Abstract
Demodex mites are microscopic, cigar-shaped, follicular mites often regarded as commensal microfauna in mammals. Although Demodex spp. can cause dermatologic disease in any immunocompromised mammal, they are rarely reported in laboratory mice. Recent identification of Demodex musculi in a colony of immunodeficient mice with dermatitis afforded us the opportunity to investigate the comparative sensitivity of 4 antemortem diagnostic techniques to detect D. musculi—superficial skin scrape (SSS), tape impression (TI), fur pluck (FP), and deep skin scrape (DSS)—which we performed on 4 anatomic sites (face, interscapular region [IS], caudal ventrum [CV], and caudal dorsum [CD]) in 46 mice. DSS had an overall detection rate of 91.1% (n = 112 tests), with the highest detection rates in IS (93.5%), CV (89.1%), and CD (90.0%). The detection rates for SSS (62.5%; n = 112 tests), TI (57.5%; n = 138 tests), and FP (62.7%; n = 158 tests) were all lower than for DSS. IS was the most reliable site. Results from combined FP and DSS samples collected from IS and CV yielded 100% detection, whereas the face was not a desirable sampling site due to inadequate sample quality and low detection rate. Demodex eggs and larvae were observed from FP more often than DSS (19.0% of 158 tests compared with 14.3% of 112 tests). In a subset of samples, an 18S rRNA PCR assay was equivalent to DSS for detection of mites (both 100%, n = 8). We recommend collecting samples from both IS and CV by both FP and DSS to assess for the presence of D. musculi and performing further studies to assess whether PCR analysis can be used as a diagnostic tool for the detection of Demodex mites in laboratory mice.
Abbreviations: CD, caudal dorsum; CV, caudal ventrum; DSS, deep skin scrape; FP, fur pluck; IS, interscapular region; SSS, superficial skin scrape; TI, tape impression test
Demodex spp. mites are mammalian microfauna that typically inhabit the pilosebaceous unit of healthy animals, where they feed and reproduce.22,53 They are found in low numbers in normal skin and adnexa and usually do not cause clinical manifestations; however, in immunocompromised hosts, they can initiate dermatologic disease.13,16,34 Demodecid mites tend to be host-specific and have been best studied in dogs and humans. Wild Mus musculus can host 6 Demodex spp. which can be found in the skin, clitoral and preputial glands, and oral cavity.7,22,25-28 Relatively few instances of Demodex mite infestation have been reported in laboratory mice, all in immunocompromised strains and, in all cases, only Demodex musculi was identified.21,38,52,73
A colony of immunocompromised transgenic mice with a recombination activation gene 1 (RAG1) deficiency presented with exophthalmia and pruritus.52 The mice were susceptible to opportunistic infections due to compromised adaptive immunity.51,58 Investigation into the cause of clinical signs revealed opportunistic bacterial infections as well as an infestation with D. musculi, first identified on histopathology. All mice examined were infested (100% prevalence), and the colony provided us with the opportunity to evaluate the capability of various of diagnostic modalities to detect D. musculi. Our goal was to find an antemortem test that was sensitive, minimally invasive, easy to perform, and economical in terms of materials, sample collection, labor, and assessment. A sensitive diagnostic test would be useful to identify Demodex mites in mice during quarantine and routine biosecurity practices and could be used to assess for D. musculi in mice with skin disease.
Diagnosis of D. musculi is challenging because the mites are microscopic (approximately 200 μm), transparent, and live within hair follicles.22,53 Molecular diagnostic assays were not commercially available for rodent Demodex species at the initiation of the study (2014); therefore, we focused the investigation on traditional microscopic methods of parasite detection, using a PCR assay with primers directed against mite 18S rRNA on a subset of cases. In dogs, deep skin scrape (DSS) is the ‘gold standard’ for the detection of D. canis, but fur pluck (FP) with trichoscopy (microscopic evaluation of hairs placed in mineral oil on a microscope slide), although not as sensitive as DSS, can be used in heavy infestations.49 In addition, FP is useful for anatomic sites such as the face and interdigital regions where the collection of DSS samples is challenging.14,49 In humans, the gold standard for the detection of Demodex spp. is the standardized skin surface biopsy;15 this method involves using an adhesive to pull the top layers of epidermis and hairs (with roots) off the skin's surface. In the standardized skin surface biopsy, the skin's surface and hairs are removed from a small region en masse, a process that likely would cause discomfort in densely haired mammals such as mice. Therefore, we did not consider including the standardized skin surface biopsy for comparison in mice. Instead we assessed other diagnostic methods, including the superficial skin scrape (SSS) and tape impression (TI) tests, which have proven useful for detection of other parasites in animals.14,43,62,64,78
Although some fur mite species have preferred sites on hosts, studies in wild mice and our own experience with D. musculi in laboratory mice indicate that these follicular mites can infest most areas of densely haired skin.18,26,52,62 We previously reported that the sites with the highest number of mites per millimeter of skin were the middorsum, interscapular region (IS), caudal dorsum (CD), caudal ventrum (CV), and head.52 In the current study, in addition to evaluating different diagnostic methods, mite detection was compared at various anatomic sites. We hypothesized that the DSS would be the best diagnostic test and that IS would be the best anatomic site for Demodex mite detection. The findings herein give insight into the most sensitive antemortem diagnostic modalities and sampling sites for the detection of Demodex mites in laboratory mice.
Materials and Methods
Animals.
B6.Cg-Rag1tm1Mom Tyrp1B-W Tg(Tcra,Tcrb)9Rest (that is, TRP1/TCR) mice were imported to the Memorial Sloan Kettering Cancer Center from the National Cancer Institute in 2006. The TRP1/TCR colony was expanded by using in vitro fertilization and was subsequently found to be infested with D. musculi.52 At the Memorial Sloan Kettering Cancer Center, the line had been backcrossed onto the C57BL/6J strain and then crossed to several other lines.52 TRP1/TCR mice contain 3 transgenes: the tyrosinase-related protein 1 transgene (Tcra,Tcrb) 9Rest, the white-based brown radiation-induced mutation of tyrosinase-related protein (Tyrp1B-W), and a Rag1 mutation (Rag1tm1Mom).51 The Rag1-null mutation inactivates an enzyme critical to normal lymphocyte development, which results in defective adaptive immunity due to the absence of functional mature T and B lymphocytes.46
Mice were housed in an AAALAC-accredited barrier facility in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition).24 Mice were maintained in IVC (no. 19, Thoren Caging Systems, Hazelton, PA) as breeding trios or were housed by sex (n = 5 males or females per cage). Animals were housed on autoclaved aspen-chip bedding (PWI Industries, Quebec, Canada), and fed a closed-formula, natural-ingredient, γ-irradiated diet (PicoLab Mouse Diet 5053, Purina LabDiet, St Louis, MO), and received acidified reverse-osmosis–purified water (pH 2.5 to 2.8, with hydrochloric acid) in polysulfone bottles with stainless steel caps and sipper tubes (Techniplast, West Chester, PA). As necessary, select mice were fed an irradiated medicated feed containing 0.12% amoxicillin (TestDiet, St Louis, MO) to prevent opportunistic infections.52 The cage bottom was changed weekly, whereas the wire bar lid, water bottle, and filter top were changed biweekly within a vertical flow, HEPA-filtered, mass-air–displacement unit (model NU-S619-400, NuAire, Plymouth, MN). The room was maintained on a 12:12-h light:dark cycle, relative humidity of 30% to 70%, and room temperature of 72 ± 2 °F (22.2 ± 1.1 °C).
Mice were free of mouse hepatitis virus, Sendai virus, mouse parvovirus, minute virus of mice, pneumonia virus of mice, Theiler meningoencephalitis virus, mouse rotavirus (epizootic diarrhea of infant mice virus), ectromelia virus, reovirus 3, lymphocytic choriomeningitis virus, K virus, mouse adenovirus types 1 and 2, polyoma virus, mouse cytomegalovirus, mouse thymic virus, and Hantaan virus; Mycoplasma pulmonis, Citrobacter rodentium, Salmonella spp., ciliary-associated respiratory bacillus, and Clostridium piliforme; and fur mites (Myobia musculi, Myocoptes musculinis, and Radfordia affinis), pinworms (Syphacia spp. and Aspiculuris spp.), and Encephalitozoon cuniculi.
Experimental design.
D. musculi-infested, TRP-1/TCR mice (n = 46 [22 male and 24 female mice], experimental and breeders; age, 49 to 854 d [mean ± SEM, 172.5 ± 25.9 d]) were included in this study. Testing was performed immediately after carbon dioxide asphyxiation (n = 40) or under manual restraint (n = 6). Manually restrained mice were observed for adverse events for 5 min after sample collection and 24 h later. We collected samples by SSS, TI, FP, and DSS from the face, IS, CV, and CD of each mouse, unless otherwise noted. Each mouse was tested with a full complement or subset of tests once at each location and at a single time point. The same person (MN) collected and evaluated all samples.
SSS procedure.
A no. 10 or 20 scalpel blade (Bard-Parker; Aspen Surgical Products; Caledonia, MI) was run against the grain of the hairs rapidly for a distance of approximately 10 mm, with the blade applying minimal pressure to the skin's surface. Hair and skin debris were collected on a piece cellophane tape (12.7 × 25 mm; Highland Brand 5190 transparent tape, 3M Stationary Products Division, St Paul, MN), which was adhered to a standard microscope slide and evaluated. SSS (n = 112) was performed on 46 mice.
TI procedure.
Using the thumb, the researcher pressed a piece of cellophane tape (12.7 × 25 mm) to the fur and removed it rapidly by pulling perpendicularly to the skin. The tape was reapplied to and removed from the same region 3 or 4 times. Hairs and surface debris were adhered to the tape, which was mounted on a glass microscope slide and evaluated. TI (n = 138) was performed on 36 animals.
FP procedure.
A 4-in., curved Halstead mosquito hemostat was used to grasp a 1- to 3-mm clump of hair from the region to be tested. The hairs were removed from the root by rapidly pulling perpendicular to the skin's surface. This process was repeated 3 or 4 times in each location. Hairs were placed in a drop of mineral oil on a microscope slide, a coverslip was applied, and samples were evaluated. The hemostat tip was rinsed in 70% alcohol and clamped to a paper towel to remove hair and visible debris before sampling subsequent sites or animals. At the end of a sample harvest session, the hemostat was cleaned by using a scrub brush and mild detergent and rinsed with tap water and air-dried. FP (n = 158) was performed on 46 animals.
DSS procedure.
