Abstract
Background
L1-cell adhesion molecule (L1CAM) was previously reported to carry a poor prognosis in Stage I, low-risk endometrial cancer (EC). We evaluated the role of L1CAM among all stages and histologies in ECs in The Cancer Genome Atlas (TCGA).
Methods
Clinical information and RNA-Seq expression data were derived from TCGA uterine cancer cohort. Associations between L1CAM expression and clinical factors were tested with linear and logistic regression. Differences in survival between “high” and “low” expression groups (defined by median expression) of L1CAM were compared using Cox regression analysis, with p-values calculated via log-rank test. Kaplan-Meier curves were tested with the log-rank test.
Results
Patient characteristics of 545 primary tumors with RNA-Seq gene expression data were analyzed. Median age was 64 years (range 31–90). Stage I, II, III, and IV comprised 62%, 10%, 23%, and 5%, respectively. 75% were endometrioid; 21% serous. Grade 1, 2, and 3 comprised 18%, 22%, and 60%, respectively. Median follow-up was 23.0 months. High L1CAM expression was associated with advanced stage (OR 3.2), high grade (OR=6.8), serous histology (OR=16.3), positive cytology (OR=3.5), positive pelvic (OR=21.8) and para-aortic lymph nodes (OR=10.3) (all p≤0.001). High L1CAM was associated with a median overall survival (OS) of 107 months, versus not reached for low L1-expressing ECs (HR= 3.46, CI 1.97 – 6.07, p<0.001). On multivariate analysis, L1CAM expression remained an independent prognostic variable in predicting OS in EC.
Conclusions
L1CAM expression is an independent predictor of poor survival in endometrial cancer, and is associated with advanced stage, high-risk endometrial cancer.
Keywords: L1CAM, endometrial cancer, TCGA
BACKGROUND
Endometrial carcinoma (EC) is the most common gynecologic malignancy in the United States, with an estimated 54,870 cases expected to be diagnosed in 2015 [1]. Despite a good survival rate for early-stage and Type I ECs, the prognosis for advanced-stage EC has been poor [2]. No FDA-approved second-line chemotherapy drugs currently exist for metastatic EC, and recent clinical trials evaluating novel therapeutics have shown modest response rates. There are no universal biomarkers in EC, though CA-125 may be useful in advanced stage and serous EC. More importantly, no effective biomarkers currently exist to direct treatment (adjuvant radiation and/or chemotherapy) in EC, or to triage pelvic and para-aortic lymphadenectomy staging in clinical Stage I EC.
L1-cell adhesion molecule (L1CAM) is a transmembrane protein of the immunoglobulin family that has been implicated in promoting tumor cell proliferation, migration, invasion, and metastasis, in part through the activation of the extracellular signal-regulated kinase (ERK) pathway, which induces the expression of motility- and invasion-associated gene products [3–6]. Recent data implicate it with both epithelial-to-mesenchymal transition (EMT) and Wnt signaling [7]. Its expression in endometrial tumors and sera from EC patients has been reported to be associated with poor prognosis [8]. A recent analysis of 1031 Stage I EC patients identified L1CAM expression as an independent predictor of progression-free survival (PFS), overall survival (OS), and distant recurrence [9]. Furthermore, a recent report of immunohistochemistry analyses in 865 tumor samples from PORTEC-1 and PORTEC-2 showed a significantly increased risk of distant recurrence and pelvic nodal relapse with high L1CAM expression [10].
The functional role of L1CAM has been further elucidated by Altevogt et al [3,11–17]. Its main role as a cell adhesion molecule is evident in its action as a “glue between cells”. More recent studies have shown L1CAM’s downstream signaling effects on epithelial-mesenchymal transition (EMT) and tumor progression [18]. Both EMT regulators (Slug) and Wnt signaling regulators (β-catenin) have been shown to regulate L1CAM transcription [16], while other suggested roles include pro-angiogenesis [15,19].
