkinson’s disease ON and OFF as above and included the mean-centred UPDRS-III score as a fully interacted covariate.
Results
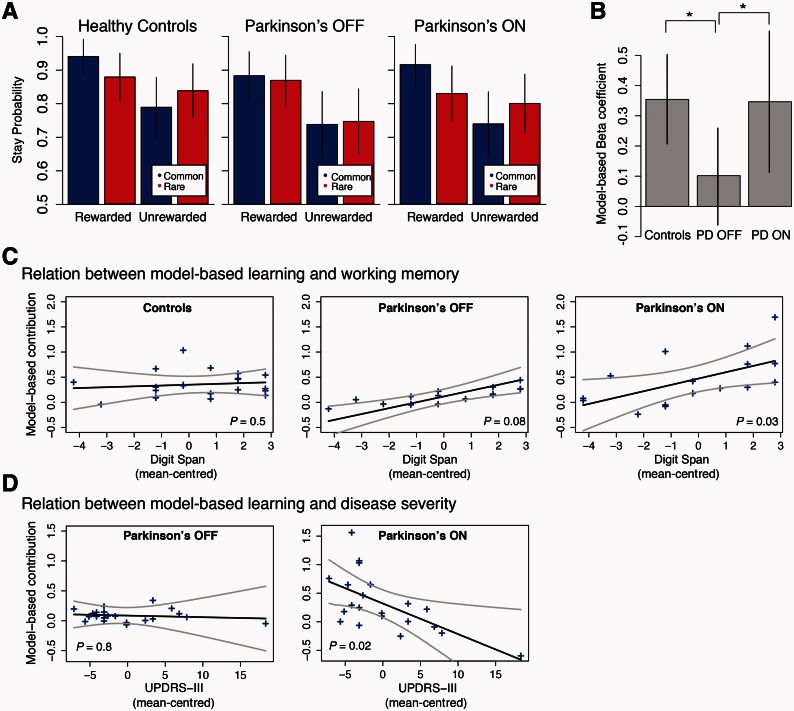
The patterns of switching as a function of reward are shown, for each group and medication condition, in Fig. 2. The results for both healthy controls and patients with Parkinson’s disease ON appear similar to those from healthy populations in previous studies, in that effects of both reward and transition are visible. However, patients tested OFF medication show a qualitatively different pattern of results. Quantifying these effects statistically, we found that patients with Parkinson’s disease OFF showed a significant effect of reward on their behaviour indicating model-free (MF) learning (P < 0.0001) but no significant effect of reward × transition (i.e. MB, P = 0.2) indicating no detectable contribution of model-based learning. In comparison, the patients with Parkinson’s disease ON showed a similar model-free contribution (MF × med: P = 0.3 indicating they are not different than patients OFF) but additionally showed a significantly greater contribution of model-based learning (MB × med: P = 0.04). The controls were also not different than patients with Parkinson’s disease OFF with respect to model-free learning (MF × disease: P = 1.0) but, similarly to the patients ON, showed a significantly greater contribution of model-based learning (MB × disease: P = 0.02) than the patients with Parkinson’s disease OFF. The full regression specification and coefficient estimates are reported in Table 2, and the distribution and group-level variability of the subject-specific model-free and model-based coefficients are reported in Supplementary Fig. 1 and Supplementary Table 4, respectively. In a second analysis, we fit the participants’ choices with a more elaborate computational model of model-based and model-free learning, producing largely consistent results (Supplementary material and Supplementary Table 1).
Figure 2.
