Abstract
Often few alternative anesthetics for exotic species are available, due to the small numbers of these animals used in research. In this study, we evaluated the depth and duration of anesthesia in Xenopus laevis after their immersion in 3 doses of etomidate (15, 22.5, and 30 mg/L) and in 3 doses of benzocaine (0.1%, 0.5%, and 1%) compared with the ‘gold standard,’ tricaine methanesulfonate (MS222; 2 g/L). We then chose an optimal dose for each alternative anesthetic according to induction time, duration of surgical plane, and time to complete recovery. The optimal etomidate and benzocaine doses (22.5 mg/L and 0.1%, respectively) as well as the MS222 dose were then used to achieve a surgical plane of anesthesia, with the addition of flunixin meglumine (25 or 50 mg/kg) administered in the dorsal lymph sac at the completion of mock oocyte harvest. Efficacy of the analgesic was assessed at 1, 3, 6, and 24 h postoperatively by using acetic acid testing (AAT). Histology of the liver, kidney, and tissues surrounding the dorsal lymph sac was performed at day 3, 14, and 28 in each group of animals. Mild to moderate myocyte degeneration and necrosis were present in tissues surrounding the dorsal lymph sac at both flunixin meglumine doses after etomidate and benzocaine anesthesia. In addition, the 50-mg/kg dose of flunixin meglumine resulted in the death of 5 of the 12 frogs within 24 h, despite an otherwise uneventful anesthetic recovery. In conclusion, benzocaine and etomidate offer alternative anesthetic regimens, according to typical requirements for an anesthetic event. Flunixin meglumine at the 25-mg/kg dose provided analgesic relief at the latest time point during etomidate dosage and at all time points during benzocaine dosage, but further characterization is warranted regarding long-term or repeated analgesic administration.
Abbreviations: AAT, acetic acid testing; MS222, tricaine methanesulfonate
African clawed frogs (Xenopus laevis) are a common amphibian species in the research environment due to their ease of maintenance, hardiness, adaptability to various housing arrangements, short generation times, and high yield of genetic material.25 Traditionally, female Xenopus have been used extensively for oocyte collection, gonadectomized male frogs have been used to study degeneration of the vocal pathway, and both sexes have been used to study cardiac morphology, cranial osteogenesis and suture morphology for craniofacial development, spinal cord regeneration, and androgen regulation of the neuromuscular junction structure and function.1,2,4,29,41
The necessity to manipulate or collect samples from Xenopus frogs can cause difficulties during handling as a result of their slippery mucous layer, consequently creating undue stress on the animals. The addition of chemical restraint not only facilitates the situation for handlers but also enhances the welfare of the animals. Chemical restraint in amphibians, ranging from sedation to surgical plane anesthesia, can be achieved through a variety of routes, including immersion, topical, injectable, and inhalant.22,35,40 Although tricaine methanesulfonate (MS222), a benzocaine derivative, is considered the ‘gold standard’ as an immersion anesthetic for X. laevis,18,21 its various disadvantages, including respiratory irritation in personnel handling the powdered form and the need to buffer the solution,14,22,26,37 prompt the consideration of alternative anesthetic regimens.
