Abstract
This is a report of a single-institution prospective study evaluating the safety of a spot-scanning dedicated, small 360-degree gantry, synchrotron-based proton beam therapy (PBT) system. Data collection was performed for 56 patients with 59 treatment sites who received proton beam therapy at Hokkaido University Hospital between March 2014 and July 2015. Forty-one patients were male and 15 were female. The median age was 66 years. The primary lesion sites were prostate (n = 17), bone/soft tissue (n = 10), liver (n = 7), lung (n = 6), central nervous system (n = 5), colon (n = 2), pancreas (n = 2), kidney (n = 2) and others (n = 5). Chemotherapy was administered in 11 patients. The prescribed total dose was from 20 to 76 GyE (Radiobiological equivalent dose, RBE = 1.1), with the median dose of 65 GyE in 4 to 35 fractions. No PBT-related Common Terminology Criteria for Adverse Events Grade 4 or 5 toxicities were observed; the incidence of early PBT-related Grade 4 adverse events was 0% (95% confidence interval 0 to 6.38%). The most common Grade 3 toxicities were hematologic toxicity (12.5%) unlikely to be related to the PBT. One patient developed a left femoral neck fracture (Grade 3) at 14.5 months after PBT for chondrosarcoma of the left pelvis. The pathological findings showed no other malignancies, suggesting that it was possibly related to the PBT. In conclusion, the spot-scanning dedicated, synchrotron-based PBT system is feasible, but further studies on its long-term safety and efficacy are warranted.
Keywords: spot-scanning, synchrotron-based, proton beam therapy, safety, toxicity, adverse event
INTRODUCTION
Proton beam therapy (PBT) is an evolving and promising technology in radiation oncology. This is a result of the superior dose conformation emerging from the Bragg peak followed by the steep gradient at the end of its range [1]. Unnecessary radiation to the surrounding tissue is markedly reduced, allowing dose escalation to the tumor, which potentially translates into better clinical outcomes. Compared with conventional passive-scattering PBT, spot-scanning has a potentially superior dose distribution and less neutron contamination [2]. Moreover, the scanned beam allows manipulation of beams from various angles and with the required energies. In combination with advanced computer science technology, intensity-modulated proton therapy (IMPT) can be achieved.
The world-first spot-scanning dedicated, small 360-degree gantry, synchrotron-based PBT, called the PROBEAT-RT, was developed by collaboration between Hokkaido University and Hitachi Co Ltd. in Japan. In this device, the real-time tumor-tracking radiotherapy (RTRT) technique was incorporated into the spot-scanning proton beam therapy technology [3]. This advanced system facilitates the precise irradiation of moving targets (movement particularly due to respiration), leading to high accuracy of dose delivery with significantly reduced doses to adjacent normal structures [4, 5]. The small spot-scanning dedicated system allows X-ray tubes and flat panels to be mounted in the gantry. The device can be used as an on-board orthogonal X-ray or cone-beam computed tomography (CBCT) for treatment verification and fluoroscopic imaging for tumor motion mitigation. We started patient treatment in 2014 at Hokkaido University Hospital after receiving approval from the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan.
The clinical benefit of spot-scanning PBT with a rotating gantry-mounted CBCT system and gating function has been shown to warrant the expectations for this device. However, dedication to spot-scanning PBT without passive scattering technology is challenging because of the potential uncertainty such as dose inhomogeneity for static tumors and, most importantly, interplay effects for tumors in moving organs [6, 7]. It is not known whether the spot-scanning PBT system discussed here is as safe as conventional passive scattering PBT systems using the total dose and fractionation schedules (for which the safety in passive scattering PBT have been reported in the literature). The aim of this study is to report the prospectively investigated safety of the PBT with a spot-scanning dedicated, small 360-degree gantry, synchrotron-based proton beam therapy system at the acute phase (within 12 months after PBT).
MATERIALS AND METHODS
Study design
This was a prospective, non-randomized single institution study. Patients who met the eligibility criteria were enrolled in this study. The inclusion criteria consisted of pathologically proved malignant tumors, or pathologically proven benign diseases where the effectiveness of X-ray treatment was verified; clinically evident malignant diseases or clinically benign diseases where the effectiveness of X-ray treatment was verified, and conforming to the Eastern Cooperative Oncology Group (ECOG) performance status of 0–3. The exclusion criteria included serious active infections in the irradiated sites; severe uncontrolled diabetes; with severe heart disease or other serious complications, including systemic lupus erythematosus (SLE), scleroderma, interstitial pneumonia; pregnancy or likely pregnancy; psychiatric symptoms or psychosis; proposed radiation treatment site previously given a tolerance dose in the past; unable to stay at rest for about 30 min; body weight over 135 kg; or unsuitable for enrollment as evaluated by the principal investigator. A well-informed written consent was obtained from the patient or a person with parental authority if the patient was younger than 20 years of age. This trial was approved by the institutional review board of our hospital and started from March 2014 at the Department of Radiation Oncology, Hokkaido University Hospital in Japan under the Clinical Trials Core Hospitals project research of Hokkaido University. The trial was registered in the University hospital Medical Information Network—Clinical Trials Registry (UMIN-CTR) (UMIN trial ID: UMIN000013197).
