Abstract
Background
Previous studies demonstrated that tendon-derived stem cells (TDSCs) were vital healing cells and that mRNA expression of anti-inflammatory cytokine IL-6 was significantly upregulated in injured tendons. The aim of the present study was to investigate the effects of IL-6 on the TDSCs in vitro.
Material/Methods
TDSCs isolated from the Achilles tendons in SD rats were co-cultured with various concentrations of IL-6. Cell proliferation, cell cycle analysis, quantitative real-time PCR, western blotting analysis, and statistical analysis were used in the study.
Results
The result showed that IL-6 strongly increased proliferation capability, and induced cell cycle activation and transition into G2/M phase from G1 phase in TDSCs. However, IL-6 treatment strongly inhibited gene expression of Scleraxis, Collagen 1, Tenomodulin, Collagen 3, Early Growth Response Protein 1, Decorin, Lumican, Biglycan and Fibromodulin in TDSCs. It also strongly inhibited protein expression of tendon cell markers like scleraxis, collagen 1, collagen 3, and tenomodulin. IL-6 treatment strongly activated the JAK/Stat3 signaling pathway in TDSCs. Furthermore, WP1066, a JAK/Stat3 signaling pathway inhibitor, abrogated the effects of IL-6 on TDSCs.
Conclusions
These findings indicated that IL-6 might exert dual effects on TDSCs in vitro: strongly enhancing their proliferation but inhibiting their tenogenic differentiation via the JAK/Stat3 pathway.
MeSH Keywords: Interleukin-6, Stem Cells, Tendons, Wounds and Injuries
Background
The tendon is a compositionally complex tissue with a predominantly mechanical function: translating muscular contractions into joint movement by transmitting forces from muscle to bone. Owing to the critical role of this tissue in body mechanics, injury and degeneration of the tendon can be highly debilitating and can result in substantial pain, disability, and healthcare costs [1]. A better understanding of biological processes of tendon repair and degeneration is required to establishing strategies that stimulate tendon repair and induce its regeneration. Recently, studies have highlighted inflammatory cell infiltration and gene expression of inflammatory cytokines in both animal and human tendon diseases [2,3], indicating that inflammation may also plays an important role in the tendon healing process [4]. Studies profiling expression of inflammatory cytokines in canine and rat tendon injury models [5,6], have found that gene expression of inflammatory cytokines including IL-6 is remarkably upregulated. Studies have also indicated that infusion of IL-6 significantly stimulates collagen synthesis in the peritendinous tissue in humans [7]. However, the exact impact of IL-6 in injured tendons has not been fully elucidated.
Recently, the presence of tendon-derived stem cells (TDSCs) has been demonstrated in various species including human, horse, rabbit, rat, and mouse [8–11]. These cells express stem cell-related markers, form adherent colonies, and show multipotency in vitro and in vivo [8,9]. Because they form tendon-like tissues in nude mouse or nude rat models [8–11], TDSCs are suggested to contribute to tendon repair.
To understand how inflammatory cytokines affect the regenerative and degenerative potentials of TDSCs, we examined the effects of IL-6 (a significant one of the cytokines that are upregulated in injured tendons) on the function of TDSCs.
Material and Methods
Animals
All aspects of the research were conducted in accordance with the guidelines set by the Institutional Animal Care and Use Committee of Nanfang Hospital, Southern Medical University. Sprague-Dawley female rats (6–8 weeks old) were purchased from Laboratory Animal Center of Southern Medical University, Guangzhou, China.
Isolation of TDSCs
Tendon-derived stem cells (TDSCs) were isolated from the Achilles tendons of SD rats as previously described [12,13]. Briefly, the Achilles tendons were dissected and incubated with 600 U/mL (3 mg/mL) type I collagenase (Sigma, #C0130) in PBS for 2 hours at 37°C with gentle shaking. The dissociated cells were plated at a density of 140 cells/cm2 in 100-mm dishes and cultured in DMEM containing 20% FBS (Gibco, South America). The TDSCs at passage 3 or 4 were used in the following experiments. We usually isolated TDSCs from 4 Achilles tendons and plated them on two 100-mm dishes. The clonogenicity and multi-lineage differentiation potential of these cells were confirmed before being used for the experiments in this study using standard assays as described previously [12].