Examples of alternative anesthetics previously explored in Xenopus include benzocaine gel, ketamine, eugenol (clove oil), isoflurane, propofol, medetomidine, and dexmedetomidine. Compared with MS222, benzocaine gel in amphibians produces a faster induction and a prolonged recovery, and its effects are weight-dependent, such that increased body weight resulted in a longer induction time.21 Ketamine can provide long-lasting anesthesia, ranging from 12 to 18 h, but published literature questions its analgesic properties in African clawed frogs.14,17,22
Eugenol, a clove-oil extract, has the ability to produce short-term anesthesia but is weight-dependent and cardiodepressive.12,16,22 Bubbling isoflurane inhalant in tank water is unsuccessful.40 Topical application of isoflurane is the most effective form to administer isoflurane; however skin irritation and variation in time to surgical plane and duration of anesthesia have been observed.30 Propofol is unsafe to use in Xenopus,14,15,40 and α2 agonists such as medetomidine and dexmedetomidine are ineffective in this species.11,40
Other anesthetic options to explore in Xenopus include drugs such as etomidate, benzocaine in solution, and alfaxalone. Etomidate, an imidazole-derivative anesthetic and hypnotic, has been used for anesthesia of Xenopus tadpoles28 and in oriental fire-bellied toads (Bombina orientalis).8 Benzocaine in solution has been used in field studies, but a dose for X. laevis has not been published.36 Alfaxalone has been studied in bullfrogs (Lithobates catesbeiana) and did not produce immobilization or anesthesia.27 Although alfaxalone is a possible alternative anesthetic for Xenopus, its classification as a schedule IV controlled substance renders it difficult and costly to obtain.10
The detection of pain in amphibians is difficult, and whether species without a cerebral or limbic cortex are able to experience pain has been debated.33 The primary mechanism of nociception in the spinal cord of amphibians is similar to that of mammals and fish, and the pain neurotransmitters and endogenous opioid peptides in the spinal cord are similar as well.23,32,39 These findings support the idea that amphibians and mammals experience nociception similarly. As with other species, distinguishing whether a frog's response to a noxious stimulus is a clinical finding indicating stress or actual pain, both of which should be treated, is essential.6 Although opioid and nonopioid analgesia have been studied more extensively in leopard frogs (Rana pipiens) by using the acetic acid test (AAT),24,34 providing and assessing analgesia in Xenopus is an important requirement for the welfare of the animals, especially given their increasing numbers in the research setting.13
Although an extensive list of analgesic choices exists, including drugs such as morphine, butorphanol, and buprenorphine, flunixin meglumine may provide better analgesia and has the additional benefit of being a nonscheduled substance.7,31 Flunixin meglumine (trade name Banamine), is a NSAID that is most often used in agricultural animals. A dose of 25 mg/kg administered in the dorsal lymph sac of X. laevis achieved significant analgesic effects in as little as 5 h after administration; in addition, the authors recommended further characterization and exploration of any adverse side effects.7
In the current study, we had 2 goals. The first was to determine whether etomidate or benzocaine produce similar anesthetic events to those of MS222 in African clawed frogs. Providing alternative anesthetic regimens that avoid the occupational health and safety risks of MS222 and the patient risks associated with incorrectly buffered solutions (particularly skin irritation and inflammation) is key to achieving this goal. The second goal was to further characterize the analgesic properties of flunixin meglumine administered to Xenopus frogs at increased dosages and when used in combination with etomidate, benzocaine, or MS222. In addition, we used histology to examine whether these anesthetic regimens were associated with significant pathology.
Materials and Methods
Animals.
All work was performed on an IACUC-approved protocol at an AAALAC-accredited program at Texas A & M University. Adult male (n = 36; weight, 22 to 59 g) and female (n = 36; weight, 24 to 130 g) African clawed frogs (X. laevis) older than 1 y were obtained from an inhouse breeding program. The frogs were determined to be in good health by physical exam (American Association of Anesthesiologists score of 1)9 and had participated in no confounding studies prior to investigation. The frogs were randomly allocated into 12 groups of 6 frogs each; 7 groups (total, 42 frogs) were used to investigate each anesthetic regimen in the absence of analgesic relief, whereas the remaining 5 groups (total, 30 frogs) received the optimal doses of each anesthetic as determined in the first experiment and were used to further characterize flunixin meglumine analgesia. Each frog underwent a single anesthetic event, which included the creation of a surgical incision in the lower abdomen.
Housing.
Frogs were group-housed (Xenoplus Stand Alone housing systems, Tecniplast, West Chester, PA) at 5 to 8 frogs per tank prior to the study and after surgical incisions had healed. Initially frogs were returned to group housing after complete recovery from anesthesia, but due to incision site complications during group housing after experimental manipulation, frogs were singly housed for the remainder of the experiment in static tanks, with weekly water changes and enrichment provided in the form of an opaque yellow plastic lid that they could use for shelter. The room was set on a 12:12-h light:dark cycle, with a room temperature of 68 to 72 °F (20 to 22 °C). The water was obtained from the municipal supply and filtered by using UV light, a carbon filter, and a biofilter. The system water was maintained at 68 to 72 °F (20 to 22 °C), with a pH of 6.2 to 8.5, conductivity ranging from 500 to 3000 μS, and monitoring for the presence of ammonia and chlorine. Frogs were fed a commercially available pelleted diet (Frog Brittle [45% protein], Nasco, Fort Atkinson, WI) 3 times each week, and uneaten food was removed from the tanks daily.
