Abstract
Anti-TNFα therapy, known to suppress T-cell immunity, is increasingly gaining popularity for treatment of autoimmune diseases including inflammatory bowel diseases (IBD). T-cell suppression increases the risk of B-cell EBV-lymphoproliferative diseases and lymphomas. Since EBV-lytic activation is essential for development of EBV-lymphomas and there have been reports of EBV-lymphomas in patients treated with anti-TNFα therapy, we investigated if patients treated with anti-TNFα antibodies demonstrate greater EBV-lytic activity in blood. Peripheral blood mononuclear cells from 10 IBD patients solely on anti-TNFα therapy compared to 3 control groups (10 IBD patients not on immunosuppressive therapy, 10 patients with abdominal pain but without IBD, and 10 healthy subjects) were examined for the percentage of T-cells, EBV load and EBV-lytic transcripts. Patients on anti-TNFα therapy had significantly fewer T-cells, greater EBV load, and increased levels of transcripts from EBV-lytic genes of all kinetic classes compared to controls. Furthermore, exposure of EBV-infected B-cell lines to anti-TNFα antibodies resulted in increased levels of BZLF1 mRNA; BZLF1 encodes for ZEBRA, the viral latency-to-lytic cycle switch. Thus, IBD patients treated with anti-TNFα antibodies have greater EBV loads likely due to enhanced EBV-lytic gene expression and anti-TNFα antibodies may be sufficient to activate the EBV lytic cycle. Findings from this pilot study lay the groundwork for additional scientific and clinical investigation into the effects of anti-TNFα therapy on the life cycle of EBV, a ubiquitous oncovirus that causes lymphomas in the setting of immunocompromise.
Keywords: inflammatory bowel disease, anti-TNFα, Epstein-Barr virus, lytic activation, lymphomas
INTRODUCTION
Epstein-Barr virus (EBV), a ubiquitous γ-herpesvirus found in greater than 90% of the adult population [Sankaran-Walters et al., 2011], is associated with 1% of tumors worldwide [Michelow et al., 2012; Dowd et al., 2013]. EBV is etiologically linked to several malignancies including nasopharyngeal carcinoma, Burkitt lymphoma, and various forms of B-cell lymphomas including Hodgkin and non-Hodgkin lymphomas [Crawford, 2001]. The most common type of EBV-lymphomas in the western world occurs in the setting of T cell-immunocompromise, most often after hematopoietic or solid organ transplantation [Gottschalk et al., 2005]. The risk of such lymphomas is also increased in patients with autoimmune diseases such as rheumatoid arthritis and inflammatory bowel diseases (IBD) who are treated with T cell-immunosuppressive/immunomodulatory therapies [Kamel et al., 1993; Dayharsh et al., 2002; Sokol et al., 2012]. While immunomodulatory agents such as methotrexate, azathiopurine and 6-mercaptopurine have been the mainstay of therapy for autoimmune diseases, anti-TNFα antibodies are now being used with increasing frequency [Kozuch and Hanauer, 2008].
Use of anti-TNFα antibodies alone or in combination with other immunomodulatory agents has been found to be associated with an increased risk of non-Hodgkin lymphomas in IBD patients compared to patients treated with immunomodulatory agents alone [Siegel et al., 2009; Herrinton et al., 2011]. While several cases of EBV-lymphomas following anti-TNFα therapy have been described [Komatsuda et al., 2008; Park et al., 2008; Mariette et al., 2010], whether treatment with anti-TNFα antibodies is associated with an increased risk of EBV-lymphomas is unclear, particularly since several of these patients were simultaneously treated with other immunomodulatory agents. Furthermore, evidence for increase in blood EBV-load following anti-TNFα therapy is conflicting [Lavagna et al., 2007; Fernandez Salazar et al., 2013; Magro et al., 2013], and its contribution to EBV lytic activation, if any, is unknown.
Similar to other herpesviruses, EBV can exist in a latent or lytic state [Knipe and Howley, 2013]. Periodically, EBV undergoes lytic activation when the majority of viral genes including the immediate early, early and late lytic genes are expressed in a specific kinetic order to replicate the viral genome and produce infectious virus [Knipe and Howley, 2013]. This switch from latency to lytic cycle is mediated by two immediate-early genes, BZLF1 and BRLF1 which code for the transcriptional activating proteins, ZEBRA and RTA, respectively. Activation of this viral lytic replication pathway is important for the development of EBV-related malignancies. Indeed lytic gene expression was seen in 92% of biopsies of EBV-lymphoproliferative diseases/lymphomas in immunosuppressed patients [Montone et al., 1996]. Furthermore, methotrexate, a medication frequently used to treat patients with rheumatoid arthritis, was shown to promote EBV-positive lymphomas by its immunosuppressive properties as well as by activating lytic cycle of EBV [Feng et al., 2004]. Moreover, mice with severe combined immunodeficiency did not develop EBV-lymphoproliferative diseases/lymphomas if EBV lytic gene activation was defective [Hong et al., 2005], underscoring the importance of EBV lytic activation for development of EBV-lymphomas during immunocompromise.