Effect of Parkinson’s disease and dopaminergic medications on model-based learning. (A) Probability of repeating the same first stage choice as a function of the transition on the previous trial (common versus rare) and the outcome (rewarded versus unrewarded). Both controls and patients ON showed an effect of the interaction between reward and transition, the signature of model-based learning. In contrast, the patients OFF showed no effect of transition probability, they showed only model-free learning. (B) Comparison of model-based contribution to behaviour across groups as measured by the reward × transition interaction coefficient in the logistic regression (the model-based beta coefficient). Controls and patients ON showed comparable levels of model-based behaviour, whereas patients OFF showed no significant model-based contribution to their behaviour. (C) Relation between model-based learning and working memory: individual participants’ model-based effect sizes (arbitrary units) are plotted separately for control participants and for patients OFF and ON levodopa against mean-centred total digit span score. The regression line is computed from the group-level effect of working memory. There was a significant positive effect of working memory on expression of model-based learning in the Parkinson’s patients ON levodopa only. (D) Relation between model-based learning and disease severity—as indexed by UPDRS-III measured when OFF medications—on model-based learning: the regression line is computed from the group-level effect of UPDRS-III, where a higher score indicates more severe symptoms. There was a significant negative effect of motor symptom severity on expression of model-based learning in the patients ON medication. Error bars represent (A) SEM and (B) 95% confidence intervals. Grey lines indicate two standard errors, estimated from the group-level mixed effects regression (C and D). P-values are for the regression coefficient of the group-level interaction between the degree of model-based contribution to behaviour and either working memory or UPDRS-III in the respective participant groups (C and D).
Table 2.
Results of the logistic regression analysis with the between participants factor Disease and the within-participant factor Medication
| Predictor | Estimate (SE) | P-value |
|---|---|---|
| Intercept | 1.97 (0.23) | <0.001 |
| Reward (i.e. MF) | 0.61 (0.13) | <0.001 |
| Transition type | 0.04 (0.07) | 0.6 |
| Reward × Transition (i.e. MB)a | 0.10 (0.08) | 0.2 |
| Disease | 0.42 (0.33) | 0.2 |
| Med | 0.05 (0.20) | 0.8 |
| MF × Disease | 0.01 (0.19) | 0.97 |
| MF × Med | −0.16 (0.14) | 0.3 |
| Transition × Disease | 0.15 (0.09) | 0.1 |
| Transition × Med | 0.07 (0.09) | 0.5 |
| MB × Diseaseb | 0.25 (0.11) | 0.02 |
| MB × Medc | 0.24 (0.12) | 0.04 |
aThe Reward × Transition interaction reflects the model-based behaviour in the PD OFF. Here, the PD OFF show no significant contribution of model-based learning.
bThe Reward × Transition × Disease interaction reflects the model-based behaviour in the healthy controls who show a significant contribution of model-based learning compared to the PD OFF.
cThe Reward × Transition × Med interaction reflects the model-based behaviour in the PD ON who show a significant contribution of model-based learning compared to the PD OFF.
MF = model-free learning; MB = model-based learning; PD = Parkinson’s disease.
Analysis of covariates revealed that better working memory was associated with a higher model-based contribution in patients with Parkinson’s disease ON (P = 0.03), but did not reach significance in patients OFF (P = 0.08) or controls (P = 0.5) (Fig. 2C). There was no effect of working memory on model-free learning in either the patients or controls. We also found that worse motor function (i.e. a higher UPDRS-III measured OFF, reflecting worse disease severity) was associated with less model-based learning in patients with Parkinson’s disease ON (P = 0.02) but not in patients OFF (P = 0.8) (Fig. 2D). The measures of mood and personality that differed between patients and controls (Geriatric Depression Scale and BIS-11) were not associated with model-based contribution to learning.
Discussion
Habit and goal-directed learning depend on neighbouring dopamine-rich striatal regions (Yin and Knowlton, 2006), but the influential prediction error theory of dopamine is thought to account only for the role of dopamine signals in habitual, model-free learning (Schultz et al., 1997). Motivated by these observations, we sought to directly test whether dopamine also plays a role in model-based learning, and whether Parkinson’s disease is primarily characterized by a deficit in model-free learning—as has often been assumed, or by a model-based learning deficit. We found that dopamine-deficient Parkinson’s disease patients had a model-based learning deficit that was fully restored by dopamine replacement and was associated with poor working memory performance. Surprisingly, we also found that model-free learning was intact in patients and unaffected by medication state, which is consistent with one previous study showing that patients were able to form stimulus-response associations (de Wit et al., 2011).
Model-based learning has been shown to rely on frontostriatal networks. Neuroimaging studies in humans have shown that the caudate, medial orbitofrontal and dorsomedial prefrontal cortex are associated with model-based learning (Daw et al., 2011; Doll et al., 2012; Voon et al., 2015). These regions are either directly or indirectly modulated by dopamine, which could account for the strong association between model-based learning and dopamine that we observed.