Proton beam therapy
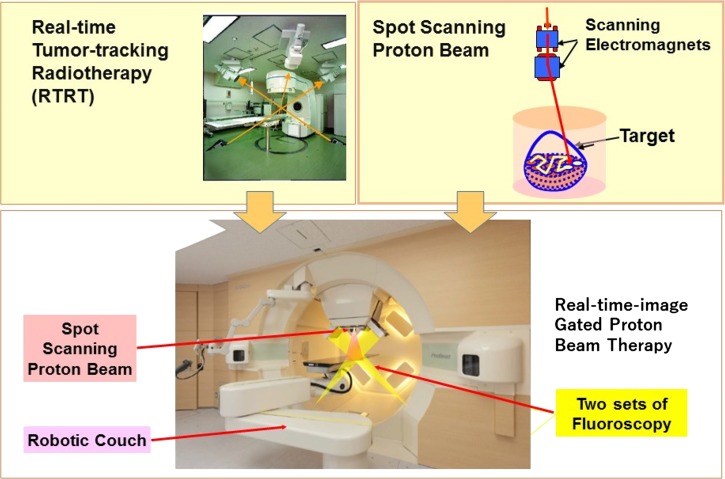
The PROBEAT-RT system (Hitachi Co. Ltd, Hitachi, Japan) is a synchrotron-based proton therapy system dedicated to discrete spot-scanning techniques. It consists of the accelerator, beam transport system, compact 360-degree rotating gantry with a robotic couch, and two sets of fluoroscopes (Fig. 1). The details of the treatment system were described in a previous study [4, 5]. Briefly summarized, proton beam energy is from 70 MeV to 220 MeV, resulting in a penetration value of 4–30 g/cm2. The accelerated proton current is 2 nC; the maximal field size is 30 × 40 cm2 at the isocenter, and the spot size is 7.9 mm and 2.9 mm at the highest and lowest energies, respectively. Image verification was performed by rotating two gantry-mounted orthogonal sets of X-ray fluoroscopes. CBCT was useful in reducing patient set-up errors, and for tumors in moving organs, real-time-image gated proton beam therapy (RGPT) was used. In the RGPT, orthogonal fluoroscopic image-tracking of a gold fiducial marker was performed for the gating of the spot-scanning PBT. Images were recorded every 0.033 s, and the proton beam was delivered with a delay of ~0.05 s and an accuracy of ±2.0 mm during the beam delivery (Fig. 2). For the gated PBT delivery, the parameters were determined by reference to the reports of Tsunashima et al. [8, 9].
Fig. 1.
The real-time tumor-tracking radiotherapy (RTRT) technique was incorporated into the spot-scanning proton beam therapy technology. The spot-scanning dedicated, small 360-degree gantry, synchrotron-based proton beam therapy system operated at Hokkaido University. Two orthogonal sets of X-ray fluoroscopes consisting of X-ray tubes and flat panel detectors are mounted in the rotating gantry.
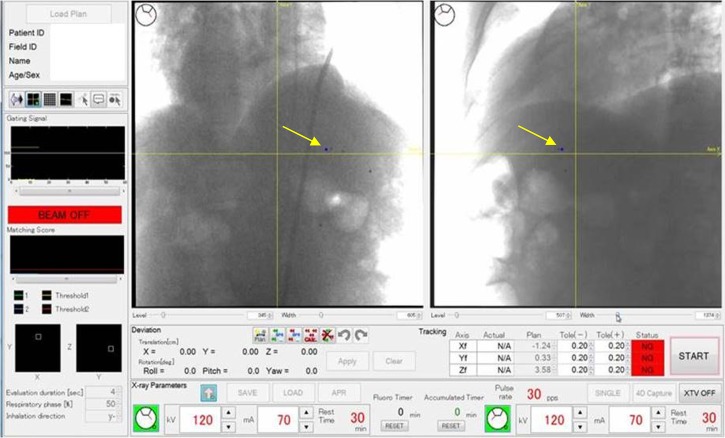
Fig. 2.
Console display of the real-time-image gated proton beam therapy (RGPT) system for a patient with a liver tumor. Two sets of fluoroscopic images are shown with the projection of the planned position of the internal fiducial markers (arrows). The scanning pencil beam of protons is delivered only when the fiducial marker is within ± 2 mm of the planned position. The rate of recording was 30 frames per second for this patient. Other parameters of the fluoroscopy are shown in the display. During the actual irradiation, the imaged area is collimated to reduce unnecessary X-ray exposure.