In this pilot study we examined the relationship between anti-TNFα therapy and EBV lytic activation in blood cells of patients with IBD treated with anti-TNFα antibodies alone, i.e., in the absence of other immunomodulatory agents. We report higher EBV loads and increased EBV lytic gene expression in peripheral blood cells of IBD patients on anti-TNFα therapy compared to untreated IBD patients and two other control groups. We also demonstrate that anti-TNFα antibodies are sufficient to induce the EBV lytic cycle in vitro.
METHODS
Study Subjects
The study of human subjects was approved by the appropriate Institutional Review Board at Stony Brook University. A total of 40 EBV-seropositive subjects (positive for IgG antibodies to Epstein-Barr Nuclear Antigen and Viral Capsid Antigen) ranging in age from 13 years to 62 years were recruited if they fell into one of the four following categories: no medical problems (10 subjects), abdominal pain but found via upper endoscopy and colonoscopy not to have IBD (10 subjects), IBD who had never been on immunosuppressive agents (10 subjects), IBD solely on infliximab for 6 or more months (10 subjects). Patients with concurrent diagnoses unrelated to IBD were excluded from the study.
PBMC Isolation
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation from 30–35 ml of heparinized venous blood using lymphocyte separation medium (Ficoll-Hypaque; ICN) according to a standardized protocol [Bhaduri-McIntosh et al., 2008]. PBMC were counted and subjected to flow cytometry, qPCR and qRT-PCR.
Culture Conditions
EBV-infected B cell lines (lymphoblastoid cell lines) were generated from healthy subjects as described previously [Hui-Yuen et al., 2011]. Cells were cultured at 37°C under 5% CO2 in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin sodium, and 100 μg/ml streptomycin sulfate. For the experiment using anti-TNFα antibodies, cells were sub-cultured, treated with 8 μg/ml (equivalent to therapeutic doses in patients) anti-TNFα antibodies (InvivoGen) or control human IgG1 24 hr later, and harvested for qRT-PCR after another 24 hr.
Flow Cytometry
PBMC were subjected to flow cytometry as previously described [Bhaduri-McIntosh et al., 2008]. Briefly, cells were fixed and permeabilized using Cytofix/Cytoperm (BD Pharmingen, San Diego, CA) and then incubated with saturating amounts of either APC-conjugated anti-CD2 antibody (BD Pharmingen) or matched isotype control antibody. Samples were acquired using FACSCalibur (BD) and data analyzed using Flow Jo software (Treestar Inc, Ashland, OR), after gating on live cells based on forward scatter and side scatter values. Cells were determined to be CD2+ after comparing with isotype control-stained cells.
Quantitative PCR
DNA was extracted from PBMC using the QIAamp DNA blood kit (Qiagen) according to manufacturer’s instructions. EBV load was quantified based on a standard curve PCR generated using plasmid 2089 [Feederle et al., 2000] and primers designed to amplify the BALF5 gene; standard qPCR curve gave linear detection over 5 logs of target concentrations. Plasmid 2089 comprises the B95.8 EBV genome cloned into an F-factor plasmid. Primer sequences to amplify BALF5 were as follows: forward primer 5′CGGAAGCCCTCTGGACTTC3′; reverse primer 5′CCCTGTTTATCCGATGGAATG3′.
Quantitative RT-PCR
Total RNA was extracted from PBMC using the RNeasy kit (Qiagen) followed by DNase digestion (Promega) and cDNA synthesis as described previously [Hill et al., 2013]. Levels of lytic gene transcripts were determined by real-time reverse transcriptase-PCR (RT-PCR) with gene-specific primers using the iScript SYBR green RT-PCR kit (Bio-Rad). Relative transcript levels were calculated using the ΔΔCT method after normalization to 18S rRNA. Assays on individual samples were performed in triplicate. Primer sequences for 18S, BMRF1 and BFRF3 were described previously [Hill et al., 2013]. Sequences of other primers were as follows: BZLF1: forward primer 5′TTCCACAGCCTGCACCAGTG3′; reverse primer 5′GGCAGCAGCCACCTCACGGT3′; BRLF1: forward primer 5′ACTCCCGGCTGTAAATTC CT3′; reverse primer 5′CCATACAGGACACAACACCT CA3′.