Our findings are in line with previous studies showing that levodopa administered to healthy, young adults increased model-based learning (Wunderlich et al., 2012). However, because the neurobiological effect of levodopa in healthy brains is unknown, it has been unclear how or where dopamine exerts this positive effect on model-based learning. One possibility is that the modulatory effect occurs in the striatum, and that model-based learning relies on a dopaminergic reward prediction error signal shared with model-free learning. Although such prediction errors are not used in standard model-based algorithms, there are variants that might explain model-based behaviours while sharing this stage (Doll et al., 2012; Daw and Dayan, 2014). However, this interpretation is difficult to reconcile with the fact that model-free learning was preserved in patients withdrawn from dopaminergic medication.
Another possibility is that levodopa restored model-based learning through direct effects in prefrontal cortex. Indeed, prefrontal cortex atrophy, evident even in the early stages of Parkinson’s disease (Tessa et al., 2014), correlates with learning deficits (O'Callaghan et al., 2013); and frontal-based executive dysfunction is also well described in the early stages of Parkinson’s disease (Lange et al., 1992; Cools et al., 2002; Ko et al., 2013; Pereira et al., 2014). Furthermore, in patients, levodopa has positive effects on prefrontal cognitive functions such as working memory (Lewis et al., 2005), planning (Lange et al., 1992), generalization of learning (Shiner et al., 2012), and task-switching (Cools et al., 2001; Rutledge et al., 2009). Finally, improved model-based learning on levodopa could be due to an effect on motivation through modulation of tonic rather than phasic dopamine signals (Niv, 2007; Beierholm et al., 2013).
In contrast to the clear effect on model-based learning, we did not find a model-free impairment in patients with Parkinson’s disease nor any effect of dopaminergic medications on model-free learning. This is surprising given the wealth of studies examining learning in patients with Parkinson’s disease, where deficits are interpreted for the most part as impairments in habitual, stimulus-response learning (Knowlton et al., 1996; Frank et al., 2004; Shohamy et al., 2004). However, the learning tasks typically used in these studies might additionally (or instead) be measuring contributions from model-based learning. For instance, participants may develop a rule-based approach and rely on explicit processes such as working memory (Foerde et al., 2006; Collins and Frank, 2012). A more recent study of learning in patients with Parkinson’s disease used an instrumental conflict task modelled on those used to study habit learning in rodents, and also showed that patients with Parkinson’s disease had intact stimulus-response habit learning, which relates to model-free learning (de Wit et al., 2011). Though they additionally interrogated goal-directed learning with an added devaluation procedure, they did not find a significant effect of dopaminergic medications or disease on goal-directed learning.
Of course, as with any negative result, the lack of effects reported here on model-free learning must be interpreted with caution. The current study cannot rule out the possibility that an impairment of model-free learning also exists in the patients with Parkinson’s disease but is not adequately operationalized by our task. For instance, although we detect substantial model-free learning with a level of individual variability comparable to that for model-based (Supplementary Fig. 1), it is possible that this sort of learning might itself be heterogeneous in the brain, with the sort hypothesized to involve dopaminergic action in striatum not dominating our measure. Indeed, the link between the computational mechanism of model-free learning and the psychological category of habits is also more controversial than that between model-based and goal-directed learning (Dezfouli and Balleine, 2012), though on that account (according to which habitual behaviour and seemingly model-free behaviour on the sequential decision task used here are proposed to arise from a common choice mechanism which is ultimately model-based) it is not clear why we see differential effects of disease and medication on model-based and model-free choices. It is also possible that model-free learning is a very robust cognitive function, in keeping with its role as a faster but less accurate system (Keramati et al., 2011), and thus that model-free learning, but not model-based, can withstand a certain degree of dopamine deficiency. Future work will be required to more finely probe the relationship, if any, between model-free learning, habits, and striatal dopaminergic function.