Anesthetic doses.
In the initial portion of the study, 36 frogs (6 per group) were immersed in the following doses of benzocaine (0.1%, 0.5%, 1%) and etomidate (15, 22.5, 30 mg/L) to determine the optimal anesthetic dose to obtain an adequate surgical anesthetic plane for each anesthetic, without prolonged immersion time or the time until complete recovery. Another group, containing 6 frogs, was anesthetized with MS222 at 2.0 g/L to serve as the control for comparing the time until induction, duration of surgical-plane anesthesia, and time to complete recovery.
Water from the recirculating system was used as the vehicle for each anesthetic immersion. The pH of each solution was evaluated by using pH paper (Hydrion, Micro Essential, Brooklyn, NY), and solutions were titrated with sodium bicarbonate as needed to reach a neutral pH of 7.0. MS222 (Sigma Aldrich, St Louis, MO) was weighed as a solid form in a hard-ducted fume hood by using respiratory protection and gloves to avoid skin irritation before being dissolved in tank water.36 Benzocaine (Sigma Aldrich) was weighed in its solid form and then dissolved in 100% ethyl alcohol before being diluted in tank water to its final concentrations.38 Etomidate (2 mg/mL, Hospira, Lake Forest, IL) was formulated as a liquid, and it was mixed directly into the tank water to create the appropriate dosages.8
Each frog was submerged in a plastic container filled with the appropriate drug and at the correct concentration until it reached a surgical plane of anesthesia. The onset of surgical-plane anesthesia was defined as the time at which the frog could no longer maintain a righting or withdrawal reflex. The righting reflex was evaluated by assessing each frog's ability to turn onto its ventrum when placed on its back and was scored from 0 to 3 (0, unable to right itself; 1, weak attempt to right itself; 2, strong attempt to right itself but unable to flip over; and 3, able to flip itself over). The withdrawal reflex was tested by pinching a phalangeal articulation of the pelvic limb with the tips of the anesthetist's fingers for a maximum of 2 s to evaluate whether the frog withdrew the limb; responses were scored from 0 to 2 (0, none; 1, weak; and 2, strong response). Reflexes were tested at 1-min intervals until complete loss of both reflexes occurred.
After reaching a surgical plane of anesthesia, frogs were removed from the anesthetic solution, weighed, rinsed with fresh recirculating tank water to move any debris from the surgical site, and placed on their dorsum on a wet diaper pad. The depth of anesthesia was further evaluated in response to an additional physical stimulus with the creation of a 1- to 2-cm full-thickness incision at the lower right quadrant of the ventrum by using a no. 15 scalpel blade. No oocytes were removed, nor were the abdominal contents manipulated. A 2-layer closure was performed by using 4-0 polydioxanone for the muscle layers, and 4-0 nylon was used to suture the skin; both closures were achieved by using a simple interrupted pattern.3
Anesthetic depth was monitored from the initiation of surgery until complete recovery by using a pulse oximeter (Nonin, Model 9847V) to monitor heart rate; the righting and withdrawal reflexes were tested at 5-min intervals. Complete recovery was indicated when the righting and withdrawal reflexes both returned to normal (scores of 3 and 2, respectively). Postoperatively, once frogs had completely recovered, they were placed in a static tank with a gas bubbler, delivering room air, for monitoring. The time from immersion to achieving a surgical plane of anesthesia (induction), duration of surgical-plane anesthesia, and duration of anesthesia until complete recovery were all recorded.
Initially animals were monitored daily postoperatively in group housing looking for abnormal behavior including floating and reluctance to dive,14 weight loss, decreased appetite, inflammation, erythema, infection or wound dehiscence. However, group-housed frogs became stressed and reacted negatively, with excessive kicking and activity, in response to daily handling involving capture with nets and individual restraint to monitor the incision site; we therefore switched to single housing, where daily visual monitoring through the clear tank was performed instead. Once the incision site had healed, typically within 10 to 14 d, frogs were returned to group housing in the recirculating water system.
Flunixin meglumine administration.
