Abstract
Objective
The clinical significance of polymorphisms in the interleukin-28B gene encoding interferon (IFN)-λ3, which has antiviral effects, is known in chronic HCV but not in HBV infection. Thus, we measured IFN-λ3 levels in patients with HBV and investigated its clinical significance and association with nucleos(t)ide (NUC) analogue administration.
Design
Serum IFN-λ3 level was measured in 254 patients with HBV with varying clinical conditions using our own high sensitivity method. The resulting values were compared with various clinical variables. In addition, cell lines originating from various organs were cultured with NUCs, and the production of IFN-λ3 was evaluated.
Results
Higher serum IFN-λ3 levels were detected in the patients treated with nucleotide analogues (adefovir or tenofovir) compared with those treated with nucleoside analogues (lamivudine or entecavir). There were no other differences in the clinical background between the two groups. A rise in the serum IFN-λ3 levels was observed during additional administration of the nucleotide analogues. In vitro experiments showed that the nucleotide analogues directly and dose-dependently induced IFN-λ3 production only in colon cancer cells. Furthermore, the supernatant from cultured adefovir-treated colon cancer cells significantly induced IFN-stimulated genes (ISGs) and inhibited hepatitis B surface antigen (HBsAg) production in hepatoma cells, as compared with the supernatant from entecavir-treated cells.
Conclusions
We discovered that the nucleotide analogues show an additional pharmacological effect by inducing IFN-λ3 production, which further induces ISGs and results in a reduction of HBsAg production. These findings provide novel insights for HBV treatment and suggest IFN-λ3 induction as a possible target.
Keywords: CHRONIC VIRAL HEPATITIS, INTERFERON, ANTIVIRAL THERAPY
Significance of this study.
What is already known on this subject?
Both nucleoside and nucleotide analogues inhibit the HBV reverse transcriptase function; however, their other effects are unknown.
Clinical importance of the interleukin-28B gene, which encodes interferon (IFN)-λ3, has been established in HCV infection but not HBV infection.
What are the new findings?
Serum IFN-λ3 levels increased after administration of nucleotide analogues in both patients with HBV and HIV, suggesting that the nucleotide analogues themselves were considered to induce IFN-λ3.
Among the various tested cell lines that potentially produce IFN-λ3, the nucleotide analogues were found to directly induce IFN-λ3 at the mRNA and protein levels only in colon cancer cells.
The supernatant (containing induced IFN-λ3) from adefovir-treated colon cancer cells robustly induced IFN-stimulated genes (ISGs) and suppressed hepatitis B surface antigen production in hepatoma cell lines.
How might it impact on clinical practice in the foreseeable future?
We discovered a novel additional pharmacological effect of nucleotide analogues involving the induction of IFN-λ3 production in GI cells, which in turn induced ISGs in hepatic cells. The discovery of this mechanism is a new advancement for HBV treatment, suggesting the possibility of developing an anti-HBV treatment targeting IFN-λ3 induction.
Introduction
Over 240 million people are infected with HBV worldwide. HBV is a life-threatening infectious virus involving a risk of cirrhosis and hepatocellular carcinoma (HCC), and it is responsible for more than 786 000 deaths annualy.1 2 Nucleos(t)ide analogues (NUCs) safely control the HBV replication and thus reduce the risk of HCC development3 and HBV-associated mortality.4 However, the effects of NUCs on serum hepatitis B surface antigen (HBsAg) levels are limited because NUCs have no direct effect on covalently closed circular DNA, which acts as a viral template.2 Therefore, long-term treatment is necessary for HBsAg loss,5 which is correlated with low incidence of HCC.6 On the other hand, treatment with interferon (IFN)-α is a finite course that has direct anti-HBV and immunomodulatory effects. However, only limited cases have been reported that achieved HBsAg loss.7 8 Consequently, the inability to completely eliminate HBV is a problem associated with current treatments.5 9 Thus, new treatment strategies are desired.
Using a genome-wide association study (GWAS), we and other researchers have identified single-nucleotide polymorphisms in the promoter region of the interleukin-28B gene (IL-28B) to be strongly associated with the effect of hepatitis C combination therapy with pegylated IFN-α (PEG-IFN)/ribavirin.10–12 On the other hand, although there have been a number of reports on the association between hepatitis B and IL-28B gene polymorphisms, they have not led to consistent opinions.13–15 These studies are not easily comparable because of heterogeneous patient backgrounds, such as different HBV genotypes or different treatment regimens. We hypothesised, however, that the inconsistent results might have been mainly due to investigation performed at the gene level because gene expression is generally affected by transcriptional or post-transcriptional modifications. Thus, in this study we analysed the IFN-λ3 protein levels in patients with HBV. We focused specifically on IFN-λ3 because it was IL-28B that was identified in the hepatitis C GWAS, and not IL-29 (IFN-λ1) or IL-28A (IFN-λ2).10–12 Furthermore, IFN-λ3 induces IFN-stimulated genes (ISGs) more strongly and has a more pronounced antiviral effect than IFN-λ1 or IFN-λ2.16
At present, there exist three independent reports on serum IFN-λ levels.17–19 However, the data have not led to a unified conclusion because different assays were used for measurements and there were only few subjects. Therefore, we developed our own IFN-λ3 measurement system, which detects IFN-λ3 in a highly sensitive and specific manner. Additionally, it exhibits clear linearity in the 1–100 pg/mL range, which corresponds to serum IFN-λ3 concentrations.20
In this study, we measured serum IFN-λ3 levels in patients with HBV using this assay and investigated the clinical significance of IFN-λ3 and its association with NUC administration.
Materials and methods
Patients
Serum and DNA samples were obtained from 254 consecutive Japanese patients with HBV who visited the National Center for Global Health and Medicine, Kohnodai Hospital, Yamanashi University Hospital, or Shinshu University Hospital between January 2011 and March 2013. All patients who agreed to participate in this study were included, and all were negative for HCV. For serial measurements of IFN-λ3, frozen sera from our cohort of patients with HBV were used. A separate series of sera from patients with HIV infection, receiving antiretroviral therapy with or without tenofovir disoproxil fumarate (TDF), was obtained from the AIDS Clinical Center, National Center for Global Health and Medicine. All serum and DNA samples were stored at −80°C until use.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from all patients.
IL28B genotyping, and measurement of HBsAg and HBV DNA
See the online supplementary materials and methods.
gutjnl-2016-312653supp001.pdf (550.9KB, pdf)
Measurement of IFN-λ1, IFN-λ2, IFN-λ3, IFN-α and IFN-β
IFN-λ3 level was measured in the serum and supernatants using a chemiluminescence enzyme immunoassay developed in-house, as previously reported.19 20 See the online supplementary materials and methods for IFN-λ1, IFN-λ2, IFN-α and IFN-β.