Our finding, of an association between model-based learning and working memory capacity in patients with Parkinson’s disease, helps bridge two seemingly independent constructs of impaired cognition in Parkinson’s disease—reward learning and executive function. Previous studies have focused on dissociating these domains on the basis of hypothesized separate neural substrates for each—striatal dopamine for reward learning (Schultz et al., 1997; Frank et al., 2004; Schonberg et al., 2010), and frontostriatal (Cools et al., 2002; Lewis et al., 2003, 2005; Monchi et al., 2007; Ko et al., 2013; Nagano-Saito et al., 2014) and extra-striatal non-dopaminergic networks for executive function (Hilker et al., 2005; Weintraub et al., 2010; Ye et al., 2014, 2015). Our finding that increased working memory capacity was associated with a greater contribution of model-based learning in patients with Parkinson’s disease but not in healthy controls could suggest that working memory is recruited as a compensatory mechanism to support model-based learning. Similarly, in healthy younger adults, better working memory predicts model-based performance (Eppinger et al., 2013; Otto et al., 2013b; Smittenaar et al., 2013). However, similar to our results here, the effect of working memory most often became apparent only under challenge; in particular, better working memory predicted robustness to stress or transcranial magnetic stimulation. In a study of ageing in healthy participants, the relationship between working memory and model-based performance was age-related: while present in younger adults, working memory was not related to model-based performance among healthy older adults (Eppinger et al., 2013) (again, consistent with our results). More generally, normal prefrontal cortex function (Smittenaar et al., 2013) and executive functioning (Otto et al., 2013a, 2015) are necessary for model-based learning. These findings support the idea that the neural substrates underlying reward learning and executive function are co-dependent rather than independent. A main limitation of this study is that we cannot directly comment on the neural substrates of the model-based learning deficit or on the likely crucial role that dopamine plays in modulating striatal-prefrontal cortex connections to support model-based learning.
In conclusion, our findings demonstrate that learning deficits in Parkinson’s are not merely a matter of reduced reward prediction but, rather, are related to an inability to pursue reward based on more complete representations of the environment. These findings emerged from a computational characterization of these two forms of learning and are generally consistent with previous results from a different task (de Wit et al., 2011). Together, these findings challenge the general assumption that patients with Parkinson’s disease cannot form stimulus-response associations and therefore cannot form habits (Knowlton et al., 1996). This is congruent with observations of patients with Parkinson’s disease who function well in simple environments where they maintain an ability to respond to informative cues, but have marked difficulties with executive functions such as multi-tasking and planning, which depend on the building and maintenance of a model of the environment (Brown and Marsden, 1991). An important goal for future research, especially in the context of treatment of cognitive symptoms in Parkinson’s disease, is to further understand the interdependence of striatal learning processes and executive function, and to identify where exogenous dopamine is exerting its positive effect on model-based learning so we can target treatments to these regions while avoiding possible detrimental effects of medications on others (Cools et al., 2001).
Supplementary Material
Acknowledgements
We thank Cate Hartley for the task and stimuli; Ross Otto, Bradley Doll, Katherine Duncan for assistance with analyses; Caroline Marvin, Kendall Braun and Peter Myers for help with participant testing and Stanley Fahn, Roy Alcalay and Cheryl Waters for help with patient recruitment. We also thank the patients and controls who participated in the study and the Clinical Trials Transportation Program for their assistance.
Glossary
Abbreviation
- UPDRS
Unified Parkinson’s Disease Rating Scale
Funding
This work was supported by National Institutes of Health R01DA038891 (D.S. and N.D.D.) and a University of British Columbia Clinician Investigator Award (M.E.S.).
Supplementary material
Supplementary material is available at Brain online.