The researcher squeezed the region of skin to be sampled (1×1 to 1.5×1.5 cm2) between the thumb and forefinger of one hand as a no. 10 scalpel blade, at an angle of approximately 30 to 45° with regard to and with moderate pressure on the skin, was run against the grain of the hairs 3 or 4 times. The collected debris was placed in a drop of mineral oil on a microscope slide, a coverslip was applied, and the sample was evaluated. A new blade was used at each site for each DSS on each animal. DSS (n = 112) was performed on 46 mice.
Multiple samples from a single animal were mounted on microscope slides, with 2 FP, 2 DSS, 4 TI, or 3 SSS samples mounted per slide.
Anatomic regions evaluated.
Anatomic sites were selected according to the literature and a recent study evaluating the topographic distribution of Demodex spp.26,52 A maximum of 14 tests were performed on each animal: face (nasal bridge and interocular region) by TI and FP; IS by SSS, TI, FP, and DSS; CV by SSS, TI, FP, and DSS; and CD by SSS, TI, FP, and DSS). We tested fewer animals (n = 20) on the CD because preliminary results from FP and DSS indicated that mite yields were low at this site, but histology showed sufficient mites for testing.52 In addition, a subset of mice (n = 10) did not undergo TI collection. Each anatomic region tested was divided into left and right sections, and when 4 tests were performed at a single anatomic site, regions were divided into quadrants to ensure that individual tests were performed on naïve sites. Test sampling for SSS, TI, FP, and DSS were alternated between left and right or rotated between left cranial, left caudal, right cranial, and right caudal areas, to avoid sampling site bias. The researcher donned disposable nitrile gloves; gloves were changed between animals, and a fresh paper towel was placed on the workstation beneath each mouse, to avoid cross-contamination.
Microscopic evaluation of samples.
Microscope slides were evaluated by using a biologic light microscope (model CX31, Olympus, Center Valley, PA) at 100× and 200× magnification. A low light level and reduced iris diaphragm aperture for enhanced contrast were used to improve visualization of the transparent mites. Each sample was reviewed twice by a single observer (MN), who was aware of the anatomic site and test type. A sample was deemed positive when it contained at least one mite or egg. In all samples, mites and eggs were counted. In addition, for SSS, TI, and FP, hairs were counted under 50× magnification. A minimum of 100 hairs was selected as the lower limit for an adequate sample, to maximize detection and remain within the recommended range of hairs as described for FP sample collection in dogs.49 Select mites were photographed and measured by using an infield micrometer to confirm the identity of the Demodex spp. Mite length was measured from the tip of the gnathosoma to the end of the opisthosoma.
Comparisons between sample sites with regard to mite detection and number of mites per sample.
When at least one mite or egg was observed in any sample, the sample and animal were deemed positive for D. musculi. The Demodex mite detection rate was determined for each test method or anatomic site as the number of positive tests divided by the total number of tests performed multiplied by 100%. The combined number of mites and eggs in each sample is presented as a median number according to the diagnostic method and anatomic site.
To determine which combinations of tests and sites yielded the highest detection rate, we compared the possible positive test result permutations with the actual results by analyzing possible 2- and 4-way test–site combinations. Initially we considered 4 methods and 4 sites for comparison, but we excluded the face from this analysis because it had the lowest detection rate and mite yield, thus resulting in 33, that is 27, potential 2-way combinations of tests and anatomic sites, including DSS at 3 anatomic sites (IS, CD, and CV) and SSS, TI, and FP each at 3 anatomic sites. After 2-way analysis, DSS was determined to have a high detection rate, and we selected the IS and CV sites for additional 4-way test–site comparisons. For the sixteen 4-way test–site combinations evaluated, the possible permutations of positive and negative results for FP–IS, FP–CV, DSS–IS, and DSS–CV are presented in addition to the actual results from the 46 test mice.
PCR analysis.
Fresh–frozen (n = 2) and formalin-fixed (n = 5) skin and samples from TI tests (n = 2; each containing an individual Demodex mite) from 8 different mice were analyzed. The clinical and pathologic findings of these mice have been described previously; their case identification numbers can be used to obtain this information.52 DNA extraction from samples, except individual mites, was performed as follows. Sample vials were thawed and incubated overnight at 56 °C in 200 μL of digestion buffer (50 mmol/L Tris-HCl, pH 8.5; 1 mmol/L EDTA) with 4 μL of recombinant proteinase K solution (10 mg/mL, Roche Applied Science, Mannheim, Germany). After overnight incubation, proteinase K was inactivated by heating at 95 °C for 10 min, and the samples were centrifuged for 10 min at 16,000 × g. DNA was extracted from the samples by using a commercial kit (DNeasy Blood and Tissue Kit, Qiagen, Hilden, Germany) according to the manufacturer's instructions. Extracted DNA samples were stored at –80 °C prior to testing, were shipped for PCR processing in a chilled shipment box, and were thawed on ice before analysis.
TI samples with individual mites were extracted as previously described for individual canine Demodex mites.69 D. canis served as the positive control for the PCR analysis. An individual mite was harvested from a canine DSS sample. Extraction and amplification of D. canis DNA was performed as previously described.69
PCR amplification was performed in a final reaction volume of 50 μL, containing 5 μL of DNA solution, PCR buffer (1.5 mM MgCl2, 0.2 mM of each dNTP), 0.3 μM of each primer, and 2 U of EcoTaq polymerase (Ecogen, Barcelona, Spain). Primer pairs were Mite 410 (5′ TCC AAG GAA GGC AGC AGG CA 3′) and Mite 941 (5′ CGC GGT AGT TCG TCT TGC GAC G 3′; sequences provided by Susan Compton [Yale University]). These primers amplify a nonspecies-specific sequence in the 18S rRNA gene of both Demodex spp. and Myobia musculi. The thermocycling profile was 95 °C for 3 min; followed by 40 cycles at 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s; and final extension at 72 °C for 5 min. Water was included as a negative control in each PCR assay. PCR products were visualized in a 1.2% agarose gel with molecular markers (DNA Molecular Weight Marker IX, Roche Applied Science).
The primers amplified a 537-bp fragment. The PCR product was sequenced by using a commercial kit (BigDye Terminator Cycle Sequencing Ready Reaction Kit, version 3.1, Life Technologies; Carlsbad, CA), by using the same primers as described earlier. Amplification products were purified (SEQ96 Montage Sequencing Reaction Cleanup Kit, Millipore, Billerica, MA) and sequenced on an automated sequencer (PRISM 3730, Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. The sequence obtained was examined and compared with those in the GenBank database by using the Basic Local Alignment Search Tool.3 The sequences with the strongest agreement are reported in the Results section.
Data and statistical analyses.
Summary statistics were calculated for the numbers of mites and hairs by site and diagnostic test (SSS, TI, and FP). The number of mites was compared among all test–site combinations in aggregate by using the Kruskal–Wallis test, and mite numbers were rank-ordered from highest to lowest among test–sites. The Demodex detection rate was calculated as the percentage of samples collected by using a particular method or from a specific anatomic site that contained any D. musculi eggs or mites. For all 14 test–site combinations, the Demodex detection rate was rank-ordered to indicate the test–site combinations with the highest and lowest detection rates. Paired results of detection rates from different tests on the same animal were compared by using the McNemar test, which compares 2 related groups and examines agreement between the tests. The McNemar test was used because multiple tests were performed on the same subject at the same time point. DSS was used as the test for comparison, because it serves as the standard in dogs.49 Differences in the median number of mites counted in each sample were compared between anatomic sites by using the Wilcoxon signed rank test. The Spearman rank correlation coefficient between the number of mites and the number of hairs per test–site combination was calculated for individual mice. A P value of less than 0.05 indicates a correlation coefficient significantly different from 0. All analyses were performed by using SAS version 9.4 (SAS Institute; Cary, NC).
Results
Demodex detection rate and mite yield compared by diagnostic method and anatomic site.
All 46 mice were confirmed mite-infested by testing positive by at least one method at a single site. The Demodex detection rate of each diagnostic method was compared with the detection rate of DSS and is provided in Table 1. The rank order for highest to lowest overall detection rate by test type, according to the total number of tests performed, was DSS > FP > SSS > TI. The detection rates of SSS, TI, and FP were not significantly different from one another, but they were all significantly (P < 0.05) lower than the sensitivity for DSS, for which 91.1% of the tests were positive. When results from all tests of each type in the same mouse were analyzed, the percentage of mice positive for at least one test of each type ranged from 76% to 98%. Testing multiple sites on an individual mouse increased the detection rate, such that FP and DSS had equivalent detection rates of 98%. In the single animal that had negative DSS on both IS and CV (CD was not tested in this animal), FP tests were positive at both sites. In addition, DSS detected significantly (P < 0.05) more mites (approximately 7fold higher counts) per sample compared with all other methods, for which mite yields did not differ significantly from one another.
Table 1.
Demodex detection rate and mite yield by test type
| Superficial skin scrape | Tape impression | Fur pluck | Deep skin scrapea.b | |
| No. of mice tested | 46 | 36 | 46 | 46 |
| Total no. of tests performed | 112 | 138 | 158 | 112 |
| No. of positive tests / total no. tests performed (% detection rate)a | 70/112 (62.5)e | 80/138 (57.5)d | 99/158 (62.7)d | 102/112 (91.1) |
| No. of mice with at least 1 site positive /total no. of mice tested (% positive)d | 35/46 (76.0)c | 32/36 (88.9)e | 45/46 (98.0) | 45/46 (98) |
| Median (interquartile range) no. of mites and eggs per samplee | 1.0 (0.3–2.3)e | 1.3 (0–2.9)e | 1.3 (0.7–2.7)e | 8.8 (3.5–17.0) |
The McNemar test method was used to compare the number of mice with at least one site positive after deep skin scraping, which served as the reference, with the number of animals positive by other methods. A positive test was recorded when at least a single mite or mite egg was detected, and an animal was positive when it had at least one positive test. The McNemar test also was used to compare the detection rates for superficial skin scraping, tape impression, and fur pluck with that for deep skin scraping.
Wilcoxon signed-rank testing was used to compare the median number of mites after superficial skin scraping, tape impression, and fur pluck with that from deep skin scraping.
P ≤ 0.01
P ≤ 0.001
P ≤ 0.0001
A comparison of detection rate according to anatomic site is provided in Table 2. When the total number of tests performed at each site was considered, IS, CV, and CD supported similar detection rates, ranging from 72.5% to 76.4%. The detection rate for IS was significantly (P < 0.05) higher than for CD, and the rate for the face was significantly lower (29.3%; P < 0.05) than for any of the other sites. When sites were compared among animals, IS, CV, and CD each yielded at least one positive test per animal; the face had a significantly (P < 0.05) lower detection rate, with only 43% of mice testing positive. In addition, IS yielded significantly (P < 0.05) more mites per sample than other sites whereas the face had the lowest mite yield.
