Abstract
Periodontal disease, which includes gingivitis and periodontitis, is highly prevalent in adults and disease severity increases with age. The relationship between periodontal disease and oral cancer has been examined for several decades, but there is increasing interest in the link between periodontal disease and overall cancer risk, with systemic inflammation serving as the main focus for biological plausibility. Numerous case-control studies have addressed the role of oral health in head and neck cancer, and several cohort studies have examined associations with other types of cancers over the past decade. For this review, we included studies that were identified from either 11 published reviews on this topic or an updated literature search on PubMed (between 2011 and July 2016). A total of 50 studies from 46 publications were included in this review. Meta-analyses were conducted on cohort and case-control studies separately when at least 4 studies could be included to determine summary estimates of the risk of cancer in relation to 1) periodontal disease or 2) tooth number (a surrogate marker of periodontal disease) with adjustment for smoking. Existing data provide support for a positive association between periodontal disease and risk of oral, lung, and pancreatic cancers; however, additional prospective studies are needed to better inform on the strength of these associations and to determine whether other cancers are associated with periodontal disease. Future studies should include sufficiently large sample sizes, improved measurements for periodontal disease, and thorough adjustment for smoking and other risk factors.
Keywords: cancer, meta-analysis, periodontal disease, periodontitis, review
INTRODUCTION
There has been a rapid increase in interest in understanding the relationship between periodontal disease and cancer risk; twice as many original observational studies were published with measures of periodontal disease (i.e., not including studies using tooth number as surrogate of periodontal disease) in the past few years (n = 12; 2011–2016) as were published during the 20 years prior to 2011 (n = 6) (Web Tables 1 and 2, available at http://aje.oxfordjournals.org/). The reason for the large increase in publications on this topic is largely due to new interest generated by the research on the human microbiome (alongside advancing technologies) and perhaps also due to the development of animal models of periodontal disease that have enabled researchers to measure the impact of periodontal pathogens on the local and systemic immune response (1). Human studies have confirmed the impact of periodontal disease on the systemic immune response, demonstrating that levels of serum markers of inflammation, especially C-reactive protein, increase with advanced periodontal disease (2, 3). The link between systemic inflammation and cancer is well established and may contribute to the strong and consistent positive associations between obesity and cancer (4). Interest in the role of inflammation on cancer, as well as the many parallels that exist between risk factors for heart disease and cancer (e.g., smoking, obesity, lack of physical activity, insulin resistance, and diabetes) (5), has motivated different lines of research to address the role of periodontal disease in cancer risk.
Periodontal disease, also known as gum disease, encompasses gingivitis and periodontitis. Gingivitis, or inflammation of the gums which leads to bleeding gums, is considered an early form of periodontal disease. Periodontitis develops over time with accumulation of dental plaque, bacterial dysbiosis, formation of periodontal pockets, gum recession, tissue destruction, and alveolar bone loss, which can ultimately lead to tooth loss. Although periodontal treatment, such as rooting and scaling, can slow the progression of periodontal disease by removing plaque at the root of the teeth and reducing inflammation, once tissue and bone loss has occurred, it is permanent.
In the United States, national health surveys have reported a high prevalence of periodontitis on the basis of oral health examinations; the prevalence of periodontitis in dentate adults over the age of 30 years is estimated to be around 47% and increases to 70% in individuals 65 years of age or older (6). Risk factors for periodontal disease include race, sex, income, education, and smoking. In the United States, the highest prevalence of periodontal disease is observed in Mexican Americans (70% in adults over 30 years), followed by non-Hispanic blacks (60%) and non-Hispanic whites (42%). The prevalence of moderate and severe periodontitis, which has been more strongly linked to systemic inflammation and the immune response, is also very high in adults over the age of 65 years (64% using the Centers for Disease Control and Prevention-American Academy of Periodontology case definition) (7). Elevated rates of periodontitis are also found in Europe; in Germany, prevalence of moderate and severe periodontitis was reported to be 76% in adults aged 65–74 years (8) when using the same definition of periodontitis (9). The prevalence of periodontal disease measurements (i.e., pocket depth and clinical attachment level) obtained from a dental examination survey conducted in France was similar to that reported in the United States (10). Earlier surveys conducted in the United States underestimated the prevalence of periodontitis because of incomplete dental measurements (6, 7).
One of the biggest challenges in studying periodontitis and chronic diseases with observational studies resides in the measurement of periodontal disease. Historically, tooth loss was used as a marker for periodontal disease, given that tooth loss that occurs in adults is largely due to periodontitis; however, number of teeth lost is also a marker of oral health and can result from dental caries, accidents, or orthodontic treatment. Thus, tooth loss can represent a number of oral conditions, is often only a crude marker of periodontitis, and may vary dramatically depending on the population. For example, in the Health Professionals Follow-up Study, a large population of male and primarily Caucasian health professionals, more than half (58%) of the men reporting having 0–16 teeth remaining did not report having periodontitis (11). Tooth loss is also a marker of socioeconomic status.
In observational studies, periodontal disease has been measured by using self-report of periodontitis (e.g., periodontal disease with bone loss) or using individual oral health assessments, such as measurements of pocket depth, clinical attachment level, and alveolar bone loss from panoramic radiographs. The large variation in methods and criteria used for the assessment and categorization of periodontitis may explain some of the discrepancies observed across observational studies. Recent studies, including the Third National Health and Nutrition Examination Survey (NHANES III) (12) and the Buffalo OsteoPerio Study (an ancillary study of the Women's Health Initiative) (13), have examined the association between periodontitis and cancer risk by using direct oral measurements and radiographs, respectively, to obtain more precise measures of periodontitis. Nevertheless, other limitations exist in observational studies on periodontal disease and cancer risk, which include small number of cases and potential confounding by smoking and other risk factors. Whether the impact of periodontal disease on cancer risk is a direct effect or a consequence of shared genetic and/or environmental factors is extremely difficult to untangle. These limitations have caused much controversy in the field of periodontitis and heart disease and will require interdisciplinary collaborations to address many remaining questions. Understanding the biological underpinnings of the associations and evaluating whether they are direct or indirect effects are critical to determine whether reductions in risk can be achieved through public health interventions.