For the second portion of the experiment, flunixin meglumine (25 or 50 mg/kg) was administered into the right dorsal lymph sac by using a 22-gauge needle immediately after the completion of surgery. This portion of the experiment involved 5 groups (n = 6 frogs per group), which received either MS222 or etomidate (22.5 mg/L) with either 25 or 50 mg/kg of flunixin meglumine or 0.1% benzocaine with 25 mg/kg of flunixin meglumine.
To test the effectiveness of pain control, AAT was performed at 1, 3, 6, and 24 h after complete recovery from anesthesia. Testing consisted of applying a single drop of acetic acid from a needleless 1-mL syringe (Terumo) in increasing concentrations to the dorsum of the thigh until the frog exhibited a wiping (positive) response to the irritation. Acetic acid was placed for a contact time of 3 to 5 s and then rinsed off with tank water. The acetic acid was alternated between legs for each increasing dose, at concentrations of 0%, 5%, 10%, 20%, and 50%. Responses were scored as either positive or negative for each increasing dose, until a positive response occurred or the maximal concentration (50% acetic acid) was reached.15
Histology.
One frog from each study group was euthanized at 3, 14, and 28 d after surgery, for a total of 36 frogs necropsied. Euthanasia was performed by using MS222 overdose (that is, exposure to 2 g/L for greater than 20 min) followed by pithing, after which tissues were fixed in 10% formalin solution. Representative samples including liver, kidney, and tissues surrounding the right dorsal lymph sac were processed and evaluated histologically by using hematoxylin and eosin staining, with particular attention for abnormalities or effects in the liver, kidney, and right dorsal lymph sac tissue where the flunixin meglumine was administered.
Statistics.
Stata 11 for Windows (StataCorp, College Station, TX) was used to perform multivariable linear regression, with anesthesia type and frog weight included as independent variables. Successful and unsuccessful anesthesia parameters were compared between agents by using the χ2 test (with P < 0.05 taken to indicate significant differences).
The average and 95% confidence interval were calculated for induction time, duration of surgical-plane anesthesia, and time to complete recovery by evaluating MS222 as the baseline regimen to generate inclusion and exclusion parameters for characterizing etomidate and benzocaine doses as comparable anesthetic alternatives. SuperANOVA was used to calculate 1 SD and to perform 1-way and 2-way ANOVA of the anesthetic parameters, and StatView 4.1 was used to perform unpaired t tests to determine whether analgesia affected anesthetic parameters when combined with each anesthetic.