Table 2.
Demodex detection rate and mite yield by anatomic site
| Facea,b | Interscapular region | Caudal ventrum | Caudal dorsum | |
| No. of mice tested | 46 | 46 | 46 | 20 |
| Total no. of tests performed | 92 | 174 | 174 | 80 |
| No. of positive tests / total no. of tests performed | 27/92 (29.3) | 133/174 (76.4)f,g | 133/174 (76.4)f | 58/80 (72.5)c |
| (% detection rate)a | ||||
| No. of mice with at least one positive test / | 20/46 (43) | 46/46 (100)f | 46/46 (100)f | 20/20 (100)d |
| total no. of animals tested (% positive)a | ||||
| Median (interquartile range) no. of mites | 0 (0–1) | 4.4 (2–9.5)f,h,i | 2.5 (1–4.5)f,h | 2.3 (1.5–4.4)e |
| and eggs per sampleb |
The McNemar test was used to compare the number of mice with at least one test type positive for face with the number of animals positive at other test sites. A positive test was recorded when at least a single mite or mite egg was detected, and an animal was positive when it had at least one positive test. In addition, the McNemar test was used to compare the Demodex detection rates of the interscapular region, caudal ventrum, and caudal dorsum with the detection rate of the face.
The Wilcoxon signed-rank test was used to compare the number of mites between anatomic sites.
P ≤ 0.05; dP ≤ 0.01; eP ≤ 0.001; and fP ≤ 0.0001 compared with value for face.
P ≤ 0.01 and hP ≤ 0.0001 compared with value for caudal dorsum.
P ≤ 0.001 compared with value for caudal ventrum.
When the test–site combinations were rank-ordered, DSS–IS (93.5%), DSS–CD (90.0%), and DSS–CV (89.1%) yielded the highest detection rates (Table 3). The lowest detection rates were for TI–face and FP–face (19.6% and 39.1%, respectively). Other test–site combinations yielded detection rates ranging from 60.0% to 83.3%.
Table 3.
Demodex detection rate (%) for each test–site combination.
| Face | Interscapular region | Caudal ventrum | Caudal dorsum | |
| Superficial skin scrape | NP | 63.0 | 61.0 | 65.0 |
| Tape impression | 19.6 | 72.2 | 83.3 | 75.0 |
| Fur pluck | 39.1 | 76.1 | 74.0 | 60.0 |
| Deep skin scrape | NP | 93.5 | 89.1 | 90.0 |
NP, not performed
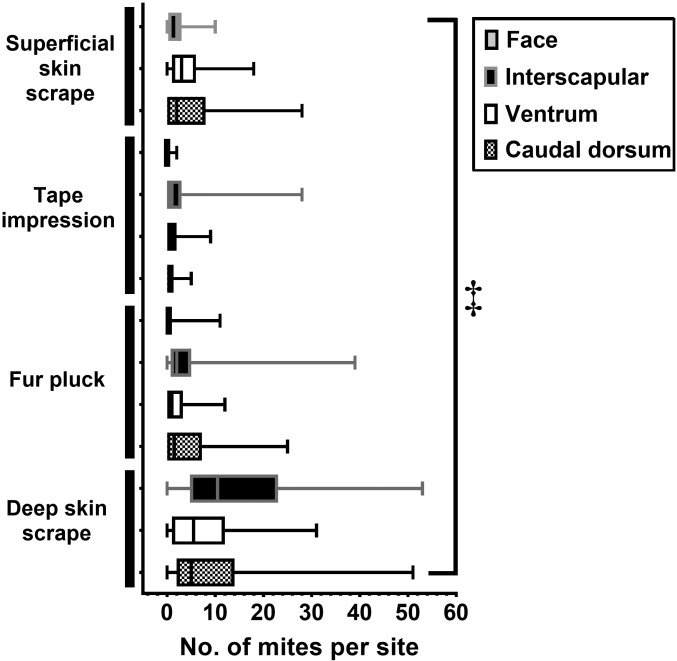
The mite yields for all test–site combinations differed significantly (P < 0.05) from one another when compared with each other in aggregate (maximum, 53 mites per sample; median, 10.5 mites per sample; Figure 1), with the greatest numbers of mites detected by DSS–IS, which also had the greatest range of mites or eggs detected when compared with all other test–site combinations. The lowest median number of mites was detected on the face (both TI and FP had a median of 0 mites per sample).
Figure 1.
Mite yield per sample by site, displayed as a box plot. The middle line represents the median, the bounds of the box represent the upper and lower quartiles, and the whiskers represent the lowest and highest numbers of mites and eggs for each test–site. The face was sampled by fur pluck and tape impression only. All values were significantly different from one another (‡, P ≤ 0.0001, Kruskal–Wallis test).
Diagnostic sample quality and analysis.
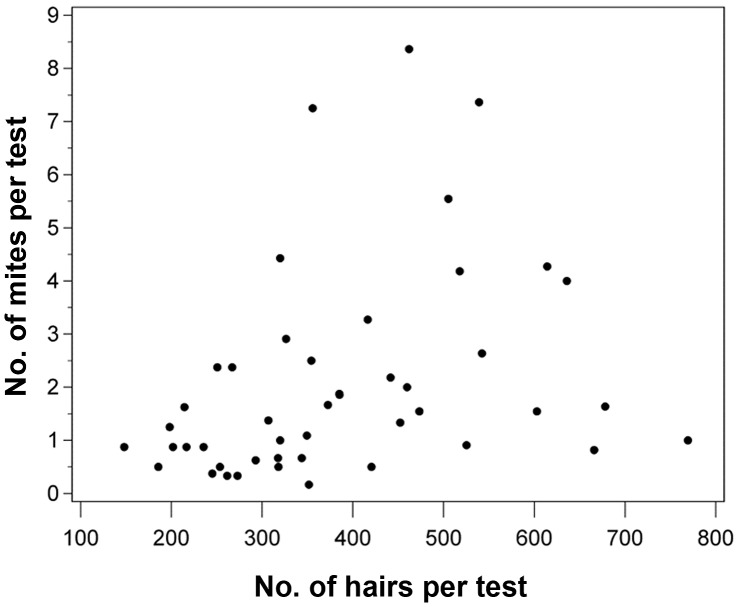
To assess sample quality, we determined whether samples had an adequate number of hairs (n ≥ 100; Table 4). Despite multiple sample collections per site by using forceps and tape, some samples had fewer than the desired number of hairs; specifically 16 of 46 TI–face samples, 4 of 46 FP–face samples, 5 of 46 SSS–IS samples, and 2 of 46 SSS–CV samples failed to meet the 100-hair criterion. The percentage of representative tests that met the criterion ranged from 89% to 100% for most sites, but only 65% of the TI samples from the face met this criterion. The Spearman correlation coefficient between the number of mites per sample and the number of hairs per sample for all tests where hairs were counted (that is, SSS, TI, and FP) was 0.47 (P = 0.001; Figure 2). For individual test types, the Spearman correlation coefficient was significantly different from 0 (P < 0.05 for SSS, TI, and FP), with the TI test having the strongest correlation between the number of mites and the number of hairs plucked per sample (Table 4)
Table 4.
Test adequacy for the face, interscapular region, caudal ventrum, and caudal dorsum based on the number of hairs per sample for each test type.
| Parametera | Face | Interscapular region | Caudal ventrum | Caudal Dorsum | |
| Superficial skin scrape | No. of tests performed | NP | 46 | 46 | 20 |
| Median (IQR) no. of hairsb,c | NP | 294 (190–344) | 311 (230–467) | 367 (306–458) | |
| % of tests with ≥100 hairs | NP | 89 | 96 | 100 | |
| Tape impression | # of tests performed | 46 | 36 | 36 | 20 |
| Median (IQR) no. of hairsb,d | 132 (73–251) | 381 (317–502) | 370 (292–499) | 512 (377–699) | |
| % of tests with ≥100 hairs | 65 | 100 | 100 | 100 | |
| Fur pluck | # of tests performed | 46 | 46 | 46 | 20 |
| Median (IQR) no. of hairsb,c | 364 (203–501) | 455 (351–610) | 459 (302–590) | 733 (594–1116) | |
| % of tests with ≥100 hairs | 91 | 100 | 100 | 100 |
IQR, interquartile range; NP, not performed
The hairs in each SSS, TI, and FP sample were counted. A test was considered adequate when it contained a minimum of 100 hairs per sample.
Spearman correlation coefficients between the number of mites (see Table 1) and the number of hairs counted per test were calculated: SSS, 0.3; TI, 0.53; and FP, 0.33.
P ≤ 0.05
P ≤ 0.001
Figure 2.
Graphical representation of the Spearman rank correlation between the number of mites per test site and the number of hairs counted at each test site for SSS, TI, and FP samples from each mouse. The Spearman correlation coefficient was 0.47 (P = 0.001).
