Abstract
Obesity is a public health problem in both developed and developing countries, and the negative effects of obesity on reproductive physiology have been highlighted recently. We evaluated the effects of porcine obesity index, sex hormones, and peptide hormones on litter size in various breeds of minipigs. Blood samples were collected from sedated 8-,10-, and 12-mo-old minipigs to measure preovulatory levels of sex hormones (follicle-stimulating hormone, luteinizing hormone, estradiol, progesterone, testosterone, and prolactin) and peptide hormones (insulin-like growth factor, glucagon, cortisol, growth hormone, free thyroxine, free triiodothyronine, insulin, and leptin). We also measured weight, abdominal circumference, neck circumference, and body length and then calculated the porcine obesity index. Data were analyzed by one-way ANOVA, and means were compared by least significance difference testing. Pearson correlation between parameters and litter size was analyzed. Prepregnancy porcine obesity index and litter size were negatively correlated in primiparous minipigs. Litter size was influenced by luteinizing hormone, estradiol, progesterone, testosterone, prolactin, follicle-stimulating hormone, cortisol, insulin-like growth factor 1, growth hormone, free thyroxine, insulin, and leptin. In conclusion, prepregnancy obesity reduces litter size in primiparous minipigs.
Abbreviations: FSH, follicle-stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine; GH, growth hormone; IGF1,insulin-like growth factor 1; LH, luteinizing hormone; POI, porcine obesity index
In recent years, minipigs have become widely accepted as an alternative to rodent species for reproductive, metabolic, pharmacologic, and toxicologic studies.5,15 Compared with NHP (age at sexual maturity, 3 to 4 y; duration of estrous cycle, 30 d),minipigs become sexually mature much earlier (age, 4 to 5 mo), and their estrous cycle is shorter (20 d). The gestation period is also shorter in minipigs (4 mo) than NHP (5.5 mo), with the major period of organogenesis occurring between 11 and 35 d in minipigs compared with 20 to 50 d in cynomolgus macaques.15
Litter size in swine breeds, including minipigs, varies due to effects on the pituitary–ovarian axis during follicle development.8,9,18 Several variables, including circulating sex hormones, the month of parturition, and sow age and parity, exert marked effects on minipig reproduction and litter size.3,15 Primiparous minipigs typically have small litters and few weaned piglets.15 As lab animals, the litter size of minipigs is important for production and reproducibility: a large litter size can increase homogeneity and thus reduce the number of animals used. To increase the litter size of minipigs, insight into factors that disrupt reproductive physiology might provide important therapeutic implications.
The negative effects of obesity on reproductive physiology have been studied for many years. Obesity is a state of excess adipose tissue, which plays critical roles in the regulation of sex hormone availability due to its ability to store lipid steroids, such as androgens.16 In addition, obesity is associated with increased risk of pregnancy loss.6 Obesity may interfere with various ovarian and extraovarian functions, reducing both ovulatory and fertility rates in otherwise healthy women.17 Obesity during pregnancy is problematic, not only due to the adverse effects on maternal health and pregnancy outcome but also because of growing evidence regarding persistent and deleterious effects on the developing child.20
Prepregnancy or pregnancy obesity is associated with multiple adverse reproductive outcomes,10 but the underlying mechanisms in minipigs remain largely unknown. This study primarily aimed to evaluate the effects of prepregnancy obesity and obesity-related hormones (sex hormones and peptide hormones) measured before ovulation—including follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, progesterone, testosterone, and prolactin, insulin-like growth factor (IGF1), glucagon, cortisol, growth hormone (GH), free thyroxine (FT4), free triiodothyronine (FT3), insulin, and leptin—on litter size in 3 breeds of primiparous minipigs.
Materials and Methods
Animals and housing.
In this study, 74 nulliparous female minipigs (age, 8 to 12 mo) were obtained from the Lab Animal Center of Southern Medical University (approval nos. SYXK[YUE]2016-0167, SCXK[YUE]2016-0041); the animals were naturally bred and comprised 24 Tibet minipigs, 24 Bama minipigs, and 26 Wuzhishan minipigs. The minipigs tested free of swine pathogens (foot and mouth disease virus, swine fever virus and so on). All animals were kept in groups of 4 to 6 per room in the same environment (temperature, 16 to 26 °C; humidity, 40% to 70%), including drinking water and nutrient levels. Animals were reared by free-choice feeding of commercial chow (Keao Xieli Feed, Beijing, China).
Experimental design and materials.
Experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals.13 An experimental protocol was approved by the IACUC of the Animal Experiment Center of Southern Medical University (no. S-20121101-01). In this study, blood samples were collected aseptically from the vena cava of anesthetized (3% pentobarbital sodium injected at a dose of 30 mg/kg into a marginal vein) nulliparous minipigs before estrus (at 8, 10, and 12 mo of age).