Despite compelling evidence for the prognostic role of L1CAM in early stage ECs, data regarding its role in advanced stage and high-risk ECs is scarce. We therefore set out to determine the relevance of L1CAM in The Cancer Genome Atlas (TCGA) in uterine cancer which includes both Type I and Type II ECs, as well as encompasses all stages of EC. Our analysis included data from 545 primary endometrial tumors, for which clinical information was available, including a median follow-up of 23 months. L1CAM expression appears to be an independent predictor for poor survival, with overexpression in advanced stage and high grade ECs, serous histology, positive cytology, deep myometrial invasion, and positive pelvic and para-aortic lymph nodes. These data add to the strength of L1CAM as a potential biomarker in EC, and given its detectability in serum and presence as a transmembrane protein, L1CAM may become an attractive candidate as a serum biomarker and potential therapeutic target in highrisk EC.
MATERIALS AND METHODS
RNA-Seq expression (combining level 3 data from Illumina GA and HiSeq platforms) and clinical data for uterine cancer patients were downloaded from the TCGA data portal (http://cancergenome.nih.gov/). The methods of biospecimen procurement, RNA isolation, and RNA sequencing were previously described by the Cancer Genome Atlas Research Network [20]. RSEM (RNA-Seq by Expectation-Maximization) expression values were used for statistical analysis. Box-plots were used to visualize expression differences for discrete variables. Univariate logistic regression analysis of L1-CAM expression as a categorical dependent variable was used, with a median L1CAM expression of greater or less than 5.37, in association with clinicopathologic characteristics. Differences in overall survival between “high” and “low” expression groups (defined by median value of L1CAM expression) were compared using Kaplan-Meier curves, with p-values calculated via log-rank test, using the Survival package in R. Univariate Cox regression analysis was used to estimate survival based on L1CAM expression and clinicopathologic factors. Multivariate Cox analysis was used to compare the influence of L1CAM expression on survival along with other clinical characteristics (stage, grade, myometrial invasion, para-aortic lymph node status, pelvic lymph node status, peritoneal cytology, and histological subtype).
Of note, lymphovascular space invasion (LVSI) was not reported in the uterine cancer TCGA. Thus, we were unable to evaluate L1CAM expression in “high-intermediate risk” patients, as defined by GOG 99 to vary with the presence of LVSI, deep myometrial invasion, grade, and age [21].
RESULTS
Patient characteristics
From the TCGA uterine cancer data, 545 primary tumors with both clinical and gene expression data were analyzed in December 2015. Patient characteristics are reported in Table 1. Median age of this patient cohort was 64 years (range 31–90 years). Seventy-three percent were White, 21% were African-American, and 4% Asian, with less than 3% identified as Native American/Alaskan Native and Native Hawaiian/Pacific Islander. Stage I, II, III, and IV comprised 62%, 10%, 23%, and 6%, respectively. The majority of tumors (75%, n=399) were of endometrioid histology, and 21% (n=116) were serous. Grade 1, 2, and 3 comprised 18%, 22%, and 60%, respectively. Slightly less than half of all patients had deep myometrial invasion (45%), and 15% had positive peritoneal washings. Approximately 10% and 6% of all patients had positive pelvic and para-aortic lymph nodes, respectively. Median follow-up for subjects alive at last contact was 23.0 months (range 0 to 192 months).
TABLE 1.
