Abstract
Streptococcus agalactiae (Group B Streptococcus, GBS) is a Gram-positive bacterial pathogen that causes invasive infections of both children and adults. During pregnancy, GBS is a significant cause of infection of the fetal membranes (chorioamnionitis), which can lead to intra-amniotic infection, preterm birth, stillbirth, and neonatal sepsis. Recently, breastfeeding has been thought to represent a potential mode of GBS transmission from mother to newborn, which might increase the risk for late-onset sepsis. Little is known, however, about the molecular components of breast milk that may support or prevent GBS colonization. In this study, we examine how human milk oligosaccharides (HMOs) affect the pathogenesis of GBS. HMOs from discrete donor samples were isolated and profiled by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (MS). Growth and biofilm assays show that HMOs from mothers of specific milk groups can modulate the growth and biofilm formation of GBS. High-resolution field-emission gun scanning electron microscopy (SEM) and confocal laser scanning microscopy confirmed the quantitative biofilm assays and demonstrated cell arrangement perturbations in bacterial cultures treated with specific oligosaccharides. These findings demonstrate that HMOs affect the growth and cell biology of GBS. Finally, this study provides the first example of HMOs functioning as anti-biofilm agents against GBS.
Keywords: Group B Streptococcus, GBS, Antimicrobial, Anti-Biofilm, Bacteriostatic, Human Milk Oligosaccharides, HMO
Graphical abstract
Human milk is the model source of nutrition for the developing infant. Professional bodies, such as the World Health Organization,1 American Academy of Pediatrics,2 and the U. S. Department of Health and Human Services3 recommend exclusive breastfeeding for the first six months of life followed by integration into a mixed diet through two years of age and beyond.
In addition to providing nourishment, human milk is among the earliest vehicles for intestinal bacterial colonization.4 There are an estimated 400 species of bacteria present in human milk at any given time.5-6 Although the majority of these bacteria are symbiotic or commensal species with roles varying from metabolic to immune enhancing, the transmission of bacteria via human milk consumption also causes newborn diseases.7-9 Nevertheless, breast milk consumption leads to critical modulation of the host immune development, which promotes both tolerance and immunity. This protection is due, in part, to human milk oligosaccharides (HMOs), the third largest macromolecular component of human milk.10-11
Structurally, HMOs incorporate just five monosaccharide residues. Still, there are approximately 200 unique structures that have been observed.12 Interestingly, not all women produce the same oligosaccharides. Instead, oligosaccharide structure and composition vary across Lewis blood group and secretor status and throughout the duration of nursing.13 Lewis blood groups are based on the presence of certain antigens synthesized by fucosyl transferases, and secretor status is determined by the activity of enzymes responsible for secreting antigens into body secretions.14 HMOs distinguish human milk from that of other mammals and, consequently, assist in conferring benefits during the perinatal period that are not observed through formula feeding. For example, HMOs serve as prebiotics that select for the growth of symbiotes.11 Moreover, HMOs are anti-adhesive antimicrobial agents or free flowing receptor decoys that block the attachment of pathogens to mucosal surfaces, preventing bacterial adhesion and colonization, the first stage of infection.10
The project described herein originated from the observation that human milk is a potential channel for vertical transmission of Streptococcus agalactiae, more commonly known as Group B Streptococcus (GBS).15-17 GBS is a Gram-positive pathogen that is a common cause of neonatal sepsis and meningitis.18-19 In addition to neonatal disease, GBS is a leading cause of both human and bovine mastitis.20-21
In industrialized countries, prenatal screening coupled with targeted antibiotic prophylaxis during labor and delivery has reduced the rate of GBS early onset neonatal sepsis (i.e. sepsis that occurs during the first week of life).22-23 This approach, however, is not universally practiced, particularly in low and middle-income countries where the quality of perinatal health care is variable.24 Moreover, there is no preventative or curative solution to GBS infection occurring after the first week of life (late onset GBS disease).
Like many bacterial species, an important aspect of GBS pathogenesis is it’s ability to form biofilms, a well-known virulence pathway that provides increased resistance to antimicrobial agents as well as host defenses.25 Based on the established evidence that HMOs possess antimicrobial activity, we hypothesized milk oligosaccharides could modulate both the bacterial growth and biofilm production of GBS. Previous research has demonstrated the importance of GBS capsule polysaccharide biosynthesis in mediating biofilm formation, supporting our hypothesis that oligosaccharides could influence biofilm establishment.26-27 Additionally, GBS capsular polysaccharides from type Ib and II are similar in structure to certain human milk oligosaccharides.28 A study by Pritchard and coworkers showed mouse antibodies for GBS capsular polysaccharides bind to HMOs.28 Moreover, the Bode and Le Doare groups have reported that HMOs inhibit the proliferation of GBS in vitro29-31 (Figure 1).
Figure 1.
Effects of human milk oligosaccharides on Group B Streptococccus.
A. Previous research (Bode, 2015 and 2017; Le Doare, 2016):
HMOs act as antibacterial agents against GBS
B. This work:
HMOs from donors assigned specific Lewis blood groups act as bacteriostatic and anti-biofilm agents
Considering that recent data supports breastfeeding as a contributory factor to late-onset GBS transmission, there is a compelling need to understand what molecular components of human milk, which is generally protective for the neonate, might positively or negatively impact pathogen transmission. Moreover, our research team has a core interest in discovering and examining compounds produced by the host, which are protective against GBS. Herein, we report the antimicrobial properties of oligosaccharide isolates from donor human milk samples as well as their effect on biofilm formation and biofilm architecture.
METHODS
PURIFICATION OF HMOS
Human milk was obtained from five healthy, lactating women between 3 days and 3 months postnatal and stored at −20°C. The de-identified milk was provided by Dr. J-H Weitkamp from the Vanderbilt Department of Pediatrics under a collection protocol approved by the Vanderbilt University Institutional Review Board (IRB#100897). Milk samples and the respective components from subsequent purification steps were kept separate. The lipid components were removed by skimming after centrifugation. Proteins were precipitated by addition of ethanol at 4°C and subsequent centrifugation. The HMO-containing supernatant was concentrated in vacuo and purified by P-2 Gel (H2O eluent) and the oligosaccharides were dried by lyophilization.