The largest number of observational studies examining oral conditions, including teeth number, and risk of cancer has been on head and neck cancers. At least historically, a link between oral disease and cancer in the oral cavity, or in the vicinity of the oral cavity, was more plausible than a link with more distant cancer sites, given the visible and measurable local inflammation, known shifts in pathogenic oral bacteria, and physical injury caused by bone loss with advanced periodontitis. However, research conducted in the past decade has led to new studies examining the potential relationship between oral disease and the risk of orodigestive cancers, lung cancer and other smoking-related cancers (e.g., kidney, bladder), and even hormonal cancers such as breast, prostate, and ovarian cancers (Web Tables 1 and 2). The motivation behind these studies lies largely on new knowledge linking periodontitis and pathogenic bacteria to a systemic impact on the body, particularly on the immune response, providing plausibility for a role in carcinogenesis of distant tumors through those mechanisms.
To summarize the evidence on this topic, we reviewed the literature on oral disease and cancer risk and performed meta-analyses on missing teeth, and separately for periodontal disease, and cancer risk for those cancer sites that had at least 4 case-control or cohort studies with adjustment for age, sex, and smoking status (at the minimum).
METHODS
Literature search and selection
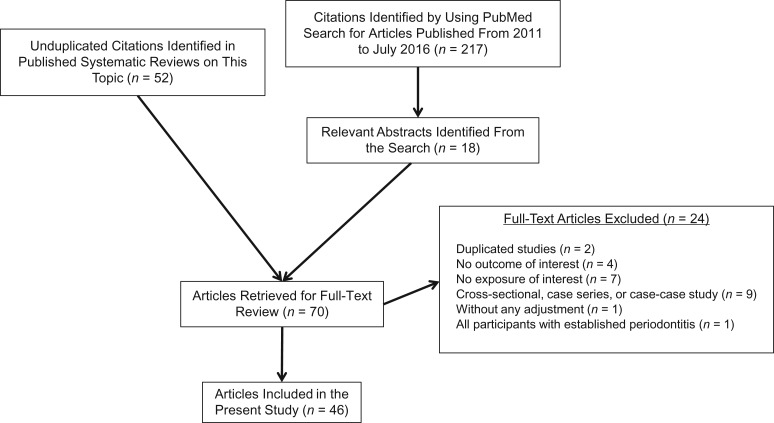
Two approaches were used to identify relevant studies: 1) a search for all published systematic reviews or related to oral health and cancer for reference mining and 2) a supplemental search for recent cohort and case-control studies published between 2011 and July 2016 (Figure 1). All electronic searches were conducted on PubMed using comprehensive search strategies (Web Table 3). We retrieved 70 full-text articles of cohort and case-control studies identified from 11 reviews and meta-analyses (14–24) and those identified from reviewing 217 relevant abstracts from the supplemental search to screen them according to predefined study eligibility criteria; only case-control and cohort studies were included (no randomized controlled trials exist) with relevant exposure categories and outcomes (Web Tables 1 and 2). A total of 46 publications met our study eligibility criteria for this review (Figure 1).
Figure 1.
Literature search and selection flow chart. Systematic reviews were used to find relevant articles (14–24). Hujoel et al. (32), Tezal et al. (37), Moergel et al. (38), and Michaud et al. (11, 70) described both periodontal disease and tooth loss exposure; Ren et al. (16) and Guha et al. (43) have 2 studies in each publication.
Data extraction, charting, and synthesis
Structured data extraction forms were used to abstract key study characteristics (Web Tables 1 and 2) and results for charts (when there were 3 studies or more for each cancer outcome with the same exposure measure) or meta-analysis. All study results are summarized narratively by each cancer outcome of interest, with the meta-analysis results if available.
Meta-analysis
Given the importance of smoking as a potential confounder for cancer outcomes, studies that presented risk estimates without adjustment for smoking status were not included in the meta-analysis to avoid aggregating confounding in the pooled results. If fewer than 4 studies achieved the inclusion criteria, no meta-analysis was conducted in this review. To minimize the clinical, methodological, and statistical heterogeneity in the meta-analyses, study results were grouped first by exposure categories (periodontal diseases or number of missing teeth) and then by study design (case-control or cohort studies). Meta-analysis using the DerSimonian-Laird random-effects model (25) was conducted comparing the presence or absence of periodontal diseases in relation to the same cancer outcome. When there were more than 6 studies with sufficient data, linear and nonlinear dose-response meta-regression analyses were performed to explore the associations between number of missing teeth and the risks of cancer using a 2-stage hierarchical regression model implemented in the “dosresmeta” R (R Foundation for Statistical Computing, Vienna, Austria) package (26, 27). The midpoints of the reported categories for the number of missing teeth were used as the “dose” in the dose-response meta-regression. Statistical heterogeneity was tested by using the Cochran Q statistic (considered significant when P < 0.10) and was quantified with the I2 index (ranging from 0% to 100%). I2 values of 25%, 50%, and 75% were typically interpreted as low, moderate, and high heterogeneity, respectively. However, these cutoffs were arbitrary and used for descriptive purposes only (28). Analyses were conducted by using Stata SE 13 software (StataCorp LP, College Station, Texas) and R version 3.2.5 (R Foundation for Statistical Computing). All P values were 2 tailed, and P < 0.05 was considered statistically significant.
RESULTS
Total cancer
Five cohort studies reported associations between periodontal disease and total cancer risk (Web Figure 1), but only 3 of these adjusted for smoking status in multivariate models (Web Table 1). The increase in total cancer risk associated with periodontal disease ranged between 14% and 20% in the studies controlling for smoking (11, 13, 29); associations were highly consistent across all 5 studies, despite substantial differences in populations (including sex-specific populations). In 2 analyses restricted to never smokers (1 was published separately (30)), a 13% (95% confidence interval (CI): 1.01, 1.27) increase was observed in a male cohort study (the Health Professionals Follow-up Study) (30)), but no association was noted in a cohort of women (hazard ratio (HR) = 0.90, 95% CI: 0.51, 1.62) (13).