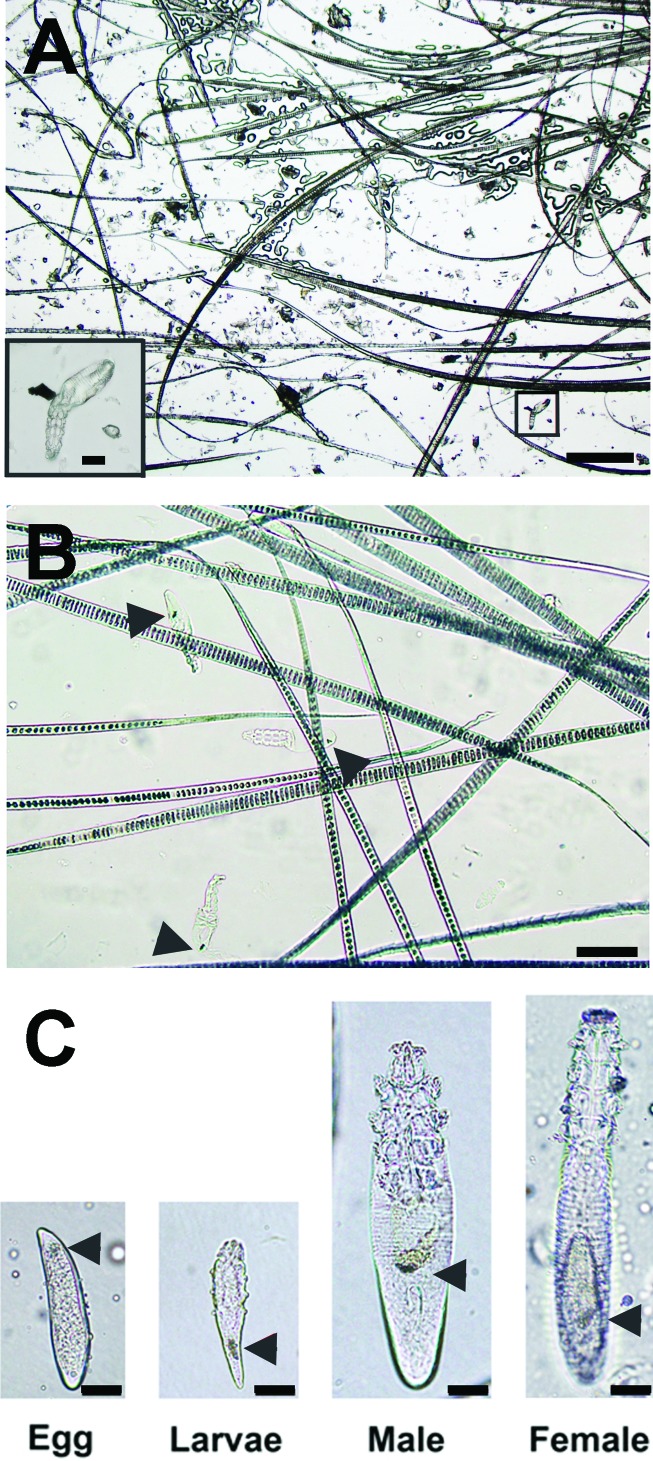
In tape-based tests (SSS and TI), adult mites and hairs were well preserved (Figure 3 A), but significant amounts of debris and air bubbles were present, making identification of mites challenging. Mineral oil-based samples (FP and DSS) had considerably less debris, making diagnosis easier than for tape-based tests (Figure 3 B). The quality of a DSS sample depended on skin squeezing (to extract mites) and the ability to obtain skin surface debris by using the blade.48 Although DSS samples contained considerable debris with broken hair shafts, indicating that a qualitatively sufficient area was scraped, sample quality did not depend on the number of hairs and, compared with other sample types, DSS samples had less debris. In FP samples, the opisthosoma (caudal region) of the mite is approximately the same width as a mouse hair bulb, making it challenging to detect mites present in hair clusters. The decreased amount of debris and lack of hair bulbs made DSS the easiest method to evaluate. Mites were easiest to identify in fresh, oil-based samples because they were often mobile, and their internal organs were visible (Figure 3 C). Guanine concretions (Figure 3 B and C, arrowheads) were useful for detecting live or recently dead mites. In addition, the coverslip could be adjusted in oil-based samples to dissociate hair clusters and mobilize hairs to ascertain whether hair strands had obscured mites. Mites were more likely to be alive (moving, opaque) when samples were viewed within 1 to 6 h of collection, although live mites were observed as long as 53 h after sampling in oil-based tests. Mites became transparent after death, making them more challenging to detect (Figure 3 B).
Figure 3.
Appearance of tape-based and oil-based samples. (A) Tape impression test under low magnification (scale bar, 300 μm). The mite, highlighted in the box, is shown at higher power in the inset (scale bar, 30 μm). (B) High-power magnification (scale bar, 100 μm) of FP. Arrowheads indicate guanine concretions in transparent, dead mites. (C) Life stages of D. musculi as observed in mineral oil from either DSS or FP (scale bar, 20 μm). From left to right, egg, hexapod larvae, adult male, and adult female. Nymphs were not observed on ectoparasite tests. Arrowheads indicate guanine concretions.
Adult female mites (n = 15) ranged in size from 170 to 220 μm, whereas adult males (n = 11) were shorter, at 135 to 175 μm. Dimensions and morphologic features were consistent with D. musculi.22,26 Dead mites are shorter than live mites, presumably due to muscle contraction upon death, which alters the exoskeleton. Although the exoskeleton of adult mites can last for months in tape-based samples or for weeks in oil-based samples, eggs and larvae were labile and were not identified in samples older than 6 to 7 d. Adult mites were found on all 46 mice, but eggs or larvae were detected in fewer than half of the animals (n = 21). In particular, eggs and larvae were not detected in tape-based tests (SSS or TI), but they were visible on oil-based tests (DSS and FP). Of the 21 mice from which eggs and larvae were detected, 46 (20 FP, 16 DSS) of 128 (35.9%) tests contained eggs or larvae. When the proportions of FP and DSS tests with eggs or larvae were compared, FP was better (P < 0.05) than DSS for detecting eggs or larvae. Nymphs were not detected on any test from any mouse.
Comparison of detection rates for test–site combinations.
Two- and 4-way test–site combinations were evaluated to determine which of the combinations yielded the highest and lowest mite detection rates. The face was excluded because it yielded the lowest detection rate and the fewest mites, thus resulting in 33, or 27, potential 2-way test combinations comprising DSS with SSS, TI, and FP, each at 3 anatomic sites (Table 5). Of the 2-way test–site combinations considered, 9 of 27 yielded 100% detection and 6 of these included DSS–IS. When DSS–IS was included in the 2-way comparison, 93% to 100% detection occurred; when DSS–CV was included, 93% to 100% detection was present; and when DSS–CD was included, 89% to 100% detection was obtained. Therefore, the IS and CV sites were selected for 4-way test–site combination analysis due to their higher range of detection rates in the 2-way analysis. Of the 16 possible permutations of results with 4-way diagnostic test–site combinations (Table 6), when including FP–IS, FP–CV, DSS-IS, and DSS-CV, 10 combinations were reflected in the actual results. The resulting frequency in a 4-way test–site combination analysis of FP-IS, FP-CV, DSS-IS, and DSS-CV yielded 100% detection. Among these test results, all mice had at least 2 positive tests, but most had either 3 (22 of 46) or 4 (18 of 46) positive tests.
Table 5.
Comparison of 2-way test–site combinations
| Test A | Test B | Total no. of tests performed | No. of positive tests (detection rate) |
| DSS–IS | SSS–IS | 46 | 43 (93%) |
| DSS–IS | SSS–CV | 46 | 43 (93%) |
| DSS–IS | SSS–CD | 20 | 20 (100%) |
| DSS–IS | TI–IS | 34 | 34 (100%) |
| DSS–IS | TI–CV | 34 | 34 (100%) |
| DSS–IS | TI–CD | 18 | 18 (100%) |
| DSS–IS | FP–IS | 46 | 46 (100%) |
| DSS–IS | FP–CV | 46 | 45 (98%) |
| DSS–IS | FP–CD | 20 | 20 (100%) |
| DSS–CV | SSS–IS | 46 | 43 (93%) |
| DSS–CV | SSS–CV | 46 | 44 (96%) |
| DSS–CV | SSS–CD | 20 | 19 (95%) |
| DSS–CV | TI–IS | 34 | 33 (97%) |
| DSS–CV | TI–CV | 34 | 34 (100%) |
| DSS–CV | TI–CD | 18 | 18 (100%) |
| DSS–CV | FP–IS | 46 | 45 (98%) |
| DSS–CV | FP–CV | 46 | 45 (98%) |
| DSS–CV | FP–CD | 20 | 19 (95%) |
| DSS–CD | SSS–IS | 20 | 19 (95%) |
| DSS–CD | SSS–CV | 20 | 19 (95%) |
| DSS–CD | SSS–CD | 20 | 19 (95%) |
| DSS–CD | TI–IS | 18 | 17 (94%) |
| DSS–CD | TI–CV | 18 | 17 (94%) |
| DSS–CD | TI–CD | 18 | 16 (89%) |
| DSS–CD | FP–IS | 20 | 20 (100%) |
| DSS–CD | FP–CV | 20 | 19 (95%) |
| DSS–CD | FP–CD | 20 | 19 (95%) |
CD, caudal dorsum; CV, caudal ventrum; DSS, deep skin scrape; FP, fur pluck; IS, interscapular region; SSS, superficial skin scrape; TI, tape impression
Table 6.
Assessment of 4-way test–site combinations
| Permutation | Possible combinations | Number of positive mice (frequency, %) | |||
| DSS–IS | DSS–CV | FP–IS | FP–CV | ||
| 1 | − | − | − | − | 0 (0) |
| 2 | − | + | − | − | 0 (0) |
| 3 | + | − | − | − | 0 (0) |
| 4 | − | − | + | − | 0 (0) |
| 5 | − | − | − | + | 0 (0) |
| 6 | − | + | − | + | 0 (0) |
| 7 | − | − | + | + | 1 (2) |
| 8 | − | + | + | − | 1 (2) |
| 9 | − | + | + | + | 1 (2) |
| 10 | + | − | − | + | 1 (2) |
| 11 | + | − | + | − | 1 (2) |
| 12 | + | − | + | + | 2 (4) |
| 13 | + | + | − | − | 2 (4) |
| 14 | + | + | − | + | 8 (17) |
| 15 | + | + | + | − | 11 (24) |
| 16 | + | + | + | + | 18 (39) |
| Total | 46 (100) | ||||
CV, caudal ventrum DSS, deep skin scrape; FP, fur pluck; IS, interscapular region
Effects of skin testing.
Samples from 6 of 46 (13%) of the test animals were collected antemortem. Momentary discomfort, primarily related to manual restraint, occurred occasionally in these mice. In addition, some breeding females vocalized when fur was plucked from the ventrum, presumably due to mammary gland sensitivity. Sampled animals began grooming to remove oil and debris and burrowing in the bedding immediately after being returned to their home cages, perhaps to assist in removal of the mineral oil residue. Bleeding was not observed during DSS in mice, as occurs in other veterinary species.49
PCR analysis for Demodex mite detection.