Statistical Analyses
P values were calculated by comparing the means of two groups of interest using unpaired Student t test.
Ethical Considerations
Informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
RESULTS
Characteristics of IBD Patients and Control Subjects
Our goal was to investigate EBV load and lytic activation in the blood of patients treated with anti-TNFα antibodies alone. We therefore recruited IBD patients treated with anti-TNFα antibodies without the confounding presence of immunomodulatory agents. Furthermore, we only recruited patients who had been on anti-TNFα therapy for at least 6 months. Table I shows that compared to control groups, patients on anti-TNFα therapy had a preponderance of males. The mean ages were similar except for IBD control subjects who were younger (P ≤0.01). Both groups with IBD were similar with regard to type of disease (Crohn’s Disease [CD] versus Ulcerative Colitis [UC]). While IBD controls had carried their diagnosis for a shorter period of time, by an average of 13 months (P ≤0.03), the number of IBD patients in remission was the same in both groups and none had severe disease activity. IBD patients on infliximab (anti-TNFα antibodies) had been on this medication for a mean duration of 28 ± 12 months.
TABLE I.
Characteristics of Study Subjects
| Healthy (n = 10) | Abd pain, no IBD (n = 10) | IBD control (n = 10) | IBD on anti-TNFα (n = 10) | |
|---|---|---|---|---|
| Sex | ||||
| Male | 1 | 4 | 6 | 8 |
| Female | 9 | 6 | 4 | 2 |
| Age (Mean ± SD) | 34 ± 8 years | 26 ± 13 years | 23 ± 8 years | 33 ± 13 years |
| Types of IBD | ||||
| CD | ------------- | 6 | 4 | |
| UC | 4 | 6 | ||
| Duration of disease (Average months ± SD) | ------------- | 23 ± 12 | 36 ± 12 | |
| Severity of disease* | ------------- | Remission 6 Mild 3 Moderate 1 | Remission 6 Mild 1 Moderate 3 | |
| Duration of anti-TNFα use | ---------------- | 28 ± 12 months | ||
CD, Crohn’s Disease; UC, ulcerative colitis.
Measured by Physician’s Global Assessment.
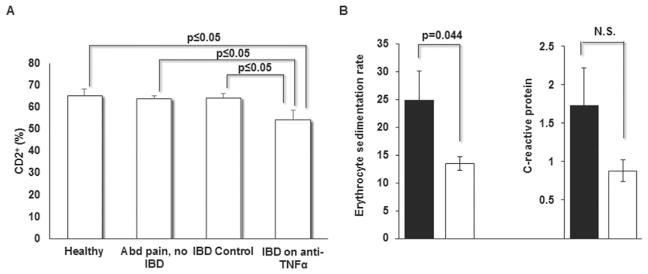
Since infliximab has been shown to suppress T cell activation and proliferation [Dahlen et al., 2013], we enumerated the frequency of peripheral T and NK cells as a general assessment of cellular immune status. As expected, flow cytometry of immunostained PBMC revealed that IBD patients on infliximab had an average of 16% fewer CD2+ cells compared to the other three groups (P ≤0.05) (Fig. 1). Sub-group analysis of the three patients on infliximab with moderate disease activity showed that these patients had on average only 8.5% fewer CD2+ cells compared to the control subjects. This is consistent with the observation that reduced frequencies of activated T cells correspond with treatment response to anti-TNFα antibodies [Dahlen et al., 2013]. There was also significant improvement in the erythrocyte sedimentation rate following treatment with anti-TNFα antibodies; however, the corresponding fall in C - reactive protein was not statistically significant.
Fig. 1.
IBD patients on anti-TNFα have fewer T and NK cells and improvement in inflammatory markers. PBMC from patients and control subjects were immunostained and CD2+ cells enumerated by FACS in A. Inflammatory markers, erythrocyte sedimentation rate and c-reactive protein, before (black bars) and after (open bars) anti-TNFα therapy are shown in B. Data represent mean +/− SEM.