Cell culture
Various cell lines, originating from lymphocytes (Raji), skin (HKA-1 and HSC-5), lungs (A-549, EBC-1 and RERF-LC-sq1), stomach (AZ-521), liver (HepG2, Huh7 and PLC/PRF/5) and colon (WiDr and HT-29), were maintained in Dulbecco's modified Eagle's medium (DMEM) (Wako Pure Chemicals, Osaka, Japan) supplemented with 10% fetal calf serum or in Roswell Park Memorial Institute medium (RPMI) (Life Technologies, Grand Island, New York, USA). HT-29 cells were purchased from the American Type Culture Collection (Manassas, Virginia, USA), and the rest were purchased from the Japanese Cancer Research Resources Bank (Osaka, Japan). Peripheral blood mononuclear cells (PBMCs) from healthy volunteers were separated by density-gradient centrifugation, and PXB cells (Phoenix Bio, Hiroshima, Japan) were obtained from chimerical mice with humanised livers. Adherent and suspension cells were seeded at a density of 1×105 cells and 1×106 cells per well, respectively. Each cell line was incubated with lamivudine (LAM, 50 μM), adefovir pivoxil (ADV, 2.5 μM), entecavir (ETV, 0.25 μM) or TDF (50 μM) for 48 hours. LAM was purchased from Sigma-Aldrich (St Louis, Missouri, USA) and the other NUCs were purchased from Adooq Bioscience (Irvine, California, USA). Each molar NUC concentration was determined based on the regular clinical oral dose used for patients with HBV. Dimethyl sulfoxide (DMSO, 0.1%) was used as a negative control. IFN-α (100 U/mL; Otsuka Pharmaceutical, Tokyo, Japan), with or without 30 μg/mL poly I:C (Imgenex, San Diego, California, USA), a toll-like receptor 3 (TLR3) agonist, or 5 μg/mL R-837 (Imgenex), a TLR7 agonist, was used as a positive control. To determine the dose dependency, we used 1:3 dilutions of each NUC and positive controls. To evaluate the effect of IFN-α on IFN-λ3 production, WiDr or HT-29 cells were treated with each NUC with or without IFN-α (100 U/mL) for 48 hours. IFN-α (10 U/mL, 100 U/mL, 1000 U/mL) was used as a positive control.
Immunohistochemistry
WiDr cells were cultured in a chamber slide (Nunc, Rochester, New York, USA) with 0.1% DMSO, 50 μM LAM, 2.5 μM ADV, 0.25 μM ETV or 50 μM TDF under the above conditions for 48 hours. After drying, the cells were fixed with 4% paraformaldehyde, and endogenous peroxidase was blocked using 0.3% H2O2. An avidin-biotin complex kit was obtained from Vector Laboratories (Burlingame, California, USA). After blocking the slides with horse serum, a primary mouse anti-IFN-λ3 antibody (TA2664; 1:100), originally generated in our laboratory,20 was applied and reacted overnight at 4°C. For colour development, 0.05% (w/v) 3,3′-diamino-benzidine tetrahydrochloride (Sigma-Aldrich) was used. The cells were counterstained with haematoxylin.
Quantitative PCR for IFN-λ3
See the online supplementary materials and methods.
Analysis of ISGs
To analyse the expression of ISGs, HepG2 and Huh7 cells (1×105 cells per well) were incubated with a medium containing 0.1% DMSO or with a supernatant from WiDr cell cultures treated with 2.5 μM ADV or 0.25 μM ETV. The cells were harvested at 0 hour, 3 hours, 6 hours, 12 hours and 24 hours of stimulation, and total RNA was extracted using an RNeasy mini kit (Qiagen, Valencia, California, USA). First-strand cDNA synthesis was performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, California, USA), according to the manufacturer's protocol. The primer-probe sets for the IFN-induced dynamin-like GTPase (MX1), 2′-5′-oligoadenylate synthetase 2 (OAS2) and β-actin genes were obtained from the TaqMan assay reagent library (Applied Biosystems).
Inhibition of HBsAg production in PLC/PRF/5 cells
To analyse the effect of IFN-λ3 on HBsAg levels, PLC/PRF/5 cells (5×104 cells per well) were treated with various concentrations of recombinant IFN-λ3 (R&D Systems, Minneapolis, Minnesota, USA), with DMSO control medium, and with the supernatants of ADV-treated and ETV-treated WiDr cells for 3 days. HBsAg in the cell supernatants was quantified.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
See the online supplementary materials and methods.
Statistical analyses
Mann-Whitney U test and Kruskal-Wallis test were used to compare continuous data between patient subgroups. Pearson's χ2 test and Fisher's exact test were used to compare non-parametrical categorical data. Pearson's product-moment correlation was used to evaluate the relationship between two variables. All tests were performed using the IBM SPSS Statistics Desktop for Japan, V.19.0 (IBM Japan, Tokyo, Japan) and p values <0.05 were considered statistically significant.
Results
Patients' background
Serum and DNA samples were obtained from patients with HBV infection, including asymptomatic carriers (ASCs, n=83) and patients with chronic hepatitis (CH, n=103), liver cirrhosis (LC, n=25) and HCC (n=43). HCC was observed in 10 patients with CH and 33 patients with LC. NUCs were administered to 80 CH, 22 LC and 40 HCC cases. The NUC treatments were as follows: LAM (n=6), ETV (n=111), TDF (n=1), LAM+ADV (n=22), ETV+ADV (n=1) and multiple NUCs (n=1). Patients who were treated with IFN-α at the time of sampling were excluded. The serum albumin, cholinesterase, total cholesterol, HBsAg levels and platelet counts were lower in the patients with advanced liver disease. An advanced age and the male sex were correlated with advanced liver disease (table 1). We compared IL-28B gene polymorphisms among the patients at different clinical stages and found that TT, the major homozygous genotype of IL-28B, was less prevalent in patients at more advanced stages of the disease (80.7%, 73.8%, 52.0% and 79.1% in the ASCs and patients with CH, LC and HCC, respectively, p=0.031).
Table 1.