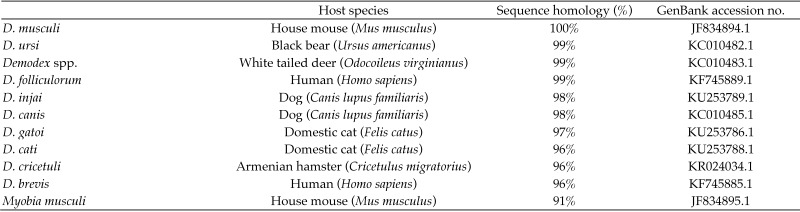
Using nonspecies-specific primers that recognize a conserved region of 18S rRNA gene for both Demodex spp. and Myobia musculi detected Demodex mites in frozen skin (lanes 1 and 2), formalin-fixed skin (lanes 3 through 5), and as individual D. musculi mites preserved on tape (lanes 6 and 7; Figure 4). Samples were run twice, and a representative gel is shown. DNA from formalin-fixed skin consistently had a weaker band than fresh-frozen skin and individual mites. A single 537-bp product present in all lanes was isolated and sequenced. When this sequence was used as the query sequence for a homology search, 40 of the top 50 hits were from various Demodex rRNA gene sequences. Many results were duplicates of human mite sequences entered under different accession numbers. There was 100% homology with the 18S rRNA gene from D. musculi and 96% to 99% sequence homology with other Demodex spp., indicating that the region was highly conserved. The remaining sequences identified were from rRNA genes of other mite species in both Trombidiformes and Sarcoptiformes orders, some of which are parasitic, including Myobia musculi, with which D. musculi shares a 91% sequence similarity. The mite species with the highest sequence homologies are listed (Figure 5).
Figure 4.
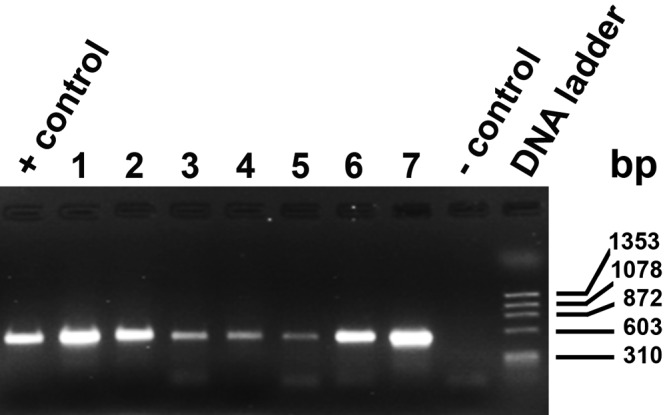
Representative PCR samples separated on an agarose gel. Lane sequence: positive control (D. canis), Lane 1, whole skin (frozen); 2, whole skin (frozen); 3, whole skin (formalin-fixed); 4, whole skin (formalin-fixed); 5, whole skin (formalin-fixed); 6, tape impression, individual mite; 7, tape impression, individual mite; negative control (water), and DNA ladder. Size markers are indicated. The PCR amplicon was 537 bp.
Figure 5.
Mite species with highly homologous 18S rRNA sequences.
A summary of PCR results compared with 4 test–site combinations is provided in Table 7 . All 8 animals were PCR-positive. In this subset, FP–IS and FP–CV had detection rates of 75% and 62.5%, respectively, but the combined FP results at both sites increased the detection rate to 100%. DSS from either site was equivalent to PCR analysis in detecting infested mice (100%) in this subset of test subjects. Despite the lower detection rates for FP in this subset, when compared with DSS and PCR analysis, the differences were not statistically significant.
Table 7.
Demodex detection rate of PCR and FP analyses compared with the detection rate of DSS in a subset of subjects
| Gel lanea | Case IDb | Sample type | PCRa | FP–IS | FP–CV | DSS–IS | DSS–CV |
| 1 | 17 | Frozen, whole skin | + | + | + | + | + |
| 2 | 18 | Frozen, whole skin | + | + | − | + | + |
| 3 | 42 | Formalin-fixed, whole skin | + | + | + | + | + |
| 4 | 43 | Formalin-fixed, whole skin | + | − | + | + | + |
| DNSa | 44a | Formalin-fixed, whole skin | + | + | − | + | + |
| DNS | 45a | Formalin-fixed, whole skin | + | + | − | + | + |
| 5 | 46 | Formalin-fixed, whole skin | + | + | + | + | + |
| 6 | 46 | Individual mite | |||||
| 7 | 20 | Individual mite | + | − | + | + | + |
| Detection rate (%)c | 100% | 75% | 62.5% | 100% | 100% | ||
CD, caudal dorsum; CV, caudal ventrum; DNS: data not shown; DSS, deep skin scrape; FP, fur pluck; IS, interscapular region; SSS, superficial skin scrape; TI, tape impression
Gel lane indicated corresponds to the gel shown in Figure 3. Results shown in the table are combined from multiple PCR assays. Samples 44 and 45 were run on another gel (not shown).
Case ID, for cross-referencing, refers to the animal identification number used in a previous publication.52
Detection rates were compared between tests by using McNemar's test. None of the values, including those for FP–IS and FP–CV, differed significantly from the DSS–IS or DSS–CV detection rate.
Discussion
Demodex mites have been reported in several immunocompromised laboratory mouse strains,21,38,52,73 but we and others have suggested that, although reported infestations are rare, the parasite is likely underrecognized and underreported.4,52,73 Just as a close relationship between Demodex populations in humans has been attributed to population movement, we propose that the frequent sharing of mice from noncommercial sources has contributed to the global spread of subclinical murine Demodex carriers.55 Although the true prevalence of Demodex mites in laboratory mouse colonies is unknown, testing of mice from noncommercial vendors imported into our institution, by using a combination of PCR analysis and FP or DSS, we found that approximately 13% of the imported colonies were infested.63 A reliable detection method is therefore warranted, but comparison of available detection methods for Demodex mites has not, to date, been conducted in rodents. Our current results shed light on how best to detect these ectoparasites by using traditional methodologies and are informative for laboratory animal personnel, diagnostic laboratories, and veterinary staff responsible for rodent health monitoring programs.
There is debate as to whether Demodex mites are commensal, mutualistic, or parasitic in mammals.34,60 A beneficial role to the mammalian host has not been clearly defined and although almost 100% of certain host species (for example, humans and dogs) have been found to harbor Demodex mites, disease can develop as a result of mite overgrowth.13,16,60 Mice obtained from commercial vendors are produced from parental strains that have been rederived through cesarean or embryo transfer and are therefore presumably free of all ectoparasites. To our knowledge and in our experience, Demodex mite infestations do not cause significant morbidity in the majority of infested mouse strains and, until recently, the presence of Demodex mites went unrecognized or was perhaps considered by some to be inconsequential for SPF rodent colonies. Evidence suggests that immunocompetent mice in laboratory colonies can be subclinical carriers of Demodex mites.21,52,63,73 Most imported Demodex-infested lines we evaluated were immunocompetent and were free of skin disease;63 however, in addition to previously published reports, we have observed that some presumed immunocompetent mouse strains can have dermatologic manifestations. Importantly, subclinical infestations may have a biologic impact and can potentially confound specific research studies. For example, infestations with other mite species can cause elevated immunoglobulin production and immune activation leading to amyloidosis in select strains.18,47,57,65 A colony's Demodex status is important in facilities that house immunocompromised animals, because they may be susceptible to clinical disease.21,38,52,73
Demodecid mites typically are located deep within hair follicles or sebaceous glands, and diagnosis requires a method in which the mites can be extracted from or observed within the follicle or gland. Trichoscopy of FP and DSS samples and histopathology have been used for diagnosis of Demodex mites in wild, laboratory, pet, and zoo-maintained rodents; however, detection methods had not been compared previously.8,29,42,54,73,75 In addition to the detection of ectoparasites in imported rodents, a sensitive diagnostic test is needed to evaluate treatment efficacy when treatment or eradication of Demodex infestation with parasiticides is elected. Although a sensitive detection method, the examination of histologic skin sections has the disadvantage that skin biopsies are invasive and are typically performed postmortem, multiple biopsies may be necessary, analysis can be labor-intensive, and results may be delayed.38,52,73 An alternative sensitive antemortem diagnostic method would be ideal. Our current results indicate that DSS, when combined with FP, at 2 different anatomic sites (IS and CV) yields 100% detection for D. musculi in a moderately infested mouse strain.
The diagnostic methods used and test type recommendations in humans and veterinary species vary depending on the anatomic site being assessed. DSS is the most commonly used method in dogs, although FP is recommended for diagnosis in specific body regions, such as the periorbital and interdigital areas.50 In some cases, biopsy with histopathology is used when negative findings are obtained by using traditional methods or when the skin is thickened or scarred.50 In other veterinary species, when DSS are performed correctly, the epidermis is scraped down to the dermis, and bleeding occurs with sufficient sampling depth.50 In contrast, when DSS samples are collected in mice, the skin does not bleed, likely because the murine dermis is poorly vascularized.77
Recently, alternatives to DSS have been proposed because it is mildly invasive. In dogs, FP or TI used with skin compression have variable sensitivity but are less costly and invasive compared with DSS.41,56,66 In domestic cats, Demodex mites have been identified through coproscopy (fecal flotation), presumably because cats are avid groomers.40,44,72 Because mice are also fastidious groomers, we have occasionally detected Demodex mites in fecal flotations, but, as in cats, fecal floatation does not appear to be as sensitive as DSS.44 Fecal PCR analysis for Demodex potentially could be useful when pooled samples are analyzed. Additional studies are necessary to ascertain the sensitivity of fecal PCR assays as compared with conventional sampling methods. In humans, Demodex mites are associated with several ocular and dermatologic conditions and are most commonly found on the face and in eyelash follicles.16 Although the standardized skin surface biopsy is the gold standard for testing lightly haired face and body areas in humans, lash epilation is routinely used for cases of Demodex-associated blepharitis.37 More recently, handheld confocal laser scanning microscopy has been proposed for surface mite detection in humans, but neither standardized skin surface biopsy nor confocal laser microscopy is practical for testing rodent colonies for Demodex mites.33,70
Our results indicate that, of the traditional microscopy methods, DSS had the highest detection rate by far, although any of the other methods tested can be used to detect Demodex mites in mice. The enhanced sensitivity of DSS most likely results from the expulsion of mites from the follicle as the skin is squeezed during sample collection. In addition, compared with other methods, DSS samples contain fewer hairs that can conceal mites, making sample analysis easier. Because SSS and TI are often used to detect other parasites in mice, such as fur mites and select pinworm species (for example, Syphacia obvelata),20,30,62,78 and given that Demodex mites are likely more prevalent than previously considered, why aren't they detected more frequently? We speculate that they are not typically an excluded pathogen, and routine careful assessments for Demodex mites are not performed. Fur mites or eggs and pinworm eggs are much larger and more opaque than Demodex mites, making detection easier. Samples for fur mite diagnostics are typically examined at 50× magnification, which would likely preclude identification of Demodex mites because of their small size (approximately 200 μm) and transparency. For comparison, a Demodex adult female is approximately the size of an adult fur mite appendage. In facilities where SSS and TI are performed routinely to detect other parasites, increasing the magnification to 100× and adjusting contrast during microscopic assessment may facilitate the detection of Demodex mites.