No associations were noted for number of missing teeth and total cancer risk (Web Figure 2A).
Lung cancer
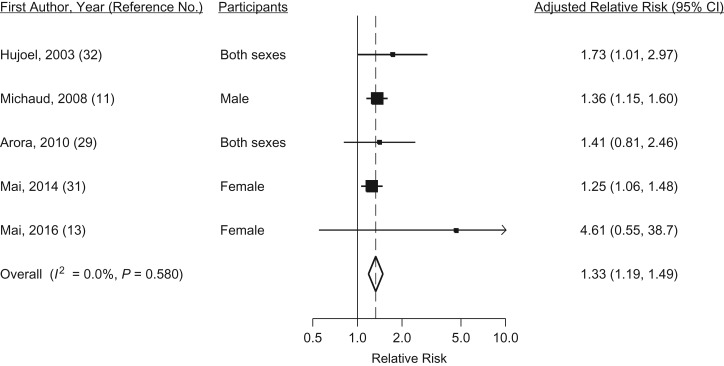
Positive associations between periodontal disease and lung cancer risk have been observed in 5 cohort studies (Web Figure 1B), all of which adjusted for smoking status. The relative risks observed ranged between 1.25 and 4.61, and the pooled relative risk was 1.33 (95% CI: 1.19, 1.49) (Figure 2), with no statistical heterogeneity (I2 = 0, P = 0.58). Given that smoking increases the risk of periodontal disease and that it is very strongly associated with the risk of lung cancer, some analyses have been conducted among never smokers only. In the Health Professionals Follow-up Study cohort, no association with periodontal disease was observed for lung cancer in never smokers (HR = 0.92, 95% CI: 0.49, 1.71) (30) and, similarly, never smokers with periodontal disease did not have a higher risk of lung cancer in the Women's Health Initiative Observational Study (31). Among smokers in the Women's Health Initiative Observational Study cohort, a positive association was observed for women with higher smoking intensity and periodontal disease compared with those with higher smoking dose and no periodontitis (HR = 1.29, 95% CI: 1.09, 3.87), suggesting that an interaction between smoking and periodontal disease may exist (31).
Figure 2.
Random-effects meta-analysis of 5 cohort studies assessing the associations between periodontal disease and lung cancer risk. CI, confidence interval.
Two out of 4 cohort studies observed a statistically significant higher risk of lung cancer with a higher number of missing teeth after adjustment for smoking dose (11, 32–34). One large case-control study also reported a positive association with teeth number and lung cancer risk (35).
Head and neck cancer
Only 1 cohort study reporting smoking-adjusted associations for periodontal disease and head and neck cancer has been published to date; in that study, an increase in risk was modest and not statistically significant (HR = 1.15, 95% CI: 0.73, 1.81) (11). In the same cohort, a strong association was observed for periodontitis and oropharyngeal and esophageal cancers combined (HR = 2.25, 95% CI: 1.30, 3.90) after restricting the analysis to never smokers (30). Another cohort study conducted in Taiwan reported a positive association for periodontal disease and head and neck cancer but did not control for smoking (HR = 1.20, 95 CI: 1.09, 1.33) (36).
Five case-control studies reported findings for periodontal disease and head and neck cancers (Web Table 1); however, 2 of these studies (37, 38) provided data only for continuous measures of periodontal disease (bone loss measures) and thus could not be included for comparison (Web Figure 3). These studies provided data from a range of different periodontal measures, including some more accurate measures of bone loss due to periodontitis (37, 38) and pocket cavity depth (39). Overall, findings are inconsistent, with earlier studies showing weak inverse associations (odds ratio (OR) = 0.8) (40) and more recent studies reporting extremely strong associations when using more detailed assessment of periodontal disease (e.g., OR > 10) (38). The 2 studies with radiographic bone loss measures reported strong positive associations with bone loss and risk of oral cancer (OR = 4.52, 95% CI: 3.03, 6.75, for each millimeter increase in alveolar bone loss (37), and OR = 2.4, 95% CI: 3.04, 11.7, for mean bone loss comparison (38)).
Because of large clinical heterogeneity and incompatible measures of periodontal disease, a meta-analysis was not performed.
A substantial number of case-control studies have examined the relation between number of missing teeth and risk of head and neck cancers (Web Figures 4 and 5). The majority of these studies reported that higher number of missing teeth was associated with an increase in oral cancer (Web Figure 4); in contrast, there were no apparent dose-response relationships for larynx and pharyngeal cancers (Web Figure 5C and 5D).
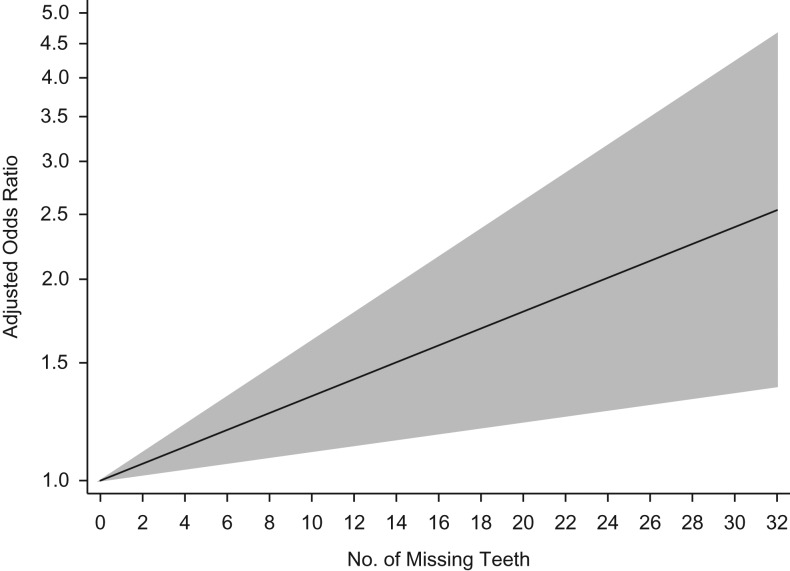
Seven case-control studies were included for dose-response meta-regression analyses for oral cancer. The linear dose-response meta-regression for teeth number and oral cancer risk was statistically significant; a 0.03 (95% CI: 0.01, 0.05) increase in the odds ratio was observed for each additional tooth lost (Figure 3), with moderate heterogeneity (I2 = 67.5%, P = 0.003). In dose-response meta-regression analyses of 6 case-control studies, no significant linear or nonlinear dose-response relationship between teeth number and head and neck cancer risk was observed (data not shown) (35, 41–44).