Levels of various sex hormones and peptide hormones were measured before ovulation. A total volume of 10 mL of blood was obtained from each animal and stabilized with heparin sodium. Plasma was obtained for analysis of FSH, LH, estradiol, progesterone, testosterone, prolactin, IGF1, glucagon, cortisol, GH, FT4, FT3, insulin, and leptin by ELISA (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). After hormone testing, all nulliparous minipigs in this study entered the breeding program. All pigs became pregnant and gave birth when 12 to 16 mo old; litter sizes were calculated after the birth of piglets.
We also examined body mass index, weight, neck circumference, and body length before ovulation to determine the influence of these variables on and their correlation with litter size. A porcine obesity index (POI) was used to quantitate obesity in pigs and was calculated as follows:20
where BS corresponds to body size (that is, length [in centimeters]), and A and N are the radii (in centimeters) of the abdomen and neck, respectively.
Statistical analysis.
To investigate the effects of breed and age on each measured variable, data were analyzed by ANOVA. Litter size was classified according to breed. In addition, descriptive statistics were determined for all variables according to breed. We used the least significance difference method for pairwise comparison and Pearson correlation to analyze correlations between hormone levels and litter size. All statistical analyses were performed by using SPSS version 13.0 (IBM, Armonk, NY), and a P value less than 0.05 defined significance.
Results
Litter size, prepregnancy body weight, and POI.
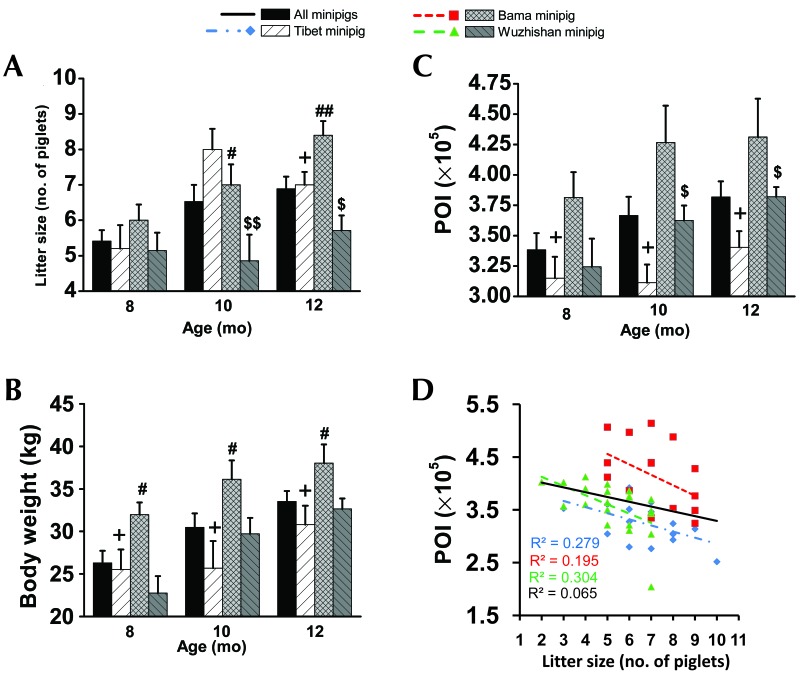
Prepregnancy body weight (Figure 1 B) and POI (Figure 1 C) increased with age in all 3 breeds of nulliparous minipigs. Bama nulliparous minipigs exhibited the highest prepregnancy body weight (32 ± 1 kg at 8mo, 36 ± 2 kg at 10 mo, and 38 ± 2 kg at 12 mo) and POI (×105; 3.8 ± 0.2 at 8 mo, 4.3 ± 0.3 kg at 10 mo, and 4.3 ± 0.3 kg at 12 mo). Tibet nulliparous minipigs had the lowest prepregnancy body weight (26 ± 2 kg at 8 mo, 26 ± 3 kg at 10 mo, and 31 ± 2 kg at 12 mo) and POI (×105; 3.2 ± 0.2 at 8 mo, 3.1 ± 0.2 at 10 mo, and 3.4 ± 0.1 at 12 mo).
Figure 1.
(A) Litter size, prepregnancy (B) body weight and (C) POI, and (D) the correlation between POI and litter size for different breeds of minipigs at 8,10, and 12 mo of age. All values (mean ± SEM) were calculated monthly. Significant differences between Tibet (n = 8 per group) compared with Bama (n = 8 per group; +, P < 0.05; ++, P < 0.005), Tibet compared with Wuzhishan (n = 8 [8 mo], 9 [10 mo], and 9 [12 mo]; #, P < 0.05; ##, P < 0.005), and Bama compared with Wuzhishan ($, P < 0.05; $$, P < 0.005) are indicated.