MS AND MS/MS ANALYSIS OF HMO SAMPLES
Dried HMO samples were reconstituted in water to approximately 1 mg/mL. The HMO solutions were deposited on a matrix-assisted laser desorption/ionization (MALDI) target plate as follows: 1 μL HMO was spotted followed by 0.2 μL 10 mM NaCl and 1 μL DHB matrix (60 mg/mL in 50% methanol). The spots were allowed to air dry and were analyzed in positive ion mode on a 9.4T Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometry (MS) (Bruker Solarix). Mass spectra were acquired in the positive ion mode from m/z 300-2500. Sodium ion adducts of HMO’s were detected with a mass accuracy of >2 ppm.
MS/MS analysis was performed for selected ions with a linear ion trap mass spectrometer equipped with a MALDI source (LTQ XL, Thermo Scientific). Selected sodium adduct ions of interest were isolated with a 1 amu window and fragmented via CID using a collision energy of 35 eV.
BACTERIAL STRAINS AND CULTURE CONDITIONS
S. agalactiae strain CNCTC 10/84 (ATCC) was cultured on tryptic soy agar plates supplemented with 5% sheep blood (blood agar plates) at 37°C in ambient air overnight. Bacteria were subcultured from blood agar plates into Todd-Hewitt broth (THB) and incubated under shaking conditions at 37°C in ambient air overnight. The following day, bacterial density was measured spectrophotometrically using optical density measurements at 600 nm (OD600), and bacterial numbers were determined using the predetermined coefficient of 1 OD600= 109 CFU/mL.
BACTERIAL GROWTH AND VIABILITY ANALYSES
S. agalactiae strain CNCTC 10/84 cells were cultured overnight in THB and then subcultured by inoculating 106 cells per 5 mL of THB or THB supplemented with 1% glucose. Cultures were grown under shaking conditions in THB alone or supplemented with 5 mg/mL HMOs isolated from the various human milk samples (Donors 1-5) or in THB supplemented with 1% glucose or THB supplemented with 1% glucose and 5 mg/mL HMOs isolated from the various human milk samples (Donors 1-5) at 37°C in ambient air. Bacterial growth was evaluated by spectrophotometric reading of OD600 and bacterial viability was evaluated by serial dilution and plating onto blood agar plates and quantifying viable colony forming units per mL of culture (CFU/mL).
BACTERIAL BIOFILM ASSAYS
S. agalactiae strain CNCTC 10/84 was grown overnight as described above prior to subculturing 106 bacterial cells into 200 μL THB supplemented with 1% glucose (to promote biofilm formation) in 96-well tissue culture plates (Corning, Inc.). Bacterial cells were added to wells containing media alone or wells supplemented with 5 mg/mL HMOs isolated from the various human milk samples (Donors 1-5). Cultures were incubated under static conditions at 37°C in ambient air for 24h.
Optical density (OD600) was measured for each sample as a measure of bacterial growth. The medium was aspirated, and each well was washed once with phosphate-buffered saline (PBS, pH 7.4) to remove non-adherent cells. Wells were then stained with a 10% crystal violet solution for 15 minutes. After staining, the wells were again washed with PBS and then allowed to dry at room temperature for 30 minutes. After drying, crystal violet staining was solubilized with an 80% ethanol/20% acetone solution. The absorbance (OD560) was measured for each sample as a measure of biofilm formation. The data shown represents 5 independent experiments, each with 3 technical replicates.
FIELD-EMISSION GUN SCANNING ELECTRON MICROSCOPY
Bacterial cells were analyzed by scanning electron microscopy as previously described with some modifications.32 Briefly, bacteria were cultured in THB supplemented with 1% glucose in wells containing 12 mm glass coverslips coated with poly-L-lysine (Corning, Bedford MA) at 37°C for 24 hours. At 24 hours, supernatants were removed and samples were fixed with 2.0% paraformaldehyde, 2.5% gluteraldehyde in 0.05 M sodium cacodylate buffer for 24 hours. Secondary fixation with 0.1% osmium tetroxide was performed for 5 minutes prior to sequential dehydration with increasing concentrations of ethanol. After ethanol dehydration, samples were dried at the critical point using a critical point dryer machine (Tousimis), mounted onto aluminum sample stubs, and sputter-coated with 80/20 gold-palladium. Afterward, samples were painted with a thin strip of colloidal silver (Electron Microscopy Sciences) at the edge to facilitate charge dissipation. Samples were imaged with an FEI Quanta 250 field-emission gun scanning electron microscope. Images shown are representative of three separate experiments.
CONFOCAL LASER SCANNING MICROSCOPY ANALYSES
Bacterial cells were cultured as above in wells containing THB supplemented with 1% glucose and containing glass coverslips coated with poly-L-lysine. Cultures were grown under static conditions for 24 hours at 37°C. At 24 hours, coverslips were washed with PBS prior to staining with LIVE/DEAD BacLight bacterial viability kit, which includes both Syto 9 (green) and propidium iodide (red) (Life Technologies) to visualize bacterial cells and calcofluor white (blue) (Sigma-Aldrich) to visualize carbohydrate capsule/matrix within the biofilm.
Coverslips were stained for 15 minutes followed by 2 washes with PBS. Both, SYTO 9 and propidium iodide stain nucleic acids, but propidium iodide is only able to penetrate damaged cell membranes and competes with SYTO 9 staining within dead bacterial cells. When used concurrently, stained bacteria with intact cell membranes will fluoresce green and cells with damaged cells will stain fluorescent red. Calcofluor white binds to β-1,3 and β-1,4 polysaccharides such as chitin and cellulose has been shown to stain the extrapolymeric substances in biofilms of Streptococcus species and other bacteria.33-35 Coverslips were then mounted on glass microscope slides using Aqua Poly/Mount (Polysciences, Inc.) Samples were imaged with a Zeiss LSM 710 Meta Inverted confocal laser-scanning microscope with Zen 2011 software.
BACTERIAL BIOFILM ASSAYS WITH ANTIMICROBIAL PEPTIDE
Bacterial biofilm assays were carried out as previously described in the bacterial biofilm assay section with some modifications. Bacterial cells were added to wells containing media alone, media and Polymixin B (1 to 10 μg/mL), media supplemented with HMOs (5 mg/mL), or media, HMOs (5 mg/mL), and Polymixin B (1 to 10 μg/mL). The data shown represents 3 independent experiments, each with 3 technical replicates.