Treatment planning and dose prescription
Target and organ-at-risk (OAR) were delineated based on the disease type and anatomical location of the tumor in the individual patient. Dose prescription and OAR constraints were determined by physicians according to institutional protocols. The VQA treatment planning system (Hitachi, Ltd, Tokyo, Japan) was used to generate appropriate treatment plans and evaluate dose–volume histograms and target coverage.
The relative biological effectiveness (RBE) value was determined to be 1.1 by a previous study and used in the treatment planning [10]. The lateral margin for the clinical target volume (CTV) was CTV plus 3.0 mm + 4% of the largest width of the CTV at the isocenter plane perpendicular to the beam direction in the water equivalent thickness (WET). The margin for the CTV along the beam direction was 1.0 mm + 3.5% of the distance between the proximal surface of the CTV and the distal surface of the CTV in WET. RGPT was used if the tumor was within a movable organ such as lung, liver, pancreas or prostate. The margin for the internal organ margin was not added between the CTV and the planning target volume (PTV) in RGPT. Regarding the total dose and fractionation schedules for the spot-scanning PBT, these were in principle selected from the schedules where the safety and efficacy have been reported in the literature for passive scattering PBT. The dose schedules listed in Supplemental data 1 are an English translation of the dose fractionation schedules that are approved by the Japanese Society of Radiation Oncology (JASTRO) for PBT. The references for Supplemental data 1 are shown in Supplemental data 2. Most of the patients were treated using one of the treatment schedules in Supplemental data 1, and some were treated with dose fractionation schedules used safely in the X-ray radiotherapy in our institution. Dose constrains are in principle based on the Emami et al. data [11]. When the daily dose is different from 2.0 GyE (RBE 1.1) and a hypofractionation schedule is used, dose constraints used for stereotactic radiotherapy in Japan were used as the reference [12]. Basically, the dose was prescribed at D99 for the CTV, with the D99 for the CTV selected; this dose distribution was close to D95 for the PTV in X-ray treatment in an in-house simulation study. The margin for the CTV was considered for reduction only when the dose to the OAR was above the dose constraint for specific organs.
Data collection and endpoint assessment
Adverse events were assessed weekly during the treatment, monthly until 3 months, and every 3 months thereafter. The study follow-up period was set to 12 months after the end of the PBT. Adverse events were graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Data collection, management, and analysis were performed by the Clinical Research and Medical Innovation Center of Hokkaido University Hospital. The primary endpoint was the incidence of early PBT-related serious adverse events (SAEs) defined as Grade 4 or higher. All SAEs were reported and the cause–effect relationship between SAE and PBT was categorized into ‘Definite; causal relationship with PBT is plausible, which cannot be explained by exacerbation of original disease, comorbidity, other medicine or treatment, etc.’, ‘Probable; causal relationship with PBT is reasonable, which is not likely due to exacerbation of original disease, comorbidity, other medicine or treatment, etc.’, ‘Possible; causal relationship with PBT is reasonable, but can be explained by exacerbation of original disease, comorbidity, other medicine or treatment, etc.’, ‘Unlikely; causal relationship with PBT is improbable, which can be explained by exacerbation of original disease, comorbidities, medicine or other treatment, etc.’, ‘Not related; there is no cause–effect relation with PBT, and it can be clearly explained by other reasons’ and ‘Unassessable; data to be judged is insufficient, more detailed data is required, or evaluation is difficult’. The attending physician judged the cause–effect relationship and when it was either ‘Definite’, ‘Probable’ or ‘Possible’, it was judged there was a causal relationship.
Statistical analysis
Assuming the incidence proportion of early PBT-related Grade 4 adverse events was 3%, a sample of size 100 would be required to determine at least 1 case with 95% probability. The incidence proportion and its 95% confidence interval were calculated using the Clopper-Pearson method; SAS software (version 9.4, SAS Institute Inc., Cary, North Carolina, USA) was used for the statistical analysis.
RESULTS
Patients
Ninety-seven patients were registered until August 2016. From May 2016, the nation-wide registration program for patients treated with PBT was started in Japan. It was decided to integrate this trial with the registration program, and registration was closed in August 2016. At this point, 56 patients with 59 treatment sites who had passed 12 months or more from the end of the PBT were recruited into the study. The contract with our data center for this trial was terminated at the same time for budgetary reasons, and the data collection for the remaining 41 patients was terminated; these patients were excluded from the analysis.
Forty-one patients (73.2%) were male and 15 female; the median age was 66 years (range 1 to 87 years); and the primary lesion sites were prostate (n = 17), bone/soft tissue (n = 10), liver (n = 7), lung (n = 6), central nervous system (CNS) (n = 5), colon (n = 2), pancreas (n = 2), kidney (n = 2), and others (n = 5). All tumors were malignant except for two patients with benign tumors: a craniopharyngioma and a cerebral arteriovenous malformation. Chemotherapy was administered concurrently for one patient and in adjuvant settings for 10 patients.