Patient characteristics (n=254)
| ASC | CH | LC | HCC | p Value | |
|---|---|---|---|---|---|
| n | 83 | 103 | 25 | 43 | |
| Age (years)* | 50 (26–83) | 49 (19–85) | 62 (35–74) | 63 (41–86) | <0.001 |
| Sex (M:F) | 36:47 | 63:40 | 18:7 | 31:12 | 0.002 |
| HBV genotype (A:B:C:D:ND) |
1:15:60:2:5 | 4:19:73:1:6 | 0:2:22:0:1 | 0:1:36:0:6 | NS |
| HBeAg+† | 2 (2.4%) | 31 (30.1%) | 8 (32.0%) | 9 (20.9%) | <0.001 |
| Alb (g/dL)* | 4.5 (3.2–5.2) | 4.4 (3.3–5.3) | 4.4 (2.1–4.6) | 4.2 (2.1–5.1) | <0.001 |
| AST (U/L)* | 21 (11–87) | 22 (13–238) | 24 (14–153) | 25 (14–99) | 0.006 |
| ALT (U/L)* | 18 (9–93) | 20 (8–375) | 18 (11–110) | 19 (8–57) | NS |
| ChE (U/L)* | 338 (189–848) | 314 (127–578) | 278 (74–382) | 265 (38–443) | <0.001 |
| T-cho (mg/dL)* | 194 (130–297) | 186 (94–315) | 171 (101–48) | 169 (85–257) | 0.013 |
| BUN (mg/dL)* | 12 (7–40) | 13 (1–39) | 14 (11–26) | 16 (9–31) | <0.001 |
| Cre (mg/dL)* | 0.65 (0.36–3.65) | 0.77 (0.37–6.2) | 0.78 (0.43–1.33) | 0.77 (0.45–1.99) | 0.002 |
| WBC (×102/μL)* | 50 (28–150) | 48 (21–93) | 44 (28–94) | 42 (15–85) | NS |
| Hb (g/dL)* | 13.8 (9.2–16.7) | 14.3 (9.9–18.0) | 14.0 (8.6–17.4) | 13.4 (8.7–16.4) | NS |
| Plt (×104/μL)* | 19.7 (9.5–49.7) | 17.8 (6.3–32.2) | 10.7 (4.7–20.3) | 11.7 (2.5–29.3) | <0.001 |
| AFP (ng/mL)* | 2.5 (0.7–80.1) | 2.5 (0.8–417.4) | 2.6 (1.5–23.0) | 3.0 (0.8–4834) | NS |
| HBsAg (IU/mL)* | 572 (1.1–64 510) | 1130 (1–50 460) | 823 (1–4326) | 199 (1–3212) | 0.001 |
| HBV DNA* (log copies/mL) |
3.2 (UD–9.0) | 2.1 (UD–9.1) | 2.1 (UD–7.3) | UD (UD–6.8) | <0.001 |
| IL-28B (TT:non-TT) | 67:16 | 76:27 | 13:12 | 34:9 | 0.031 |
| No treatment | 83 | 23 | 3 | 3 | <0.001 |
| Treatment with NUCs | 0 | 80 | 22 | 40 |
*Data are presented as the median (range).
†Data are presented as a positive number (%).
AFP, α-fetoprotein; Alb, albumin; ALT, alanine aminotransferase; ASC, asymptomatic carrier; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CH, chronic hepatitis; ChE, cholinesterase; Cre, creatine; Hb, haemoglobin; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; LC, liver cirrhosis; ND, not determined; NS, non-significant; NUC, nucleos(t)ide analogue; Plt, platelet count; T-cho, total cholesterol; TT, major homozygous genotype of IL-28B; UD, under detection threshold; WBC, white blood cell.
Serum IFN-λ3 levels in patients with HBV
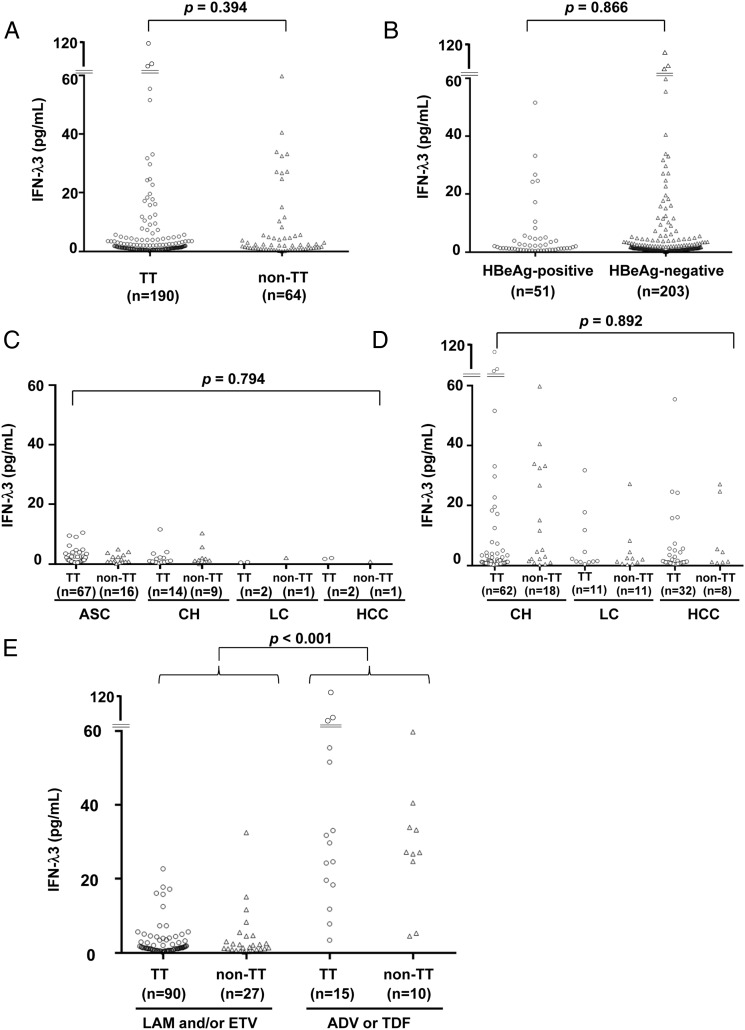
We compared serum IFN-λ3 levels in the patients with different IL-28B genotypes (TT vs non-TT; figure 1A) and hepatitis B e antigen statuses (positive vs negative; figure 1B) but did not find differences between the respective groups. We also investigated a correlation between the serum IFN-λ3 levels and the disease progression. In all treatment-naïve patients, the serum IFN-λ3 levels were no greater than 20 pg/mL, and there was no difference depending on the clinical condition (n=112; figure 1C). The serum IFN-λ3 levels showed a weak negative correlation with age (correlation coefficient (CC)=−0.205) and weak positive correlations with HBsAg (CC=0.278) and HBV DNA (CC=0.215) levels; however, no correlation was observed with the other test values (see online supplementary table S1). No significant changes in the serum IFN-λ3 levels were observed during the course of acute hepatitis B (see online supplementary figure S1A and B). Taken together, these results suggest that serum IFN-λ3 levels do not reflect the pathogenesis of HBV. However, compared with the treatment-naïve patients (figure 1C), higher serum IFN-λ3 levels were found in the patients undergoing NUC treatment (figure 1D).
Figure 1.
Serum interferon (IFN)-λ3 levels in patients with HBV (n=254). Serum IFN-λ3 levels in patients with different IL-28B genotypes (A) and HBeAg positivity statuses (B). Serum IFN-λ3 levels and the disease statuses (C) in treatment-naïve patients (n=112) and (D) in patients treated with nucleos(t)ide analogues (NUCs) (n=142). (E) Serum IFN-λ3 levels in patients treated with different NUCs (n=142). ADV, adefovir pivoxil; ASC, asymptomatic carrier; CH, chronic hepatitis; ETV, entecavir; HBeAg, hepatitis B e antigen; HCC, hepatocellular carcinoma; LAM, lamivudine; LC, liver cirrhosis; TDF, tenofovir disoproxil fumarate; TT, major homozygous genotype of IL-28B.