Patient characteristics of TCGA cohort
| Characteristic | Total | % |
|---|---|---|
| Age at diagnosis (y) Median age (range) | 64 (31–90) | |
| Race | ||
| White | 373 | 72.7 |
| African-American | 107 | 20.9 |
| Asian | 20 | 3.9 |
| Native American/Alaska Native | 4 | 0.8 |
| Native Hawaiian/Pac Islander | 9 | 1.8 |
| BMI | ||
| Median BMI (range) | 32.2 (17.4–81.6) | |
| Menopausal status | ||
| Premenopausal | 35 | 7.0 |
| Perimenopausal | 16 | 3.2 |
| Postmenopausal | 435 | 86.5 |
| Indeterminate | 17 | 3.4 |
| Stage | ||
| I | 332 | 62.3 |
| II | 51 | 9.6 |
| III | 121 | 22.7 |
| IV | 29 | 5.4 |
| Histology | ||
| Serous endometrial adenocarcinoma | 113 | 21.2 |
| Mixed serous and endometrioid | 21 | 3.9 |
| Endometrioid endometrial adenocarcinoma | 399 | 74.9 |
| Grade | ||
| 1 | 98 | 18.4 |
| 2 | 118 | 22.1 |
| 3 | 317 | 59.5 |
| Myometrial invasion | ||
| Superficial (<50%) | 255 | 55.3 |
| Deep (≥50%) | 206 | 44.7 |
| Pelvic peritoneal cytology | ||
| Negative | 341 | 84.8 |
| Positive | 61 | 15.2 |
| Pelvic Lymph nodes | ||
| Negative | 489 | 89.7 |
| Positive | 56 | 10.3 |
| Para-aortic Lymph nodes | ||
| Negative | 514 | 94.3 |
| Positive | 31 | 5.7 |
L1CAM expression and association with clinicopathologic variables
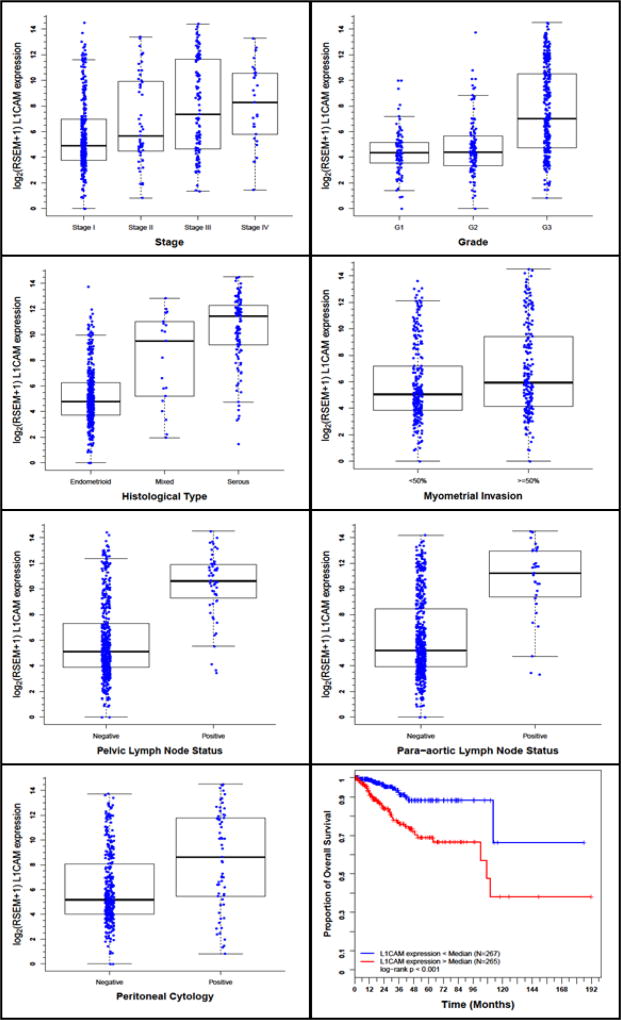
A total of 545 EC samples with L1CAM expression data across all patient characteristics were analyzed from TCGA. Normal endometrial samples were exluded. L1CAM expression varied significantly with prognostic clinicopathologic variables, including stage, grade, depth of invasion, histology, peritoneal cytology, and lymph node metastases (Figure 1a–g). Univariate L1CAM expression as a categorical dependent variable (based on median expression value of 5.37) was associated with poor prognostic clinicopathologic characteristics (Table 2). High L1CAM expression (above median value derived from 532 patients having survival information) was associated with advanced stage (OR=3.2 for Stage I-II vs III-IV), high grade (OR=6.8 for Grade 1 and 2 vs 3), deep myometrial invasion (OR=1.85), serous histology (OR=16.3), positive peritoneal cytology (OR=3.5), and positive pelvic (OR=21.8) and para-aortic lymph nodes (OR=10.3) (all p-values ≤0.001). Similarly, L1CAM as a continuous independent variable was also associated with poor prognostic clinicopathologic characteristics (Supplementary Table A).