The prescribed total dose was from 20 to 76 GyE (RBE = 1.1), with the median dose 65 GyE and the median fraction number 26 (range, 4–35). Internal fiducial markers were implanted in 26 patients, and RGTP was used for 28 lesions in these 26 patients. Details of the demographic characteristics and treatments are shown in Tables 1 and 2. All patients were treated with schedules that have been reported to be safe, as reported in the PBT literature, except one patient who needed to have the treatment interrupted because of out-of-field tumor progression. There were no dates when treatment had to be rescheduled due to mechanical problems.
Table 1.
Demographic characteristics (n = 56)
| No. of patients (%) | |
|---|---|
| Age, median (range) | 66 years (1–87 years) |
| Gender | |
| Male | 41 (73.2%) |
| Female | 15 (26.8%) |
| Disease | |
| Malignant tumors | 54 (96.4%) |
| Craniopharyngioma | 1 (1.8%) |
| Cerebral arteriovenous malformation | 1 (1.8%) |
| Primary tumor | |
| Prostate | 17 (30.4%) |
| Bone/soft tissue | 10 (17.8%) |
| Liver | 7 (12.5%) |
| Lung | 6 (10.7%) |
| Central nervous system | 5 (8.9%) |
| Colon | 2 (3.6%) |
| Pancreas | 2 (3.6%) |
| Kidney | 2 (3.6%) |
| Others | 5 (8.9%) |
| Aim of treatment | |
| Curative | 52 (92.9%) |
| Palliative | 4 (7.1%) |
| Prescribed dose, median (range) | 65 GyE (20–76 GyE) |
| No. of fractions, median (range) | 26 (4–35) |
| Use of RGPT | 28 (50.0%) |
| Chemotherapy | |
| Concurrent | 1 (1.8%) |
| Adjuvant | 10 (17.9%) |
Table 2.
Treatment characteristics classified by tumor sites (n = 59)
| Tumor site | Disease status | Dose (GyE)/fraction | RGPT technique | |
|---|---|---|---|---|
| Primary tumor | Metastatic tumor | |||
| Prostate (16) | 16 | – | 70/30 (14), 65/26 (1), 60/30 (1) | 15 |
| Liver (12) | 7 | 5 | 76/20 (4), 60/20 (4), 60/8 (3), 30/10 (1) | 11 |
| Bone/soft tissue (10) | 9 | 1 | 70/28 (5), 70/35 (1), 54/27 (1), 50/25 (1), 46/23 (1), 30/10 (1) | – |
| Lymph node (8) | – | 8 | 50/25 (4), 60/30 (1), 60/24 (1), 50.4/28 (1), 20/10 (1) | – |
| Lung (5) | 5 | – | 70/10 (5) | 2 |
| Central nervous system (5) | 5 | – | 54/30 (2), 50.4/28 (1), 41.4/23 (1), 24/4 (1) | – |
| Pancreas (2) | 1 | 1 | 54/30 (1), 30/10 (1) | – |
| Urethra (1) | 1 | – | 60/30 (1) | – |
| Total | 44 | 15 | 28 | |
RGPT = real-time gated, spot-scanning proton therapy using fiducial markers.
The 12-month follow-up completion rate was 87.5%. Of 56 patients, 4 had died before the completion of the 12-month follow-up after the PBT. A further three patients did not attend follow-ups after the visit at 9 months.
Adverse events
Acute toxicities were mild (Grade 1–2). The most common Grade 1–2 toxicities were radiation dermatitis (n = 31), elevated alanine aminotransferase (ALT) (n = 13), aspartate aminotransferase (AST) (n = 10), and thrombocytopenia (n = 10). Adverse events of Grade 3 or higher are detailed in Table 3. Grade 3 or 4 hematologic adverse events were observed in 7 patients (12.5%) within the 12 months after the PBT. Of these, 6 patients received chemotherapy before and/or after the PBT and one patient had liver cirrhosis with pre-existing thrombocytopenia Grade 2–3; therefore, we concluded there were no Grade 3 or higher hematologic adverse events related to the PBT.
Table 3.