Serum IFN-λ3 levels depending on NUC type
We compared serum IFN-λ3 levels among the patients undergoing treatment with the different types of NUCs (n=142). Higher serum IFN-λ3 levels were found in the patients treated with the nucleotide analogues (ADV or TDF; 27.2 pg/mL (range: 3.4–113.2), n=25) compared with those treated with the nucleoside analogues (LAM or ETV; 1.4 pg/mL (range: 0.1–32.5), n=117) (figure 1E). These associations were not found in serum levels of IFN-λ1 or IFN-λ2 (see online supplementary figure S2). The clinical backgrounds of the patents in the two groups revealed no differences, except the serum IFN-λ3 levels and the duration of NUC treatment (table 2). However, there was no correlation between the NUC treatment duration and serum IFN-λ3 levels (see online supplementary figure S3A and B).
Table 2.
Clinical backgrounds by different NUCs treatment (n=142)
| LAM and/or ETV | ADV or TDF | p Value | |
|---|---|---|---|
| N | 117 | 25 | |
| Age (years)* | 59 (31–86) | 62 (37–80) | NS |
| Sex (M:F) | 78:39 | 14:11 | NS |
| HBV genotype (A:B:C:D:ND) |
2:15:92:0:8 | 0:2:21:0:2 | NS |
| Alb (g/dL)* | 4.4 (2.1–5.1) | 4.4 (3.3–5.3) | NS |
| AST (U/L)* | 22 (13–238) | 21 (16–45) | NS |
| ALT (U/L)* | 19 (8–373) | 18 (8–54) | NS |
| ChE (U/L)* | 296 (38–578) | 279 (151–443) | NS |
| T-cho (mg/dL)* | 179 (85–315) | 158 (113–226) | NS |
| BUN (mg/dL)* | 14 (6–39) | 17 (1–25) | NS |
| Cre (mg/dL)* | 0.77 (0.37–2.41) | 0.79 (0.47–1.99) | NS |
| Plt (×104/μL)* | 15.6 (2.5–26.3) | 16.4 (8.1–28.7) | NS |
| AFP (ng/mL)* | 2.5 (0.8–4834) | 2.1 (0.8–5.6) | NS |
| HBsAg (IU/mL)* | 826 (1–47 205) | 464 (1–8150) | NS |
| HBeAg+† | 34 (29.1%) | 5 (20.0%) | NS |
| HBV DNA (log copies/mL)* | UD (UD–8.4) | UD (UD–3.7) | NS |
| IL-28B (TT:non-TT) | 90:27 | 15:10 | NS |
| CH:LC | 72:45 | 17:8 | NS |
| HCC† | 34 (29.1%) | 6 (24.0%) | NS |
| Duration of NUC treatment* (months) |
37 (1–135) | 92 (17–148) | <0.001 |
| Serum IFN-λ3 (pg/mL)* | 1.4 (0.1–32.5) | 27.2 (3.4–113.2) | <0.001 |
*Data are presented as the median (range).
†Data are presented as a positive number (%).
ADV, adefovir pivoxil; AFP, α-fetoprotein; Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CH, chronic hepatitis; ChE, cholinesterase; Cre, creatine; ETV, entecavir; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; IFN, interferon; IL-28B, interleukin 28B; LAM, lamivudine; LC, liver cirrhosis; ND, not determined; NS, non-significant; NUC, nucleos(t)ide analogue; Plt, platelet count; T-cho, total cholesterol; TDF, tenofovir disoproxil fumarate; TT, major homozygous genotype of IL-28B; UD, under detection threshold.
Changes in serum IFN-λ3 values before and during NUC treatment
To confirm the association between nucleotide analogue treatment and IFN-λ3, we measured serum IFN-λ3 levels in serial samples obtained before and during NUC treatment. Serial serum samples were conserved for 20 patients treated with LAM and/or ETV, 19 cases treated with an ADV-containing regimen, one case treated with TDF alone, and one case treated with multiple NUCs as described below. No elevation of the serum IFN-λ3 levels was observed during the treatment with LAM and/or ETV (figure 2A, C). However, the serum IFN-λ3 levels rose with the start of ADV (figure 2B, D) or TDF (figure 2E) treatment. The data for the patient treated with various different NUCs are shown in figure 2F. His serum IFN-λ3 level was high during the treatment with LAM+ADV when he first visited our hospital. However, after the ADV administration was stopped due to the onset of renal damage, his IFN-λ3 level suddenly declined and remained low during the treatment with LAM+ETV. After switching to TDF due to the appearance of ETV-resistant viral clones, his serum IFN-λ3 level rose again and then declined slightly after the TDF dose was reduced.
Figure 2.
Serial measurements of serum interferon (IFN)-λ3 levels during treatment with different nucleos(t)ide analogues (NUCs). Changes in serum IFN-λ3 levels in representative patients during (A) lamivudine (LAM)-containing and/or entecavir (ETV)-containing (n=20) or (B) adefovir pivoxil (ADV)-containing regimes (n=19). Serum IFN-λ3 levels are shown in representative patients treated with (C) ETV alone (a 58-year-old man, HBeAg+, genotype C, IL-28B TT), (D) additional ADV (a 67-year-old man, HBeAg+, genotype C, IL-28B TT), (E) tenofovir disoproxil fumarate (TDF) alone (a 42-year-old man, HBeAg−, genotype D) and (F) multiple NUCs (a 78-year-old man, HBeAg−, genotype C, IL-28B TT). (G) Serum IFN-λ3 levels in patients with HIV infection before and during anti-HIV treatment. The black, grey and dotted lines represent patients with HIV alone with TDF treatment, HIV and HBV co-infection with TDF treatment, and HIV alone without TDF treatment, respectively. Three representative patients are shown per group. ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBeAg, hepatitis B e antibody; M, month, y, year.
Next, we investigated whether this phenomenon would also be observed in patients with HIV treated with NUCs. As expected, the serum IFN-λ3 levels rose after initiating treatment with TDF, but no such elevation was observed in the patients treated without TDF (figure 2G, see online supplementary figure S4A–C). These observations strongly suggested that the nucleotide analogues are themselves responsible for the induction of IFN-λ3, regardless of the presence of HBV or HIV infection.