Figure 1.
L1CAM expression and its association with clinicopathologic characteristics, including a. Stage, b. Grade, c. Histology, d. Depth of myometrial invasion, e. peritoneal cytology, f. pelvic lymph node metastases, g. para-aortic lymph node metastases, h. Impact of L1CAM expression on overall survival in EC patients in TCGA cohort.
TABLE 2.
Univariate L1CAM expression* association with clinicopathologic characteristics (logistic regression)
| Clinicopathologic variable | Total N |
Odds Ratio in L1- CAM expression |
p-value |
|---|---|---|---|
| Stage (I or II vs. III or IV) | 533 | 3.217 (2.148 – 4.818) | < 0.001 |
| Grade (Grade 1 or 2 vs. 3) | 533 | 6.819 (4.605 – 10.10) | < 0.001 |
| Histology (Serous vs. endometrioid) | 533 | 16.31 (8.883 – 29.93) | < 0.001 |
| Myometrial invasion (continuous %) | 461 | 1.012 (1.006 – 1.018) | < 0.001 |
| Myometrial invasion (deep vs. superficial) | 461 | 1.848 (1.274 – 2.679) | 0.001 |
| Peritoneal cytology (positive vs. negative) | 402 | 3.510 (1.888 – 6.528) | < 0.001 |
| Pelvic lymph nodes (positive vs. negative) | 545 | 21.78 (6.715 – 70.65) | < 0.001 |
| Para-aortic lymph nodes (positive vs. negative) | 545 | 10.33 (3.101 – 34.40) | < 0.001 |
| Age (continuous) | 533 | 1.040 (1.023 – 1.056) | < 0.001 |
| BMI (continuous) | 504 | 0.966 (0.947 – 0.986) | < 0.001 |
categorical dependent variable, greater or less than the median expression level
Survival outcomes and multivariate analysis
A total of 532 EC samples with both L1CAM expression and survival data were available for analysis. High L1CAM expression was associated with poor survival, with a median overall survival of 107 months, versus not reached for low L1CAM-expressing endometrial tumors (HR= 3.46, CI 1.97–6.07, p<0.001) (Figure 1h, Table 3a). Among Stage I EC patients alone (n=331), high L1CAM expression was associated with a trend towards worse survival, though was not statistically significant (Supplemental Figure A). Other clinicopathologic variables associated with poor survival include advanced stage, high grade, positive pelvic washings and pelvic/para-aortic lymph nodes, serous histology, deep myometrial invasion, and age (Table 3a).
Table 3.