RESULTS AND DISCUSSION
MALDI-FTICR MS/MS PROFILING OF HMOS REVEALS LEWIS BLOOD GROUPS OF DONORS
HMO expression patterns are known to vary from mother to mother and depend largely on the mother’s secretor and Lewis blood status.10, 29, 36-37 Recent reports have demonstrated the use of mass spectrometry and NMR analysis to categorize human milk samples by mother secretor and Lewis blood group status.38-40 Kunz and coworkers developed a high throughput mass fingerprinting technique that uses MALDI-TOF MS and MALDI-TOF MS/MS.38 Their work shows that the MS and MS/MS fragmentation peaks and intensities, major fucose-containing oligosaccharides and their fucosyl linkage types can be identified and the corresponding secretor/Lewis status assigned. Based on the analysis presented by Kunz and coworkers,38 we investigated MS/MS fragmentations of parent peaks m/z 657 and 1022. Analysis of MS/MS of m/z 657 revealed Donors 2, 3, 4, and 5 to have a fragmentation m/z 511 present, which is associated with secretor-types Le(a−b+) and Le(a−b−), whereas and Donor 1 was missing this characteristic fragment ion and thus designated as a nonsecretor-type Le(a+b−) (Figure 2A). Distinguishing between secretor-types Le(a−b+) and Le(a−b−) required analysis of MS/MS of m/z 1022, particularly the relative intensities of main fragment ions at m/z 730 and m/z 876 (Figure 2B). Based on this analysis, Donors 2 and 4 were identified as Le(a−b+) and Donors 3 and 5 were identified as Le(a−b−) (Table 1).
Figure 2.
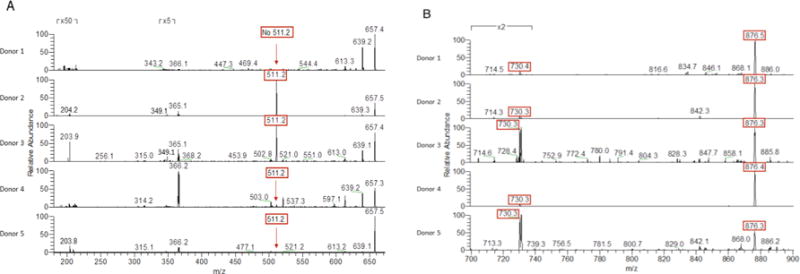
MALDI-FT-ICR-MS/MS spectra of (A) m/z 657.2 and (B) m/z 1022.2 from 700-900 of HMOs from 5 separate donors obtained in the positive ion mode. Diagnostic peaks highlighted in red.
Table 1.
Lewis blood group assignments of donor milk samples
| Donor | Lewis Blood Group |
|---|---|
| 1 | a+b− |
| 2 | a−b+ |
| 3 | a−b− |
| 4 | a−b+ |
| 5 | a−b− |
HMOS MODULATE GROWTH AND VIABILITY OF S. AGALACTIAE
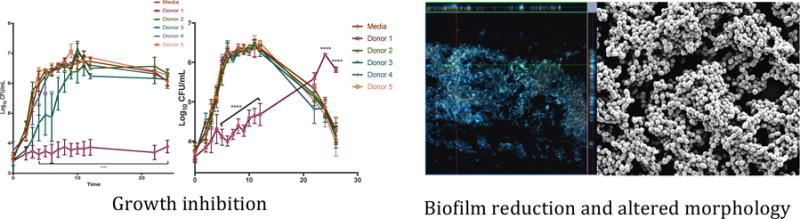
We next sought to understand how HMOs affected the growth and viability of GBS, a common pathogen in the neonatal period, in two growth environments. First, GBS cells were grown in media alone (Todd Hewitt Broth, THB) or THB containing HMOs from different donor breast milk samples. Cultures were measured spectrophotometrically at OD600 as a measurement of bacterial growth and samples were serially diluted and plated on blood agar plates to confirm bacterial cell viability. Interestingly, HMOs from donor 1 demonstrated marked anti-microbial activity against GBS compared with media alone (Figure 3), resulting in approximately 40% growth inhibition. Additionally, HMOs from donor 3 inhibited GBS growth to a lesser degree and growth curves demonstrated a significant decrease in bacterial growth for the first 8 hours of culture (Figure 3), with percent inhibition holding near 23% for hours 4-6 and dropping to 14.4% at hour 7. Donors 2, 4, and 5 showed no significant effect on GBS growth in THB.
Figure 3.
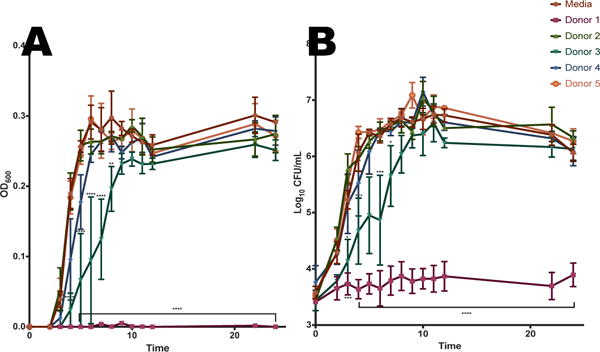
Effect of HMOs isolated from individual milk samples on growth rate/proliferation of GBS 10/84 in Todd Hewitt Broth (A) OD600 readings were taken at 0, 2-12, 22, and 24 h. Mean OD600 for each HMO sample and time point is indicated by the respective symbols. (B) Enumeration of CFU was performed at 0, 2-12, 22, and 24 h, corresponding to the OD values graphed in panel A. The mean CFU/mL was calculated for each time point and is indicated by the respective symbols. Data displayed represents the mean OD +/− SEM of 3 biological replicates, * P<0.05, ** P<0.01, *** P<0.001, ****P<0.0001 by 2-way ANOVA with post-hoc Dunnett’s mutiple comparison test, with all donor samples compared to the GBS growth in media alone.
Next, GBS cells were grown in THB containing 1% glucose or THB containing both 1% glucose and HMOs from different donor breast milk samples. Growth was measured as described previously. As before, HMOs from donor 1 demonstrated marked anti-microbial activity against GBS compared with the control (Figure 4). However, after 22 hours, growth begins, suggesting that the HMOs from donor 1 are acting as a bacteriostatic agent. HMOs from all other donors (2-5) showed no significant effect on GBS growth in THB supplemented with 1% glucose.
Figure 4.