Figure 3.
Predicted linear dose-response relationship between number of missing teeth and oral cancer risk using data from 7 case-control studies. The solid black line is the predicted linear trend, and the gray area is the 95% confidence interval of the predicted linear trend. The 7 case-control studies included in the analysis are described by Kabat et al. (71) (female), Zheng et al. (72) (male and female separately), Bundgaard et al. (73) (both sexes), Rosenquist et al. (74) (both sexes), Guha et al. (43) (Central Europe, both sexes), Guha et al. (43) (Latin America, both sexes), and Chang et al. (44) (both sexes).
Pancreatic cancer
There has been a substantial interest in periodontal disease and pancreatic cancer risk over the past decade, and the associations reported have been remarkably consistent. Web Figure 1D provides results from 5 cohort studies. All studies reported at least a 50% increase in risk for those with periodontal disease, and results from 3 of the studies were statistically significant. Unfortunately, 2 studies did not adjust for smoking (32, 45), and 1 study had a very small number of pancreatic cancer cases (12) (Web Table 1). In addition, results from a national cohort in Taiwan are not shown in Web Figure 1D as the comparison group consisted of individuals with gingivitis. An elevated risk was observed for those with periodontitis compared with gingivitis (relative risk = 1.2, 95% CI: 1.0, 2.4), although no adjustment was made for smoking status (46).
In contrast, associations between missing teeth and pancreatic cancer risk have not been consistent (Web Figure 2B).
Stomach and esophageal cancers
Findings from cohort studies on periodontal disease and risk of stomach cancer have been largely null (Web Figure 1C). Five cohort studies and 1 case-control study examined the association between teeth number and risk of stomach cancer. No associations were noted in 4 of those studies (11, 32, 33, 35), but 2 cohort studies reported positive associations for gastric noncardia subtypes (47, 48). A meta-analysis was not conducted as a number of studies did not adjust for history of smoking (refer to Methods).
Only 1 cohort study reported associations for periodontal disease and esophageal cancer risk alone, where a modest increase in risk was noted (n = 82 cases) (HR = 1.44, 95% CI: 0.98, 2.11) (11). Three cohort and 4 case-control studies examined the associations between teeth number and risk of esophageal cancer, but no dose-response trends were observed in either cohort or case-control studies (Web Figures 2C and 5B, respectively).
Breast cancer
Five cohort studies reported on associations between periodontal disease and risk of breast cancer (Web Figure 1E). Findings from these studies were null or modest in magnitude, ranging between 0.94 and 1.32; nevertheless, associations in 2 of the 5 studies did reach statistical significance (36, 49).
Colorectal cancer
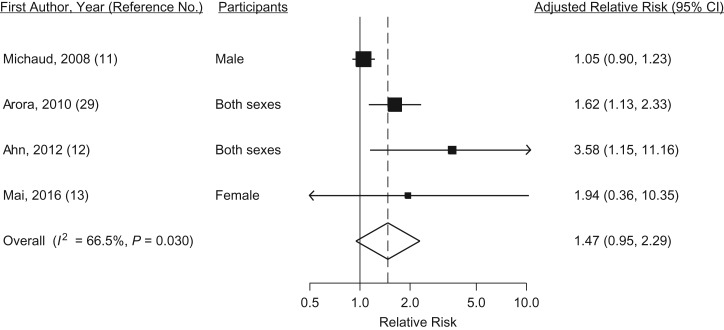
Although the current meta-analysis suggests that periodontal disease may increase the risk of colorectal cancer by 47% (Figure 4), the overall estimate was not statistically significant because of large uncertainty (95% CI: 0.95, 2.29) and heterogeneity was moderate (I2 = 66.5%, P = 0.03). One study reported no association (11), and 2 of the remaining 4 studies had a small number of cases (Web Table 1) (12, 13). Findings from 5 cohort studies with data on number of teeth and risk of colorectal cancer have been consistently null (11, 16, 32, 33).
Figure 4.
Random-effects meta-analysis of 4 cohort studies assessing the associations between periodontal disease and colorectal cancer risk. CI, confidence interval.
DISCUSSION
Recent interest in the link between periodontal disease and cancer risk has led to publication of data from several large cohort studies that provide some evidence for positive associations between periodontitis and total cancer risk, particularly for pancreatic and lung cancers, and head and neck cancer. Unfortunately, adjustment for smoking status was lacking in some studies, reducing the number of studies eligible for meta-analysis. Positive associations were observed for teeth number and oral cancer, with a significant positive dose response in the meta-regression. No consistent associations were observed for number of teeth and other cancer sites.
