Abstract
Autophagy breaks down nonessential cellular components to replenish macromolecular building blocks during starvation. Nevertheless, the downstream events regulating vesicle trafficking during this essential cellular process are not yet fully defined. Xu et al. combined approaches of crystallography, biochemistry, and cell biology to show that the guanine nucleotide exchange factor DENND3 contains an actin-binding site they call “PHenn domain” in a region previously thought to be unstructured. PHenn domain binding to microfilaments is necessary for DENND3's participation in autophagy, providing a new link between autophagic stimulation and actin microfilaments. The findings by Xu et al. shed important new light on how membrane trafficking participates in critical steps of autophagy in relationship with actin microfilaments.
Introduction
Macroautophagy (hereafter referred to as autophagy) is an evolutionary conserved catabolic process from yeast to mammals. When autophagy is triggered, a double membrane structure called “autophagosome” isolates organelles, proteins, and other cellular material destined for degradation. Lysosomes fuse with the autophagosome leading to degradation and the recycling back of its content to the cytosol (1). In addition to supporting cells during starvation conditions, autophagy has also been reported to take part in seemingly distant cellular processes such as regulation of the immune response against pathogens, neuronal homeostasis (2), brain development (3), and neuroprotection following brain injury (4). Dysfunctional autophagy has been linked to major human pathological conditions such as cancer, cardiovascular disease, and neurological disorders (5). Thus, understanding how autophagy is regulated during normal and pathological conditions is a central question in biochemical and cell biology research, as highlighted by the awarding of the Nobel Prize in Physiology or Medicine in 2016 to Yoshinori Oshumi.
From phagophore formation to lysosomal degradation, several steps in autophagy rely on vesicular trafficking. However, key mechanisms of vesicular trafficking in autophagy remain elusive or controversial, very likely because of the diverse nature of the organelles involved, as well as an incomplete picture of the regulatory molecules involved, and of their interaction networks. The cytoskeleton, particularly actin microfilaments, is also known to play important roles in the autophagic response: Nucleation and polymerization of actin microfilaments are necessary for initial phagophore formation while microfilaments provide tracks for myosin-dependent vesicle trafficking to sustain phagophore expansion. Actin polymerization is also necessary in mature autophagosomes to propel them through the cell (6). Although much is known about the role of actin in autophagy, the possible implications it might have in vesicle trafficking regulation remain largely unexplored. The new study by Xu et al. (7) provides an unexpected insight into these questions, connecting a known autophagy-regulatory molecule to the cytoskeleton through an actin-binding domain that has been hiding in plain sight.
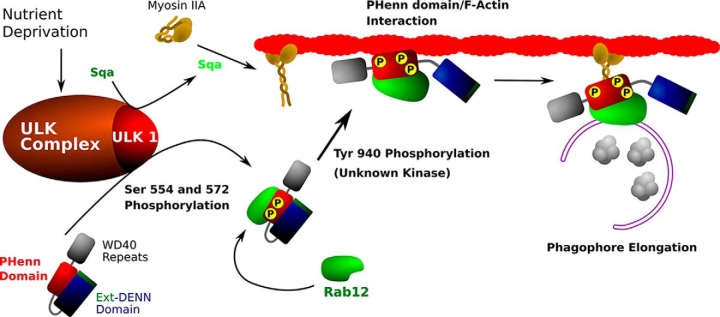
The autophagic response begins with Unc-51–like autophagy-activating kinase (ULK),2 which phosphorylates DENND3, the guanine nucleotide exchange factor (GEF) for the small GTPase Rab12, at Ser-554 and Ser-572. This phosphorylation up-regulates DENND3's GEF activity, stimulating Rab12 activity and facilitating autophagosome trafficking (8) (Fig. 1). Previous work from this group has shown that DENND3 is autoinhibited through an intramolecular interaction; ULK-independent phosphorylation of DENND3's residue Tyr-940 releases this inhibition, initiating a conformational change that further increases GEF activity (9). DENND3 contains known functional domains at the N and C terminus (the extended DENN and WD40 repeat domains, respectively), but Tyr-940 is located in what was thought to be an unstructured linker domain. As a result, it was not clear how Tyr-940 influences DENND3 activity nor how the DENND3-Rab12 activation led to downstream effects on vesicle trafficking.
Figure 1.
Model for the role of DENND3 in the autophagic response based on Xu et al. (8). DENND3 is first phosphorylated by ULK1, increasing its GEF activity toward Rab12 and promoting Rab12 binding. ULK also activates the myosin light chain kinase (MLCK)-like protein, spaghetti-squash activator (Sqa), which in turn activates myosin IIA. DENND3 is also phosphorylated at Tyr-940 by an as yet unknown kinase, leading to an open and active conformation; this also exposes an actin-binding surface. ULK1-activated myosin IIA binds the Rab12–DENND3–actin complex in the growing phagophore, enabling autophagosome translocation over microfilaments. After maturation, autophagosomes fuse with lysosomes, and their contents are degraded and recycled back to the cytosol.
To try to understand the local environment of Tyr-940, Xu et al. used a combination of secondary structure prediction tools and X-ray crystallography. They surprisingly found a large helical bundle capped by a sandwich of two β-sheets stacked perpendicularly to each other, where the β-sheets together with one α-helix form a characteristic pleckstrin homology (PH)-like fold. Notably, Xu et al. also identified a cluster of hydrophobic residues making up a hydrophobic β-turn protruding away from the structure. Using GST-pulldown assays, the authors determined that these residues were required for the intramolecular interaction between this structure and the DENN domain, leading them to name this new structure the “PHenn domain,” for PH-like domain binding to a DENN domain.