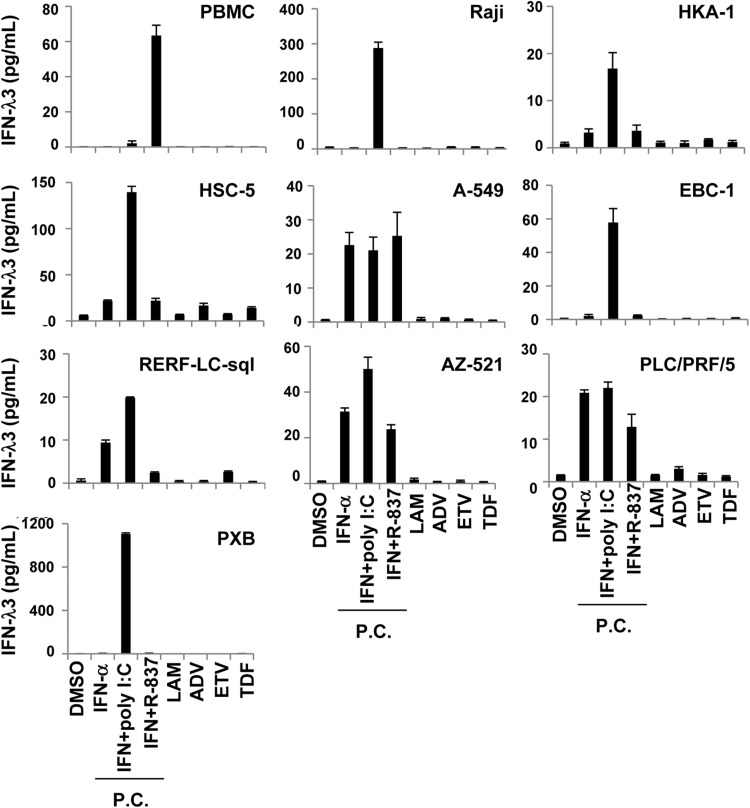
IFN-λ3 was not induced by nucleotide analogues in a number of cell types
We sought to confirm that the nucleotide analogues induce IFN-λ3 production in vitro. In this experiment, we used cell lines derived from organs previously reported to produce IFN-λ3 (lymphocytes, skin, lungs, stomach and liver).21 As primary cells, we used PBMCs sampled from healthy adults and hepatocytes sampled from chimerical mice with humanised livers. IFN-λ3 was produced in at least one of the three positive controls (see Materials and methods) for all cells tested, suggesting that all the cells used had the potential to produce IFN-λ3. However, none of the NUCs induced IFN-λ3 in any of the cells tested (figure 3). IFN-λ3 was not induced in HepG2 or Huh7 cells relative to any of the positive controls (see online supplementary figure S5).
Figure 3.
Confirmation of interferon (IFN)-λ3 production induced by nucleos(t)ide analogues. Each cell line, originating from lymphocytes (Raji), skin (HKA-1 and HSC-5), lung (A-549, EBC-1 and RERF-LC-sql), stomach (AZ-521), liver (PLC/PRF/5) and peripheral blood mononuclear cells (PBMCs) or PXB cells were incubated with dimethyl sulfoxide (DMSO, 0.1%), lamivudine (LAM, 50 μM), adefovir pivoxil (ADV, 2.5 μM), entecavir (ETV, 0.25 μM) or tenofovir disoproxil fumarate (TDF, 50 μM). After 48 h, the supernatants were subjected to a chemiluminescence enzyme immunoassay for IFN-λ3. IFN-α (100 U/mL), with or without poly I:C (30 μg/mL) or R-837 (5 μg/mL), was used as a positive control. PC, positive controls.
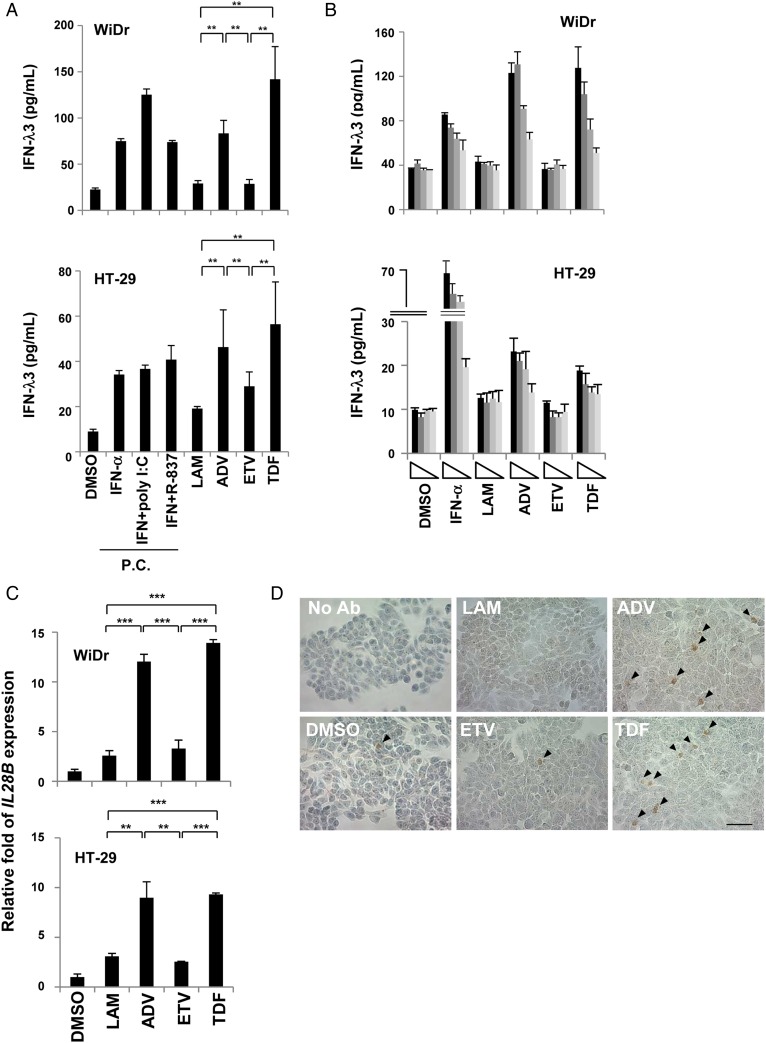
Nucleotide analogues induce IFN-λ3 only in colon cancer cell lines
Next, we used colon cancer cell lines because NUCs are administered orally and thus enter the GI tract. IFN-λ3 induction was detected in the supernatants from WiDr and HT-29 cells treated with the nucleotide analogues (figure 4A). On the other hand, the results obtained with the nucleoside analogues were comparable to those obtained with DMSO, which was the negative control. Similar tendencies were observed in IFN-λ1 and IFN-λ2 levels in the supernatant (see online supplementary figure S6). ADV and TDF induced IFN-λ3 in a dose-dependent manner (figure 4B). These results were similar to those obtained with the patients' sera. Furthermore, IL-28B mRNA was significantly upregulated by the nucleotide analogue treatments in both WiDr and HT-29 cells (figure 4C). Immunohistochemistry showed clear positive staining for IFN-λ3 in the cytoplasm, and a higher frequency of IFN-λ3-positive cells was found among the WiDr cells treated with the nucleotide analogues compared with those treated with DMSO or the nucleoside analogues (figure 4D). When no primary antibody was used, no positive staining for IFN-λ3 was observed. As indicated in figure 4A, IFN-α induced IFN-λ3 in the colon cancer cell lines, and the possibility that the NUCs indirectly induced IFN-λ3 via IFN-α or IFN-β induction could not be ruled out. Thus, in order to rule this out, we measured IFN-α and IFN-β in the same supernatant shown in figure 4A. However, the data showed that none of the NUCs induced IFN-α or IFN-β (see online supplementary figure S7A and B, respectively). Taken together, these results suggest that ADV and TDF directly induced IFN-λ3 in the colon cancer cell lines.
Figure 4.