Strengths of this meta-analysis
To date, 5 meta-analyses or reviews have been published on the associations between periodontal disease and/or tooth loss and risk of head and neck cancers (15, 19–21, 23), 3 meta-analyses on periodontal disease and other cancers (1 lung cancer (22), 1 colon cancer (16), 1 gastric cancer (24)), and 2 reviews on periodontal disease and all cancers (14, 17). None of the prior publications conducted meta-analyses on multiple cancer types collectively. Although our findings are mostly consistent with those from the previous reviews and meta-analyses, the present review provides the most comprehensive analysis by adding 13 new studies across all cancers. Furthermore, several differences in the evidence synthesis methods that were implemented in the present review can explain some discordance between present and previous findings. In the present review, in addition to evaluating the strength of associations and potential confounding, our synthesis considered consistencies and dose-response relationships across study findings to improve causal inference of the body of evidence. Moreover, a 2-stage hierarchical meta-regression model was used to estimate the summarized linear and nonlinear dose–response relationships between number of missing teeth and the risks of cancer in this review. This advanced analytical method overcomes the use of arbitrary cutoffs for number of missing teeth to compare the risks of cancer above or below the cutoffs.
Summary of evidence
A large number of studies examining number of teeth have been conducted to evaluate general oral health in relation to head and neck and esophageal cancers. However, most of these studies have been case-control studies because of the lower incidence of these cancers. A review of the literature and our summary plots for these associations suggest that there is a positive relation between number of teeth lost and oral cancer risk. In contrast, tooth loss does not appear to be related to cancer risk for most other cancer sites. Given that people lose teeth for reasons other than periodontitis, it is likely that this measure is too crude to be used as a measure of periodontal disease and is more likely a measure of overall oral health. Although we did not perform a meta-analysis for periodontal disease and oral cancer, recent studies with improved measurements of periodontitis do support strong positive associations for periodontitis and oral cancer risk (37, 38). Improving reporting of periodontitis using standardization of measurements should be made to allow for meta-analyses to be conducted.
The findings for periodontal disease and lung cancer are also strong overall, but questions remain regarding the role of smoking, as either a confounder or an effect modifier. Two studies that conducted separate analyses on never smokers did not observe any increase in risk with periodontal disease (30, 31), raising the possibility that the elevated risk is due to residual confounding by smoking. Nevertheless, it is also biologically plausible that smoking and periodontal disease act jointly to increase the risk of lung cancer, as was recently reported in a large cohort analysis (31). A previous meta-analysis on periodontal disease and lung cancer reported a similar summary risk estimate (HR = 1.24, 95% CI: 1.13, 1.36); the previous meta-analysis did not include a recent cohort study (13) and included a study that we did not include because of the lack of appropriate referent category (i.e., gingivitis) (46).
The results on periodontal disease and colorectal cancer risk are less consistent, and the meta-analysis result indicates there could be a positive association, but the uncertainty is large (wide confidence interval) and the moderate level of heterogeneity further limited interpretation of the pooled risk estimate. A previous meta-analysis on oral health and colorectal cancer combined risk estimates obtained from associations reported for periodontal disease and teeth number (defined jointly as “oral health”) and reported no overall associations (16). Given the differences that we observed in associations with cancer risk when using periodontal disease and teeth number separately, combining these measures is likely to dilute any real effect that exists. Several studies have reported associations between Fusobacterium nucleatum, which has also been considered a periodontal pathogen, and colorectal cancer (50–52); however, it is unclear if these bacteria are found at higher levels in the gut as a result of periodontal disease.
Despite the limited evidence available to allow a meta-analysis for pancreatic cancer at this time because of the small number of high quality studies, results from individual studies shown in Web Figure 1D provide consistent support for a positive association with history of periodontitis. Two additional cohort studies that were not included in our review, as they did not have measures for periodontal disease, also provide indirect support for an association for periodontal disease and pancreatic cancer risk. In 1 large cohort study, a statistically significant 2-fold increase in risk of pancreatic cancer was observed for those who had dental plaque covering more than one-third of the tooth surface versus no dental plaque at the baseline examination (among those with at least 10 teeth remaining) (53). In the second study conducted in Sweden, an increase in risk for pancreatic cancer was observed for those with a history of oral cavity diseases (54).
Additional supportive evidence for an association with pancreatic cancer comes from studies examining oral bacteria that are believed to play a key role in the pathogenesis of periodontal disease (55). A recent cohort study reported positive associations between 2 periodontal pathogens, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, and pancreatic cancer using direct bacterial DNA measurement from saliva of individuals collected years prior to diagnosis (56). In addition, 2 studies have examined relationships between antibodies to periodontal oral pathogens and cancer risk. In a European cohort study, a statistically significant 2-fold increase in pancreatic cancer risk was observed for elevated levels of antibodies to P. gingivalis (57) and, in a separate cohort study in the United States, an association with the same oral pathogen was noted for orodigestive cancer mortality, which included pancreatic cancer cases (12). Biological mechanisms have been proposed that may explain the link among periodontal disease, oral pathogenic bacteria, and pancreatic carcinogenesis (58).
Studies available at this time do not support associations for periodontal disease and breast or stomach cancer risk (Web Figure 1). In addition, data were currently too sparse to critically examine periodontal disease associations for cancers not included in this study. For ovarian cancer, 1 large cohort study reported no positive associations using self-reported measures of periodontal disease and even suggested slightly inverse associations for younger women (59). No association was observed for prostate cancer in 1 cohort study (11), but a positive association was noted in a different cohort study using dental examination for the periodontal disease assessment and with mortality as the end point (HR = 1.47, 95% CI: 1.04, 2.07) (29). In a subset of the Health Professionals Follow-up Study, periodontal disease was positively associated with smoking-related cancers, including kidney and bladder cancers, among men who had reported being never smokers, and associations were substantially stronger when combining self-reported periodontal disease with tooth loss (30). Finally, a positive association was recently reported for periodontal disease and risk of non-Hodgkin lymphoma (60).