We found that oil-based tests (FP and DSS) were superior to tape-based tests (SSS and TI) for Demodex mite detection. They are better at detecting both adult mites as well as eggs and larvae. Tape-based tests may not be useful for visualizing eggs and larvae because debris and air bubbles can obscure these smaller mite stages. The adhesive tape may be suboptimal when the adhesive has a similar refractive index to eggs and larvae. In oil-based tests, mite visualization is easier because there is less debris. Furthermore, movement of adult mites is visible in fresh oil-based samples, enhancing detection. In addition, the coverslip can be manipulated, allowing repositioning of hairs and debris and revealing concealed mites. Importantly, some of our samples were positive for only a single mite and technical staff training was required to identify individual mites in such samples. One structure that was helpful in identification of mites in oil-based tests was a small, opaque structure called the guanine concretion, which is the deposition of a pigmented nitrogenous waste product in the opisthosoma.74 The guanine concretion was refractive, making it identifiable with subtle changes in light or depth of focus and was observed in most live or recently dead mites. There are caveats to using oil-based tests, because they are more invasive than tape-based tests and ideally should be analyzed in a timely manner to detect live mites. Compared with other samples, oil-based test samples are more difficult to store because they must remain flat and cannot be stacked.
Interestingly, FP was better than DSS for detecting eggs and larvae. It is plausible that eggs and larvae are not well-anchored in the follicle and they are released more readily than mites when hairs are plucked. According to our observations, eggs and larvae are more labile than adult mites and, for easier detection of living mites, we recommend oil-based samples be read ideally within 12 h, but no later than 48 h, of collection. Dead mites are observable at later times, but the detection rate will likely decrease as mites become transparent and become more challenging to identify.
Based on experience with fur mite species, which vary in their site preferences on hosts, selection of the anatomic site for Demodex collection affects test sensitivity.18,43,62 Even though Demodex mite distribution is generalized in immunocompromised TRP1/TCR mice, the face is not an ideal location for mite detection.52 We speculate that the hair on the face is shorter and more difficult to grasp with forceps or extract with tape, resulting in nonrepresentative samples. In addition, sampling from the face would be more irritating to the subject, given that the face is highly innervated. We found that the IS region is the best single location for detecting mites: it yielded the highest detection rate with DSS (93.5%) and the most mites per sample (median, 4.4 mites). In fact, 100% of the mice we examined were positive in IS with DSS or FP or both, indicating that it may be possible to detect Demodex mites in moderately infested strains by sampling only IS. The density of mites in IS may reflect that this site is difficult to groom or that the hair turnover rate in this region is slower.52,62 Although CD had a lower detection rate than the IS and CV regions when paired with FP, CD yielded a 90% detection rate with DSS. It, therefore, remains a viable anatomic site for testing; however, it is a more difficult region to sample during manual restraint, and the pelvis made it more difficult to scrape the skin evenly. This site would be easier to sample postmortem or after chemical restraint. Although we did not assess the middorsum in the current study, this site might be useful, given that topographic analysis revealed high numbers of mites in this region, and there is abundant loose skin in this region.52
When multiple tests were performed on individual mice, the overall mite detection rate for a specific test increased in our sample. This phenomenon has also been observed in dogs.60 As in other species, false negatives are possible in healthy, immunocompetent mice.14,60 We therefore recommend that FP and DSS should be performed at multiple sites, minimally the IS and CV regions, for optimal detection.
One limitation of the study was that a single person collected and evaluated all samples. Another limitation was that a single moderately infested, immunocompromised mouse strain was used. Therefore, systematically determining the effectiveness of DSS and FP in diagnosing Demodex mite infestation in immunocompetent mice commonly used as sentinels, such as Swiss Webster or CD1 stocks, is important. We surmise that a 4 test–site combination strategy will be useful in strains with lower mite burdens, but the overall detection rate will likely be lower in healthy, immunocompetent animals.41,60 We used the FP–DSS combination to determine the extent of Demodex infestation in the animals housed in the same holding rooms as TRP1/TCR mice. Although the majority of the 40 strains tested were Demodex-negative, we found mites in 3 of 5 mice (60%) in each of 2 presumed immunocompetent strains.52 In addition, we used FP with DSS, as well as a commercially available PCR assay (Charles River Laboratories) specific to Demodex spp., to assess whether mice imported from other institutions were infested with Demodex mites. Positive results were obtained in subclinical animals by using all methods.63 Therefore, DSS and FP can be useful as well as cost-effective for testing mice for biosecurity and quarantine, when trained personnel are available to collect and assess samples.
In terms of life stage, we observed that adult females were more numerous than adult male mites in the samples evaluated. This pattern is likely related to their tendency for arrhenotoky, a form of parthenogenesis.23,36,53 We also note that the maximal length we measured for adult males and females was longer than previously published.22,26 This outcome is not unexpected because variability in length has been described in various Demodex spp., including D. musculi.5,10,26,39 Interestingly, nymphs were not present in any of the samples examined, although they were identified previously when the skin of TRP1/TCR mice was assessed by using skin fragmentation digestion.52 This finding may reflect that nymphs represent a small proportion of the overall mite population. Nymphs appear deeply embedded within sebaceous glands (data not shown) histologically, perhaps making them more difficult to retrieve by using the methods we evaluated. Another possibility is that nymphs, like eggs and larva, are more labile than mites and may not be detectable unless samples are evaluated immediately.
Given recommendations for sample collection in dogs, it was important to obtain a representative sample for each method evaluated. We determined that the number of hairs in a sample is similarly important in the mouse, because there was a significant correlation between the number of hairs and the number of mites detected in the sample. Whether a sample is representative is based on the number of hairs contained in the sample when using SSS, TI, and FP. SSS of IS and CV, TI of the face, and FP of the face had fewer than the desired number of hairs in the sample, but all other test–site combinations met our criterion of at least 100 hairs per sample. We speculate that as the number of hairs increased, the number of follicles with potential mites sampled increased; however, more densely haired samples became progressively more challenging to assess accurately. The narrow width of Demodex mites allows them to be concealed beneath hair shafts or hair bulbs. Densely haired samples are more time-consuming to analyze, and large numbers of hairs may hamper mite detection, potentially leading to false negative results. In one report, false-negative results in detecting murine fur mites was attributed to both sampling strategy and human error.62 Time-consuming, duplicative review of each sample (as in the current study) or independent evaluations by multiple technicians may reduce false-negative results. Some authors have suggested applying additives to samples as ways to enhance mite visibility in trichoscopy samples, but they have the disadvantage of being lethal to parasites or hydrophilic and thus incompatible with mineral oil.6,32
PCR analysis is increasingly used as a diagnostic tool in laboratory rodent health surveillance programs. Specific genes, such as 18S rRNA and chitin synthase, are highly conserved among Demodex spp.11,81 Research in humans, domestic animal species, and wildlife indicates that PCR assays of these genes may be used to detect Demodex mites.9,17,45,59,67,80 The DNA sequence for D. musculi 18S rRNA was posted to GenBank in 2011 after the isolation of mites from an immunocompromised laboratory mouse, and it has been compared with the DNA of various mite species.19,79,82 The primers we used in the current study were used to amplify the original D. musculi sequence submitted to GenBank, which is 100% homologous with the D. musculi rRNA sequence we isolated. In agreement with other studies, all Demodex rRNA sequences in GenBank had strong homology (96% to 99%) when compared with our amplicon.35,69 However, the M. musculi rRNA sequence had less homology (91%). Our results agree with previous reports demonstrating that individual Demodex mites can be detected by using PCR analysis, indicating that this method is highly sensitive.69,82 Although the primers we used were effective, their usefulness may be limited due to their lack of specificity. We were in the process of constructing more specific primers when PCR assays for D. musculi became commercially available in 2015.
In dogs and cats, PCR analysis has been used to identify new mite species and the etiologic mite species in clinical infestations.12,17,59,69,71 PCR analysis is not routinely used to diagnose Demodex in dogs and humans because 100% of subjects are infested (the majority subclinically), but recently, quantitative PCR analysis in humans has been proposed, because clinical manifestations are associated with Demodex mite overgrowth.9,31,76 PCR assays may be more effective as a diagnostic tool in mice because vendor-sourced mice should be free of ectoparasites. Because a commercially available PCR assay was unavailable for D. musculi at the time this study was initiated, we evaluated traditional parasitology methods as well as an inhouse, nonspecific assay. The detection rate of DSS was equivalent to that of PCR assays performed on skin biopsies or mites from TI, but FP was not as sensitive. In a separate study, we opted to compare one commercially available PCR assay with FP and DSS to determine parasiticide treatment efficacy. Our results (manuscript under review) indicate that PCR analysis of pelt swabs can be used as a highly sensitive antemortem diagnostic tool. Therefore, based on the Demodex detection rates observed in this study, we recommend that DSS of IS should always be included in a dermatologic work-up when ectoparasites are suspected. In addition, when time and resources are available, the combination of 4 test–sites (DSS–IS, DSS–CV, FP–IS, FP–CV) is ideal because of enhanced detection due to multiple site testing and the ability to detect eggs and larvae. PCR analysis and histopathology are both important tools that can be combined with antemortem tests to enhance mite detection.
The intimate relationship between Demodex mites and mammals is ancient, potentially dating back to the radiation of mammals from synapsids more than 200 million years ago.67 Although the mammalian host's immune system is tolerant of these ubiquitous mites, it has been demonstrated in both humans and dogs that significant immune system perturbations can result in response to colonization with Demodex mites.1,2,9,13,16,61 Immune responses are likely altered in Demodex-infested mice, especially those with dermatologic disease, potentially affecting research as well as animal welfare. Further investigation is needed to determine whether immunologic changes result from colonization of immunocompetent mouse strains. In conclusion, FP and DSS are valuable diagnostic methods for Demodex mite detection in laboratory mice. Future studies will determine whether PCR analysis could be used alone or as an adjunct to standard detection methods, as is the case with other parasites.20,78
Acknowledgments
We thank the members of Taha Merghoub's laboratory, especially Hong Zhong; the staff of the Center of Comparative Medicine and Pathology's (CCMP) Laboratory of Comparative Pathology, especially Jacqueline Candelier; and the CCMP's Veterinary Services staff for their assistance with this project. We also thank Clifford Desch for his valuable advice, Michelle Lepherd for teaching and training the staff how to detect Demodex mites, and Susan Compton for her insights regarding PCR assays and for providing primer sequences. This work was supported in part by a grant from the National Cancer Institute (P30 CA 008748) to the Memorial Sloan Kettering Cancer Center.