Grade 3 or higher adverse events (n = 56)
| Pt. no. | Diagnosis | Treatment site | Dose/fraction | RGPT | Adverse event | Grade | Time from the start of PBT | Relationship to PBT |
|---|---|---|---|---|---|---|---|---|
| 1 | Liposarcoma | Retroperitoneum | 46 GyE/23 fr | No | Anemia | 3 | 3.2 months | Unlikely |
| Underlying disease progression | 5 | 4.5 months | Not related | |||||
| 2 | Chondrosarcoma | Left pelvis | 70 GyE/28 fr | No | Left femoral fracture | 3 | 14.5 months | Possible |
| 4 | Urothelial carcinoma | Ureter | 50 GyE/25 fr | No | Leukopenia, neutropenia, | 4 | 9.0 months | Not related |
| thrombocytopenia | 4 | 9.0 months | Not related | |||||
| 5 | Ewing sarcoma | Mediastinum | 54 GyE/27 fr | No | Leukopenia, neutropenia, | 4 | 2.2 months | Not related |
| thrombocytopenia | 4 | 2.3 months | Not related | |||||
| Anemia | 3 | 3.3 months | Not related | |||||
| Underlying disease progression | 5 | 5.9 months | Not related | |||||
| 8 | Prostate cancer | Pelvic bone | 30 GyE/10 fr | No | Leukopenia | 3 | 11.0 months | Not related |
| Neutropenia | 4 | 11.0 months | Not related | |||||
| 11 | Pancreatic cancer | Pancreas | 50.4 GyE/28 fr | No | Hypokalemia | 3 | 0.4 months | Unlikely |
| 17 | Synovial sarcoma | Trachea | 50 GyE/25 fr | No | Leukopenia, neutropenia | 4 | 1.7 months | Not related |
| 22 | Lung cancer | Right lung | 70 GyE/10 fr | No | Left pleural effusion | 3 | 0.9 months | Not related |
| Thrombocytopenia | 3 | 3.4 months | Not related | |||||
| Left lung cancer progression | 5 | 5.8 months | Not related | |||||
| 26 | Lung cancer | Right lung | 70 GyE/10 fr | No | Left lung cancer | 3 | 11.7 months | Not related |
| 41 | Liposarcoma | Abdomen | 50 GyE/25 fr | No | Ileus | 3 | 13.0 months | Not related |
| 42 | Liver cancer | Liver | 76 GyE/20 fr | Yes | Thrombocytopenia | 3 | 6.1 months | Unlikely |
| Esophageal varix hemorrhage | 3 | 8.7 months | Unlikely | |||||
| 47 | Pancreatic cancer | Pancreas | 54 GyE/30 fr | No | Biliary stent obstruction, | 3 | 2.5 months | Not related |
| ALT elevation | 3 | 2.6 months | Unlikely | |||||
| 50 | Liver cancer | Liver | 60 GyE/20 fr | Yes | Heart failure | 5 | 5.8 months | Not related |
Pt. no. = patient number, RGPT = real-time gated, spot-scanning proton therapy using fiducial markers, PBT = proton beam therapy, fr = fractions, ALT = alanine aminotransferase.
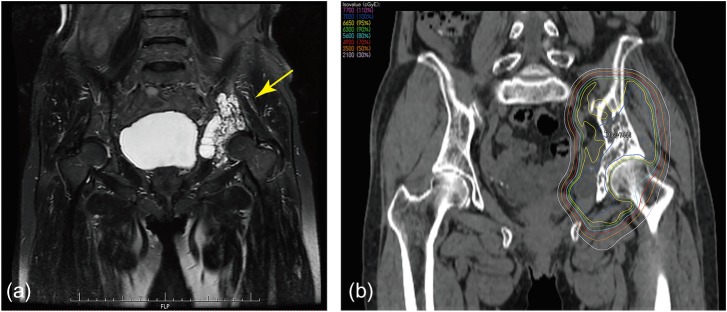
One patient developed a fracture of the left femoral neck (Grade 3) 14.5 months from the start of the PBT. This patient was a 77-year-old female with chondrosarcoma of the left pelvis (Fig. 3a). She received PBT of 70 GyE in 28 fractions to the left pelvis (Fig. 3b). The maximal dose to the femoral neck was 65.4 Gy. This patient underwent hip replacement surgery after the fracture, and the pathological findings showed osteonecrosis with no residual cancerous lesion, suggesting that it was possibly related to the PBT. No other PBT-related SAE above Grade 2 occurred within 3 months or 12 months after the treatment; the incidence of early PBT-related Grade 4 adverse events was 0% (95% confidence interval 0–6.38%).
Fig. 3.
A 77-year-old female with chondrosarcoma of the left pelvis (Pt. No. 2). (a) Coronal image of a T2-weighted MRI of the pelvis indicating the lesion in the left pelvic bone (arrow). (b) Coronal image of computed tomographic image demonstrating the proton dose distribution.
Four patients had died within 12 months of the PBT. Two patients died of underlying disease progression. One patient with bilateral lung cancer and receiving PBT to the right lung died of contralateral lung cancer progression 5.8 months from the start of the PBT. One patient with hepatocellular carcinoma receiving PBT to the liver died of heart failure 5.8 months from the start of the PBT, for reasons considered unrelated to the PBT.