The litter size of Tibet minipigs rose by 53.9% at 10 mo (8.0 ± 0.6 piglets) and 34.6% at 12 mo (7.0 ± 0.4 piglets), compared with that at 8 mo (5.2 ± 0.7 piglets). By comparison, litter size in Bama minipigs increased by16.7% at 10 mo (7.0 ± 0.6 piglets) and 40.0% at 12 mo (8.4 ± 0.4 piglets), relative to that at 8 mo (6.0 ± 0.4 piglets). In contrast, litter size of Wuzhishan was 5.1 ± 0.5 piglets at 8 mo, 4.9 ± 0.7 piglets at 10 mo, and 5.7 ± 0.4 piglets at 12 mo (Figure 1 A). Litter size differed significantly between Wuzhishan and Tibet (P = 0.007) and Bama (P = 0.048) minipigs at 10 mo of age and between Tibet and Bama (P = 0.029), Tibet and Wuzhishan (P = 0.045), and Bama and Wuzhishan (P = 0.001) minipigs at 12mo. Bama minipigs had the largest litter size at 12 mo (7.1 ± 0.4 piglets). Pearson correlation analysis (Figure 1 D) showed a negative correlation between litter size and prepregnancy POI in Tibet (Pearson r = –0.529; P = 0.029), Bama (Pearson r = –0.442; P = 0.086), and Wuzhishan (Pearson r = –0.552; P = 0.009) minipigs. However, this correlation was significant for Tibet and Wuzhishan minipigs only.
Prepregnancy levels of sex hormones and their effects on litter size in minipigs.
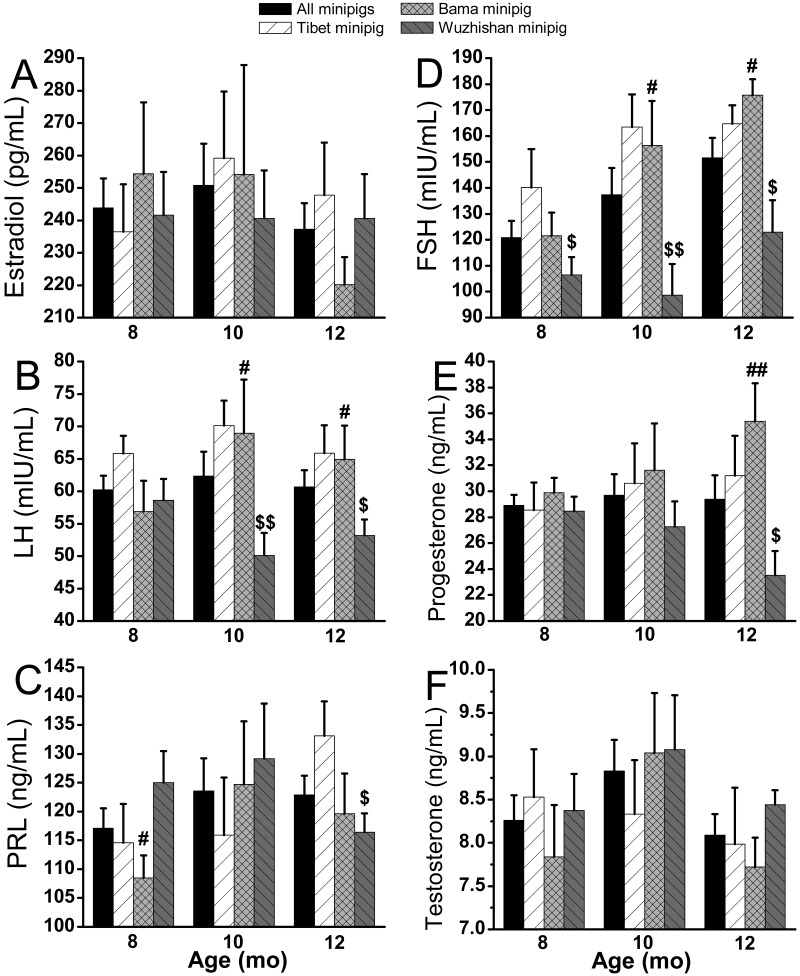
Tibet nulliparous minipigs had the highest levels of prepregnancy LH and FSH (Figure 2 B and D), except for FSH at 12 mo. Wuzhishan nulliparous minipigs manifested the lowest levels of prepregnancy LH, FSH, and progesterone (Figures 2 B, D, and E, respectively), except for LH at 8 mo. Prepregnancy levels of LH, FSH, and progesterone differed significantly (P < 0.05) between Wuzhishan minipigs and the other 2 breeds, except for FSH at 8 mo between Wuzhishan and Tibet (Figure 2 B, D, and E). Bama nulliparous minipigs had the highest prepregnancy levels of FSH and progesterone and the lowest prolactin level at 12 mo. Differences in prepregnancy FSH, LH, and progesterone among all breeds paralleled those of litter size. Prepregnancy FSH, LH, progesterone, and prolactin did not differ between Tibet and Bama minipigs (Figure 2 B through E). Neither prepregnancy estradiol nor testosterone differed between any breeds (Figure 2 A and F).