References
- 1.Akilov OE, Kazanceva SV, Vlasova IA. 2001. Particular features of immune response after invasion of different species of human Demodex mites. Russ J Immunol 6:399–404. [PubMed] [Google Scholar]
- 2.Akilov OE, Mumcuoglu KY. 2004. Immune response in demodicosis. J Eur Acad Dermatol Venereol 18:440–444. [DOI] [PubMed] [Google Scholar]
- 3.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. [DOI] [PubMed] [Google Scholar]
- 4.Barthold SW, Griffey SM, Percy DH. 2016. Pathology of laboratory rodents and rabbits, 4th ed Ames (IA): Blackwell Publishing. [Google Scholar]
- 5.Bourdeau PJ. 2010. Variation of size in Demodex canis: from the shortest to the longest forms. The 24th Annual Congress of the ECVD-ESVD, 17–19 September 2009, Bled, Slovenia. Vet Dermatol 21:213. [Google Scholar]
- 6.Bruet V, Bourdeau P. 2011. Evolution over time of Demodex mite detection in skin scrapings with Amann's lactophenol in dogs. Vet Dermatol 22:378–379. [DOI] [PubMed] [Google Scholar]
- 7.Bukva V. 1985. Demodex flagellurus sp. n. (Acari: Demodicidae) from the preputial and clitoral glands of the house mouse, Mus musculus L. Folia Parasitol (Praha) 32:73–81. [PubMed] [Google Scholar]
- 8.Bukva V. 1995. Demodex species (Acari:Demodecidae) parasitizing the brown rat, Rattus norvegicus (Rodentia): redescription of Demodex ratti and description of D. norvegicus sp. n. and D. ratticola sp. n. Folia Parasitol (Praha) 42:149–160. [PubMed] [Google Scholar]
- 9.Casas C, Paul C, Lahfa M, Livideanu B, Lejeune O, Alvarez-Georges S, Saint-Martory C, Degouy A, Mengeaud V, Ginisty H, Durbise E, Schmitt AM, Redoulès D. 2012. Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp Dermatol 21:906–910. [DOI] [PubMed] [Google Scholar]
- 10.Chesney CJ. 1999. Short form of Demodex sp. mite in the dog: occurrence and measurements. J Small Anim Pract 40:58–61. [DOI] [PubMed] [Google Scholar]
- 11.de Rojas M, Riazzo C, Callejon R, Guevara D, Cutillas C. 2012. Molecular study on 3 morphotypes of Demodex mites (Acarina: Demodicidae) from dogs. Parasitol Res 111:2165–2172. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira D, Sastre N, Ravera I, Altet L, Francino O, Bardagi M, Ferrer L. 2015. Identification of a 3rd feline Demodex species through partial sequencing of the 16S rDNA and frequency of Demodex species in 74 cats using a PCR assay. Vet Dermatol 26:239–e53. [DOI] [PubMed] [Google Scholar]
- 13.Ferrer L, Ravera I, Silbermayr K. 2014. Immunology and pathogenesis of canine demodicosis. Vet Dermatol 25:427–e65. [DOI] [PubMed] [Google Scholar]
- 14.Fondati A, De Lucia M, Furiani N, Monaco M, Ordeix L, Scarampella F. 2010. Prevalence of Demodex canis-positive healthy dogs at trichoscopic examination. Vet Dermatol 21:146–151. [DOI] [PubMed] [Google Scholar]
- 15.Forton F, Seys B. 1993. Density of Demodex folliculorum in rosacea: a case-control study using standardized skin-surface biopsy. Br J Dermatol 128:650–659. [DOI] [PubMed] [Google Scholar]
- 16.Forton FM. 2011. Papulopustular rosacea, skin immunity, and Demodex: pityriasis folliculorum as a missing link. J Eur Acad Dermatol Venereol 26:19–28. [DOI] [PubMed] [Google Scholar]
- 17.Frank LA, Kania SA, Chung K, Brahmbhatt R. 2013. A molecular technique for the detection and differentiation of Demodex mites on cats. Vet Dermatol 24:367–369, e382 –363. [DOI] [PubMed] [Google Scholar]
- 18.Galton M. 1963. Myobic mange in the mouse leading to skin ulceration and amyloidosis. Am J Pathol 43:855–865. [PMC free article] [PubMed] [Google Scholar]
- 19.GenBank. [Internet]. 2011. Demodex musculi 18S ribosomal RNA gene, partial sequence. [Cited 6 June 2014]. Available at: https://www.ncbi.nlm.nih.gov/nuccore/jF834894.
- 20.Gerwin PM, Ricart Arbona RJ, Riedel ER, Lepherd ML, Henderson KS, Lipman NS. 2017. Evaluation of traditional and contemporary methods for detecting Syphacia obvelata and Aspiculuris tetraptera in laboratory mice. J Am Assoc Lab Anim Sci 56:32–41. [PMC free article] [PubMed] [Google Scholar]
- 21.Hill LR, Kille PS, Weiss DA, Craig TM, Coghlan LG. 1999. Demodex musculi in the skin of transgenic mice. Contemp Top Lab Anim Sci 38:13–18. [PubMed] [Google Scholar]
- 22.Hirst S. 1919. Studies on Acari. No. 1. The genus Demodex, Owen. Order of trustees of the British Museum (Natural History). London: Taylor & Francis. [Google Scholar]
- 23.Hughes SE, Nutting WB. 1981. Demodex leucogasteri n. sp. from Onychomys leucogaster - with notes on its biology and host pathogenesis. Acarologia 22:181–186. [PubMed] [Google Scholar]
- 24.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 25.Izdebska JN, Rolbiecki L. 2015. A new species of the genus Demodex Owen, 1843 (Acari: Demodecidae) from the ear canals of the house mouse Mus musculus L. (Rodentia: Muridae). Syst Parasitol 91:167–173. [DOI] [PubMed] [Google Scholar]
- 26.Izdebska JN, Rolbiecki L. 2015. Two new species of Demodex (Acari: Demodecidae) with a redescription of Demodex musculi and data on parasitism in Mus musculus (Rodentia: Muridae). J Med Entomol 52:604–613. [DOI] [PubMed] [Google Scholar]
- 27.Izdebska JN, Rolbiecki L. 2016. A new genus and species of demodecid mites from the tongue of a house mouse Mus musculus: description of adult and immature stages with data on parasitism. Med Vet Entomol 30:135–143. [DOI] [PubMed] [Google Scholar]
- 28.Izdebska JN, Rolbiecki L, Fryderyk S. 2016. A new species of Demodex (Acari: Demodecidae) from the skin of the vibrissal area of the house mouse Mus musculus (Rodentia: Muridae), with data on parasitism. Syst Appl Acarol 21:1031–1039. [Google Scholar]
- 29.Jekl V, Hauptman K, Jeklova E, Knotek Z. 2006. Demodicosis in 9 prairie dogs (Cynomys ludovicianus). Vet Dermatol 17:280–283. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson EM, Pearson LM, Kuzma KM, Burkholder TH. 2014. Combined evaluation of commonly used techniques, including PCR, for diagnosis of mouse fur mites. J Am Assoc Lab Anim Sci 53:69–73. [PMC free article] [PubMed] [Google Scholar]
- 31.Kasetsuwan N, Kositphipat K, Busayarat M, Threekhan P, Preativatanyou K, Phumee A, Siriyasatien P. 2017. Prevalence of ocular demodicosis among patients at Tertiary Care Center, Bangkok, Thailand. Int J Ophthalmol 10:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kheirkhah A, Blanco G, Casas V, Tseng SC. 2007. Fluorescein dye improves microscopic evaluation and counting of Demodex in blepharitis with cylindrical dandruff. Cornea 26:697–700. [DOI] [PubMed] [Google Scholar]
- 33.Lacey N, Forton FM, Powell FC. 2013. Demodex quantification methods: limitations of confocal laser scanning microscopy. Br J Dermatol 169:212–213. [DOI] [PubMed] [Google Scholar]
- 34.Lacey N, Kavanagh K, Tseng SC. 2009. Under the lash: Demodex mites in human diseases. Biochem (Lond) 31:2–6. [PMC free article] [PubMed] [Google Scholar]
- 35.Lankton JS, Chapman A, Ramsay EC, Kania SA, Newkirk KM. 2013. Preputial Demodex species in big brown bats (Eptesicus fuscus) in eastern Tennessee. J Zoo Wildl Med 44:124–129. [DOI] [PubMed] [Google Scholar]
- 36.Lebel RR, Desch CE., Jr 1979. Karyotype and anomalous development in Demodex caprae. In: Proceedings of the 4th International Congress of Acarology, Saalfelden (Austria), August 1974. Budapest: Akadémiai Kiadó; p525–529 [Google Scholar]
- 37.Liu J, Sheha H, Tseng SC. 2010. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol 10:505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q, Arseculeratne C, Liu Z, Whitmire J, Grusby MJ, Finkelman FD, Darling TN, Cheever AW, Swearengen J, Urban JF, Gause WC. 2004. Simultaneous deficiency in CD28 and STAT6 results in chronic ectoparasite-induced inflammatory skin disease. Infect Immun 72:3706–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Löwenstein C, Beck W, Bessmann K, Mueller RS. 2005. Feline demodicosis caused by concurrent infestation with Demodex cati and an unnamed species of mite. Vet Rec 157:290–292. [DOI] [PubMed] [Google Scholar]
- 40.Lucio-Forster A, Bowman DD. 2011. Prevalence of fecal-borne parasites detected by centrifugal flotation in feline samples from 2 shelters in upstate New York. J Feline Med Surg 13:300–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marques Cury GM, Pereira ST, Botoni LS, de Oliveira Pereira RD, da Costa Telles T, Ferreira AP, de Costa-Val AP. 2013. Diagnosis of canine demodicosis: comparative study between hair plucking and adhesive tape tests. Rev Bras Parasitol Vet 20: 137–139. [Google Scholar]
- 42.McKeon GP, Nagamine CM, Ruby NF, Luong RH. 2011. Hematologic, serologic, and histologic profile of aged Siberian hamsters (Phodopus sungorus). J Am Assoc Lab Anim Sci 50:308–316. [PMC free article] [PubMed] [Google Scholar]
- 43.Metcalf Pate KA, Rice KA, Wrighten R, Watson J. 2011. Effect of sampling strategy on the detection of fur mites within a naturally infested colony of mice (Mus musculus). J Am Assoc Lab Anim Sci 50:337–343. [PMC free article] [PubMed] [Google Scholar]