Potential role of confounding by genetic susceptibility
It is possible that the positive associations observed between periodontal disease and cancer risk are driven by shared genetic susceptibility between these 2 diseases. On the basis of twin studies, it has been estimated that as much as 50% of periodontitis is heritable (61). Large efforts and searches for genetic determinants of periodontitis, including several large genome-wide association studies, have failed to produce genetic associations for chronic periodontitis (61). Similarly, a recent exome variant analysis, aimed at identifying rare variants, was also unsuccessful at identifying genetic susceptibility variants for chronic periodontitis (62). In contrast, several genes have been associated with aggressive periodontitis, which develops at an early age, is a rarer type of periodontitis (63), and is associated with high severity of disease. Genes that have been consistently associated with aggressive periodontitis include the glycosyltransferase 6 domain containing 1 gene (GLT6D1), the CDKN2B antisense RNA 1 gene (ANRIL), and the cyclooxygenase 2 gene (COX2) (61). Variants in the COX2 gene have also been associated with risk of digestive cancers (64), and polymorphisms in the cyclin-dependent kinase inhibitor 2A and 2B genes (CDKN2A, CDKN2B) have been associated with nasopharyngeal cancer (65) and glioma (66). Therefore, although it is plausible that shared genetic susceptibility for periodontitis and cancer explains some of the associations observed, this would mostly be true if the associations observed were largely driven by aggressive periodontitis, given the lack of major susceptibility genes associated with chronic periodontitis. However, at this time, existing studies on cancer have not made the distinction between aggressive and chronic periodontitis, making it difficult to evaluate the potential role for confounding by underlying shared genetic factors.
Limitations with periodontal disease measurements in observational studies
Several limitations exist in observational studies on periodontal disease and cancer risk, and these become especially problematic in meta-analyses. Periodontal disease is extremely difficult to measure and quantify in observational studies as the assessment of periodontal disease requires several periodontal measurements, and clinical definitions have changed over time. These problems are not unique to epidemiologic studies evaluating the risk of cancer; standardized principles on the reporting of periodontal disease and using standardized clinical case definitions for population-based studies have been proposed (67, 68). Unfortunately, large cohort studies enrolling thousands of participants cannot conduct periodontal examinations on all subjects, and obtaining radiographs on all participants is not feasible in these studies. Consequently, existing cohort studies with larger numbers of cancer outcomes have suboptimal and relatively crude measures of periodontitis, including self-reported questions pertaining to oral health or number of teeth (50% of studies relied on self-reports of periodontal disease status) (Web Table 1). Radiographic bone loss is one of the defining criteria for the classification of periodontitis (69), yet only 3 out of 18 studies had dental radiographs on participants. In addition, periodontitis is a complex disease with multiple classifications that require a number of oral measurements, including clinical attachment loss, probing depth, and bone loss; periodontitis can manifest as chronic or aggressive disease, generalized or localized, and with different degrees of progression (i.e., slight, moderate, and severe periodontitis). The large heterogeneity in periodontal disease creates substantial complexity in defining exposure, especially because it is unclear which measures are more relevant to immune perturbations, dysbiosis, and possibly cancer risk. Moreover, when exposure is reported by using different measures or is reported on different scales, it becomes difficult to obtain summary measures of association; for example, exclusive reporting of continuous measures of clinical attachment loss prevented us from including some studies in the meta-analysis (37). Going forward, standardizing measurements and definitions for categories in studies examining cancer outcomes should allow meta-analyses with periodontal disease as an exposure to be more comparable and complete.
Recommendations for future studies to guide policy and intervention studies
Although the relative risks associated with periodontal disease are not very large, the associations observed are significant, from a public health standpoint, because of the high prevalence of periodontal disease in the general population. Improving periodontal disease measurement, definition, and reporting will be critical to solidifying the associations being reported in epidemiologic studies. Moreover, new opportunities for cancer prevention may arise if we are able to identify individuals or populations with elevated risk of cancer according to periodontal disease status; for example, smokers with severe periodontitis may be targeted for periodontal treatment or lung cancer screening. Similarly, better understanding the underlying mechanisms, including the role of microbiota in disease, may provide new opportunities for early detection and cancer screening. Finally, policy changes may include improving dental care and treatment of periodontal disease in high-risk populations by providing dental insurance or subsidized dental treatments.
Additional research efforts are needed to clarify the role of periodontal disease in cancer. These efforts will need to include conducting observational studies using standard protocols previously outlined by experts in the field (67), pooling individual-level data to obtain summary risk estimates (rather than performing study-level meta-analyses) in order to harmonize case definitions, and including biological markers to evaluate the role of the immune response and/or presence of pathogenic microbiota.
Conclusions
At this time, existing data provide support for positive associations between periodontal disease and risk of lung, pancreatic, and head and neck cancers. Despite the heterogeneity of measurements used to evaluate periodontal disease, consistency in findings for pancreatic and lung cancer risk is noteworthy, and, at least for pancreatic cancer, observed associations across studies did not appear to be due to confounding by smoking. The literature on this field is still relatively sparse and prevented us from conducting quality meta-analysis on most cancer sites. Future studies with larger samples sizes, improved measurements for periodontal disease, and more thorough adjustment for smoking and other risk factors should help to solidify the role of periodontal disease on cancer risk and assist with identification of individuals at higher risk. Moreover, clarification on the potential causal role of periodontal disease in cancer development is critical, as it may lead to new opportunities for cancer prevention and may warrant policy changes and recommendations for dental care access and coverage.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliation: Department of Public Health and Community Medicine, Tufts University School of Medicine, Boston, Massachusetts (Dominique S. Michaud, Zhuxuan Fu, Jian Shi, Mei Chung).
This work was supported by National Cancer Institute grant R01 CA166150.
Conflict of interest: none declared.