| a – Overall survival and associations with clinicopathologic characteristics using Cox regression | |||
|---|---|---|---|
| Clinicopathologic variable | Total N | Hazard Ratio | p-value |
| L1-CAM expression (continuous) | 532 | 1.153 (1.076 – 1.236) | < 0.001 |
| L1-CAM expression (categorical, above or below median of 5.37) | 532 | 3.456 (1.968 – 6.071) | < 0.001 |
| Stage (I or II vs. III or IV) | 532 | 3.790 (2.269 – 6.329) | < 0.001 |
| Grade (Grade 1 or 2 vs. 3) | 532 | 4.333 (2.209 – 8.500) | < 0.001 |
| Peritoneal cytology (positive vs. negative) | 402 | 2.970 (1.643 – 5.371) | < 0.001 |
| Pelvic lymph nodes (positive vs. negative) | 532 | 2.112 (1.128 – 3.953) | 0.031 |
| Para-aortic lymph nodes (positive vs. negative) | 532 | 1.662 (0.665 – 4.153) | 0.31 |
| Histology (Serous vs. endometrioid) | 532 | 1.640 (1.277 – 2.105) | < 0.001 |
| Myometrial invasion (continuous %) | 460 | 1.024 (1.015 – 1.032) | < 0.001 |
| Myometrial invasion (deep vs. superficial) | 460 | 2.957 (1.686 – 5.184) | < 0.001 |
| Age (continuous) | 532 | 1.030 (1.007 – 1.054) | 0.011 |
| BMI (continuous) | 503 | 0.975 (0.947 – 1.005) | 0.088 |
| b - Multivariate survival model after variable selection | |||||
|---|---|---|---|---|---|
| HR | SE | p-val | Lower 95% |
Upper 95% |
|
| L1-CAM expression (categorical, above or below median of 5.37) | 4.572 | 2.26 | 0.002 | 1.736 | 12.046 |
| Myometrial invasion (continuous %) | 1.013 | 0.01 | 0.014 | 1.002 | 1.023 |
| Stage (I or II vs. III or IV) | 3.856 | 1.33 | < 0.001 | 1.957 | 7.598 |
In a multivariate analysis (Table 3b), L1CAM expression remained an independent prognostic variable for overall survival, with a HR of 4.56 (1.74–12.05, p=0.002), along with stage and myometrial invasion.
DISCUSSION
L1CAM has been well described as a prognostic marker in Stage I ECs based on IHC studies in large cohorts from European trials, including PORTEC-1 and 2 [10,22]. Our present analysis adds L1CAM as a prognostic marker in advanced stage and high-risk EC patients, based on a large cohort of EC patients. Recent knowledge from The Cancer Genome Atlas provides insight on various molecular subtypes of endometrial cancer and expands on the traditional twotype endometrial cancer model, in which Type I ECs are associated with PTEN, PIK3 and β-catenin aberrations, while Type II ECs are associated with TP53 and more aggressive molecular aberrations. L1CAM appears to be predominantly expressed in Type II ECs, and has recently been associated with TP53 mutations [23]. Its association with high-risk endometrial cancers, particularly with pelvic and para-aortic lymph node metastases, suggests potential future utility in several aspects of treatment and diagnosis in EC. First, its increased expression in patients with positive pelvic and para-aortic lymph nodes may be translated into a triage biomarker for pelvic and para-aortic lymph node staging. This may require confirmation of a similar correlation between serum L1CAM expression and positive lymph node metastases in EC patients, or feasibility of preoperative expression levels in endometrial biopsies. The results of the currently ongoing Pipelle Prospective endometrial carcinoma (PIPENDO) study which includes preoperative and postoperative L1CAM expression analyses, will thus be eagerly awaited [24]. Secondly, increased expression in advanced, high-risk ECs of this transmembrane protein may offer potential novel therapeutic strategies to target the extracellular portion of L1CAM in EC patients, and simultaneously allow for biomarker selection of these patients by IHC. Lastly, L1CAM expression as an independent prognostic marker suggests its potential for an endometrial cancer biomarker, especially given the lack of currently available, effective biomarkers in endometrial cancer for monitoring and treatment selection. Several drawbacks exist in this study. We were unable to study the prevalence of L1CAM expression in high-intermediate risk Stage I patients, as lymphovascular space invasion was not recorded in the TCGA; additionally, L1CAM expression was not significantly associated with survival in the Stage I subpopulation, thus limiting our conclusion of the role of L1CAM in earlystage EC. However, this subpopulation may have been underpowered, as a trend towards worse survival was present in high-expressing L1CAM tumors. Nonetheless, previously published large studies have already shown a predictive role for L1CAM in early stage EC. Secondly, our analysis utilized RNA-seq expression from EC samples as reported by the TCGA, and protein data were not available to confirm these expression levels. Nonetheless, the uniform accuracy and precision of an integrated analysis as performed by the Cancer Genome Atlas Research Network, as well as its comprehensive clinical data, add strength to this study. Lastly, data regarding recurrence sites and treatment modality are not available and limit the clinical outcome analysis of this study. In contrast, a recently published pilot series of 116 endometrial cancer samples which included advanced stage and high-risk endometrial cancers, did report an increased rate of distant recurrences associated with L1CAM expression. Of note, this analysis also included a limited TCGA analysis of 245 cases, which similarly reported associations of L1CAM with older age, advanced stage, non-endometrioid histology, and higher grade [23].