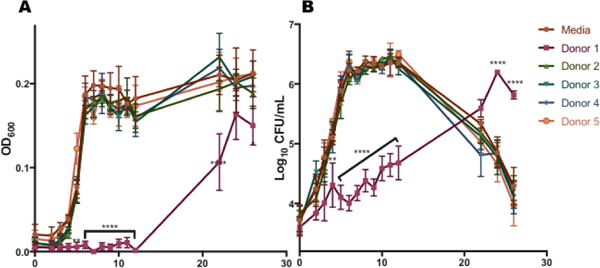
Effect of HMOs isolated from individual milk samples on growth rate/proliferation of GBS 10/84 in Todd Hewitt Broth supplemented with 1% glucose (A) OD600 readings were taken at 0, 2-12, 22, 24, and 26 h. Mean OD600 for each HMO sample and time point is indicated by the respective symbols. (B) Enumeration of CFU was performed at 0, 2-12, 22, 24, and 26 h, corresponding to the OD values graphed in panel A. The mean CFU/mL was calculated for each time point and is indicated by the respective symbols. Data displayed represents the mean OD +/− SEM of 3 biological replicates, * P<0.05, ** P<0.01, *** P<0.001, ****P<0.0001 by 2-way ANOVA with post-hoc Dunnett’s mutiple comparison test, with all donor samples compared to the GBS growth in media alone.
EVALUATION OF HMO EFFECT ON GBS BIOFILM FORMATION
In order to evaluate biofilm formation, a plate based biofilm assay was utilized that allows for measurement of bacterial growth as well as biofilm production noted by crystal violet staining. Results are expressed as a ratio of the biofilm produced to the number of bacterial cells present (biomass). GBS biofilm production was largely unaffected by the presence of HMOs in the growth media (Figure 5a). HMOs from donor 1 seemed to significantly increase the biofilm/biomass ratio of cells grown in THB alone (p=0.0008 by one-way ANOVA with post-hoc Dunnett’s multiple comparison test). However, this result was due to inhibition of cell growth (as shown in Figure 3), which is in contrast to the restored growth observed after 22 hours in THB with 1% glucose (Figure 4). When GBS cells were grown in media supplemented with glucose, which has been shown to promote biofilm formation,25 supplementation with HMOs from donor 3 significantly diminished the biofilm/biomass ratio (p = 0.0018 by one-way ANOVA with post-hoc Dunnett’s multiple comparison test) (Figure 5b). A comparison of the relative biofilm amounts produced shows that in THB alone none of the donor HMOs had a significant effect (Figure 5c). However, HMOs from donors 1 and 3 significantly reduced the amount of biofilm produced when grown in THB with 1% glucose (p < 0.05 by way ANOVA with post-hoc Dunnett’s multiple comparison test) (Figure 5d). Numerical measurements used to compare biofilm formation and IC50 values for HMOs from donors that inhibited growth are shown in Figure 5e.
Figure 5.
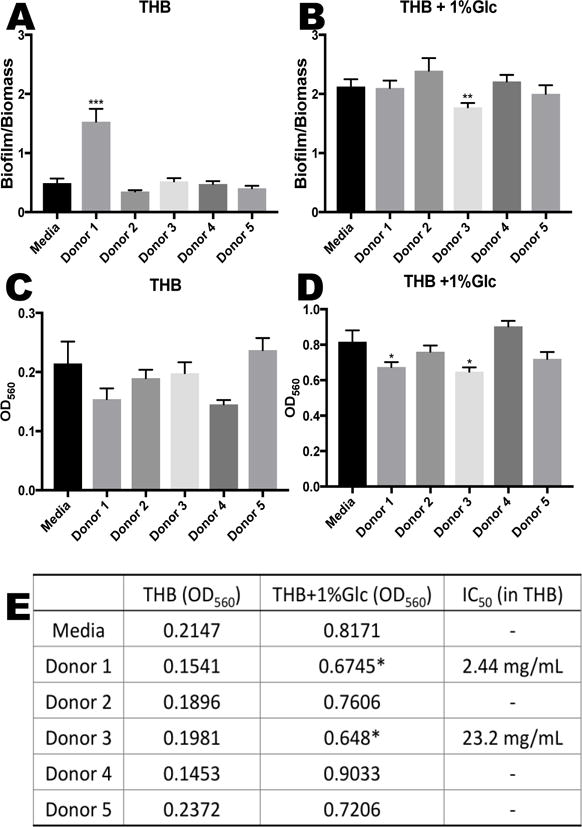
HMOs at biologically relevant breast milk concentrations (4.88 mg/mL) induce changes in biofilm formation of GBS cultures. The total biofilm to biomass ratio after 24 hours of growth was compared for (A) THB medium alone. Data represented as the mean biofilm/biomass ratio +/− SEM of 5 separate experiments, each with 3 technical replicates. *** represents p = 0.0008 by one-way ANOVA, F = 23.35 with post-hoc Dunnet’s multiple comparison test comparing each HMO group against the control sample without HMOs. (B) THB medium supplemented with 1% glucose. Data are expressed as the mean biofilm/biomass ratio +/− SEM of 5 separate experiments, each with 3 technical replicates. ** represents p = 0.0018 by one-way ANOVA, F = 3.449 with post-hoc Dunnet’s multiple comparison, compared to media alone. (C) Biofilm measurements of GBS grown in THB medium and (D) THB medium supplemented with 1% glucose. Data are expressed as the mean biofilm measurments (OD560) +/− SEM of 5 separate experiments, each with 3 technical replicates. * represents p = < 0.05 by one-way ANOVA, F = 5.935 with post-hoc Dunnet’s multiple comparison, compared to media alone. (E) Average measurement of biofilm quantities represented by optical density (OD560) of 5 separate experiments, each with 3 technical replicates. IC50 values are listed for donors that inhibited growth. * represents p = < 0.05 by one-way ANOVA, F = 5.935 with post-hoc Dunnet’s multiple comparison, compared to media alone.
MICROSCOPIC EVALUATION OF BIOFILMS GROWN IN THE PRESENCE OF HMOS
In addition to biofilm quantification, we evaluated if incubation with HMO’s resulted in any structural differences to GBS biofilms. Biofilms were grown in media supplemented with 1% glucose to enhance biofilm formation and compared to media also supplemented with HMOs from the 5 donor breast milk samples and then analyzed by high-resolution scanning electron microscopy (SEM) to evaluate changes in biofilm architecture and size. Compared to media alone, GBS cells incubated with HMOs from donor 1 demonstrated less diffuse biofilms and with smaller biofilm mushroom structures (Figure 6). Additionally, GBS biofilms grown in the presence of HMOs from donor 3 and 5 had less prominent nutrient channels compared to GBS biofilms grown in the presence of HMOs from Donors 2 and 4. This observation aligns with the breast milk donor categorization of Lewis blood groups by MALDI profiling, suggesting that HMO groups may lead to alterations in GBS biofilm structure. We then examined these biofilms at higher magnification to visualize finer details in biofilm structure. SEM analysis at high magnification revealed that while most donor samples had little effect on the cellular organization of the biofilm, samples grown with HMOs from donor 1 demonstrated changes in GBS chaining morphology. GBS strain 10/84 phenotypically forms long chains. However, donor 1 seemed to induce a truncated chain phenotype that is shorter than the control sample as well as a denser packing morphology within the biofilm (Figure 7).