REFERENCES
- 1. Papadopoulos G, Kramer CD, Slocum CS, et al. . A mouse model for pathogen-induced chronic inflammation at local and systemic sites. J Vis Exp. 2014;(90):e51556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joshipura KJ, Wand HC, Merchant AT, et al. . Periodontal disease and biomarkers related to cardiovascular disease. J Dent Res. 2004;83(2):151–155. [DOI] [PubMed] [Google Scholar]
- 3. Ebersole JL, Machen RL, Steffen MJ, et al. . Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997;107(2):347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deng T, Lyon CJ, Bergin S, et al. . Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421–449. [DOI] [PubMed] [Google Scholar]
- 5. Koene RJ, Prizment AE, Blaes A, et al. . Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eke PI, Dye BA, Wei L, et al. . Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eke PI, Dye BA, Wei L, et al. . Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–920. [DOI] [PubMed] [Google Scholar]
- 8. Holtfreter B, Kocher T, Hoffmann T, et al. . Prevalence of periodontal disease and treatment demands based on a German dental survey (DMS IV). J Clin Periodontol. 2010;37(3):211–219. [DOI] [PubMed] [Google Scholar]
- 9. Eke PI, Genco RJ. CDC Periodontal Disease Surveillance Project: background, objectives, and progress report. J Periodontol. 2007;78(7 suppl):1366–1371. [DOI] [PubMed] [Google Scholar]
- 10. Bourgeois D, Bouchard P, Mattout C. Epidemiology of periodontal status in dentate adults in France, 2002–2003. J Periodontal Res. 2007;42(3):219–227. [DOI] [PubMed] [Google Scholar]
- 11. Michaud DS, Liu Y, Meyer M, et al. . Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9(6):550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33(5):1055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mai X, LaMonte MJ, Hovey KM, et al. . Periodontal disease severity and cancer risk in postmenopausal women: the Buffalo OsteoPerio Study. Cancer Causes Control. 2016;27(2):217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fitzpatrick SG, Katz J. The association between periodontal disease and cancer: a review of the literature. J Dent. 2010;38(2):83–95. [DOI] [PubMed] [Google Scholar]
- 15. Javed F, Warnakulasuriya S. Is there a relationship between periodontal disease and oral cancer? A systematic review of currently available evidence. Crit Rev Oncol Hematol. 2016;97:197–205. [DOI] [PubMed] [Google Scholar]
- 16. Ren HG, Luu HN, Cai H, et al. . Oral health and risk of colorectal cancer: results from three cohort studies and a meta-analysis. Ann Oncol. 2016;27(7):1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sadighi Shamami M, Sadighi Shamami M, Amini S. Periodontal disease and tooth loss as risks for cancer: a systematic review of the literature. Iran J Cancer Prev. 2011;4(4):189–198. [PMC free article] [PubMed] [Google Scholar]
- 18. Sayehmiri F, Sayehmiri K, Asadollahi K, et al. . The prevalence rate of Porphyromonas gingivalis and its association with cancer: a systematic review and meta-analysis. Int J Immunopathol Pharmacol. 2015;28(2):160–167. [DOI] [PubMed] [Google Scholar]
- 19. Wang RS, Hu XY, Gu WJ, et al. . Tooth loss and risk of head and neck cancer: a meta-analysis. PLoS One. 2013;8(8):e71122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeng XT, Luo W, Huang W, et al. . Tooth loss and head and neck cancer: a meta-analysis of observational studies. PLoS One. 2013;8(11):e79074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng XT, Deng AP, Li C, et al. . Periodontal disease and risk of head and neck cancer: a meta-analysis of observational studies. PLoS One. 2013;8(10):e79017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeng XT, Xia LY, Zhang YG, et al. . Periodontal disease and incident lung cancer risk: a meta-analysis of cohort studies. J Periodontol. 2016;87(10):1158–1164. [DOI] [PubMed] [Google Scholar]
- 23. Yao QW, Zhou DS, Peng HJ, et al. . Association of periodontal disease with oral cancer: a meta-analysis. Tumour Biol. 2014;35(7):7073–7077. [DOI] [PubMed] [Google Scholar]
- 24. Yin XH, Wang YD, Luo H, et al. . Association between tooth loss and gastric cancer: a meta-analysis of observational studies. PLoS One. 2016;11(3):e0149653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DerSimonian R, Laird N. Meta-analysis in clincial trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 26. Liu Q, Cook NR, Bergström A, et al. . A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose-response data. Comput Stat Data Anal. 2009;53(12):4157–4167. [Google Scholar]
- 27. Crippa A. dosresmeta: Performing Multivariate Dose-Response Meta-Analysis 1.3.2 ed. 2015.
- 28. Higgins JP, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arora M, Weuve J, Fall K, et al. . An exploration of shared genetic risk factors between periodontal disease and cancers: a prospective co-twin study. Am J Epidemiol. 2010;171(2):253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Michaud DS, Kelsey KT, Papathanasiou E, et al. . Periodontal disease and risk of all cancers among male never smokers: an updated analysis of the Health Professionals Follow-up Study. Ann Oncol. 2016;27(5):941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mai X, LaMonte MJ, Hovey KM, et al. . History of periodontal disease diagnosis and lung cancer incidence in the Women's Health Initiative Observational Study. Cancer Causes Control. 2014;25(8):1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hujoel PP, Drangsholt M, Spiekerman C, et al. . An exploration of the periodontitis-cancer association. Ann Epidemiol. 2003;13(5):312–316. [DOI] [PubMed] [Google Scholar]
- 33. Ansai T, Takata Y, Yoshida A, et al. . Association between tooth loss and orodigestive cancer mortality in an 80-year-old community-dwelling Japanese population: a 12-year prospective study. BMC Public Health. 2013;13:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tu YK, Galobardes B, Davey Smith G, et al. . Associations between tooth loss and mortality patterns in the Glasgow Alumni Cohort. Heart. 2007;93(9):1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hiraki A, Matsuo K, Suzuki T, et al. . Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1222–1227. [DOI] [PubMed] [Google Scholar]
- 36. Chung SD, Tsai MC, Huang CC, et al. . A population-based study on the associations between chronic periodontitis and the risk of cancer. Int J Clin Oncol. 2016;21(2):219–223. [DOI] [PubMed] [Google Scholar]