References
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 2010; 35: 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, et al. Linear mixed-effects models using ‘Eigen’ and S4. Version 1.0. 2013. [Google Scholar]
- Beato R, Levy R, Pillon B, Vidal C, du Montcel ST, Deweer B, et al. Working memory in Parkinson's disease patients: clinical features and response to levodopa. Arq Neuropsiquiatr 2008; 66: 147–51. [DOI] [PubMed] [Google Scholar]
- Beierholm U, Guitart-Masip M, Economides M, Chowdhury R, Duzel E, Dolan R, et al. Dopamine modulates reward-related vigor. Neuropsychopharmacology 2013; 38: 1495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003; 24: 197–211. [DOI] [PubMed] [Google Scholar]
- Broussolle E, Dentresangle C, Landais P, Garcia-Larrea L, Pollak P, Croisile B, et al. The relation of putamen and caudate nucleus 18F-Dopa uptake to motor and cognitive performances in Parkinson's disease. J Neurol Sci 1999; 166: 141–51. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Dual task performance and processing resources in normal subjects and patients with Parkinson's disease. Brain 1991; 114 (Pt 1A): 215–31. [PubMed] [Google Scholar]
- Collins AG, Frank MJ. How much of reinforcement learning is working memory, not reinforcement learning? A behavioral, computational, and neurogenetic analysis. Eur J Neurosci 2012; 35: 1024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex 2001; 11: 1136–43. [DOI] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson's disease: the role of the prefrontal cortex revealed by PET. Brain 2002; 125: 584–94. [DOI] [PubMed] [Google Scholar]
- Daw ND, Dayan P. The algorithmic anatomy of model-based evaluation. Philos Trans R Soc Lond B Biol Sci 2014; 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans' choices and striatal prediction errors. Neuron 2011; 69: 1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci 2005; 8: 1704–11. [DOI] [PubMed] [Google Scholar]
- de Wit S, Barker RA, Dickinson AD, Cools R. Habitual versus goal-directed action control in Parkinson disease. J Cogn Neurosci 2011; 23: 1218–29. [DOI] [PubMed] [Google Scholar]
- Deserno L, Huys QJM, Boehme R, Buchert R, Heinze H-J, Grace AA, et al. Ventral striatal dopamine reflects behavioral and neural signatures of model-based control during sequential decision making. Proc Natl Acad Sci USA 2015; 112: 1595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfouli A, Balleine BW. Habits, action sequences and reinforcement learning. Eur J Neurosci 2012; 35: 1036–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Balleine B. The role of learning in the operation of motivational systems. In: Gallistel CR, editor. Steven’s handbook of experimental psychology: learning, motivation and emotion. 3rd ed. New York: John Wiley & Sons; 2002. p. 497–534. [Google Scholar]
- Dolan RJ, Dayan P. Goals and Habits in the Brain. Neuron 2013; 80: 312–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Simon DA, Daw ND. The ubiquity of model-based reinforcement learning. Curr Opin Neurobiol 2012; 22: 1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Walter M, Heekeren HR, Li S-C. Of goals and habits: age-related and individual differences in goal-directed decision-making. Front Neurosci 2013; 7: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Knowlton BJ, Poldrack RA. Modulation of competing memory systems by distraction. Proc Natl Acad Sci USA 2006; 103: 11778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science 2004; 306: 1940–3. [DOI] [PubMed] [Google Scholar]
- Hilker R, Thomas AV, Klein JC, Weisenbach S, Kalbe E, Burghaus L, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology 2005; 65: 1716–22. [DOI] [PubMed] [Google Scholar]
- Houk J, Adams J, Barto A. A model of how the basal ganglia generates and uses neural signals that predict reinforcement. In: Houk J, Davis J, Beiser D, editors. Models of information processing in the basal ganglia. Cambridge: MIT Press; 1995. p. 249–70. [Google Scholar]
- Keramati M, Dezfouli A, Piray P. Speed/accuracy trade-off between the habitual and the goal-directed processes. PLoS Comput Biol 2011; 7: e1002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science 1996; 273: 1399–402. [DOI] [PubMed] [Google Scholar]
- Ko JH, Antonelli F, Monchi O, Ray N, Rusjan P, Houle S, et al. Prefrontal dopaminergic receptor abnormalities and executive functions in Parkinson's disease. Hum Brain Mapp 2013; 34: 1591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain 2013; 136: 2419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM. L-dopa withdrawal in Parkinson's disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology 1992; 107: 394–404. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson's disease. Neuropsychologia 2005; 43: 823–32. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson's disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci 2003; 23: 6351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson's disease during executive processing depends on striatal involvement. Brain 2007; 130: 233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A, Habak C, Mejia-Constain B, Degroot C, Monetta L, Jubault T, et al. Effect of mild cognitive impairment on the patterns of neural activity in early Parkinson's disease. Neurobiol Aging 2014; 35: 223–31. [DOI] [PubMed] [Google Scholar]