Figure 6.
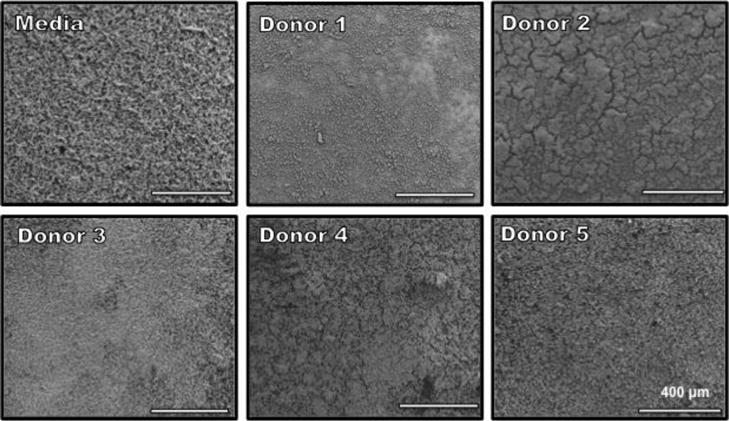
Scanning electron micrographs of biofilm formation after 24 h. GBS 10/84 cells were grown in THB + 1% glucose supplemented with individual donor samples for 24 hours at 37°C. Images are shown at ×250 magnification.
Figure 7.
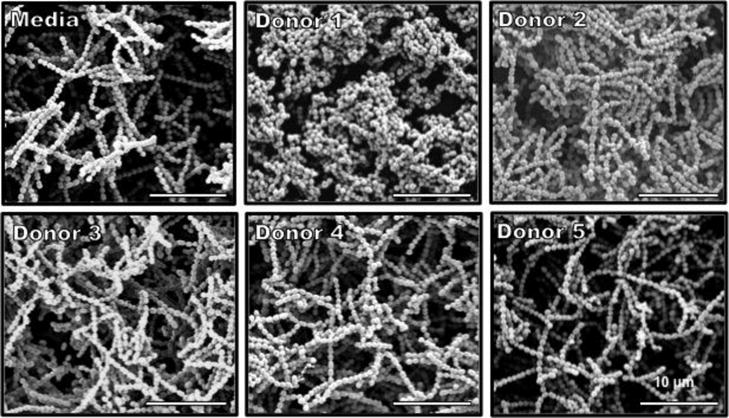
Scanning electron micrographs of biofilm formation after 24 h. GBS 10/84 cells were grown in THB + 1% glucose supplemented with HMOs from individual donor samples for 24 hours at 37°C. Images are shown at ×1,000 magnification.
Confocal laser scanning microscopy was used to analyze structural and compositional aspects of GBS 10/84 biofilms. Bacterial biofilms were grown in media alone (THB + 1% glucose) or media supplemented with HMOs from 5 donor breast milk samples. Biofilms grown in the presence of donor 3 showed a decrease in thickness of the biofilm relative to biofilms grown in media alone as seen by a comparison of the x- and y-axis views of Figure 8. Additionally, comparison of the first and last z-stack images show more intense carbohydrate (blue) content at the apical surface of the biofilm relative to the base, which is mostly composed of dead (red) and live (green) cells (Figure 9).
Figure 8.
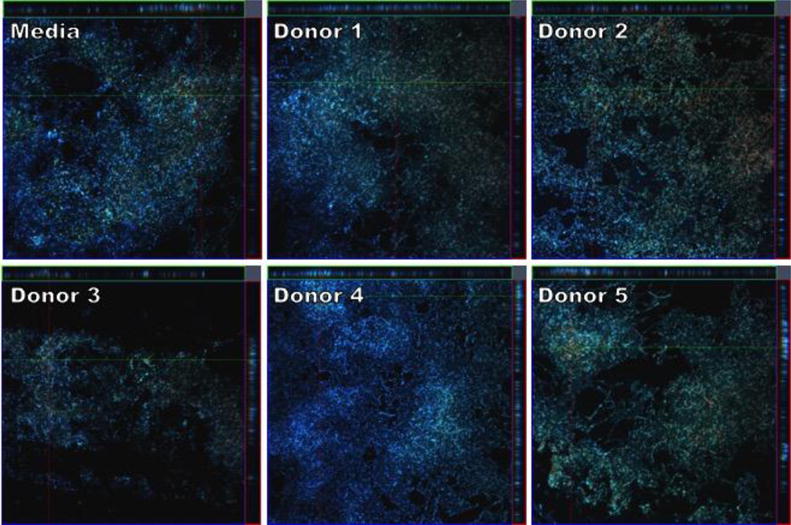
CLSM micrographs comparing biofilm formation of GBS 10/84 grown in THB supplemented with 1% glucose or THB supplemented with 1% glucose and HMOs isolated from milk donors. Bacteria were grown under static conditions at 37°C for 24 hours on glass coverslips. Biofilms were stained immediately prior to analysis with SYTO-9 (green, live bacterial cells), propidium iodide (red, dead bacterial cells), and Calcofluor White (blue, carbohydrates) at 600× magnification. Images shown represent a z-stack series of images of the three stains where the larger panel is a “bird´s eye” view of the biofilms and the right and upper panels are side views of the x- and y-axis sections respectively.
Figure 9.