Figure 2.
Levels of prepregnancy sex hormones in different breeds of minipigs at 8, 10, and 12 mo of age. All values (mean ± SEM) were calculated monthly. Significant differences between Tibet (n = 8 per group) compared with Bama (n = 8 per group; +, P < 0.05; ++, P < 0.005), Tibet compared with Wuzhishan (n = 8 [8 mo], 9 [10 mo], and 9 [12 mo]; #, P < 0.05; ##, P < 0.005), and Bama compared with Wuzhishan ($, P < 0.05; $$, P < 0.005) are indicated.
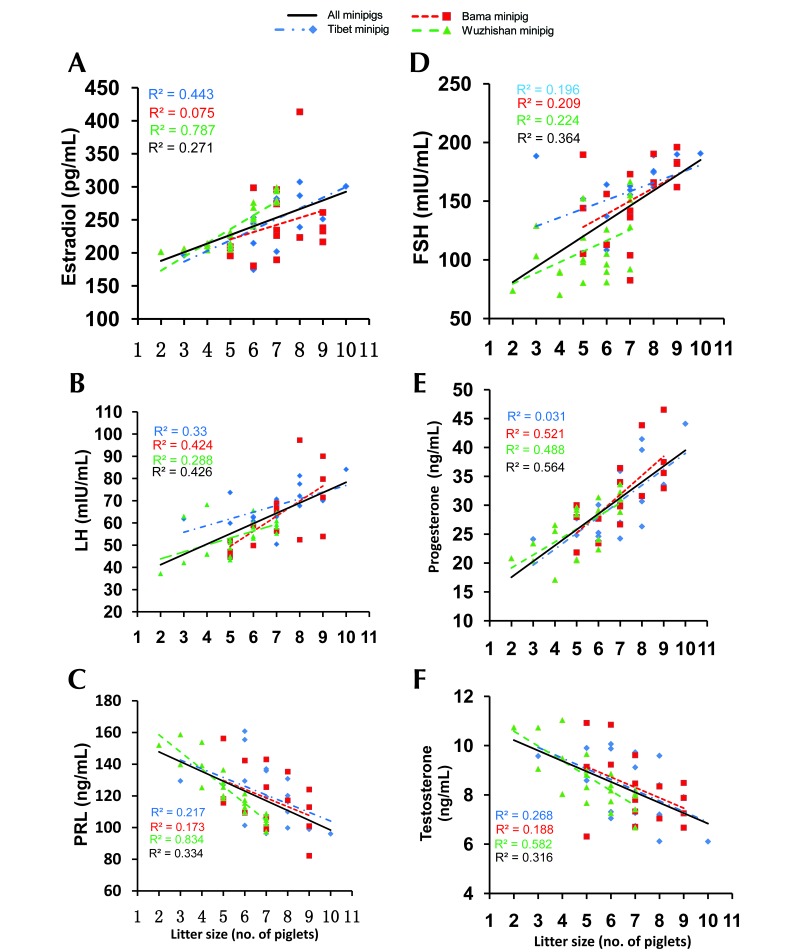
Correlations between litter size and prepregnancy levels of sex hormone were analyzed (Figure 3). Significant positive correlations emerged between litter size and prepregnancy estradiol, FSH, LH, or progesterone in all 3 breeds, except for estradiol in Bama minipigs and FSH and progesterone in Tibet minipigs (Figure 3 A, B, D, and E). Significant negative correlation occurred between prepregnancy prolactin (Figure 3 C) and testosterone (Figure 3 F) and litter size in Tibet and Wuzhishan minipigs. No significant relationship was observed between litter size and either prolactin or testosterone in Bama minipigs.
Figure 3.
Relationship of prepregnancy sex hormones to litter size in different breeds of minipigs at 8, 10, and 12 mo of age. The line represents linear regression of data, with R2 ≥ 0.16 (Pearson r ≥ 0.4) and P < 0.05 indicating significant correlation. Tibet, n = 8 per group; Bama, n = 8 per group; Wuzhishan, n = 8 (8 mo), 9 (10 mo), 9 (12 mo).
Prepregnancy levels of peptide hormones and their effects on litter size.