DISCUSSION
The world-first spot-scanning dedicated, small 360-degree gantry synchrotron-based proton beam therapy, PROBEAT-RT system, integrated with the real-time tumor tracking system has been developed at Hokkaido University. We started treating patients using PBT with non-gated and gated functions in March 2014. The current study demonstrates that the PBT system here successfully administers treatments without early serious adverse events for a variety of disease sites, based on the prospectively collected patient database.
The PROBEAT-RT system was jointly developed by Hokkaido University and Hitachi Co. Ltd. The project was sponsored by the Japanese government under the Funding Program for World-Leading Innovative Research and Development on Science and Technology (the ‘FIRST’ program). The spot-scanning PBT delivers the dose to the target by moving the beam on a layer-by-layer basis until the entire tumor volume is irradiated, using a magnetic-controlled computer system. With the absence of customized equipment such as a compensator and a collimator, which are necessary in passive-scattering PBT, the inventive spot-scanning dedicated machinery here enables two orthogonal X-ray tubes with flat panels to be mounted just beside the nozzle with the rotating gantry. The device can provide pre-treatment image verification by both traditional orthogonal X-ray and CBCT. Further, in combination with the positioning of internal fiducial markers, it can track and irradiate the tumor with the incorporated gating function. The new treatment method this makes possible is termed real-time-image-gated, spot-scanning proton therapy (RGPT), and it has shown very promising results in target coverage and reduction of normal tissue doses with lung and liver tumors [4, 5]. This technique also contributed to a reduction in the total size and cost of the equipment and thus provides an attractive method in proton treatment of moving tumors [5]. The facility has been developed since 2009 and has been in operation since March 2014. There were no days when we were unable to treat patients and the ratio of uptime, the rate of operation of the proton machinery without problems over the whole operation time, was calculated to be 98.5% during this period.
In Japan, proton therapy was first put to clinical use at the National Institute of Radiological Sciences (NIRS), Chiba, and the Particle Radiation Medical Science Center (PARMS), University of Tsukuba using passive scattering technology [13]. Generally speaking, no severe complications have been reported with the RBE value of 1.1 and with various dose fractionation schedules [14, 15]. Recently, several studies successively reported the use of novel scanning PBT of several tumor sites. A study at the Pual Scherrer Institute (PSI), Switzerland on base-of-skull tumors (one of the tumor types that would potentially benefit most from PBT) demonstrated excellent local control and survival outcomes with acceptable toxicity [16–19]. Grade ≥3 toxicities reported were CNS necrosis and optic neuropathy. At the University of Texas MD Anderson Cancer Center (MDACC), none of 15 patients with chordoma/chondrosarcomas treated with spot-scanning PBT developed Grade 3–5 adverse events, despite the prescription dose of 66–70 GyE [20]. Iwata et al. has shown that RBE values of passive scattering proton beams in four cell lines examined were 1.01 to 1.22 (average, 1.14) and almost identical to those of spot-scanning beams in their own institution [21].
Selection of the dose fractionation schedule in this kind of prospective study for new radiotherapy technology is difficult. Here, using dose fractionation schedules where the safety was reported for passive scattering PBT, no patient in our study experienced Grade ≥3 CNS toxicity during the observation period. However, since the prescribed radiation dose in this study was administered to a wide variety of treatment regions, we cannot conclusively show equality of the scanning method to the passive scattering method. This study should be regarded as a feasibility study for the spot-scanning dedicated PBT system with dose fractionation schedules where safety has been reported for passive scattering PBT. This study supports the concept of multi-institutional clinical studies that include institutions where passive scattering PBT systems as well as our spot-scanning PBT system in principle administer and employ the same total dose and fractionation schedules.
Dosimetric studies have shown a potential sparing of bone marrow by spot-scanning PBT [22, 23]. In previous work we conducted an in silico study in 13 postoperative gynecologic malignancy patients. Compared with IMRT, Grade 3 or higher hematologic adverse events (HT3+) were significantly lower in spot-scanning PBT estimated by the Lyman normal tissue complication probability (NTCP) model [22]. The mean NTCP value for estimated HT3+ was 4% and 19% for SSPT and IMRT, respectively (P-value = 0.0002). In the current study, Grade 3 or 4 hematologic adverse events occurred in six patients. However, the presence of co-administered chemotherapy or pre-existing hematologic dysfunction hampered the diagnosis of PBT-related HT3+.