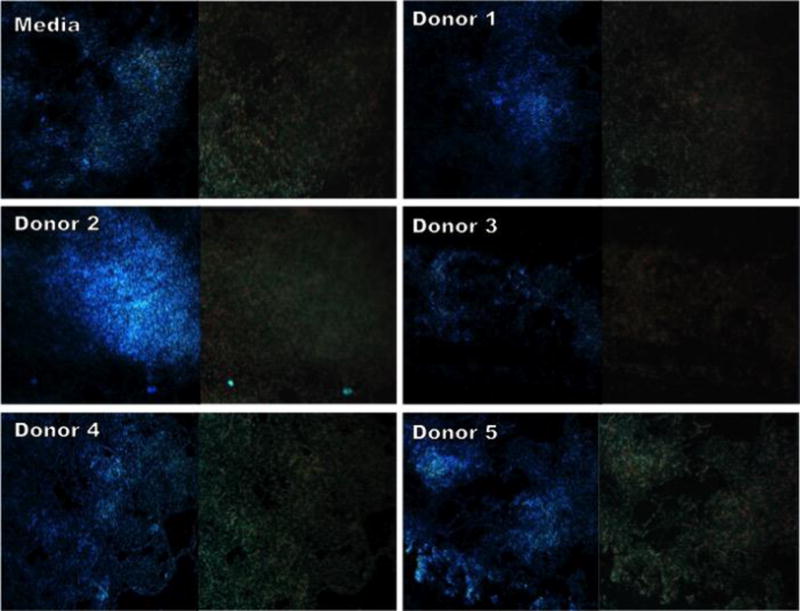
CLSM micrographs comparing apical and base sections of GBS 10/84 biofilms grown in THB supplemented with 1% glucose or THB supplemented with 1% glucose and HMOs isolated from milk donors. Bacteria were grown under static conditions at 37°C for 24 hours on glass coverslips. Images shown represent the apical surface (left image) and base of biofilm (right image) from a z-stack series. Biofilms were stained with SYTO-9 (green, live bacterial cells), propidium iodide (red, dead bacterial cells), and Calcofluor White (blue, carbohydrates) and imaged at 600× magnification.
While there is preliminary evidence that late-onset GBS transmission is associated with breast-feeding, we have demonstrated that HMOs isolated from distinct donors exhibit antimicrobial properties. Moreover, we have demonstrated that HMOs also disrupt the formation of biofilms produced by this pathogen. To the best of our knowledge, this is the first example of HMOs serving as anti-biofilm agents.
Given the discovery of the modulatory effects of HMOs against GBS, it is peculiar to note the diversity of effects displayed by glycans from each donor (Table 2). HMOs from donor 1 (non-secretor) significantly inhibited the growth of GBS in both THB (Figure 3) and THB supplemented with 1% glucose (Figure 4) and changed the morphology of the biofilm (Figure 6). HMOs from donor 3 (secretor) significantly inhibited GBS growth in THB for the first 8 hours of a 24-hour growth period (Figure 3) and significantly decreased in vitro biofilm production as measured by the biofilm/biomass ratio (Figure 6). Additionally, donors 3 and 5 affected the visual nutrient channel formation of GBS biofilms as show by SEM (Figure 5). HMOs from donors 2 and 4 showed no significant antibacterial or anti-biofilm effects.
Table 2.
Summarization of the effect of HMOs on GBS
| Donor | Lewis Blood Group | Antibacterial Activity | Anti-biofilm activity |
|---|---|---|---|
| 1 | a+b− | bacteriostatic (THB, THB + 1% glc) | altered morphology |
| 2 | a−b+ | - | - |
| 3 | a−b− | bacteriostatic (THB) | decreased biofilm produced |
| 4 | a−b+ | - | - |
| 5 | a−b− | - | - |
This data provides insight, at the molecular level, to the protective measures available from the host to decrease risk of GBS transmission. Previously, human milk based biologics have demonstrated modulatory effects on streptococcal biofilm formation. For example, lactoferrin and IgA inhibit biofilm formation while lactose and casein enhance biofilm formation in Streptococcus mutans.41 Our study reveals pooled HMOs from distinct donors modulate biofilm formation in GBS. Furthermore, our work indicates pooled HMOs can inhibit bacterial growth. Interestingly, genetic analyses in related streptococcal species, such as S. mutans, reveal that accumulation of galactose metabolism intermediates can inhibit bacterial growth.42 Thus, it remains possible that exposure of GBS to HMOs may serve to alter carbohydrate metabolism leading to accumulation of toxic intermediates which ultimately repress bacterial growth or biofilm formation.
The combinatorial approach to study antimicrobial and anti-biofilm properties of HMOs from both secretors and non-secretors has provided insight into how the milk of different mothers might influence infant health in relationship to GBS. The data presented in this paper add to previous studies supporting the importance and potential inhibitory effect of HMOs in defending against GBS colonization.
We speculate the anti-biofilm activity could be related to metabolism, as it is possible the addition of exogenous sugars saturates the bacterial community to provide excess nutrients. This action would negate advantages associated with biofilm formation. Alternatively, HMOs may interfere with the bacterial communication pathways (i.e. quorum sensing) necessary for biofilm formation. A final suggestion is that HMOs are synergistic with or enhance naturally occurring antimicrobials (such as AMPs) that may be present.
While research into the mechanism of action is ongoing in our laboratory, we have conducted a study to probe the postulated synthetic lethality of HMOs and AMPs. As shown in Figure 10, when exposed to a cocktail of HMOs from donors 2-5 and polymixcin B, GBS growth is not enhanced relative to the control. The cocktail of donor 1 HMOs and polymyxin B completely inhibited growth of the colony. Interestingly, when the same experiment was conducted in the presence of 1% glucose, HMOs from donor 1 demonstrated no synergistic effects.
Figure 10.
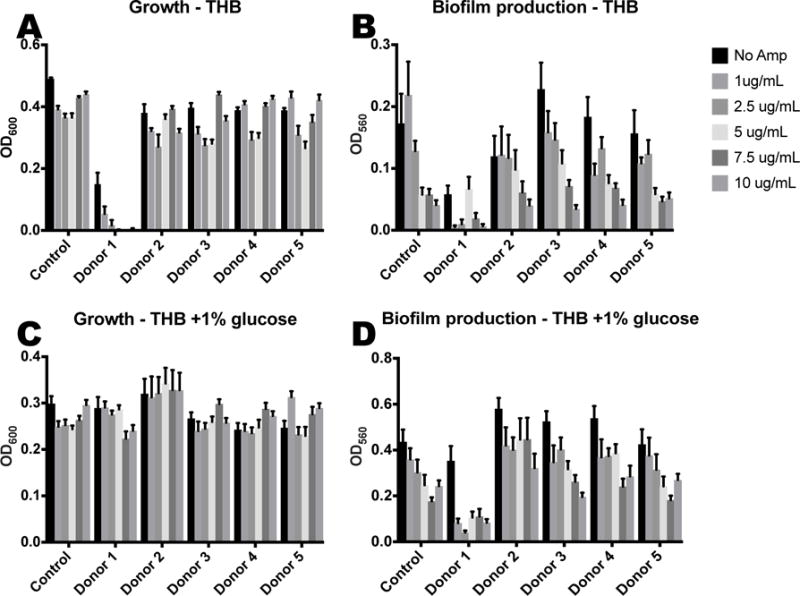
HMOs at physiologial breast milk concentrations (4.88 mg/mL) coupled with AMPs decrease growth and biofilm formation of GBS cultures. (A) Growth of GBS (OD600) and (B) biofilm measurments of GBS after 24 hours in THB medium with increasing concentrations of Polymixin B. (C) Growth of GBS (OD600) and (D) biofilm measurments of GBS after 24 hours in THB medium supplemented with 1% glucose and increasing concentrations of Polymixin B. Data represented as the mean biofilm/biomass ratio +/− SEM of 3 separate experiments, each with 3 technical replicates.