Nucleotide analogues directly induce interferon (IFN)-λ3 in colon cancer cells. (A) Colon cancer cells (WiDr and HT-29) were incubated with dimethyl sulfoxide (DMSO, 0.1%), lamivudine (LAM, 50 μM), adefovir pivoxil (ADV, 2.5 μM), entecavir (ETV, 0.25 μM) or tenofovir disoproxil fumarate (TDF, 50 μM). After 48 hours, the supernatants were subjected to a chemiluminescence enzyme immunoassay for IFN-λ3. IFN-α (100 U/mL), with or without poly I:C (30 μg/mL) or R-837 (5 μg/mL), was used as a positive control. (B) WiDr and HT-29 cells were treated with each nucleos(t)ide analogue (NUC) diluted 1:3 (maximum concentration of each NUC is shown above). (C) WiDr and HT-29 cells were treated under the same conditions as above for 24 hours, and total RNA was extracted. The IL-28B mRNA expression level was determined using real-time PCR, as described in the online supplementary materials and methods. At least three independent experiments were conducted in triplicate, and representative data are shown. (D) WiDr cells were treated with 0.1% DMSO, 50 μM LAM, 2.5 μM ADV, 0.25 μM ETV or 50 μM TDF for 48 hours, and immunohistochemistry was performed. ‘No Ab’ indicates that the cells were treated without the primary antibody. Arrowheads indicate positive staining. Scale bar represents 50 μm. *p<0.05, **p<0.01 and ***p<0.001. PC, positive controls.
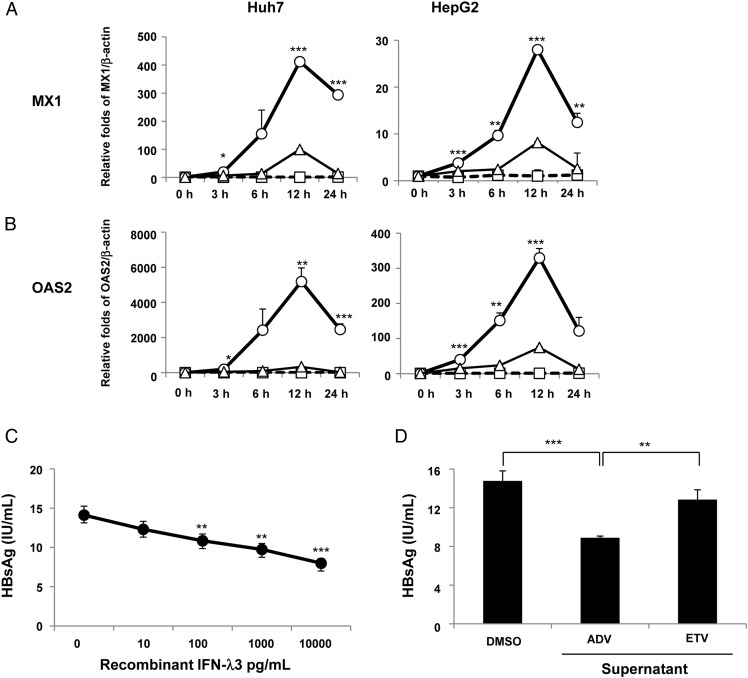
Induction of ISGs in hepatoma cells by the supernatant of ADV-treated colon cancer cells
We evaluated whether the supernatants obtained from the cultures of the ADV-treated or ETV-treated WiDr cells could induce ISGs in HepG2 or Huh7 cells. Unlike the DMSO control medium and the supernatants from the ETV-treated cells, the supernatant from the WiDr cells treated with ADV for 48 hours robustly upregulated both MX1 (figure 5A) and OAS2 (figure 5B), which peaked at 12 hours in both HepG2 and Huh7 cells.
Figure 5.
Upregulation of interferon-stimulated genes (ISGs) and inhibition of hepatitis B surface antigen (HBsAg) production by the supernatants of nucleos(t)ide analogue-treated WiDr cells. HepG2 and Huh7 cells were treated using a medium containing dimethyl sulfoxide (DMSO, 0.1%) or supernatants from adefovir pivoxil (ADV, 2.5 μM)-treated WiDr cells or entecavir (ETV, 0.25 μM)-treated WiDr cells, and mRNA expression levels of IFN-induced dynamin-like GTPase (MX1) (A) and 2′-5′-oligoadenylate synthetase 2 (OAS2) (B) were determined at each time point using a real-time PCR. The circles (○), triangles (△) and squares (□) represent the supernatants from 2.5 μM ADV-treated WiDr cells, 0.25 μM ETV-treated WiDr cells or the medium containing 0.1% DMSO, respectively. The interferon (IFN)-λ3 levels in the supernatants of the ADV-treated WiDr cells and ETV-treated WiDr cells were 113.3 pg/mL and 43.7 pg/mL, respectively. (C) Three days after the treatment of PLC/PRF/5 cells with or without recombinant IFN-λ3 (0 pg/mL, 10 pg/mL, 100 pg/mL, 1000 pg/mL and 10 000 pg/mL) or (D) with the supernatants from 2.5 μM ADV-treated WiDr cells, 0.25 μM ETV-treated WiDr cells or the medium containing 0.1% DMSO, HBsAg levels were examined in the supernatants. The IFN-λ3 levels in the supernatants of the ADV-treated WiDr cells and ETV-treated WiDr cells were 107.3 pg/mL and 25.4 pg/mL, respectively. At least three independent experiments were performed in triplicate, and representative data are shown. *p<0.05, **p<0.01 and ***p<0.001. PC, positive controls.
Reduction of HBsAg production in PLC/PRF/5 cells by recombinant IFN-λ3 and the supernatant from ADV-treated colon cancer cells
We evaluated whether recombinant IFN-λ3 and IFN-λ3 induced by ADV would inhibit the HBsAg production in PLC/PRF/5 cells, which constitutively produce HBsAg, but not HBV DNA. Recombinant IFN-λ3 dose-dependently inhibited HBsAg production in PLC/PRF/5 cells (figure 5C) and, in particular, significantly decreased HBsAg production at a concentration of 100 pg/mL, which corresponds to the serum IFN-λ3 level in the patients treated with the nucleotide analogues. Furthermore, the supernatant from the WiDr cells treated with ADV for 48 hours significantly inhibited HBsAg production in PLC/PRF/5 cells, as compared with the DMSO control medium or the supernatants from the WiDr cells treated with ETV (figure 5D). These reductions in HBsAg production are unlikely to be caused by cell death because neither recombinant IFN-λ3 nor the supernatant affected the cell viability as measured by the MTT assay (see online supplementary figure S8A and B).
Discussion
In order to elucidate the clinical significance of IFN-λ3 for hepatitis B, we measured serum IFN-λ3 levels in patients with HBV with varying clinical conditions. We discovered that serum IFN-λ3 was upregulated by nucleotide analogue administration. In cell culture experiments, we found that the nucleotide analogues directly induced IFN-λ3 in colon cancer cell lines. Furthermore, the induced IFN-λ3 in turn induced ISGs in hepatoma cells and inhibited HBsAg production. Based on these results, we conclude that the nucleotide analogues show a novel additional pharmacological effect of inducing IFN-λ3.