The lack of an adequate biomarker, as well as relatively modest therapeutic gains in the field of endometrial cancer, suggest continued efforts to optimize and refine both surgical and chemotherapeutic treatments. L1CAM has risen as a promising target for both therapeutic and biomarker utility. Its reported molecular associations with EMT suggest a more invasive molecular phenotype. Our study adds to previous findings of L1CAM expression as a poor prognostic marker in endometrial cancer patients, especially in high-risk ECs. Further studies are needed to confirm the prognostic value and feasibility of serum L1CAM, to validate L1CAM as a predictive marker in a larger, prospective cohort of high risk EC patients, as well as to further elucidate the mechanisms by which L1CAM is associated with metastasis in EC patients.
Supplementary Material
RESEARCH HIGHLIGHTS.
L1CAM expression is associated with high-risk, Type II endometrial cancer.
L1CAM is an independent predictor for survival in endometrial cancer.
L1CAM is an attractive target for novel therapy in high-risk endometrial cancer.
Acknowledgments
The results shown here are in whole or part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Obel JC, Friberg G, Fleming GF. Chemotherapy in endometrial cancer. Clin Adv Hematol Oncol. 2006;4:459–468. [PubMed] [Google Scholar]
- 3.Mechtersheimer S, Gutwein P, Agmon-Levin N, Stoeck A, Oleszewski M, et al. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. Journal of Cell Biology. 2001;155:661–673. doi: 10.1083/jcb.200101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gast D, Riedle S, Schabath H, Schlich S, Schneider A, et al. L1 augments cell migration and tumor growth but not beta3 integrin expression in ovarian carcinomas. Int J Cancer. 2005;115:658–665. doi: 10.1002/ijc.20869. [DOI] [PubMed] [Google Scholar]
- 5.Gast D, Riedle S, Issa Y, Pfeifer M, Beckhove P, et al. The cytoplasmic part of L1-CAM controls growth and gene expression in human tumors that is reversed by therapeutic antibodies. Oncogene. 2008;27:1281–1289. doi: 10.1038/sj.onc.1210747. [DOI] [PubMed] [Google Scholar]
- 6.Arlt MJ, Novak-Hofer I, Gast D, Gschwend V, Moldenhauer G, et al. Efficient inhibition of intraperitoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res. 2006;66:936–943. doi: 10.1158/0008-5472.CAN-05-1818. [DOI] [PubMed] [Google Scholar]
- 7.Colas E, Pedrola N, Devis L, Ertekin T, Campoy I, et al. The EMT signaling pathways in endometrial carcinoma. Clin Transl Oncol. 2012;14:715–720. doi: 10.1007/s12094-012-0866-3. [DOI] [PubMed] [Google Scholar]
- 8.Fogel M, Gutwein P, Mechtersheimer S, Riedle S, Stoeck A, et al. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet. 2003;362:869–875. doi: 10.1016/S0140-6736(03)14342-5. [DOI] [PubMed] [Google Scholar]
- 9.AG Zeimet SA-A, et al. Large international multicenter evaluation of the clinical significance of L1-CAM expression in FIGO stage I, type 1 endometrial cancer. Journal of Clinical Oncology. 2011;29 Abstract 5091. [Google Scholar]
- 10.Bosse T, Nout RA, Stelloo E, Dreef E, Nijman HW, et al. L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: pooled PORTEC trial results. Eur J Cancer. 2014;50:2602–2610. doi: 10.1016/j.ejca.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Sebens Muerkoster S, Kotteritzsch J, Geismann C, Gast D, Kruse ML, et al. alpha5-integrin is crucial for L1CAM-mediated chemoresistance in pancreatic adenocarcinoma. Int J Oncol. 2009;34:243–253. [PubMed] [Google Scholar]