When examining changes to biofilm, we observed that as the amount of AMP is increased, biofilm production is decreased. Thus far, we have no observed enhancement by HMOs from any donor. Thus, we conclude that HMOs, depending on donor, may have the potential to increase susceptibility to an AMP. However, we have not observed any HMO-AMP synergy that reduces biofilm production.
Future studies toward the effects of individual HMOs on GBS may uncover unique structural motifs responsible for HMO antimicrobial and anti-biofilm properties. Moreover, expanding the scope of GBS strains to include others of clinical relevance will provide further insight to the protective mechanisms of human milk glycans. Expanding the number of human milk oligosaccharide samples will fully define the extent of variability in effects of HMOs on GBS pathogenesis. Finally, we are currently studying cellular trafficking, RNA sequencing and proteomics, and additional HMO-antimicrobial cocktails, in order to tease out additional information regarding a mechanism of action. Results to this end, will be reported in due course.
Acknowledgments
SDT would like to acknowledge Vanderbilt University, the Department of Pediatrics at Vanderbilt University Medical Center, and the Institute of Chemical Biology for financial support. JAG is supported by the Department of Veterans Affairs CDA-2 1IK2BX001701. DLA acknowledges a financial award from the Amgen Foundation and has been supported by the Mitchum E. Warren, Jr. Graduate Research Fellowship. RSD has been supported by NIH grants T32AI007474-20 and 2T32HD060554-06A2. Work was also supported through the Vanderbilt University Core Services including use of the Cell Imaging Shared Resource were provided by the Vanderbilt Institute for Clinical and Translational Research program supported by the National Center for Research Resources, Grant UL1 RR024975-01, and the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06. Dr. Michelle Reyzer and Prof. Richard Caprioli are acknowledged for assistance with mass spectral analysis. Donor mothers are acknowledged for their generous contribution. The reviewers are acknowledged for their helpful suggestions.
Footnotes
AUTHOR CONTRIBUTIONS
SDT, JAG, DLA, and RSD designed the research program. JHW collected milk samples. DLA and RSD performed the research. Each author analyzed the data. The manuscript was written through contributions from all authors. All authors have given approval to the final version of the manuscript.
References
- 1.Exclusive breastfeeding for six months best for babies everywhere. Vol. 2011 World Health Organization; Jan 15, 2011. [Google Scholar]
- 2.Eidelman AI, Schanler RJ, Johnston M, Landers S, Noble L, Szucs K, Viehmann L, Feldman-Winter L, Lawrence R, Kim S, Onyema N, Breastfeeding S. Breastfeeding and the Use of Human Milk. Pediatrics. 2012;129(3):E827–E841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 3.Mass S. Supporting breastfeeding in the United States: the Surgeon General’s call to action. Current Opinion in Obstetrics & Gynecology. 2011;23(6):460–464. doi: 10.1097/GCO.0b013e32834cdcb3. [DOI] [PubMed] [Google Scholar]
- 4.Walker WA, Iyengar RS. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res. 2015;77(1-2):220–8. doi: 10.1038/pr.2014.160. [DOI] [PubMed] [Google Scholar]
- 5.Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012;96(3):544–51. doi: 10.3945/ajcn.112.037382. [DOI] [PubMed] [Google Scholar]
- 6.McCracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001;3(1):1–11. doi: 10.1046/j.1462-5822.2001.00090.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones CA. Maternal transmission of infectious pathogens in breast milk. J Paediatr Child Health. 2001;37(6):576–82. doi: 10.1046/j.1440-1754.2001.00743.x. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz S, Friedberg I, Ivanov IV, Davidson LA, Goldsby JS, Dahl DB, Herman D, Wang M, Donovan SM, Chapkin RS. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012;13(4):r32. doi: 10.1186/gb-2012-13-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donovan SM, Wang M, Li M, Friedberg I, Schwartz SL, Chapkin RS. Host-microbe interactions in the neonatal intestine: role of human milk oligosaccharides. Adv Nutr. 2012;3(3):450S–5S. doi: 10.3945/an.112.001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22(9):1147–62. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newburg DS. Glycobiology of human milk. Biochemistry (Mosc) 2013;78(7):771–85. doi: 10.1134/S0006297913070092. [DOI] [PubMed] [Google Scholar]
- 12.Urashima T, Kitaoka M, Asakuma S, Messer M. Milk Oligosaccharides. In: McSweeney P, Fox PF, editors. Advanced Dairy Chemistry: Volume 3: Lactose, Water, Salts and Minor Constituents. Springer New York; New York, NY: 2009. pp. 295–349. [DOI] [Google Scholar]
- 13.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 14.Kudo T, Iwasaki H, Nishihara S, Shinya N, Ando T, Narimatsu I, Narimatsu H. Molecular genetic analysis of the human Lewis histo-blood group system. II. Secretor gene inactivation by a novel single missense mutation A385T in Japanese nonsecretor individuals. J Biol Chem. 1996;271(16):9830–7. doi: 10.1074/jbc.271.16.9830. [DOI] [PubMed] [Google Scholar]
- 15.Brandolini M, Corbella M, Cambieri P, Barbarini D, Sassera D, Stronati M, Marone P. Late-onset neonatal group B streptococcal disease associated with breast milk transmission: molecular typing using RAPD-PCR. Early Hum Dev. 2014;90(Suppl 1):S84–6. doi: 10.1016/S0378-3782(14)70025-8. [DOI] [PubMed] [Google Scholar]
- 16.Le Doare K, Kampmann B. Breast milk and Group B streptococcal infection: vector of transmission or vehicle for protection? Vaccine. 2014;32(26):3128–32. doi: 10.1016/j.vaccine.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filleron A, Lombard F, Jacquot A, Jumas-Bilak E, Rodiere M, Cambonie G, Marchandin H. Group B streptococci in milk and late neonatal infections: an analysis of cases in the literature. Arch Dis Child Fetal Neonatal Ed. 2014;99(1):F41–7. doi: 10.1136/archdischild-2013-304362. [DOI] [PubMed] [Google Scholar]