Cell proliferation
To perform cell proliferation assays, TDSCs were plated at 103 cells/well in a 96-well plate and allowed to adhere overnight. DMEM containing 10% FBS medium was supplemented with 0, 0.1, 1, 10, and 100 ng/mL rat IL-6 (PEPROTRCH, #400-19) for 1, 3, and 5 days. Proliferation activity was then determined using manual counting and a CCK8 cell counting kit (Dojindo, #KL640) following the manufacturer’s protocol. All assays were carried out in triplicate for each sample.
Cell cycle analysis
TDSCs that were either untreated or treated with IL-6 for 3 days were washed once in phosphate-buffered saline (PBS) and fixed with 500 μL of cold 70% ethanol in PBS for 2 hours or overnight at 4°C. The cells were then centrifuged at 2000 rpm for 5 min, washed again in phosphate-buffered saline (PBS) and resuspended in 100 μL RNase A (KeyGENBioTECH, #KGA511), and incubated at 37°C for 30 min. Afterwards, after mixed with 400 μL propidium iodide (PI) (KeyGENBioTECH, #KGA511), the cells were incubated at 4°C for 30 min, and then analyzed by flow cytometry (FACScan; Becton Dickinson, San Francisco, California, USA). All assays were carried out in triplicate for each sample.
RNA isolation and gene expression assay
After appropriate treatments, total RNA was isolated using TRIzol (Invitrogen, USA) following the manufacturer’s protocol and reverse-transcribed into cDNA. The resulting cDNA was subjected to a quantitative polymerase chain reaction (qPCR) assay. The qPCR was performed with a LightCycle480 real-time PCR System (Roche) using SYBR green reagents (TaKaRa, #AK8307). The average threshold cycle value (Ct value) was calculated from triplicate reactions. Standard curves were generated using 10-fold serial dilutions of cDNA of each gene with a correlation coefficient of >0.98. Relative expression levels were calculated based on a standard curve and normalized to glyceraldehyde 3-phosphate dehydrogenase (Gapdh). The primer sequences used in this research are listed in Table 1.
Table 1.
Primers used for quantitative real-time PCR.
| Gene | Forward primers | Reverse primers | Accession No. |
|---|---|---|---|
| Scx | 5′-AGAACACCCAGCCCAAACA-3′ | 5′-CGGTCTTTGCTCAACTTTCT-3′ | NM_001130508 |
| Col1 | 5′-GTGCTAAGGGTGAAGCTGGT-3′ | 5′-CATCAGCACCAGGGTTTCCAG-3′ | NM_053304 |
| Tnmd | 5′-GTCACATTCTAAATGCAGAAG-3′ | 5′-CTCCCCCAAAACAGGACAAT-3′ | NM_022290 |
| Col3 | 5′-CTGGAGATAAGGGTGAAGGT-3′ | 5′-GAGGGCCTCCTTCACCTTTCT-3′ | NM_032085 |
| Mkx | 5′-CTATCGCACAGGTAAGCCCA-3′ | 5′-CCCACGTATCAGTTTCTCCCA-3′ | XM_017600733 |
| Egr1 | 5′-AACAACCCTACGAGCACCTG-3′ | 5′-ACCAGCGCCTTCTCGTTATT-3′ | NM_012551 |