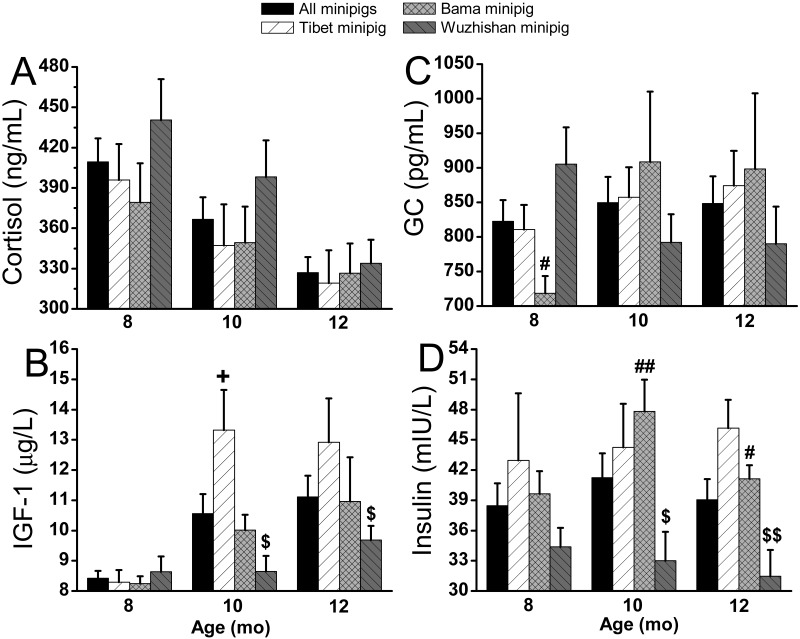
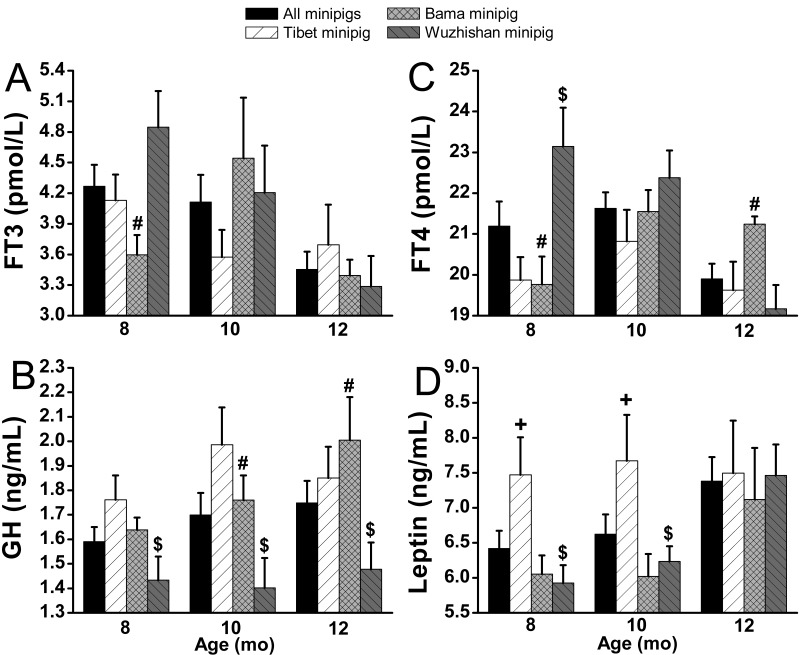
Prepregnancy levels of plasma cortisol (Figure 4 A) and FT3 (Figure 5 A) decreased with age, whereas IGF1 (Figure 4 B), glucagon (Figure 4 C), GH (Figure 5 B), and leptin (Figure 5 D) increased in all 3 breeds of nulliparous minipigs. Tibet minipigs had the highest prepregnancy levels of IGF1, GH, insulin (Figure 4 D), and leptin, except for insulin at 10 mo, GH at 12 mo, and IGF1 at 8 mo. Prepregnancy levels of IGF1, GH, insulin, and leptin were similar between Tibet nulliparous minipigs at different ages, but IGF1 increased from 8.3 ± 0.4 to 13.3 ± 1.3 (60.5%) at 10 mo. However, prepregnancy levels of IGF1, GH, and insulin in Wuzhishan minipig were lowest (P < 0.05) among the 3 breeds, except for insulin and IGF1 at 8 mo. The prepregnancy level of cortisol showed no significant difference among the 3 breeds. Prepregnancy levels of all peptide hormones evaluated did not differ significantly between Tibet and Bama nulliparous minipigs, except for IGF1 at 10 mo and leptin at 8 and 10 mo.
Figure 4.