IBD Patients Treated With Anti-TNFα Antibodies Demonstrate Higher EBV Load
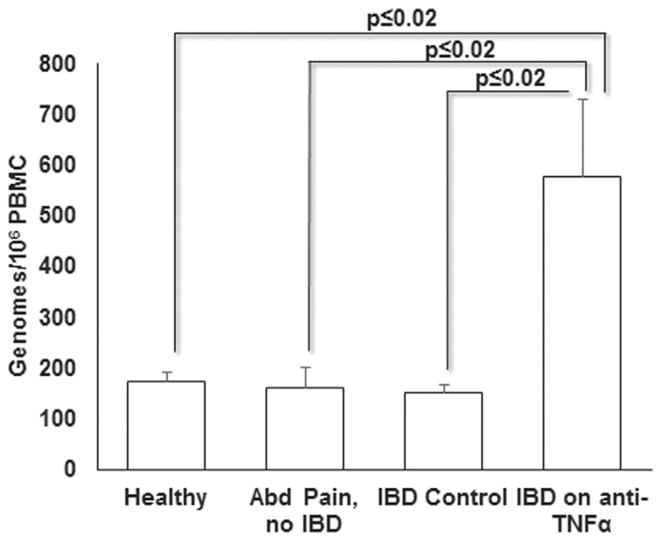
To determine EBV load in PBMC, we amplified the EBV DNA polymerase gene BALF5 using qPCR. Although EBV genomes were detected in all subjects recruited in the study, IBD patients on anti-TNFα antibodies had on average 578 genomes (range 116–1341 genomes) per 106 PBMC as compared to the other three groups which collectively had on average 161 genomes (range 5–430 genomes) per 106 PBMC (Fig. 2). This corresponded to a > 3.5-fold increase in viral load in the anti-TNFα group, a statistically significant increase compared to the EBV load in each of the three control groups. The three patients with moderate disease activity on infliximab had an average of 386 genomes per 106 PBMC which was below the study group average but still greater than the control groups.
Fig. 2.
IBD patients on anti-TNFα antibodies demonstrate greater EBV load. EBV load (mean genome copies +/− SEM) per 106 PBMC was determined in patients and control subjects by qPCR using the standard curve method.
IBD Patients on Anti-TNFα Antibodies Demonstrate Higher Levels of EBV Lytic Gene Transcripts Belonging to All Kinetic Classes
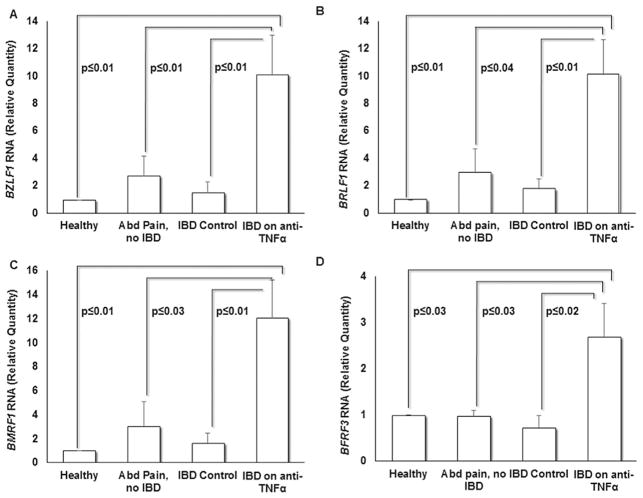
As IBD patients on anti-TNFα antibodies revealed greater EBV load, we asked whether this increase in EBV genomes stemmed from increased EBV lytic activation. We therefore compared relative levels of EBV lytic gene transcripts amongst the four study groups by amplifying mRNA derived from the EBV immediate early lytic genes BZLF1 and BRLF1, early lytic gene BMRF1, and late lytic gene BFRF3. These genes were chosen as representatives of the three kinetic classes of EBV lytic genes. We found that on average, IBD patients on anti-TNFα antibodies had greater than 5.5-fold increase in BZLF1 and BRLF1 transcript levels compared to the three control groups (P ≤0.01) (Fig. 3A–B). BZLF1 and BRLF1, encoding for transcription factors ZEBRA and RTA respectively, mediate EBV latency-to-lytic switch [Knipe and Howley, 2013]. Transcript levels of BMRF1 and BFRF3 were on average sixfold and threefold higher, respectively in the anti-TNFα group compared to the other study groups (P ≤0.04) (Fig. 3C–D). The early lytic gene BMRF1, which is transcriptionally activated by ZEBRA, encodes for the DNA polymerase processivity factor (Early Antigen-Diffuse) and is essential for EBV replication [Neuhierl and Delecluse, 2006]. BFRF3 is a late lytic gene encoding a structural capsid protein which aids in packaging EBV DNA leading to the release of infectious EBV particles [Serio et al., 1997]. Thus, in addition to demonstrating higher EBV loads, patients on anti-TNFα also demonstrated higher levels of EBV lytic transcripts.