| Fmod | 5′-CCCGTGATTGTCCCCAAGAA-3′ | 5′-CAGGTACTTGAGGTTGCGGT-3′ | NM_080698 |
| Lum | 5′-GCTTCACCGGGCTTCAATAC-3′ | 5′-AAATGAGTTTCCAGGCACGC-3′ | NM_031050 |
| Dcn | 5′-CCTAAAGGAGCTGCCCGAAA-3′ | 5′-GCCGCCCAGTTCTATGACAA-3′ | NM_024129 |
| Bgn | 5′-CTGCATTGAGATGGGTGGGA-3′ | 5′-GGTAGTTGAGCTTCAGGCCA-3′ | NM_017087 |
Immunoblot analysis
The TDSCs were plated on a density of 4×105/well in a 60-mm dish and cultured in DMEM containing 10% FBS in the presence or absence IL-6 at the concentration of 10 ng/mL for 3 days and then lysed in SDS sample buffer. Scleraxis (Scx), tenomodulin (Tnmd), collagen 1 (Col1), collagen 3 (Col3) and GAPDH contents were examined by Immunoblot assays using the corresponding antibodies. The anti-scleraxis rabbit polyclonal antibody (Abcam, #ab58655) and anti-tenomodulin rabbit polyclonal antibody (Abcam, #ab203676) were purchased from Abcam (USA). The anti-collagen 1 rabbit polyclonal antibody (Proteintech, #14695-1-AP), anti-collagen 3 rabbit polyclonal antibody (Proteintech, #13548-1-AP), anti-Stat3 rabbit polyclonal antibody (Proteintech, #10253-2-AP) and anti-GAPDH rabbit polyclonal antibody (Proteintech, #10494-1-AP) were purchased from Proteintech (China). The anti-Phospho-Stat3(Tyr705) antibody (Affinity, #AF3295) were purchased from Affinity (China).
Application of the Stat3 inhibitor
To demonstrate that IL-6 exerts functions through the JAK/Stat3 pathway, the TDSCs were plated at a density of 4×105/well on a 60-mm dish and allowed to adhere overnight. DMEM containing 10% FBS medium was supplemented with 10 ng/mL IL-6 with or without Stat3 inhibitor WP1066 (Selleck, #S2796, China) at the concentration of 5 μM for 3 days. After the aforementioned treatment, the cells were collected and used in further experiments.
Statistical analysis
Results were analyzed using the Statistical Package for the Social Sciences (SPSS) 20 (IBM), and expressed as mean ± standard deviation (SD). Student’s t-test or one-way ANOVA followed by Dunnett test was used to identify the differences. The threshold for significance for all tests was set as P<0.05.
Results
Effects on cell proliferation
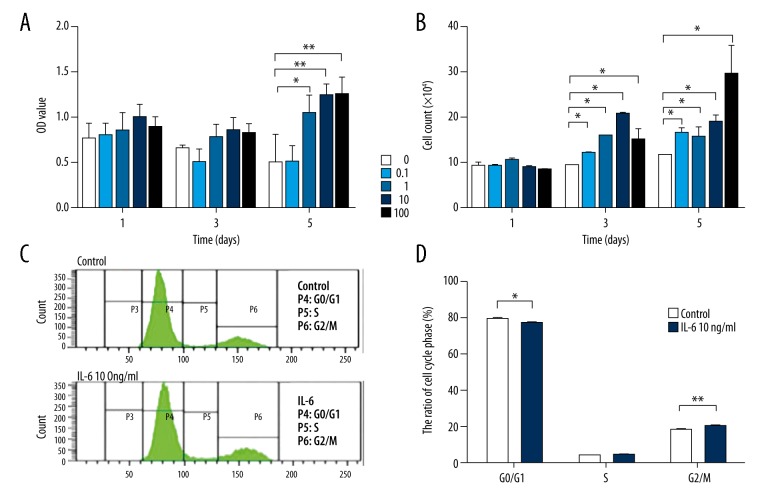
A statistically significant increase was found in cell proliferation of IL-6-treated cells as compared to controls. The effects were statistically significant after 5 days in the groups treated with 1, 10, or 100 ng/mL IL-6 (the control group was 0.4955±0.3183, the IL-6 at 0.1 lg/mL group was 0.4976±0.192, the IL-6 at 1 lg/mL group was 1.0372±0.2084, P<0.05; the IL-6 at 10 lg/mL group was 1.2337±0.1346, P<0.01; and the IL-6 at 100 lg/mL group was 1.2456±0.1969, P<0.01) (Figure 1A, 1B).