- 18.Schuchat A, Wenger JD. Epidemiology of group B streptococcal disease. Risk factors, prevention strategies, and vaccine development. Epidemiol Rev. 1994;16(2):374–402. doi: 10.1093/oxfordjournals.epirev.a036159. [DOI] [PubMed] [Google Scholar]
- 19.Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1):21–47. doi: 10.1128/CMR.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez E, de Andres J, Manrique M, Pareja-Tobes P, Tobes R, Martinez-Blanch JF, Codoner FM, Ramon D, Fernandez L, Rodriguez JM. Metagenomic Analysis of Milk of Healthy and Mastitis-Suffering Women. J Hum Lact. 2015;31(3):406–15. doi: 10.1177/0890334415585078. [DOI] [PubMed] [Google Scholar]
- 21.Keefe GP. Streptococcus agalactiae mastitis: a review. Can Vet J. 1997;38(7):429–37. [PMC free article] [PubMed] [Google Scholar]
- 22.Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, Hadler JL, Danila R, Cieslak PR, Schuchat A. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342(1):15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 23.Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1–22. [PubMed] [Google Scholar]
- 24.Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005;115(2 Suppl):519–617. doi: 10.1542/peds.2004-1441. [DOI] [PubMed] [Google Scholar]
- 25.Rosini R, Margarit I. Biofilm formation by Streptococcus agalactiae: influence of environmental conditions and implicated virulence factors. Front Cell Infect Microbiol. 2015;5:6. doi: 10.3389/fcimb.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuzeville S, Dramsi S, Madec JY, Haenni M, Payot S. Antigen I/II encoded by integrative and conjugative elements of Streptococcus agalactiae and role in biofilm formation. Microb Pathog. 2015;88:1–9. doi: 10.1016/j.micpath.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Xia FD, Mallet A, Caliot E, Gao C, Trieu-Cuot P, Dramsi S. Capsular polysaccharide of Group B Streptococcus mediates biofilm formation in the presence of human plasma. Microbes Infect. 2015;17(1):71–6. doi: 10.1016/j.micinf.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Pritchard DG, Gray BM, Egan ML. Murine monoclonal antibodies to type Ib polysaccharide of group B streptococci bind to human milk oligosaccharides. Infect Immun. 1992;60(4):1598–602. doi: 10.1128/iai.60.4.1598-1602.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bode L. The functional biology of human milk oligosaccharides. Early Human Development. 2015;91(11):619–622. doi: 10.1016/j.earlhumdev.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Andreas NJ, Al-Khalidi A, Jaiteh M, Clarke E, Hyde MJ, Modi N, Holmes E, Kampmann B, Mehring Le Doare K. Role of human milk oligosaccharides in Group B Streptococcus colonisation. Clin Transl Immunology. 2016;5(8):e99. doi: 10.1038/cti.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin AE, Autran CA, Szyszka A, Escajadillo T, Huang M, Godula K, Prudden AR, Boons GJ, Lewis AL, Doran KS, Nizet V, Bode L. Human milk oligosaccharides inhibit growth of group B Streptococcus. J Biol Chem. 2017 doi: 10.1074/jbc.M117.789974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaddy JA, Tomaras AP, Actis LA. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun. 2009;77(8):3150–60. doi: 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domenech M, Garcia E, Prieto A, Moscoso M. Insight into the composition of the intercellular matrix of Streptococcus pneumoniae biofilms. Environ Microbiol. 2013;15(2):502–16. doi: 10.1111/j.1462-2920.2012.02853.x. [DOI] [PubMed] [Google Scholar]
- 34.Cowan SE, Gilbert E, Liepmann D, Keasling JD. Commensal interactions in a dual-species biofilm exposed to mixed organic compounds. Appl Environ Microbiol. 2000;66(10):4481–5. doi: 10.1128/aem.66.10.4481-4485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakeman CA, Moore JL, Noto MJ, Zhang Y, Singleton MD, Prentice BM, Gilston BA, Doster RS, Gaddy JA, Chazin WJ, Caprioli RM, Skaar EP. The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat Commun. 2016;7:11951. doi: 10.1038/ncomms11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stahl B, Thurl S, Henker J, Siegel M, Finke B, Sawatzki G. Detection of four human milk groups with respect to Lewis-blood-group-dependent oligosaccharides by serologic and chromatographic analysis. Adv Exp Med Biol. 2001;501:299–306. doi: 10.1007/978-1-4615-1371-1_37. [DOI] [PubMed] [Google Scholar]
- 37.Grollman EF, Ginsburg V. Correlation between Secretor Status and Occurrence of 2′-Fucosyllactose in Human Milk. Biochem Bioph Res Co. 1967;28(1):50–&. doi: 10.1016/0006-291x(67)90404-4. [DOI] [PubMed] [Google Scholar]
- 38.Blank D, Gebhardt S, Maass K, Lochnit G, Dotz V, Blank J, Geyer R, Kunz C. High-throughput mass finger printing and Lewis blood group assignment of human milk oligosaccharides. Anal Bioanal Chem. 2011;401(8):2495–510. doi: 10.1007/s00216-011-5349-9. [DOI] [PubMed] [Google Scholar]
- 39.Totten SM, Zivkovic AM, Wu S, Ngyuen U, Freeman SL, Ruhaak LR, Darboe MK, German JB, Prentice AM, Lebrilla CB. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J Proteome Res. 2012;11(12):6124–33. doi: 10.1021/pr300769g. [DOI] [PubMed] [Google Scholar]
- 40.van Leeuwen SS, Schoemaker RJ, Gerwig GJ, van Leusen-van Kan EJ, Dijkhuizen L, Kamerling JP. Rapid milk group classification by 1H NMR analysis of Le and H epitopes in human milk oligosaccharide donor samples. Glycobiology. 2014;24(8):728–39. doi: 10.1093/glycob/cwu036. [DOI] [PubMed] [Google Scholar]
- 41.Allison LM, Walker LA, Sanders BJ, Yang Z, Eckert G, Gregory RL. Effect of Human Milk and its Components on Streptococcus Mutans Biofilm Formation. J Clin Pediatr Dent. 2015;39(3):255–61. doi: 10.17796/1053-4628-39.3.255. [DOI] [PubMed] [Google Scholar]
- 42.Zeng L, Das S, Burne RA. Utilization of lactose and galactose by Streptococcus mutans: transport, toxicity, and carbon catabolite repression. J Bacteriol. 2010;192(9):2434–44. doi: 10.1128/JB.01624-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