Fig. 3.
Greater EBV lytic gene expression is seen in IBD patients on anti-TNFα antibodies. PBMC were subjected to qRT-PCR using primers targeting immediate early lytic genes BZLF1 (A) and BRLF1 (B), the early lytic gene BMRF1 (C) and the late lytic gene BFRF3 (D). Results represent means of relative amounts of RNA normalized to 18S rRNA levels +/− standard errors of the means of three technical replicates.
Anti-TNFα Antibodies Activate the Lytic Molecular Switch in B Cells Latently Infected With EBV
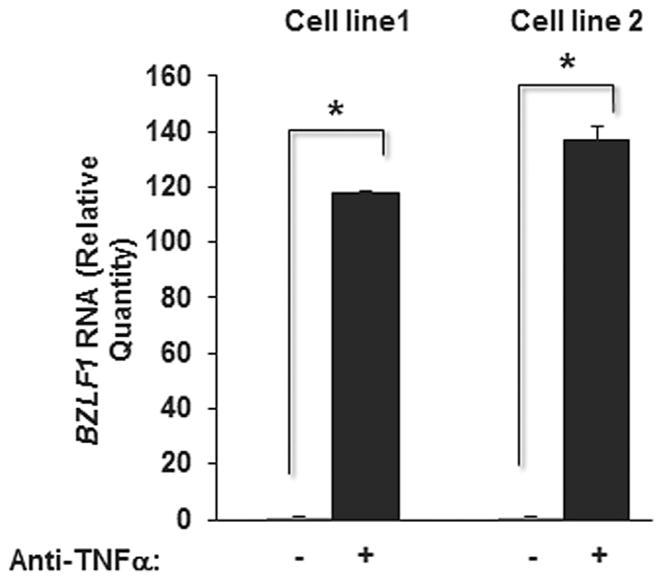
Increased lytic gene expression could result from impaired T cell surveillance or anti-TNFα-mediated lytic cycle activation in EBV-infected B cells. To address if anti-TNFα antibodies are capable of activating the EBV lytic cycle, we exposed previously established EBV-infected B cell lines to anti-TNFα antibodies and measured transcript levels of BZLF1, the critical molecular lytic switch. As shown in Figure 4, addition of anti-TNFα antibodies resulted in significantly higher levels of BZLF1 transcripts compared to control in both cell lines, indicating that anti-TNFα antibodies are sufficient to activate the EBV latency-to-lytic cycle switch.
Fig. 4.
Anti-TNFα antibodies induce the EBV lytic switch in latently infected B cells. EBV-infected B cell lines were treated with anti-TNFα antibodies (or control human IgG1 antibody), harvested after 24 hr and subjected to qRT-PCR using primers targeting transcripts from the EBV latency-to-lytic cycle switch gene BZLF1. Results represent means of relative amounts of RNA normalized to 18S rRNA levels +/− standard errors of the means of three technical replicates.
DISCUSSION
In this study, we report that treatment with anti-TNFα antibodies is associated with increased EBV load in peripheral blood cells, this increase is at least partially a result of increased EBV lytic cycle activation and anti-TNFα antibodies are sufficient to activate the EBV latency-to-lytic switch. While anti-TNFα antibody-mediated suppression of immune surveillance may also contribute to EBV lytic activation, this remains to be determined. Although a pilot study using a small number of subjects, to our knowledge, this is the first to examine whether anti-TNFα antibodies are associated with a shift in the balance between EBV latency and lytic cycles.
Evidence for increased EBV loads in autoimmune patients treated with TNFα blockers is conflicting. A few studies found substantial fractions of patients while others reported a minority of patients treated with anti-TNFα antibodies to have higher EBV loads [Reijasse et al., 2004; Lavagna et al., 2007; Magro et al., 2013]. A prominent confounding factor in these studies was concurrent treatment with immunomodulating agents. Therefore, our primary recruitment criterion was to include patients with IBD who were treated solely with anti-TNFα without concomitant immunomodulator therapy. Because there are very few such patients, we were only able to recruit 10 within the time constraints that we had. We also chose to include 1) IBD patients lacking immunomodulatory therapy and 2) patients with abdominal pain without IBD to control for the possibility of IBD causing EBV lytic cycle activation. While these factors also contributed to the small sample size of our study, nevertheless, we felt that it was important to have clearly-defined study and control groups to aid in defining the contribution of TNFα-antagonists to EBV load and lytic cycle activation.