Figure 1.
Increased cell proliferation was found in IL-6-treated TDSCs. (A) CCK8 assay was done to detect cell viability in TDSCs treated with various concentrations (0, 0.1, 1, 10, and 100 ng/mL) of IL-6 for 1, 3, and 5 days, respectively. (B) Manual cell count was performed to detect cell viability in TDSCs treated with various concentrations (0, 0.1, 1, 10, and 100 ng/mL) of IL-6 for 1, 3, and 5 days, respectively. (C, D) Cell cycle was detected in TDSCs with or without 10 ng/mL IL-6 treatment for 3 days. Values are average and SD for 3 samples. * P<0.05; ** P<0.01.
Effects on cell cycle
IL-6 altered the cell cycle of TDSCs. After TDSCs were incubated with 10 ng/mL IL-6 for 3 days, flow cytometry showed activated G1 phase and an increased number of cells in G2/M phase in the IL-6-treated cells. The percentages of control and 10 ng/mL IL-6-treated TDSCs in G1 phase were 78.8±0.95% and 76.2±0.51%, respectively, showing a statistically significant difference between the groups (P<0.05). In contrast, the percentages of control and 10 ng/mL IL-6-treated TDSCs in G2/M phase were 17.6±0.57% and 19.7±0.36%, respectively (P<0.05). The percentages of TDSCs in S phase with and without IL-6 treatment were nearly identical. (Figure 1C, 1D)
Effects on tenogenic differentiation
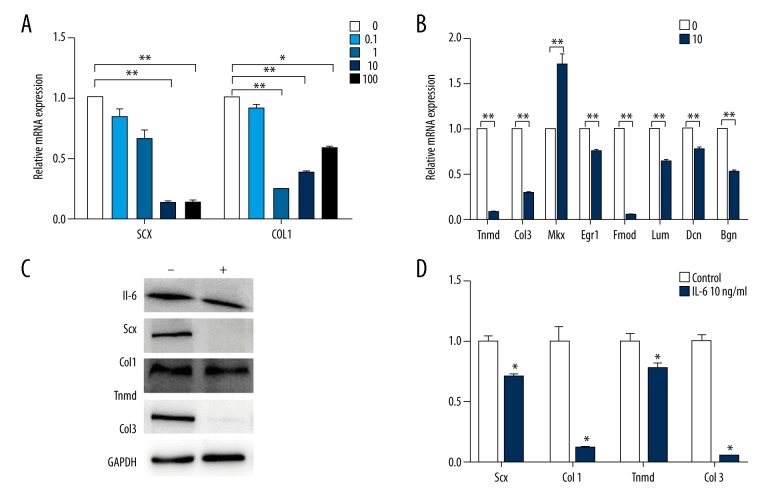
After the cells were treated with 0, 0.1, 1, 10, or 100 ng/mL IL-6 for 3 days, a significant reduction was shown in the mRNA expression levels of Scx and Col1 (Figure 2A). At the protein level, the western-blotting consistently showed a significant reduction in the expression of Scx and Col1 in the cells incubated with 10 ng/mL IL-6 (Figure 2C). Quantitative analyses of the protein bands certainly indicated a significant difference in the protein level expression of Scx and Col1 between IL-6-treated and control groups (P<0.05) (Figure 2D).
Figure 2.