- 44.Milley C, Dryden M, Rosenkrantz W, Griffin J, Reeder C. 2017. Comparison of parasitic mite retrieval methods in a population of community cats. J Feline Med Surg 19:657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milosevic MA, Frank LA, Brahmbhatt RA, Kania SA. 2013. PCR amplification and DNA sequencing of Demodex injai from otic secretions of a dog. Vet Dermatol 24:286–e66. [DOI] [PubMed] [Google Scholar]
- 46.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. 1992. RAG1-deficient mice have no mature B and T lymphocytes. Cell 68:869–877. [DOI] [PubMed] [Google Scholar]
- 47.Morita E, Kaneko S, Hiragun T, Shindo H, Tanaka T, Furukawa T, Nobukiyo A, Yamamoto S. 1999. Fur mites induce dermatitis associated with IgE hyperproduction in an inbred strain of mice, NC/Kuj. J Dermatol Sci 19:37–43. [DOI] [PubMed] [Google Scholar]
- 48.Mueller RS. 2008. Quick tests in veterinary dermatology—we can do this here and now. Proceedings of the 33rd World Small Animal Veterinary Congress. Dublin, Ireland: International Veterinary Information Service; p 174–176. [Google Scholar]
- 49.Mueller RS. 2012. An update on the therapy of canine demodicosis. Compend Contin Educ Vet 34:E1–E4. [PubMed] [Google Scholar]
- 50.Mueller RS, Bensignor E, Ferrer L, Holm B, Lemarie S, Paradis M, Shipstone MA. 2012. Treatment of demodicosis in dogs: 2011 clinical practice guidelines. Vet Dermatol 23:86–96, e20 –e21. [DOI] [PubMed] [Google Scholar]
- 51.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. 2008. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 112:362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nashat MA, Luchins KR, Lepherd ML, Riedel ER, Izdebska JN, Lipman NS. 2017. Characterization of Demodex musculi infestation, associated comorbidities, and its topographical distribution in a mouse strain with defective adaptive immunity. Comp Med 67:315–329. [PMC free article] [PubMed] [Google Scholar]
- 53.Nutting WB. 1976. Hair follicle mites (Demodex spp.) of medical and veterinary concern. Cornell Vet 66:214–231. [PubMed] [Google Scholar]
- 54.Nutting WB, Emejuaiwe SO, Tisdel MO. 1971. Demodex gapperi sp. n. (Acari: Demodicidae) from the red-backed vole, Clethrionomys gapperi. J Parasitol 57:660–665. [PubMed] [Google Scholar]
- 55.Palopoli MF, Fergus DJ, Minot S, Pei DT, Simison WB, Fernandez-Silva I, Thoemmes MS, Dunn RR, Trautwein M. 2015. Global divergence of the human follicle mite Demodex folliculorum: Persistent associations between host ancestry and mite lineages. Proc Natl Acad Sci USA 112:15958–15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pereira AV, Pereira SA, Gremião ID, Campos MP, Ferreira AM. 2012. Comparison of acetate tape impression with squeezing versus skin scraping for the diagnosis of canine demodicosis. Aust Vet J 90:448–450. [DOI] [PubMed] [Google Scholar]
- 57.Pochanke V, Hatak S, Hengartner H, Zinkernagel RM, McCoy KD. 2006. Induction of IgE and allergic-type responses in fur miteinfested mice. Eur J Immunol 36:2434–2445. [DOI] [PubMed] [Google Scholar]
- 58.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. 2010. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 207:637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravera I, Altet L, Francino O, Bardagí M, Sánchez A, Ferrer L. 2010. Development of a real-time PCR to detect Demodex canis DNA in different tissue samples. Parasitol Res 108:305–308. [DOI] [PubMed] [Google Scholar]
- 60.Ravera I, Altet L, Francino O, Sánchez A, Roldán W, Villanueva S, Bardagí M, Ferrer L. 2013. Small Demodex populations colonize most parts of the skin of healthy dogs. Vet Dermatol 24:168–e37. [DOI] [PubMed] [Google Scholar]
- 61.Ravera I, Ferreira D, Gallego LS, Bardagi M, Ferrer L. 2015. Serum detection of IgG antibodies against Demodex canis by western blot in healthy dogs and dogs with juvenile generalized demodicosis. Res Vet Sci 101:161–164. [DOI] [PubMed] [Google Scholar]
- 62.Ricart Arbona RJ, Lipman NS, Wolf FR. 2010. Treatment and eradication of murine fur mites: II. Diagnostic considerations. J Am Assoc Lab Anim Sci 49:583–587. [PMC free article] [PubMed] [Google Scholar]
- 63.Ricart Arbona RJ, Nashat MA, Wolf FR, Lipman NS. 2016. Estimated prevalence of Demodex spp. in a 1600-cage mouse colony and in imported mice from other academic institutions. Abstract presented at the AALAS National Meeting, 30–3 November 2016. Charlotte, North Carolina. J Am Assoc Lab Anim Sci 55: 698. [Google Scholar]
- 64.Rice KA, Albacarys LK, Metcalf Pate KA, Perkins C, Henderson KS, Watson J. 2013. Evaluation of diagnostic methods for Myocoptes musculinus according to age and treatment status of mice (Mus musculus). J Am Assoc Lab Anim Sci 52:773–781. [PMC free article] [PubMed] [Google Scholar]
- 65.Roble GS, Boteler W, Riedel E, Lipman NS. 2012. Total IgE as a serodiagnostic marker to aid murine fur mite detection. J Am Assoc Lab Anim Sci 51:199–208. [PMC free article] [PubMed] [Google Scholar]
- 66.Saridomichelakis MN, Koutinas AF, Farmaki R, Leontides LS, Kasabalis D. 2007. Relative sensitivity of hair pluckings and exudate microscopy for the diagnosis of canine demodicosis. Vet Dermatol 18:138–141. [DOI] [PubMed] [Google Scholar]
- 67.Sastre N, Francino O, Curti JN, Armenta TC, Fraser DL, Kelly RM, Hunt E, Silbermayr K, Zewe C, Sanchez A, Ferrer L. 2016. Detection, prevalence, and phylogenetic relationships of Demodex spp and further skin prostigmata mites (Acari, Arachnida) in wild and domestic mammals. PLoS One 11:e0165765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sastre N, Ravera I, Ferreira D, Altet L, Sánchez A, Bardagí M, Francino O, Ferrer L. 2013. Development of a PCR technique specific for Demodex injai in biological specimens. Parasitol Res 112:3369–3372. [DOI] [PubMed] [Google Scholar]
- 69.Sastre N, Ravera I, Villanueva S, Altet L, Bardagi M, Sanchez A, Francino O, Ferrer L. 2012. Phylogenetic relationships in 3 species of canine Demodex mite based on partial sequences of mitochondrial 16S rDNA. Vet Dermatol 23:509–e101. [DOI] [PubMed] [Google Scholar]
- 70.Sattler EC, Maier T, Hoffmann VS, Hegyi J, Ruzicka T, Berking C. 2012. Noninvasive in vivo detection and quantification of Demodex mites by confocal laser scanning microscopy. Br J Dermatol 167:1042–1047. [DOI] [PubMed] [Google Scholar]
- 71.Silbermayr K, Horvath-Ungerboeck C, Eigner B, Joachim A, Ferrer L. 2014. Phylogenetic relationships and new genetic tools for the detection and discrimination of the 3 feline Demodex mites. Parasitol Res 114:747–752. [DOI] [PubMed] [Google Scholar]
- 72.Silbermayr K, Joachim A, Litschauer B, Panakova L, Sastre N, Ferrer L, Horvath-Ungerboeck C. 2013. The first case of Demodex gatoi in Austria, detected with fecal flotation. Parasitol Res 112:2805–2810. [DOI] [PubMed] [Google Scholar]
- 73.Smith PC, Zeiss CJ, Beck AP, Scholz JA. 2016. Demodex musculi infestation in genetically immunomodulated mice. Comp Med 66:278–285. [PMC free article] [PubMed] [Google Scholar]
- 74.Stromberg BE, Nutting WB. 1972. Adaptive features of the exoskeleton and pigment deposits in Demodex spp. (Demodicidae). Acarologia 14:605–611. [Google Scholar]
- 75.Tani K, Iwanaga T, Sonoda K, Hayashiya S, Hayashiya M, Taura Y. 2001. Ivermectin treatment of demodicosis in 56 hamsters. J Vet Med Sci 63:1245–1247. [DOI] [PubMed] [Google Scholar]
- 76.Tenorio-Abreu A, Sanchez-Espana JC, Naranjo-Gonzalez LE, Gonzalez-Gallego MC, Hidalgo-Grass C, Ruiz-Frutos C. 2016. [Development of a PCR for the detection and quantification of parasitism by Demodex folliculorum infestation in biopsies of skin neoplasms periocular area.] Rev Esp Quimioter 29:220–223. [Article in Spanish]. [PubMed] [Google Scholar]
- 77.Treuting PM, Dintzis SM, Montine KS. 2017. Comparative anatomy and histology: a mouse and human atlas, 2nd ed London (United Kingdom): Academic Press. [Google Scholar]
- 78.Weiss EE, Evans KD, Griffey SM. 2012. Comparison of a fur mite PCR assay and the tape test for initial and posttreatment diagnosis during a natural infection. J Am Assoc Lab Anim Sci 51:574–578. [PMC free article] [PubMed] [Google Scholar]
- 79.Yabsley MJ, Clay SE, Gibbs SEJ, Cunningham MW, Austel MG. 2013. Morphologic and molecular characterization of a Demodex (Acari: Demodicidae) species from white-tailed deer (Odocoileus virginianus). Int Sch Res Notices 2013:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao YE, Hu L, Ma JX. 2013. Phylogenetic analysis of Demodex caprae based on mitochondrial 16S rDNA sequence. Parasitol Res 112:3969–3977. [DOI] [PubMed] [Google Scholar]
- 81.Zhao YE, Wang ZH, Xu Y, Xu JR, Liu WY, Wei M, Wang CY. 2012. Cloning and sequence analysis of chitin synthase gene fragments of Demodex mites. J Zhejiang Univ Sci B 13:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao YE, Xu JR, Hu L, Wu LP, Wang ZH. 2012. Complete sequence analysis of 18S rDNA based on genomic DNA extraction from individual Demodex mites (Acari: Demodicidae). Exp Parasitol 131:45–51. [DOI] [PubMed] [Google Scholar]