In assessing the demographics of our study subjects, we found that there were gender differences between the four groups (Table I). This was partly due to difficulty identifying IBD patients solely on anti-TNFα therapy as well as locating IBD control subjects not on any immunomodulatory treatment. Since there is no known association between gender and EBV load or development of EBV-lymphomas, the differences in gender distribution are likely not to be confounding factors. The mean ages were similar except for IBD control subjects who were younger (P ≤0.01). This age range is in line with the natural epidemiology of IBD in which most patients are diagnosed in adolescence and childhood [Gasparetto and Guariso, 2013]. Furthermore, the shorter duration of disease in IBD controls was expected as anti-TNFα therapy is typically reserved for refractory IBD because of safety concerns. Adverse effects of anti-TNFα antibodies have included fatal cases of tuberculosis, hepatitis B virus reactivation, and increased risk of cancer [Lavagna et al., 2007]. However of note, the fraction of patients in remission was identical in both the IBD groups. It is also important to point out that the EBV loads calculated for healthy controls in our study are similar to those observed by Balandraud et al. who reported copy numbers ranging from 0–43.7 per 1.5 × 105 PBMC [Balandraud et al., 2003]. Furthermore, suppression in the T and NK cell fraction is consistent with apoptosis of T cells (as well as macrophages) that has been demonstrated in IBD patients on anti-TNFα antibodies [ten Hove et al., 2002; Van den Brande et al., 2003; Di Sabatino et al., 2004]. In summary, our results indicate that any future study with a prospective study design would need to recruit IBD patients at initial diagnosis and obtain laboratory information on multiple parameters including EBV copy number, T cell number, cellular or viral markers and EBV serologic status before and after commencement of anti-TNFα therapy.
Most lymphomas in IBD patients on immunosuppressive therapy are of B-cell origin with almost 50% being positive for EBV [Sokol et al., 2012; Fernandez Salazar et al., 2013]. Also, IBD patients treated with anti-TNFα ±immunomodulators were found to have a higher risk for NHL compared to those only on immunomodulators [Siegel et al., 2009]. However, how many of these tumors were EBV-positive is not known. Indeed, the risk of development of non-Hodgkin lymphomas is high when EBV load exceeds 5,000 copies/106 mononuclear cells [Reijasse et al., 2004]. What fraction of this load is derived from replication of latent EBV genomes versus replication of lytic genomes which can yield 100–1000-fold amplification of the genome in a linear form [Serio et al., 1997], is unclear. Our discovery of EBV lytic cycle activation including increased transcripts from a late lytic gene indicates that increased EBV loads in patients on anti-TNFα therapy were derived from lytic EBV genomes. While none of the subjects in our study had EBV loads greater than 1,341 copies/106 mononuclear cells, our study had a relatively short follow-up period. Our finding that anti-TNFα therapy is associated with a shift towards EBV lytic activation, despite the small study group, provides the basis for larger prospective studies on patients treated with anti-TNFα therapies.
Acknowledgments
Grant sponsor: Research Foundation for the State University of New York
We thank our study subjects for their participation. This study was supported by funds from the Research Foundation for the State University of New York (to S.B.-M.).
Abbreviations
- IBD
inflammatory bowel disease
- EBV
Epstein-Barr virus
- CD
crohn’s disease
- UC
ulcerative colitis
Footnotes
Conflict of interest disclosure: The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
S.L. and S.B.-M. designed the research; S.L., S.K., and S.S. performed the experiments; S.L. and S.B.-M. analyzed the data and wrote the manuscript; and R.R. and A.C. provided access to study subjects.