IL-6 showed inhibitory effect on tenogenic differentiation of TDSCs. (A) RT-PCR analysis of SCX and COL1 in TDSCs treated with various concentrations (0, 0.1, 1, 10, and 100 ng/mL) of IL-6 for 3 days. (B) RT-PCR analysis of other tenogenic genes in TDSCs treated with 10 ng/mL IL-6 or not for 3 days. (C, D) Western blot analysis of Scx, Col1, Tnmd, and Col3 proteins in TDSCs treated with 10 ng/mL IL-6 or not for 3 days. Values are average and SD for 3 samples. * P<0.05; ** P<0.01.
IL-6 also downregulated the expression of other pivotal genes, Tenomodulin (Tnmd) and Collagen 3 (Col3) while it upregulated Mohawk (Mkx) expression (Figure 2B). The western blotting also showed a significant reduction in the protein expression of Tnmd and Col3 in the cells incubated with 10 ng/mL IL-6 (Figure 2C). The quantitative analyses of the protein bands also indicated a significant difference in the expression of Tnmd and Col3 between IL-6-treated and control groups (P<0.05) (Figure 2D). Examination of other gene expression including Lumican (Lum), Decorin (Dcn), early growth response gene 1 (Egr1), Fibromoduline (Fmod), and Biglycan (Bgn) in the IL-6-treated cells found a significant reduction in all of them (Figure 2B).
IL-6 promotes proliferation but inhibits tenogenic differentiation via the JAK/Stat3 pathway of TDSCs
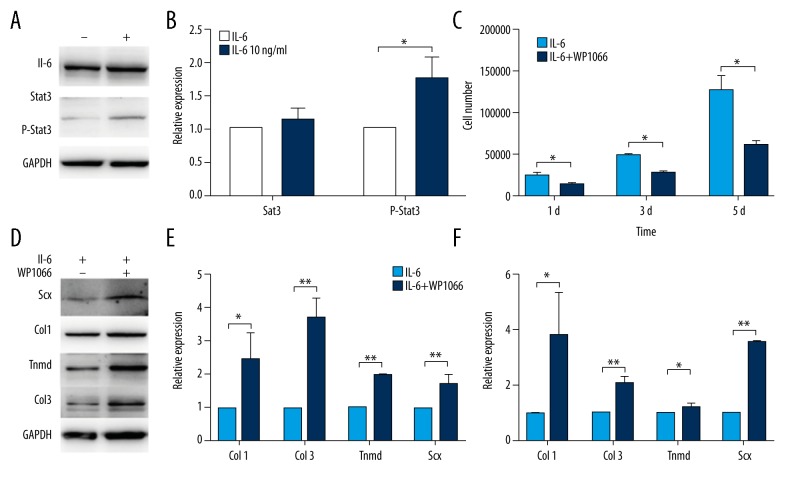
The western blotting consistently showed a significant increase in the expression of Phospho-Stat3 in the cells incubated with 10 ng/mL IL-6 for 3 days and there was no significant difference in the expression of Stat3 (Figure 3A). Quantitative analyses of the protein bands certainly indicated a significant difference in the protein level expression of Phospho-Stat3 between IL-6-treated and control groups (P<0.05) (Figure 3B).
Figure 3.
IL-6 promotes proliferation but inhibits tenogenic differentiation via the JAK/Stat3 pathway of TDSCs. (A, B) Western blot analysis of Stat3 and P-Stat3 in TDSCs treated with 10ng/mL IL-6 or not for 3 days. (C) Manual cell count was performed to detect cell viability in TDSCs treated with 10ng/mL IL-6 along with 5 μM WP1066 or not for 3 days. (D, E) Western blot analysis of Scx, Col1, Tnmd, and Col3 proteins in TDSCs treated with 10ng/mL IL-6 along with 5 μM WP1066 or not for 3 days. (F) RT-PCR analysis of tenogenic genes in TDSCs treated with 10ng/mL IL-6 along with 5 μM WP1066 or not for 3 days. Values are average and SD for 3 samples. * P<0.05; ** P<0.01.