Prepregnancy (A) cortisol, (B) IGF1, (C) glucagon, and (D) insulin in different breeds of minipigs at 8, 10, and 12 mo of age. All values (mean ± SEM) were derived monthly. Significant differences between Tibet (n = 8 per group) compared with Bama (n = 8 per group; +, P < 0.05; ++, P < 0.005), Tibet compared with Wuzhishan (n = 8 [8 mo], 9 [10 mo], and 9 [12 mo]; #, P < 0.05; ##, P < 0.005), and Bama compared with Wuzhishan ($, P < 0.05; $$, P < 0.005) are indicated.
Figure 5.
Prepregnancy level of (A) FT4, (B) FT3, (C) GH, and (D) leptin in different breeds of minipigs at 8, 10, and 12 mo of age. All values (mean ± SEM) were calculated monthly. Significant differences between Tibet (n = 8 per group) compared with Bama (n = 8 per group; +, P < 0.05; ++, P < 0.005), Tibet compared with Wuzhishan (n = 8 [8 mo], 9 [10 mo], and 9 [12 mo]; #, P < 0.05; ##, P < 0.005), and Bama compared with Wuzhishan ($, P < 0.05; $$, P < 0.005) are indicated.
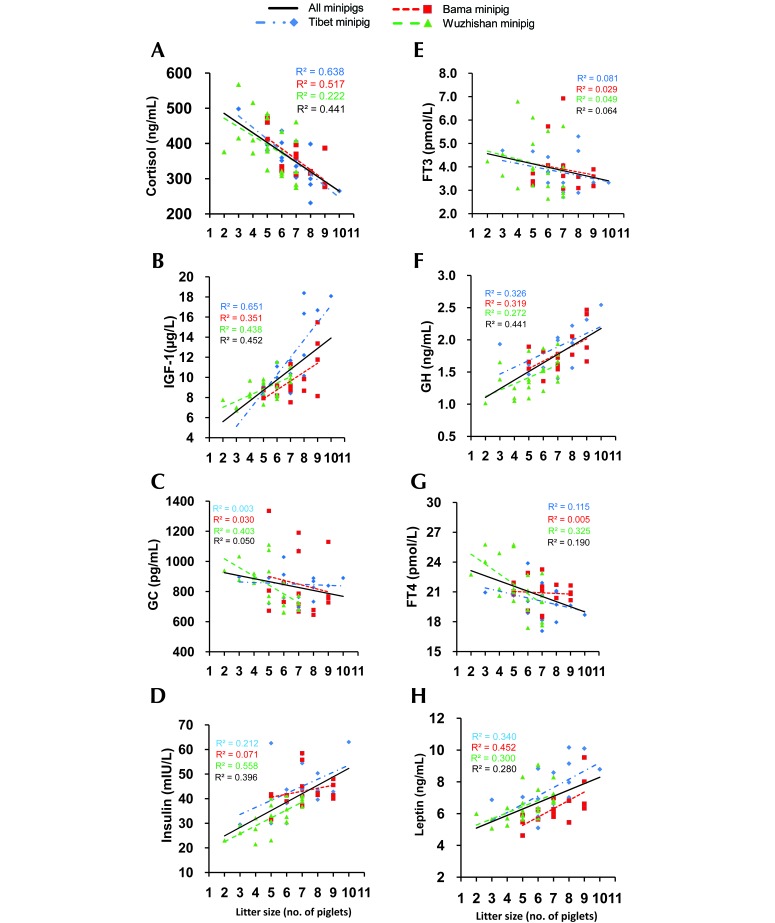
The correlation of litter size with prepregnancy peptide hormones was analyzed (Figure 6). All minipigs showed significant negative correlation between prepregnancy cortisol and litter size (Figure 6 A), whereas prepregnancy IGF1, GH, insulin, and leptin were associated with significant positive effects (Figure 6 B, D, F, and H). Litter size was not significantly correlated with prepregnancy levels of glucagon, FT4, or FT3 in the minipig breeds we evaluated.
Figure 6.
Relationship of prepregnancy level of (A) cortisol, (B) IGF1, (C) glucagon, (D) insulin, (E) FT3, (F) GH, (G) FT4, and (H) leptin to litter size indifferent breeds of minipigs at 8, 10, and 12 mo of age. The line represents linear regression of data, with R2 ≥ 0.16 (Pearson r ≥ 0.4) and P < 0.05 indicating significant correlation. Tibet, n = 8 per group; Bama, n = 8 per group; Wuzhishan, n = 8 (8 mo), 9 (10 mo), and 9 (12 mo).