References
- Balandraud N, Meynard JB, Auger I, Sovran H, Mugnier B, Reviron D, Roudier J, Roudier C. Epstein-Barr virus load in the peripheral blood of patients with rheumatoid arthritis: Accurate quantification using real-time polymerase chain reaction. Arthritis Rheum. 2003;48:1223–1228. doi: 10.1002/art.10933. [DOI] [PubMed] [Google Scholar]
- Bhaduri-McIntosh S, Rotenberg MJ, Gardner B, Robert M, Miller G. Repertoire and frequency of immune cells reactive to Epstein-Barr virus-derived autologous lymphoblastoid cell lines. Blood. 2008;111:1334–1343. doi: 10.1182/blood-2007-07-101907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DH. Biology and disease associations of Epstein-Barr virus. Philos Trans R Soc Lond B Biol Sci. 2001;356:461–473. doi: 10.1098/rstb.2000.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlen R, Strid H, Lundgren A, Isaksson S, Raghavan S, Magnusson MK, Simren M, Sjovall H, Ohman L. Infliximab inhibits activation and effector functions of peripheral blood T cells in vitro from patients with clinically active ulcerative colitis. Scand J Immunol. 2013;78:275–284. doi: 10.1111/sji.12081. [DOI] [PubMed] [Google Scholar]
- Dayharsh GA, Loftus EV, Jr, Sandborn WJ, Tremaine WJ, Zinsmeister AR, Witzig TE, Macon WR, Burgart LJ. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology. 2002;122:72–77. doi: 10.1053/gast.2002.30328. [DOI] [PubMed] [Google Scholar]
- Di Sabatino A, Ciccocioppo R, Cinque B, Millimaggi D, Morera R, Ricevuti L, Cifone MG, Corazza GR. Defective mucosal T cell death is sustainably reverted by infliximab in a caspase dependent pathway in Crohn’s disease. Gut. 2004;53:70–77. doi: 10.1136/gut.53.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Palermo T, Brite J, McDade TW, Aiello A. Seroprevalence of Epstein-Barr virus infection in U.S. children ages 6–19, 2003–2010. PLoS One. 2013;8:e54921. doi: 10.1371/journal.pone.0064921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feederle R, Kost M, Baumann M, Janz A, Drouet E, Hammerschmidt W, Delecluse HJ. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. Embo J. 2000;19:3080–3089. doi: 10.1093/emboj/19.12.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng WH, Cohen JI, Fischer S, Li L, Sneller M, Goldbach-Mansky R, Raab-Traub N, Delecluse HJ, Kenney SC. Reactivation of latent Epstein-Barr virus by methotrexate: A potential contributor to methotrexate-associated lymphomas. J Natl Cancer Inst. 2004;96:1691–1702. doi: 10.1093/jnci/djh313. [DOI] [PubMed] [Google Scholar]
- Fernandez Salazar L, Rojo S, De Lejarazu RO, Castro E, Higuera E, Gonzalez JM. No increase in Epstein-Barr virus viral load in a group of 30 asymptomatic patients with Crohn’s disease. Am J Gastroenterol. 2013;108:1933–1935. doi: 10.1038/ajg.2013.250. [DOI] [PubMed] [Google Scholar]
- Gasparetto M, Guariso G. Highlights in IBD epidemiology and its natural history in the paediatric age. Gastroenterol Res Pract. 2013;2013:829040. doi: 10.1155/2013/829040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- Herrinton LJ, Liu L, Weng X, Lewis JD, Hutfless S, Allison JE. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2011;106:2146–2153. doi: 10.1038/ajg.2011.283. [DOI] [PubMed] [Google Scholar]
- Hill ER, Koganti S, Zhi J, Megyola C, Freeman AF, Palendira U, Tangye SG, Farrell PJ, Bhaduri-McIntosh S. Signal transducer and activator of transcription 3 limits Epstein-Barr virus lytic activation in B lymphocytes. J Virol. 2013;87:11438–11446. doi: 10.1128/JVI.01762-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong GK, Gulley ML, Feng WH, Delecluse HJ, Holley-Guthrie E, Kenney SC. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. J Virol. 2005;79:13993–14003. doi: 10.1128/JVI.79.22.13993-14003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui-Yuen J, McAllister S, Koganti S, Hill E, Bhaduri-McIntosh S. Establishment of Epstein-Barr virus growth-transformed lymphoblastoid cell lines. J Vis Exp. 2011 doi: 10.3791/3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel OW, van de Rijn M, Weiss LM, Del Zoppo GJ, Hench PK, Robbins BA, Montgomery PG, Warnke RA, Dorfman RF. Brief report: Reversible lymphomas associated with Epstein-Barr virus occurring during methotrexate therapy for rheumatoid arthritis and dermatomyositis. N Engl J Med. 1993;328:1317–1321. doi: 10.1056/NEJM199305063281806. [DOI] [PubMed] [Google Scholar]