- 12.Geismann C, Arlt A, Bauer I, Pfeifer M, Schirmer U, et al. Binding of the transcription factor Slug to the L1CAM promoter is essential for transforming growth factor-beta1 (TGF-beta)-induced L1CAM expression in human pancreatic ductal adenocarcinoma cells. Int J Oncol. 2011;38:257–266. [PubMed] [Google Scholar]
- 13.Gutwein P, Stoeck A, Riedle S, Gast D, Runz S, et al. Cleavage of L1 in exosomes and apoptotic membrane vesicles released from ovarian carcinoma cells. Clin Cancer Res. 2005;11:2492–2501. doi: 10.1158/1078-0432.CCR-04-1688. [DOI] [PubMed] [Google Scholar]
- 14.Schirmer U, Fiegl H, Pfeifer M, Zeimet AG, Muller-Holzner E, et al. Epigenetic regulation of L1CAM in endometrial carcinoma: comparison to cancer-testis (CT-X) antigens. BMC Cancer. 2013;13:156. doi: 10.1186/1471-2407-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, et al. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol. 2005;168:633–642. doi: 10.1083/jcb.200408051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeifer M, Schirmer U, Geismann C, Schafer H, Sebens S, et al. L1CAM expression in endometrial carcinomas is regulated by usage of two different promoter regions. BMC Mol Biol. 2010;11:64. doi: 10.1186/1471-2199-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huszar M, Pfeifer M, Schirmer U, Kiefel H, Konecny GE, et al. Up-regulation of L1CAM is linked to loss of hormone receptors and E-cadherin in aggressive subtypes of endometrial carcinomas. J Pathol. 2010;220:551–561. doi: 10.1002/path.2673. [DOI] [PubMed] [Google Scholar]
- 18.Kiefel H, Bondong S, Hazin J, Ridinger J, Schirmer U, et al. L1CAM: a major driver for tumor cell invasion and motility. Cell Adh Migr. 2012;6:374–384. doi: 10.4161/cam.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedli A, Fischer E, Novak-Hofer I, Cohrs S, Ballmer-Hofer K, et al. The soluble form of the cancer-associated L1 cell adhesion molecule is a pro-angiogenic factor. Int J Biochem Cell Biol. 2009;41:1572–1580. doi: 10.1016/j.biocel.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas Research N. Kandoth C, Schultz N, Cherniack AD, Akbani R, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 22.Zeimet AG, Reimer D, Huszar M, Winterhoff B, Puistola U, et al. L1CAM in Early-Stage Type I Endometrial Cancer: Results of a Large Multicenter Evaluation. J Natl Cancer Inst. 2013 doi: 10.1093/jnci/djt144. [DOI] [PubMed] [Google Scholar]
- 23.Van Gool IC, Stelloo E, Nout RA, Nijman HW, Edmondson RJ, et al. Prognostic significance of L1CAM expression and its association with mutant p53 expression in high-risk endometrial cancer. Mod Pathol. 2016;29:174–181. doi: 10.1038/modpathol.2015.147. [DOI] [PubMed] [Google Scholar]
- 24.Visser NC, Bulten J, van der Wurff AA, Boss EA, Bronkhorst CM, et al. PIpelle Prospective ENDOmetrial carcinoma (PIPENDO) study, pre-operative recognition of high risk endometrial carcinoma: a multicentre prospective cohort study. BMC Cancer. 2015;15:487. doi: 10.1186/s12885-015-1487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.