We cultured cells with 10 ng/mL IL-6 with or without Stat3 inhibitor WP1066 at the concentration of 5 μM for 3 days. A statistically significant reduction was found in cell proliferation of WP1066-treated group as compared to controls (P<0.05) (Figure 3C). Meanwhile, a significant increase was shown in the mRNA expression levels of Col1, Col3, Scx, and Tnmd between WP1066-treated and control groups (P<0.05) (Figure 3F). At the protein level, the western blotting consistently showed a significant increase in the expression of Col1, Col3, Scx and Tnmd in the WP1066-treated group (P<0.05) (Figure 3D, 3E).
Discussion
To develop novel targeted therapies for tendon injury, it is necessary to define the molecular changes and mechanisms governing the tendon healing process. The cellular process of tendon repair may be divided into 3 phases: inflammation, proliferation and differentiation, and remodeling [14]. The injured site is initially filled with blood and inflammatory cells, and later on occupied over time by fibroblastic cells that are mixed populations migrating from the paratenon, endotenon, sheaths and/or surrounding tissues such as synovium [14]. These cells can produce new tendon cells that synthesize collagen matrix and organize dense collagen fibers, thus resulting in restoration of damaged tendon structure [14]. However, neo-forming tendons do not represent the original tendon structure but contain scar characterized by high cell density, disoriented cell arrangement, thin and randomly aligned collagen fibers, high vascularity, and mucoid matrix [15]. Failure in structural recovery results in biomechanical properties and possibly in re-rupture.
Anderson et al. reported that infusion of IL-6 into the peritendinous tissue of the Achilles tendon stimulated significantly collagen synthesis in the peritendinous tissue in humans [7]. Lin et al. also showed that mechanical and organizational properties of injured tendons from IL6−/− mice were inferior to those from control mice, indicating that IL-6 which could not be compensated for plays an important role in tendon healing [7]. However, Lin et al. showed that IL6−/− mice exhibited no significant differences in collagen fiber distribution or maximum stress but a smaller cross-sectional area compared with control mice [17]. Thus, the exact impact of increased IL-6 on injured tendon has not been fully elucidated.
In the regenerative phase, healing cells migrate into the injury/repair site, actively proliferate and deposit abundant extracellular matrix (ECM) in the tissue [18]. Therefore, proliferation and migration of tendon-derived stem cells play a pivotal role in tendon healing. As reported in other cell types, IL-6 and the anti-inflammatory cytokine IL-10 induce the activation of the STAT3 signaling pathway, which is implicated in cell proliferation and survival [19]. Our results showed that proliferation of TDSCs was significantly increased when the cells were cocultured with IL-6. This indicated that upregulation of IL-6 may promote tendon healing by stimulating proliferation of TDSCs. IL-6 also promotes blood vessel proliferation by VEGF-dependent angiogenesis via the STAT3 pathway in other cell types [20]. IL-6 might play a role in the proliferation phase of tendon healing via STAT3 activation by stimulation of cell proliferation and thus supporting survival. Particularly, we found that IL-6 induced cell cycle activation and transition into G2/M phase from G1 phase. IL-6, which promotes proliferation of tendon-derived stem cells, has obviously a positive impact on tendon healing.