Discussion
The characteristics of their reproductive system support the use of minipigs as a large animal model for reproductive system disease, obstetrics, neonatal disease, and other studies. Obesity is assumed to reduce fertility.17,19 In many lab animals with obesity, particularly genetic forms, females show complete infertility, whereas males demonstrate marked impairment of reproductive function.10,11,16 Previous work has revealed that prepregnancy obesity poses risks to both pregnant women and their infants. Prepregnancy overweight or obesity is a risk factor for diabetes, hypertension, and preeclampsia in pregnancy.23 The majority of previous studies relating obesity to health risks has focused on chronic diseases. However, we are learning more about components of prepregnancy obesity that relate to reproductive risks.
In our study, prepregnancy POI and litter size significantly differed among the 3 evaluated breeds of primiparous minipigs. In particular, Bama minipigs were the most obese but had the largest litter size. Breed played a key role in prepregnancy POI and litter size, given that all minipigs in our study program were housed in the same conditions, including drinking water and nutrient levels. Our study identified a significant negative correlation between prepregnancy POI and litter size, indicating that prepregnancy obesity reduced litter size in these 3 breeds of primiparous minipigs. This study is the first to highlight the relationship between prepregnancy obesity and litter size in primiparous minipigs, and this finding has important implications for all stages of reproductive health care for nulliparous minipigs.
Sex hormones are the primary physiologic hormones that directly influence reproductive activity and the reproductive process in animals. Changes in these substances markedly affect reproductive performance. In our study, the prepregnancy levels of sex hormones had significant correlations with litter size. Within the appropriate physiologic ranges, high levels of FSH, LH, estradiol, and progesterone before pregnancy and low levels of prolactin and testosterone—which aid in the development and maturation of follicles, during ovulation, and in implantation of the fertilized egg—increase litter size in minipigs. LH is responsible for the release of eggs from follicles (ovulation) and their development, maintenance, and function of the corpus luteum or yellow body. Progesterone improves the synchronization of estrus, especially in primiparous sows.14 However, litter size is determined by ovulation rate, fertilization rate, and prenatal survival rate,22 and the ovulation rate determines the upper limit of litter size.12 Ovarian follicles depend on the secretion of adenohypophyseal gonadotropins (FSH and LH) for their growth and maturation. With the stimulation of FSH, follicles develop and grow, and as FSH secretion reduces, the secretion of LH can promote the ovulation of mature follicles.7 These events might help to explain why Wuzhishan nulliparous minipigs had the smallest litter size, as they also had the lowest levels of prepregnancy FSH and LH, whereas Bama minipigs the largest litters at 12 mo.
Because obesity can reduce litter size, obesity-related peptide hormones may exert effects on litter size. Our findings show that prepregnancy levels of cortisol, IGF1, GH, insulin, and leptin had strong effects on litter size. High prepregnancy levels of circulating cortisol were associated with smaller litter size, whereas high concentrations of IGF1, GH, insulin, and leptin were associated with larger litter size. Prepregnancy levels of IGF1, GH, and insulin were lowest and cortisol was highest in Wuzhishan minipigs and perhaps resulted in their having the smallest litter size among the 3 breeds. Prolonged stress or prolonged elevation of cortisol reportedly impair reproductive processes in sows.21 Downregulating cortisol levels in minipigs and increasing their IGF1, GH, and insulin concentrations might help to increase litter size under normal physiologic conditions.
Our study focuses on the effect of prepregnancy levels of leptin on litter size, because a deficiency in this hormone leads to obesity.2 Previous studies reported that female obese (ob/ob) mice lacking leptin are infertile and have markedly impaired reproductive function.2,4 During the development and maturity of the reproductive system, leptin transmits energy storage signals to centers in the brain. Through the neuroendocrine system, this hormone promotes reproductive system development and maturation, maintains pregnancy, and supports other reproductive functions. In recent years, leptin has been suggested to be a metabolic signal in the reproductive system.1 This information supports our finding that prepregnancy leptin deficiency decreased litter size in primiparous minipigs. If higher prepregnancy leptin concentrations lead to a larger litter size in primiparous minipigs, then manipulation of the leptin level might provide an alternative means for treating delayed primiparity.
In conclusion, prepregnancy obesity reduces litter size in primiparous minipigs. Controlling body weight gain in minipigs (for example, through appropriate pregnancy nutrition or selective breeding) might consequently increase litter size and thus provide an important animal model for obstetrics, neonatology, and reproductive research.
Acknowledgments
We are grateful to Dr. Pei Fan and Yongquan Han for editing this paper. This study was supported by the 973 Program (2011CBA01006), International Cooperation of Science (2011DFA33290), and Science and Technology Planning Project of Guangdong Province (2016A030303008), Guangdong Natural Science Foundation of China (2017A030313192), Guangdong Provincial Science and Technology Plan Project (2017A030303019), Guangdong Medical Science and Technology Research Foundation (A2017124), and general financial grant from China Postdoctoral Science Foundation (2017M622727).