- 37. Tezal M, Sullivan MA, Hyland A, et al. . Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2406–2412. [DOI] [PubMed] [Google Scholar]
- 38. Moergel M, Kammerer P, Kasaj A, et al. . Chronic periodontitis and its possible association with oral squamous cell carcinoma—a retrospective case control study. Head Face Med. 2013;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moraes RC, Dias FL, Figueredo CM, et al. . Association between chronic periodontitis and oral/oropharyngeal cancer. Braz Dent J. 2016;27(3):261–266. [DOI] [PubMed] [Google Scholar]
- 40. Winn DM, Blot WJ, McLaughlin JK, et al. . Mouthwash use and oral conditions in the risk of oral and pharyngeal cancer. Cancer Res. 1991;51(11):3044–3047. [PubMed] [Google Scholar]
- 41. Talamini R, Vaccarella S, Barbone F, et al. . Oral hygiene, dentition, sexual habits and risk of oral cancer. Br J Cancer. 2000;83(9):1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garrote LF, Herrero R, Reyes RM, et al. . Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer. 2001;85(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guha N, Boffetta P, Wunsch Filho V, et al. . Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am J Epidemiol. 2007;166(10):1159–1173. [DOI] [PubMed] [Google Scholar]
- 44. Chang JS, Lo HI, Wong TY, et al. . Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013;49(10):1010–1017. [DOI] [PubMed] [Google Scholar]
- 45. Chang JS, Tsai CR, Chen LT, et al. . Investigating the association between periodontal disease and risk of pancreatic cancer. Pancreas. 2016;45(1):134–141. [DOI] [PubMed] [Google Scholar]
- 46. Wen BW, Tsai CS, Lin CL, et al. . Cancer risk among gingivitis and periodontitis patients: a nationwide cohort study. QJM. 2014;107(4):283–290. [DOI] [PubMed] [Google Scholar]
- 47. Abnet CC, Qiao YL, Mark SD, et al. . Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control. 2001;12(9):847–854. [DOI] [PubMed] [Google Scholar]
- 48. Abnet CC, Kamangar F, Dawsey SM, et al. . Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand J Gastroenterol. 2005;40(6):681–687. [DOI] [PubMed] [Google Scholar]
- 49. Freudenheim JL, Genco RJ, LaMonte MJ, et al. . Periodontal disease and breast cancer: prospective cohort study of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2016;25(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Castellarin M, Warren RL, Freeman JD, et al. . Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kostic AD, Chun E, Robertson L, et al. . Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kostic AD, Gevers D, Pedamallu CS, et al. . Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang J, Roosaar A, Axell T, et al. . A prospective cohort study on poor oral hygiene and pancreatic cancer risk. Int J Cancer. 2016;138(2):340–347. [DOI] [PubMed] [Google Scholar]
- 54. Ljung R, Sjoberg Bexelius T, Martin L, et al. . Oral disease and risk of pancreatic cancer. Epidemiology. 2011;22(5):749–750. [DOI] [PubMed] [Google Scholar]
- 55. Nishihara T, Koseki T. Microbial etiology of periodontitis. Periodontol 2000. 2004;36:14–26. [DOI] [PubMed] [Google Scholar]
- 56. Fan X, Alekseyenko AV, Wu J, et al. . Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study [published online ahead of print October 14, 2016]. Gut. (doi:10.1136/gutjnl-2016-312580). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Michaud DS, Izard J, Wilhelm-Benartzi CS, et al. . Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62(12):1764–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Michaud DS. Role of bacterial infections in pancreatic cancer. Carcinogenesis. 2013;34(10):2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Babic A, Poole EM, Terry KL, et al. . Periodontal bone loss and risk of epithelial ovarian cancer. Cancer Causes Control. 2015;26(6):941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bertrand KA, Shingala J, Evens A, et al. . Periodontal disease and risk of non-Hodgkin lymphoma in the Health Professionals Follow-Up Study. Int J Cancer. 2017;140(5):1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vaithilingam RD, Safii SH, Baharuddin NA, et al. . Moving into a new era of periodontal genetic studies: relevance of large case-control samples using severe phenotypes for genome-wide association studies. J Periodontal Res. 2014;49(6):683–695. [DOI] [PubMed] [Google Scholar]
- 62. Kasbohm E, Holtfreter B, Völker U, et al. . Exome variant analysis of chronic periodontitis in 2 large cohort studies. J Dent Res. 2016;96(1):73–80. [DOI] [PubMed] [Google Scholar]
- 63. Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dong J, Dai J, Zhang M, et al. . Potentially functional COX-2 -1195G>A polymorphism increases the risk of digestive system cancers: a meta-analysis. J Gastroenterol Hepatol. 2010;25(6):1042–1050. [DOI] [PubMed] [Google Scholar]
- 65. Bei JX, Li Y, Jia WH, et al. . A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet. 2010;42(7):599–603. [DOI] [PubMed] [Google Scholar]
- 66. Shete S, Hosking FJ, Robertson LB, et al. . Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41(8):899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Holtfreter B, Albandar JM, Dietrich T, et al. . Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: proposed standards from the Joint EU/USA Periodontal Epidemiology Working Group. J Clin Periodontol. 2015;42(5):407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7 suppl):1387–1399. [DOI] [PubMed] [Google Scholar]
- 69. American Academy of Periodontology Task Force report on the update to the 1999 classification of periodontal diseases and conditions. J Periodontol. 2015;86(7):835–838. [DOI] [PubMed] [Google Scholar]
- 70. Michaud DS, Joshipura K, Giovannucci E, et al. . A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99(2):171–175. [DOI] [PubMed] [Google Scholar]
- 71. Kabat GC, Hebert JR, Wynder EL. Risk factors for oral cancer in women. Cancer Res. 1989;49(10):2803–2806. [PubMed] [Google Scholar]
- 72. Zheng TZ, Boyle P, Hu HF, et al. . Dentition, oral hygiene, and risk of oral cancer: a case-control study in Beijing, People's Republic of China. Cancer Causes Control. 1990;1(3):235–241. [DOI] [PubMed] [Google Scholar]
- 73. Bundgaard T, Wildt J, Frydenberg M, et al. . Case-control study of squamous cell cancer of the oral cavity in Denmark. Cancer Causes Control. 1995;6(1):57–67. [DOI] [PubMed] [Google Scholar]
- 74. Rosenquist K, Wennerberg J, Schildt EB, et al. . Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125(12):1327–1336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.