- Niv Y. Cost, benefit, tonic, phasic: what do response rates tell us about dopamine and motivation? Ann N Y Acad Sci 2007; 1104: 357–76. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C, Moustafa AA, de Wit S, Shine JM, Robbins TW, Lewis SJG, et al. Fronto-striatal gray matter contributions to discrimination learning in Parkinson's disease. Front Comput Neurosci 2013; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C, Shine JM, Lewis SJG, Hornberger M. Neuropsychiatric symptoms in Parkinson's disease: fronto-striatal atrophy contributions. Parkinsonism Relat Disord 2014; 20: 867–72. [DOI] [PubMed] [Google Scholar]
- Otto AR, Gershman SJ, Markman AB, Daw ND. The curse of planning: dissecting multiple reinforcement-learning systems by taxing the central executive. Psychol Sci 2013a; 24: 751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto AR, Raio CM, Chiang A, Phelps EA, Daw ND. Working-memory capacity protects model-based learning from stress. Proc Natl Acad Sci USA 2013b; 110: 20941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto AR, Skatova A, Madlon-Kay S, Daw ND. Cognitive control predicts use of model-based reinforcement learning. J Cogn Neurosci 2015; 27: 319–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Iddon JL, Hodges JR, Summers BA, Robbins TW. Spatial and non-spatial working memory at different stages of Parkinson's disease. Neuropsychologia 1997; 35: 519–32. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Svenningsson P, Weintraub D, Bronnick K, Lebedev A, Westman E, et al. Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology 2014; 82: 2017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos E, Desmond M, Sulzer D. L-3,4-dihydroxyphenylalanine increases the quantal size of exocytotic dopamine release in vitro. J Neurochem 1996; 66: 629–36. [DOI] [PubMed] [Google Scholar]
- R Development Core and Team. R: A language and environment for statistical computing. Version 3.1.3. 2015.
- Rutledge RB, Lazzaro SC, Lau B, Myers CE, Gluck MA, Glimcher PW. Dopaminergic drugs modulate learning rates and perseveration in Parkinson's patients in a dynamic foraging task. J Neurosci 2009; 29: 15104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science (New York, NY) 1991; 251: 947–50. [DOI] [PubMed] [Google Scholar]
- Schonberg T, O'Doherty JP, Joel D, Inzelberg R, Segev Y, Daw ND. Selective impairment of prediction error signaling in human dorsolateral but not ventral striatum in Parkinson's disease patients: evidence from a model-based fMRI study. Neuroimage 2010; 49: 772–81. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 1997; 275: 1593–9. [DOI] [PubMed] [Google Scholar]
- Shiner T, Seymour B, Wunderlich K, Hill C, Bhatia KP, Dayan P, et al. Dopamine and performance in a reinforcement learning task: evidence from Parkinson's disease. Brain 2012; 135: 1871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Daw N. Habits and reinforcement learning. In: Gazzaniga M, Mangun G, editors. Cognitive neurosciences. 5th ed Cambridge: MIT Press; 2014. p. 591–604. [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA, Poldrack RA. Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain 2004; 127: 851–9. [DOI] [PubMed] [Google Scholar]
- Smittenaar P, FitzGerald THB, Romei V, Wright ND, Dolan RJ. Disruption of dorsolateral prefrontal cortex decreases model-based in favor of model-free control in humans. Neuron 2013; 80: 914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement learning: an introduction. IEEE Trans Neural Netw 1998; 9: 1054. [Google Scholar]
- Tessa C, Lucetti C, Giannelli M, Diciotti S, Poletti M, Danti S, et al. Progression of brain atrophy in the early stages of Parkinson's disease: a longitudinal tensor-based morphometry study in de novo patients without cognitive impairment. Hum Brain Mapp 2014; 35: 3932–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Derbyshire K, Ruck C, Irvine MA, Worbe Y, Enander J, et al. Disorders of compulsivity: a common bias towards learning habits. Mol Psychiatry 2015; 20: 345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Mavandadi S, Mamikonyan E, Siderowf AD, Duda JE, Hurtig HI, et al. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology 2010; 75: 448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Oehlberg KA, Katz IR, Stern MB. Test characteristics of the 15-item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry 2006; 14: 169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich K, Smittenaar P, Dolan RJ. Dopamine enhances model-based over model-free choice behavior. Neuron 2012; 75: 418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, et al. Improving response inhibition in Parkinson's disease with atomoxetine. Biol Psychiatry 2015; 77: 740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, et al. Selective serotonin reuptake inhibition modulates response inhibition in Parkinson's disease. Brain 2014; 137: 1145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci 2006; 7: 464–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.