References
- 1.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. 1996. Leptin is a metabolic signal to the reproductive system. Endocrinology 137:3144–3147. [DOI] [PubMed] [Google Scholar]
- 2.Barb CR, Hausman GJ, Czaja K. 2005. Leptin: a metabolic signal affecting central regulation of reproduction in the pig. Domest Anim Endocrinol 29:186–192. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard G, McLaughlin RM, Ellersieck MR, Krause GF, Franklin C, Reddy CS. 1995. Retrospective evaluation of production characteristics in Sinclair miniature swine—44 y later. Lab Anim Sci 45:408–414. [PubMed] [Google Scholar]
- 4.Cunningham MJ, Clifton DK, Steiner RA. 1999. Leptin's actions on the reproductive axis: perspectives and mechanisms. Biol Reprod 60:216–222. [DOI] [PubMed] [Google Scholar]
- 5.Ellendorff F, Parvizi N, Elsaesser F, Smidt D. 1977. The miniature pig as an animal model in endocrine and neuroendocrine studies of reproduction. Lab Anim Sci 27:822–830. [PubMed] [Google Scholar]
- 6.Fedorcsák P, Storeng R, Dale PO, Tanbo T, Abyholm T. 2000. Obesity is a risk factor for early pregnancy loss after IVF or ICSI. Acta Obstet Gynecol Scand 79:43–48. [PubMed] [Google Scholar]
- 7.Guthrie HD, Bolt DJ. 1990. Changes in plasma follicle-stimulating hormone, luteinizing hormone, estrogen and progesterone during growth of ovulatory follicles in the pig. Domest Anim Endocrinol 7:83–91. [DOI] [PubMed] [Google Scholar]
- 8.Howard PK, Chakraborty PK, Camp JC, Stuart LD, Wildt DE. 1982. Correlates of ovarian morphology, estrous behavior, and cyclicity in an inbred strain of miniature swine. Anat Rec 203: 55–65. [DOI] [PubMed] [Google Scholar]
- 9.Howard PK, Chakraborty PK, Camp JC, Stuart LD, Wildt DE. 1983. Pituitary–ovarian relationships during the estrous cycle and the influence of parity in an inbred strain of miniature swine. J Anim Sci 57:1517–1524. [DOI] [PubMed] [Google Scholar]
- 10.Jungheim ES, Travieso JL, Carson KR, Moley KH. 2012. Obesity and reproductive function. Obstet Gynecol Clin North Am 39:479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. 2007. Trends in prepregnancy obesity in 9 states, 1993–2003. Obesity (Silver Spring) 15:986–993. [DOI] [PubMed] [Google Scholar]
- 12.Kunavongkrit A, Heard TW. 2000. Pig reproduction in South East Asia. Anim Reprod Sci 60-61:527–533. [DOI] [PubMed] [Google Scholar]
- 13.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 14.Martinat-Botté F, Bariteau F, Badouard B, Terqui M. 1985. Control of pig reproduction in a breeding programme. J Reprod Fertil Suppl 33:211–228. [PubMed] [Google Scholar]
- 15.McAnulty PA, Dayan AD, Ganderup NC, Hastings KL. 2012. The minipig in biomedical research. Florida (FL): CRC Press. [Google Scholar]
- 16.Moran LJ, Dodd J, Nisenblat V, Norman RJ. 2011. Obesity and reproductive dysfunction in women. Endocrinol Metab Clin North Am 40:895–906. [DOI] [PubMed] [Google Scholar]
- 17.Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. 2003. Obesity and reproductive disorders in women. Hum Reprod Update 9:359–372. [DOI] [PubMed] [Google Scholar]
- 18.Pope WF, Xie S, Broermann DM, Nephew KP. 1990. Cases and consequences of early embryonic diversity in pigs. J Reprod Fertil Suppl 40:251–260. [PubMed] [Google Scholar]
- 19.Sarwer DB, Allison KC, Gibbons LM, Markowitz JT, Nelson DB. 2006. Pregnancy and obesity: a review and agenda for future research. J Womens Health (Larchmt) 15:720–733. [DOI] [PubMed] [Google Scholar]
- 20.Taylor PD, Poston L. 2007. Developmental programming of obesity in mammals. Exp Physiol 92:287–298. [DOI] [PubMed] [Google Scholar]
- 21.Turner AI, Tilbrook AJ. 2006. Stress, cortisol, and reproduction in female pigs. Soc Reprod Fertil Suppl 62:191–203. [PubMed] [Google Scholar]
- 22.van der Lende T, Schoenmaker GJW. 1990. The relationship between ovulation rate and litter size before and after day 35 of pregnancy in gilts and sows: an analysis of published data. Livestock production science 26:217–229. [Google Scholar]
- 23.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. 2013. Prepregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]