Our study found that IL-6 reduced expression of tenogenic differentiation markers (Scx and Tnmd) [21] and Egr-1, a transcriptional factor that plays an important role in tendon [22], indicating IL-6 might suppress tenogenic differentiation of TDSCs. We found IL-6 also reduced the main tendon-associated collagens (Col1 and Col3). Similarly, gene expression of Fmod and Lum, two leucine-rich repeat proteins influencing collagen fibrillogenesis [23], was also inhibited in TDSCs by IL-6. Importantly, as Fmod is a critical component of niche, reduction of Fmod affects differentiation of TDSCs [8]. Previous studies also showed that overexpression of Fmod enhanced tendon healing either in vivo or in vitro [24]. Contrarily, Fmod-deficient mice developed abnormal tendon and ectopic ossification [24]. And Lum has a combined effect together with Fmod at different developmental stages [23]. These effects of IL-6 might change the microenvironment of the niche of TDSCs and thus alter their fate, impairing recovery of biomechanical properties of regenerating tendon. However, we have only evaluated the effect of IL-6 alone on the TDSCs. There are many other growth factors and cytokines that modulate the differentiation of TDSCs after tendon injury, which likely work in concert with IL-6 to induce changes during tendon repair. Previous studies have reported that the mRNA levels of inflammatory cytokines such as IL-1β are remarkably upregulated in injured tendons [5]. IL-1β irreversibly inhibits tenogenic differentiation of injured tendon-derived progenitor cells, indicating that inflammatory cytokines strongly affect function of tendon progenitor cells appearing in injured tendons [26]. As immunoregulatory cytokine, IL-6 may have synergic actions with IL-β and other cytokines to affect the differentiation of TDSCs. The role of IL-6 on differentiation of TDSCs after tendon injury in vivo remains unclear and needs to be further investigated. Katsma et al. reported that chronic treatment with physiologically relevant levels of IL-6 suppresses expression of Col1a1 and LOX while also altering expression of select MMPs but does not alter Achilles tendon collagen synthesis in vivo [27]. However, as the animal model used in Katsma et al. was normal rat Achilles tendon, it is still unclear how IL-6 affects TDSCs and tendon repair after tendon injury.
In contrast, we detected upregulated expression of Mkx, a homeobox gene involved in tendon development [28]. Ito et al. reported that in Mkx mutant mice tendons were hypoplastic throughout the body and collagen fibril diameters smaller, indicating that Mkx plays a critical role in tendon development by regulating production of type I collagen [28]. Although the role of Mkx in tendon repair has not been elucidated yet, upregulation of Mkx by IL-6 may have a role in collagen fibril formation during tendon healing. The specific significance of upregulation of Mkx by IL-6 needs to be further investigated.
Our present study showed that IL-6 exerted dual effects on TDSCs in vitro. On one hand, it enhanced proliferation of TDSCs but on the other hand, it inhibited their tenogenic differentiation. Lin et al. also showed that complex mechanisms might regulate the effect of IL-6 in vivo because mechanical and organizational properties of injured tendons from IL6−/− mice were inferior to those from control mice [16]. Although it is still unclear whether the 2 effects are regulated via the same pathway, we suggest that timely control of the negative effect of IL-6 but interventional promotion of its positive effect may benefit tendon regeneration.
Conclusions
IL-6 exerts dual effects on tendon-derived stem cells in vitro: strongly enhancing their proliferation but inhibiting their tenogenic differentiation via the JAK/Stat3 pathway.
Acknowledgments
We thank Professor Liang Ping for his revision of this manuscript.