- Knipe DM, Howley PM. Fields Virology. Vol. 2 Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2013. [Google Scholar]
- Komatsuda A, Wakui H, Nimura T, Sawada K. Reversible infliximab-related lymphoproliferative disorder associated with Epstein-Barr virus in a patient with rheumatoid arthritis. Mod Rheumatol. 2008;18:315–318. doi: 10.1007/s10165-008-0053-0. [DOI] [PubMed] [Google Scholar]
- Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: A review of medical therapy. World J Gastroenterol. 2008;14:354–377. doi: 10.3748/wjg.14.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavagna A, Bergallo M, Daperno M, Sostegni R, Costa C, Leto R, Crocella L, Molinaro G, Rocca R, Cavallo R, Pera A. Infliximab and the risk of latent viruses reactivation in active Crohn’s disease. Inflamma Bowel Dis. 2007;13:896–902. doi: 10.1002/ibd.20131. [DOI] [PubMed] [Google Scholar]
- Magro F, Santos-Antunes J, Albuquerque A, Vilas-Boas F, Macedo GN, Nazareth N, Lopes S, Sobrinho-Simoes J, Teixeira S, Dias CC, Cabral J, Sarmento A, Macedo G. Epstein-Barr virus in inflammatory bowel disease-correlation with different therapeutic regimens. Inflamm Bowel Dis. 2013;19:1710–1716. doi: 10.1097/MIB.0b013e318281f31c. [DOI] [PubMed] [Google Scholar]
- Mariette X, Tubach F, Bagheri H, Bardet M, Berthelot JM, Gaudin P, Heresbach D, Martin A, Schaeverbeke T, Salmon D, Lemann M, Hermine O, Raphael M, Ravaud P. Lymphoma in patients treated with anti-TNF: Results of the 3-year prospective French RATIO registry. Ann Rheum Dis. 2010;69:400–408. doi: 10.1136/ard.2009.117762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelow P, Wright C, Pantanowitz L. A review of the cytomorphology of Epstein-Barr virus-associated malignancies. Acta Cytol. 2012;56:1–14. doi: 10.1159/000334235. [DOI] [PubMed] [Google Scholar]
- Montone KT, Hodinka RL, Salhany KE, Lavi E, Rostami A, Tomaszewski JE. Identification of Epstein-Barr virus lytic activity in post-transplantation lymphoproliferative disease. Mod Pathol. 1996;9:621–630. [PubMed] [Google Scholar]
- Neuhierl B, Delecluse HJ. The Epstein-Barr virus BMRF1 gene is essential for lytic virus replication. J Virol. 2006;80:5078–5081. doi: 10.1128/JVI.80.10.5078-5081.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Kim CG, Kim JY, Choe JY. Spontaneous regression of EBV-associated diffuse lymphoproliferative disease in a patient with rheumatoid arthritis after discontinuation of etanercept treatment. Rheumatol Int. 2008;28:475–477. doi: 10.1007/s00296-007-0467-6. [DOI] [PubMed] [Google Scholar]
- Reijasse D, Le Pendeven C, Cosnes J, Dehee A, Gendre JP, Nicolas JC, Beaugerie L. Epstein-Barr virus viral load in Crohn’s disease: Effect of immunosuppressive therapy. Inflamm Bowel Dis. 2004;10:85–90. doi: 10.1097/00054725-200403000-00004. [DOI] [PubMed] [Google Scholar]
- Sankaran-Walters S, Ransibrahmanakul K, Grishina I, Hung J, Martinez E, Prindiville T, Dandekar S. Epstein-Barr virus replication linked to B cell proliferation in inflamed areas of colonic mucosa of patients with inflammatory bowel disease. J Clin Virol. 2011;50:31–36. doi: 10.1016/j.jcv.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serio TR, Kolman JL, Miller G. Late gene expression from the Epstein-Barr virus BcLF1 and BFRF3 promoters does not require DNA replication in cis. J Virol. 1997;71:8726–8734. doi: 10.1128/jvi.71.11.8726-8734.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: A meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874–881. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Beaugerie L, Maynadie M, Laharie D, Dupas JL, Flourie B, Lerebours E, Peyrin-Biroulet L, Allez M, Simon T, Carrat F, Brousse N, Group CS. Excess primary intestinal lymphoproliferative disorders in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2063–2071. doi: 10.1002/ibd.22889. [DOI] [PubMed] [Google Scholar]
- ten Hove T, van Montfrans C, Peppelenbosch MP, van Deventer SJ. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn’s disease. Gut. 2002;50:206–211. doi: 10.1136/gut.50.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, van Montfrans C, Hommes DW, Peppelenbosch MP, van Deventer SJ. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s disease. Gastroenterology. 2003;124:1774–1785. doi: 10.1016/s0016-5085(03)00382-2. [DOI] [PubMed] [Google Scholar]