NUCs target only the action of reverse transcriptase, which transcribes the pregenomic RNA to HBV DNA,5 and their other effects are unknown. The predicted time of the HBsAg loss during NUC administration depends on the NUC type, with TDF estimated to have the shortest time.22 These differences among the NUCs have been explained by their different potencies in inhibition of reverse transcriptase.22 Meanwhile, it has been reported that TDF prevents the simian immunodeficiency virus infection,23 and that no incidental acute HBV infections were found in patients with HIV during the course of TDF treatment, although the prevention was not complete when using LAM.24 These clinical phenomena could be explained by the novel additional effect of the nucleotide analogues in inducing IFN-λ3 and consequently inducing ISGs, as shown in the current study.
IFN-λs, also known as type III IFNs, have different receptors compared with type I IFNs (IFN-α and IFN-β). However, the two types of IFNs use similar intracellular signalling pathways and have similar antiviral activity.21 25 26 Although it has not been completely explained why hosts require IFNs with such similar functions,21 one of the characteristic features of type III IFNs is organ-specific expression of their receptors. Receptors for type I IFNs exist ubiquitously, whereas those for type III IFNs are largely restricted to cells of epithelial origin, excluding dendritic cells.21 27 From this perspective, there is a distinct possibility that type III IFNs contribute to mucosal immunity as a first line of defence against viral invasion. The level of ISG expression induced by an IFN-λ is correlated with the level of type III IFN receptor expression, with the GI tract being an organ with one of the strongest reactions to IFN-λ.27 In fact, protection from intestinal rotavirus infection was severely impaired in mice lacking a functional type III IFN receptor,28 and exogenous IFN-λ administration ameliorated murine rotavirus and norovirus infections, even in the absence of adaptive immunity.28 29 Furthermore, rotavirus infection has been observed to induce IFN-λ in intestinal cells.29 This evidence supports the possibility that the nucleotide analogues induce IFN-λ3 in GI cells, as observed in this study. The precise mechanisms of IFN-λ3 induction by nucleotide analogues in the GI tracts are unknown. However, pattern-recognition receptors might recognise nucleotide analogues as pathogen-associated molecular patterns and induce IFN-λ3 as the first line of defence.30
This phenomenon of colon IFN-λ3 induction by the nucleotide analogues is clinically important because IFN-λ3 induced in the GI tract reaches the liver via the portal vein. IFN-λ directly inhibits the replication of HBV31 and dengue virus.32 IFN-λ also induces ISGs,25 which contribute to inhibition of viral mRNA translation, as well as to RNA degradation and synthesis.33 The current study demonstrated that the induced IFN-λ3 further induced ISGs in hepatoma cell lines. Furthermore, recombinant IFN-λ3 and the supernatant from ADV-treated WiDr cells significantly inhibited HBsAg production in PLC/PRF/5 cells. Therefore, IFN-λ3 reaching the liver via the portal vein could exhibit an anti-HBV effect directly or indirectly, via ISGs. Thus, this phenomenon could be an immunomodulatory effect caused by the nucleotide analogues themselves, as noted in a recent review.9 However, we cannot rule out the possibility that the supernatant from the ADV-treated colon cancer cells may have contained other substances, besides IFN-λ3, contributing to the inhibition of HBsAg production, which deserves further study.
ETV and TDF both similarly decrease the HBV DNA levels with low rates of antiviral drug resistance. However, HBsAg loss was achieved only in a small portion of patients on single-drug treatment.5 34 In other words, even if TDF provides the novel additional effect demonstrated in the current study, the effect from a single-drug treatment is not sufficient, and additional strategies are required for HBsAg loss. Type I and type III IFNs exhibit different kinetics of ISG induction. The ISG induction by type I IFNs is strong but transient, whereas type III IFNs cause less strong but more sustained ISG induction.16 Thus, co-administration of these two types of cytokines is expected to result in a complementary antiviral effect. In addition, IFN-α had an additive effect on IFN-λ3 production with nucleotide analogues in vitro (see online supplementary figure S9A and B). These enhanced IFN-λ3 levels together with IFN-α might further enhance ISG expression. Therefore, a combination of nucleotide analogues with PEG-IFN could theoretically be a promising treatment method. A direct comparison is difficult due to the differences in clinical background factors and treatment regimens, but actual simultaneous administration of PEG-IFN and ADV or TDF has resulted in a high probability of HBsAg loss,35 36 whereas co-administration of PEG-IFN and first-generation NUCs, especially LAM, has generated results similar to those obtained with PEG-IFN alone.7 8 Prospective studies to demonstrate the upregulation of ISGs in the liver and its association with HBsAg changes during treatment with nucleotide analogues or combination with PEG-IFN-α would be warranted to confirm our hypothesis.
IFNL4 is associated with treatment-response in chronic hepatitis C as well as IL28B.37 A strong linkage disequilibrium between rs8099917 (IL28B) and rs368234815 (IFNL4) was reported in the Japanese population (γ2=0.94).38 In addition, functional IFN-λ4 is eliminated through pseudogenisation in most individuals of Japanese descent.39 Therefore, we did not investigate IFNL4 and IFN-λ4 in this study. However, further studies are needed in the European and the African populations because the allele frequency of rs368234815 in the Japanese population was quite different from what was obtained in those populations as shown in the 1000 Genomes data set39 and the HapMap samples (see online supplementary table S2).
In conclusion, we discovered a novel additional pharmacological effect of orally administered nucleotide analogues, which were found to directly induce IFN-λ3 in GI cells. Consequently, IFN-λ3 causes the induction of ISGs, which in turn results in the inhibition of HBsAg production in hepatic cells. This discovery may provide new insights into anti-HBV therapy and suggests the possibility of developing an anti-HBV therapy targeting IFN-λ3 induction.
Acknowledgments
The authors thank Editage (http://www.editage.jp) for English language editing.
Footnotes
Contributors: KM and MM conceived and designed the experiments. AM, ET, TI, MS, NE, MH, SK, HG, SO, YS and HY collected patient samples and performed the analyses. KM, MA, MS, NN, TS, MH, YIK and TD performed the experiments. KM and MM wrote the manuscript.