Charged particle radiotherapies, particularly carbon ion radiotherapy (CIRT), is considered a promising treatment for bone and soft tissue sarcomas due to the excellent physical dose distribution and biological intratumoral dose [24–27]. There have been cases of bone fracture after particle beam treatment for chondrosarcoma (CS). Indelicato et al. reported only one thoracic fracture requiring fusion surgery among proton therapy treatment of 51 patients with spinal CS [24]. Sumiyoshi et al. reported a case of low-grade CS at the left ileum treated with CIRT 70.4 GyE in 16 fractions that developed femoral neck fracture at 21 months after treatment [28]. The patient underwent left hemiarthroplasty and the pathological report showed osteonecrosis, indicating a radiation-induced mechanism. Outani et al. reported five of 7 CS patients who received CIRT developed pelvic compression fractures and avascular necrosis was seen in 4 of these 5 patients [29]. All tumors were located at the periacetabular region similar to the patient reported here. The dose constraint recommended for the femoral head/neck is TD 5/5 = 52 Gy and TD 50/5 = 65 Gy by Emami et al. [11], and <5% of the femoral head/neck would receive 60 Gy according to RTOG 0630 (a Phase II trial of image-guided preoperative radiotherapy for primary soft tissue sarcomas of the extremities). The patient in the report here received a higher dose than the recommended threshold as the possibility of microscopic tumor involvement around the femoral head could not be disregarded.
The limitations of this study were the inclusion of only a small number of patients and data heterogeneity due to the variety of tumor sites and dose schedules. The safety of this treatment was not formally proven because this study had terminated before the completion of all patients’ follow-up. We performed additional analysis involving all 97 patients after the 12-month follow-up of the last patient, and found that 93 patients (96%) completed planned treatment, with a median prescribed total dose of 66 GyE (RBE = 1.1). Two patients had cancelled PBT before treatment initiation. The 12-month follow-up completion rate of treated patients was 84%. Seven patients were confirmed to have died within 12 months after the PBT. There were no PBT-related Grade 4 or higher SAEs in any of the followed patients within 3 months or 12 months after the treatment; therefore, we believe that the results here suggest that spot-scanning dedicated PBT using the small 360-degree gantry synchrotron-based PBT was very well tolerated, with acceptable toxicity and without SAEs. The toxicity outcomes were comparable with those of other charged particle therapy trials; however, the results should not be used to predict accurate outcomes and do not allow direct comparisons with other studies. The efficacy and long-term safety in a larger group of patients with tumor-specific uniform treatment regimens is under investigation.
Conclusions
We treated 56 patients with 59 lesions using a spot-scanning dedicated, small 360-degree gantry, synchrotron-based proton beam therapy (PROBEAT-RT) system and evaluated the safety of the treatment in the prospective study here, with a complete follow-up rate of 87.5%. The proton beam therapy using the PROBEAT-RT system irradiated various treatment sites without SAEs related to the proton beam therapy within 3 or 12 months post treatment. The efficacy and long-term safety of the system are under further evaluation.
ACKNOWLEDGEMENTS
Results from this study were presented in a poster at the 56th Annual Conference of the Particle Therapy Co-operative Group (PTCOG56), Yokohama, Japan.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
This research is supported by the Clinical Trials Core Hospitals project of the Japanese Ministry of Health, Labour and Welfare and the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15H04899.
REFERENCES
- 1. Brown A, Suit H. The centenary of the discovery of the Bragg peak. Radiother Oncol 2004;73:265–8. [DOI] [PubMed] [Google Scholar]
- 2. Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys 2006;65:1–7. [DOI] [PubMed] [Google Scholar]
- 3. Shirato H, Shimizu S, Kunieda T et al. Physical aspects of a real-time tumor-tracking system for gated radiotherapy. Int J Radiat Oncol Biol Phys 2000;48:1187–95. [DOI] [PubMed] [Google Scholar]
- 4. Matsuura T, Miyamoto N, Shimizu S et al. Integration of a real-time tumor monitoring system into gated proton spotscanning beam therapy: an initial phantom study using patient tumor trajectory data. Med Phys 2013;40:071729. [DOI] [PubMed] [Google Scholar]
- 5. Shimizu S, Miyamoto N, Matsuura T et al. A proton beam therapy system dedicated to spot-scanning increases accuracy with moving tumors by real-time imaging and gating and reduces equipment size. PLoS One 2014;9:e94971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seco J, Robertson D, Trofimov A et al. Breathing interplay effects during proton beam scanning: simulation and statistical analysis. Phys Med Biol 2009;54:N283–94 [DOI] [PubMed] [Google Scholar]