Footnotes
Source of support: This study was supported by National Natural Science Foundation of China (81601900), and Science and technology project of Guangdong Province (2016A020214010)
References
- 1.Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: Repair and regeneration. Annu Rev Biomed Eng. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- 2.Battery L, Maffulli N. Inflammation in overuse tendon injuries. Sports Med Arthrosc. 2011;19:213–17. doi: 10.1097/JSA.0b013e31820e6a92. [DOI] [PubMed] [Google Scholar]
- 3.Millar ML, Dean BJ, Dakin SG. Inflammation and the continuum model: Time to acknowledge the molecular era of tendinopathy. Br J Sports Med. 2016 doi: 10.1136/bjsports-2016-096419. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Abate M, Silbernagel KG, Siljeholm C. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther. 2009;11:235. doi: 10.1186/ar2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manning CN, Havlioglu N, Knutsen E, et al. The early inflammatory response after flexor tendon healing: A gene expression and histological analysis. J Orthop Res. 2014;32:645–52. doi: 10.1002/jor.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugg KB, Lubardic J, Gumucio JP, et al. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res. 2014;32:944–51. doi: 10.1002/jor.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen MB, Pingel J, Kjaer M, et al. Interleukin-6: A growth factor stimulating collagen synthesis in human tendon. J Appl Physiol (1985) 2011;110:1549–54. doi: 10.1152/japplphysiol.00037.2010. [DOI] [PubMed] [Google Scholar]
- 8.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–27. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovati AB, Corradetti B, Lange CA, et al. Characterization and differentiation of equine tendon-derived progenitor cells. J Biol Regul Homeost Agents. 2011;25:S75–84. [PubMed] [Google Scholar]
- 11.Rui YF, Lui PP, Li G, et al. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16:1549–58. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 12.Asai S, Otsuru S, Candela ME, et al. Tendon progenitor cells in injured tendons have strong chondrogenic potential: The CD105-negative subpopulation induces chondrogenic degeneration. Stem Cells. 2014;32:3266–77. doi: 10.1002/stem.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K, Zhang S, Li Q, et al. Effects of celecoxib on proliferation and tenocytic differentiation of tendon-derived stem cells. Biochem Biophys Res Commun. 2014;450:762–66. doi: 10.1016/j.bbrc.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Maffulli N. Tendon injury and tendinopathy: Healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 15.Galatz LM, Gerstenfeld L, Heber-Katz E, et al. Tendon regeneration and scar formation: The concept of scarless healing. J Orthop Res. 2015;33:823–31. doi: 10.1002/jor.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin TW, Cardenas L, Glaser Dl, et al. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2006;39:61–69. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Lin TW, Cardenas L, Soslowsky LJ. Tendon properties in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2005;38:99–105. doi: 10.1016/j.jbiomech.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Tsai WC, Yu TY, Lin LP, et al. Prevention of simvastatin-induced inhibition of tendon cell proliferation and cell cycle progression by geranylgeranyl pyrophosphate. Toxicol Sci. 2016;149:326–34. doi: 10.1093/toxsci/kfv239. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed ST, Ivashkiv LB. Inhibition of IL-6 and IL-10 signaling and Stat activation by inflammatory and stress pathways. J Immunol. 2000;165:5227–37. doi: 10.4049/jimmunol.165.9.5227. [DOI] [PubMed] [Google Scholar]
- 20.Wei LH, Kuo ML, Chen CA, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–27. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 21.Gaut L, Duprez D. Tendon development and diseases. Wiley Interdiscip Rev Dev Biol. 2016;5:5–23. doi: 10.1002/wdev.201. [DOI] [PubMed] [Google Scholar]
- 22.Guerquin MJ, Charvet B, Nourissat G, et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest. 2013;123:3564–76. doi: 10.1172/JCI67521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezura Y, Chakravarti S, Oldberg A, et al. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–88. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delalande A, Gosselin MP, Suwalski A, et al. Enhanced Achilles tendon healing by fibromodulin gene transfer. Nanomedicine. 2015;11(7):1735–44. doi: 10.1016/j.nano.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Ameye L, Aria D, Jepsen K, et al. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. Faseb J. 2002;16:673–80. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 26.Zhang K, Asai S, Yu B, et al. IL-1beta irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem Biophys Res Commun. 2015;463:667–72. doi: 10.1016/j.bbrc.2015.05.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsma MS, Patel SH, Eldon E, et al. The influence of chronic IL-6 exposure, in vivo, on rat Achilles tendon extracellular matrix. Cytokine. 2017;93:10–14. doi: 10.1016/j.cyto.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Ito Y, Toriuchi N, Yoshitaka T, et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc Natl Acad Sci USA. 2010;107:10538–42. doi: 10.1073/pnas.1000525107. [DOI] [PMC free article] [PubMed] [Google Scholar]