Funding: This study was supported by the National Center for Global Health and Medicine in Japan (27-1302), Grants-in-Aid for Scientific Research (23390117), and Japan Agency for Medical Research and Development (15fk0210035h0001).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Local Ethics Committee at each associated institute (reference number NCGM-A-000208-0).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. WHO. Media Centre, Hepatitis B, 2014. Update http://www.who.int/mediacentre/en/
- 2. Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. Lancet 2014;384:2053–63. 10.1016/S0140-6736(14)60220-8 [DOI] [PubMed] [Google Scholar]
- 3. Liaw YF, Sung JJ, Chow WC, et al. . Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521–31. 10.1056/NEJMoa033364 [DOI] [PubMed] [Google Scholar]
- 4. Wong GL, Chan HL, Mak CW, et al. . Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 2013;58:1537–47. 10.1002/hep.26301 [DOI] [PubMed] [Google Scholar]
- 5. Liang TJ, Block TM, McMahon BJ, et al. . Present and future therapies of hepatitis B: From discovery to cure. Hepatology 2015;62:1893–908. 10.1002/hep.28025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simonetti J, Bulkow L, McMahon BJ, et al. . Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology 2010;51:1531–7. 10.1002/hep.23464 [DOI] [PubMed] [Google Scholar]
- 7. Marcellin P, Lau GK, Bonino F, et al. . Peginterferon α-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–17. 10.1056/NEJMoa040431 [DOI] [PubMed] [Google Scholar]
- 8. Lau GKK, Piratvisuth T, Luo KX, et al. . Peginterferon α-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95. 10.1056/NEJMoa043470 [DOI] [PubMed] [Google Scholar]
- 9. Rehermann B, Bertoletti A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology 2015;61:712–21. 10.1002/hep.27323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanaka Y, Nishida N, Sugiyama M, et al. . Genome-wide association of IL28B with response to pegylated interferon-α and ribavirin therapy for chronic hepatitis C. Nat Genet 2009;41:1105–9. 10.1038/ng.449 [DOI] [PubMed] [Google Scholar]
- 11. Ge D, Fellay J, Thompson AJ, et al. . Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009;461:399–401. 10.1038/nature08309 [DOI] [PubMed] [Google Scholar]
- 12. Suppiah V, Moldovan M, Ahlenstiel G, et al. . IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009;41:1100–4. 10.1038/ng.447 [DOI] [PubMed] [Google Scholar]
- 13. Sonneveld MJ, Wong VW, Woltman AM, et al. . Polymorphisms near IL28B and serologic response to peginterferon in HBeAg-positive patients with chronic hepatitis B. Gastroenterology 2012;142:513–20. 10.1053/j.gastro.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 14. Lampertico P, Viganò M, Cheroni C, et al. . IL28B polymorphisms predict interferon-related hepatitis B surface antigen seroclearance in genotype D hepatitis B e antigen-negative patients with chronic hepatitis B. Hepatology 2013;57:890–6. 10.1002/hep.25749 [DOI] [PubMed] [Google Scholar]
- 15. Martin MP, Qi Y, Goedert JJ, et al. . IL28B polymorphism does not determine outcomes of hepatitis B virus or HIV infection. J Infect Dis 2010;202:1749–53. 10.1086/657146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bolen CR, Ding S, Robek MD, et al. . Dynamic expression profiling of type I and type III interferon-stimulated hepatocytes reveals a stable hierarchy of gene expression. Hepatology 2014;59:1262–72. 10.1002/hep.26657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Groen RA, Mcphee F, Friborg J, et al. . Endogenous IFNλ in viral hepatitis patients. J Interferon Cytokine Res 2014;34:552–6. 10.1089/jir.2013.0068 [DOI] [PubMed] [Google Scholar]
- 18. Diegelmann J, Beigel F, Zitzmann K, et al. . Comparative analysis of the lambda-interferons IL-28A and IL-29 regarding their transcriptome and their antiviral properties against hepatitis C virus. PLoS One 2010;5:e15200 10.1371/journal.pone.0015200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aoki Y, Sugiyama M, Murata K, et al. . Association of serum IFN-λ3 with inflammatory and fibrosis markers in patients with chronic hepatitis C virus infection. J Gastroenterol 2015;50:894–902. 10.1007/s00535-014-1023-2 [DOI] [PubMed] [Google Scholar]
- 20. Sugiyama M, Kimura T, Naito S, et al. . Development of specific and quantitative real-time detection PCR and immunoassay for λ3-interferon. Hepatol Res 2012;42:1089–99. 10.1111/j.1872-034X.2012.01032.x [DOI] [PubMed] [Google Scholar]
- 21. Kotenko SV. IFN-λs. Curr Opin Immunol 2011;23:583–90. 10.1016/j.coi.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boglione L, D'Avolio A, Cariti G, et al. . Kinetics and prediction of HBsAg loss during therapy with analogues in patients affected by chronic hepatitis B HBeAg negative and genotype D. Liver Int 2013;33:580–5. 10.1111/liv.12091 [DOI] [PubMed] [Google Scholar]
- 23. Tsai CC, Follis KE, Sabo A, et al. . Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science 1995;270:1197–9. 10.1126/science.270.5239.1197 [DOI] [PubMed] [Google Scholar]
- 24. Gatanaga H, Hayashida T, Tanuma J, et al. . Prophylactic effect of antiretroviral therapy on hepatitis B virus infection. Clin Infect Dis 2013;56:1812–19. 10.1093/cid/cit145 [DOI] [PubMed] [Google Scholar]
- 25. Kotenko SV, Gallagher G, Baurin VV, et al. . IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 2003;4:69–77. 10.1038/ni875 [DOI] [PubMed] [Google Scholar]
- 26. Sheppard P, Kindsvogel W, Xu W, et al. . IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 2003;4:63–8. 10.1038/ni873 [DOI] [PubMed] [Google Scholar]
- 27. Sommereyns C, Paul S, Staeheli P, et al. . IFN-lambda (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog 2008;4:e1000017 10.1371/journal.ppat.1000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pott J, Mahlakõiv T, Mordstein M, et al. . IFN-λ determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci USA 2011;108:7944–8. 10.1073/pnas.1100552108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nice TJ, Baldridge MT, McCune BT, et al. . Interferon-λ cures persistent murine norovirus infection in the absence of adaptive immunity. Science 2015;347:269–73. 10.1126/science.1258100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilkins C, Gale M Jr. Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol 2010;22:41–7. 10.1016/j.coi.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol 2005;79:3851–4. 10.1128/JVI.79.6.3851-3854.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palma-Ocampo HK, Flores-Alonso JC, Vallejo-Ruiz V, et al. . Interferon lambda inhibits dengue virus replication in epithelial cells. Virol J 2015;12:150 10.1186/s12985-015-0383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev 2001;14:778–809, table of contents 10.1128/CMR.14.4.778-809.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lok AS, Trinh H, Carosi G, et al. . Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naïve patients with chronic hepatitis B. Gastroenterology 2012;143:619–28.e1. 10.1053/j.gastro.2012.05.037 [DOI] [PubMed] [Google Scholar]
- 35. Wursthorn K, Lutgehetmann M, Dandri M, et al. . Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology 2006;44:675–84. 10.1002/hep.21282 [DOI] [PubMed] [Google Scholar]
- 36. Marcellin P, Ahn SH, Ma X, et al. . Combination of tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology 2016;150:134–44.e10. 10.1053/j.gastro.2015.09.043 [DOI] [PubMed] [Google Scholar]
- 37. Prokunina-Olsson L, Muchmore B, Tang W, et al. . A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet 2013;45:164–71. 10.1038/ng.2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ochi H, Miki D, Hayes CN, et al. . IFNL4/IL28B haplotype structure and its impact on susceptibility to hepatitis C virus and treatment response in the Japanese population. J Gen Virol 2014;95:1297–306. 10.1099/vir.0.060103-0 [DOI] [PubMed] [Google Scholar]
- 39. Key FM, Peter B, Dennis MY, et al. . Selection on a variant associated with improved viral clearance drives local, adaptive pseudogenization of interferon lambda 4 (IFNL4). PLoS Genet 2014;10:e1004681 10.1371/journal.pgen.1004681 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2016-312653supp001.pdf (550.9KB, pdf)