According to their model, release of the intramolecular inhibition by Tyr-940 phosphorylation would expose the hydrophobic cluster. However, the authors found no evidence for this cluster to be a determinant of membrane association nor to possess lipid-binding activity. To understand what the fate of these hydrophobic residues might be, the authors returned to their structure. Aligning the PHenn domain against protein databases revealed a high structural similarity to FERM domains, known to bind F-actin; incubation of a GST-PHenn domain with preassembled F-actin confirmed that the PHenn domain is a novel actin-binding module. Site-directed mutagenesis and GST pulldown assays further identified a patch of basic amino acids responsible for actin binding. Overexpression of constructs with mutations at these residues did not recover the autophagic response in cells treated with siRNA against DENDD3, demonstrating that actin binding is required for the role of DENND3 in autophagy. Finally, GST pulldown experiments in rat lung lysates with the GST-PHenn domain or GST-Rab12 revealed that these proteins interact with the nonmuscular actin motor protein myosin IIA. Surprisingly, the PHenn domain mutants with defects in actin binding also have limited interactions with myosin IIA, suggesting that actin may bridge the binding between DENND3 and myosin IIA.
In conclusion, this article proposes an original “release of autoinhibition” mechanism for the activation of DENDD3 by ULK, which would result in Rab12 activation. The authors further show that DENDD3's role in autophagy requires its binding to actin microfilaments. The proposed model of a myosin II–DENDD3–Rab12 complex and coupling of transport on actin microfilaments and Rab12 activation paves the way for further exploration of downstream effectors in autophagy. Indeed, RLIP was shown to be a Rab12 effector and to mediate retrograde transport (10) of mast cell secretory granules. It would thus be tempting to anticipate that the mechanisms unraveled by Gehring and colleagues (7) are followed by retrograde transport of autophagosomes to allow for fusion with lysosomes. The molecular chain of events of autophagy will continue to reveal further links with membrane trafficking.
This work was funded in part by INSERM, the Fondation pour la Recherche Médicale (FRM), the French National Research Agency (NeuroImmunoSynapse ANR-13-BSV2-0018-02 and MetDePaDi ANR-16-CE16-0012), the Ecole des Neurosciences de Paris (ENP), awards of the Association Robert Debré pour la Recherche Médicale and Fondation Bettencourt Schueller (to T. G.), and Who am I? Labex (Idex ANR-11-IDEX-0005-01). The authors declare that they have no conflicts of interest with the contents of this article.
- ULK
- Unc-51–like kinase
- GEF
- guanine nucleotide exchange factor
- DENN
- differentially expressed in normal and neoplastic cells
- PH
- pleckstrin homology.
References
- 1. Boya P., Reggiori F., and Codogno P. (2013) Emerging regulation and functions of autophagy. Nat. Cell Biol. 15, 713–720 10.1038/ncb2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maday S., and Holzbaur E. L. F. (2016) Compartment-specific regulation of autophagy in primary neurons. J. Neurosci. 36, 5933–5945 10.1523/JNEUROSCI.4401-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takáts S., Nagy P., Varga Á., Pircs K., Kárpáti M., Varga K., Kovács A. L., Hegedűs K., and Juhász G. (2013) Autophagosomal syntaxin17-dependent lysosomal degradation maintains neuronal function in Drosophila. J. Cell Biol. 201, 531–539 10.1083/jcb.201211160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galluzzi L., Bravo-San Pedro J. M., Blomgren K., and Kroemer G. (2016) Autophagy in acute brain injury. Nat. Rev. Neurosci. 17, 467–484 10.1038/nrn.2016.51 [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto A., and Yue Z. (2014) Autophagy and its normal and pathogenic states in the brain. Annu. Rev. Neurosci. 37, 55–78 10.1146/annurev-neuro-071013-014149 [DOI] [PubMed] [Google Scholar]
- 6. Kast D. J., and Dominguez R. (2017) The cytoskeleton-autophagy connection. Curr. Biol. 27, R318–R326 10.1016/j.cub.2017.02.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu J., Kozlov G., McPherson P. S., and Gehring K. (2018) A PH-like domain of the Rab12 guanine nucleotide exchange factor DENND3 binds actin and is required for autophagy. J. Biol. Chem. 293, 4566–4574 10.1074/jbc.RA117.001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu J., Fotouhi M., and McPherson P. S. (2015) Phosphorylation of the exchange factor DENND3 by ULK in response to starvation activates Rab12 and induces autophagy. EMBO Rep. 16, 709–718 10.15252/embr.201440006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu J., and McPherson P. S. (2017) Regulation of DENND3, the exchange factor for the small GTPase Rab12 through an intramolecular interaction. J. Biol. Chem. 292, 7274–7282 10.1074/jbc.M116.772434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Efergan A., Azouz N. P., Klein O., Noguchi K., Rothenberg M. E., Fukuda M., and Sagi-Eisenberg R. (2016) Rab12 regulates retrograde transport of mast cell secretory granules by interacting with the RILP-dynein complex. J. Immunol. 196, 1091–1101 10.4049/jimmunol.1500731 [DOI] [PubMed] [Google Scholar]