- 7. Shimizu S, Matsuura T, Umezawa M et al. Preliminary analysis for integration of spot-scanning proton beam therapy and real-time imaging and gating. Phys Med 2014;30:555–8 [DOI] [PubMed] [Google Scholar]
- 8. Tsunashima Y, Vedam S, Dong L et al. Efficiency of respiratory-gated delivery of synchrotron-based pulsed proton irradiation. Phys Med Biol 2008;53:1947–59. [DOI] [PubMed] [Google Scholar]
- 9. Tsunashima Y, Vedam S, Dong L et al. The precision of respiratory-gated delivery of synchrotron-based pulsed beam proton therapy. Phys Med Biol 2010;55:7633–47. [DOI] [PubMed] [Google Scholar]
- 10. Maeda K, Yasui H, Matsuura T et al. Evaluation of the relative biological effectiveness of spot-scanning proton irradiation in vitro. J Radiat Res 2016;57:307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emami B, Lyman J, Brown A et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22. [DOI] [PubMed] [Google Scholar]
- 12. Onimaru R, Shirato H, Shibata T et al. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer with PTV<100 cc using a continual reassessment method (JCOG0702). Radiother Oncol 2015;116:276–80. [DOI] [PubMed] [Google Scholar]
- 13. Tsunemoto H, Morita S, Ishikawa T et al. Proton therapy in Japan. Radiat Res Suppl 1985;8:S235–43. [PubMed] [Google Scholar]
- 14. Sakurai H, Ishikawa H, Okumura T. Proton beam therapy in Japan: current and future status. Jpn J Clin Oncol 2016;46:885–92. [DOI] [PubMed] [Google Scholar]
- 15. Demizu Y, Mizumoto M, Onoe T et al. Proton beam therapy for bone sarcomas of the skull base and spine: a retrospective nationwide multicenter study in Japan. Cancer Sci 2017;108:972–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ares C, Hug EB, Lomax AJ et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys 2009;75:1111–8. [DOI] [PubMed] [Google Scholar]
- 17. Pehlivan B, Ares C, Lomax AJ et al. Temporal lobe toxicity analysis after proton radiation therapy for skull base tumors. Int J Radiat Oncol Biol Phys 2012;83:1432–40. [DOI] [PubMed] [Google Scholar]
- 18. Rombi B, Ares C, Hug EB et al. Spot-scanning proton radiation therapy for pediatric chordoma and chondrosarcoma: clinical outcome of 26 patients treated at Paul Scherrer institute. Int J Radiat Oncol Biol Phys 2013;86:578–84. [DOI] [PubMed] [Google Scholar]
- 19. Weber DC, Schneider R, Goitein G et al. Spot scanning-based proton therapy for intracranial meningioma: long-term results from the Paul Scherrer Institute. Int J Radiat Oncol Biol Phys 2012;83:865–71. [DOI] [PubMed] [Google Scholar]
- 20. Grosshans DR, Zhu XR, Melancon A et al. Spot scanning proton therapy for malignancies of the base of skull: treatment planning, acute toxicities, and preliminary clinical outcomes. Int J Radiat Oncol Biol Phys 2014;90:540–6. [DOI] [PubMed] [Google Scholar]
- 21. Iwata H, Ogino H, Hashimoto S et al. Spot scanning and passive scattering proton therapy: relative biological effectiveness and oxygen enhancement ratio in cultured cells. Int J Radiat Oncol Biol Phys 2016;95:95–102. [DOI] [PubMed] [Google Scholar]
- 22. Yoshimura T, Kinoshita R, Onodera S et al. NTCP modeling analysis of acute hematologic toxicity in whole pelvic radiation therapy for gynecologic malignancies—a dosimetric comparison of IMRT and spot-scanning proton therapy (SSPT). Phys Med 2016;32:1095–102. [DOI] [PubMed] [Google Scholar]
- 23. Dinges E, Felderman N, McGuire S et al. Bone marrow sparing in intensity modulated proton therapy for cervical cancer: efficacy and robustness under range and setup uncertainties. Radiother Oncol 2015;115:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Indelicato DJ, Rotondo RL, Begosh-Mayne D et al. A prospective outcomes study of proton therapy for chordomas and chondrosarcomas of the spine. Int J Radiat Oncol Biol Phys 2016;95:297–303. [DOI] [PubMed] [Google Scholar]
- 25. Kamada T, Tsujii H, Blakely EA et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol 2015;16:e93–100. [DOI] [PubMed] [Google Scholar]
- 26. Sugahara S, Kamada T, Imai R et al. Carbon ion radiotherapy for localized primary sarcoma of the extremitied: results of a phase I/II trial. Radiother Oncol 2012;105:226–31. [DOI] [PubMed] [Google Scholar]
- 27. Matsumoto K, Imai R, Kamada T et al. Impact of carbon ion radiotherapy for primary spinal sarcoma. Cancer 2013;119:3496–503. [DOI] [PubMed] [Google Scholar]
- 28. Sumiyoshi N, Torigoe T, Maezawa K et al. Femoral neck fracture and central migration of the artificial femoral head after carbon ion radiotherapy for chondrosarcoma in the pelvis. J Orthop Sci, 10.1016/j.jos.2016.07.014 (16 December 2017, date last accessed). [DOI] [PubMed] [Google Scholar]
- 29. Outani H, Hamada K, Imura Y et al. Comparison of clinical and functional outcome between surgical treatment and carbon ion radiotherapy for pelvic chondrosarcoma. Int J Clin Oncol 2016;21:186–93. [DOI] [PubMed] [Google Scholar]