Results
Induction time.
Weight influenced induction time (R2 = 0.6817, P = 0.043), on the basis of the multivariable linear regression analysis for frogs that did not receive analgesia. However, weight did not influence the duration of the surgical plane of anesthesia (R2 = 0.6187, P = 0.552) or recovery time (R2 = 0.7612, P = 0.094).
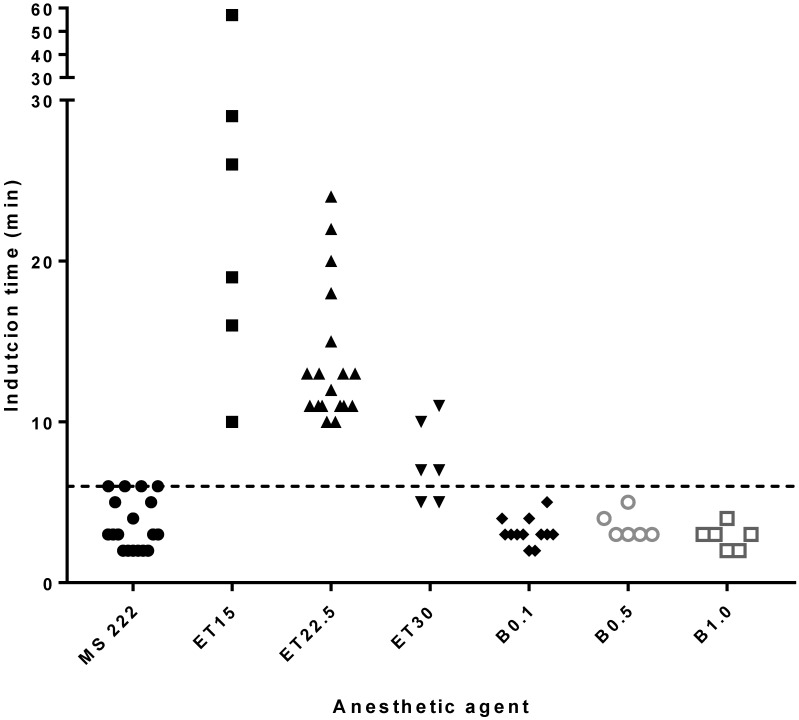
For MS222, the induction time was 3.6 ± 1.6 min (mean ± 1 SD), with upper and lower 95% confidence intervals of 2.9 and 4.4 min, respectively; 6 min was the maximal time needed to reach a surgical plane of anesthesia. The etomidate doses of 15, 22.5, and 30 mg/L took 26.2 ±16.6, 13.8 ± 4.3, and 7.5 ± 2.5 min, respectively, to reach a surgical plane, whereas the benzocaine doses of 0.1%, 0.5%, and 1% took 3.2 ± 0.8, 3.5 ± 0.8, and 2.8 ± 0.8 min, respectively, to achieve surgical anesthesia. Relative to the maximal induction time of 6 min for MS222, doses of etomidate required a longer immersion time for induction while all doses of benzocaine required a shorter immersion time (Figure 1). When comparing induction times of anesthetics to MS222 without the administration of analgesia in any frogs, etomidate 15 mg/L and etomidate 22.5 mg/L had a statistically significant difference (P < 0.05).
Figure 1.
Induction time (that is, from the point of immersion into anesthetic solution until complete loss of righting and withdrawal reflexes). The dotted line represents the 6-min maximum required for MS222 to induce anesthesia. MS222, MS222 at 2 g/L; Et, etomidate at 15, 22.5, or 30 mg/L; B, benzocaine at 0.1%, 0.5%, or 1%.
Duration of surgical-plane anesthesia.
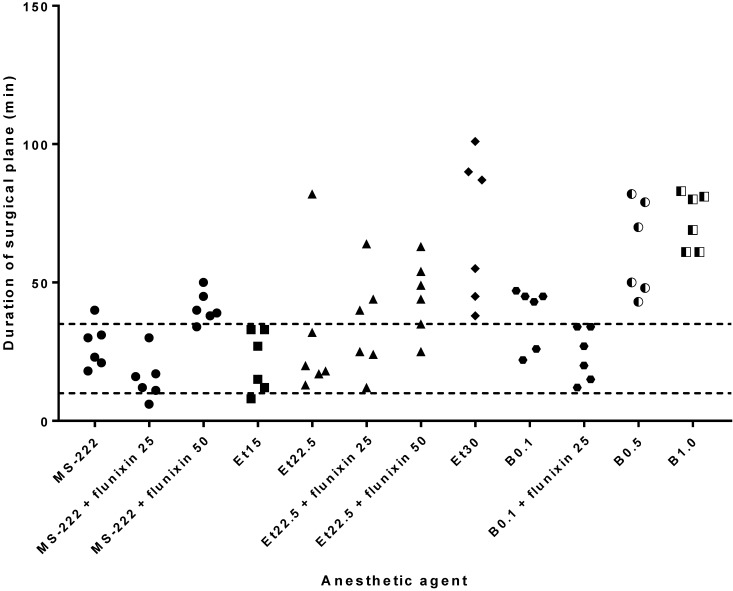
For MS222, the duration of surgical-plane anesthesia was 27.2 ± 8.1 min, with 95% confidence limits of 21.4 and 34.2 min. When the low dose (25 mg/kg) of flunixin meglumine was administered with MS222, the surgical-plane time was 15.3 ± 8.2 min, whereas at the high dose (50 mg/kg), it was 41.0 ± 5.7 min. Etomidate doses of 15, 22.5, and 30 mg/L without flunixin meglumine provided surgical-plane anesthesia for 21.3 ± 11.0, 30.3 ± 26.1, and 69.3 ± 26.5 min, respectively. With the addition of flunixin meglumine at 25 mg/kg to etomidate anesthesia at 22.5 mg/L, surgical-plane anesthesia lasted 34.8 ± 18.4 min, and with the addition of 50 mg/kg flunixin meglumine, the duration was 45.0 ± 13.6 min. Benzocaine at 0.1%, 0.5%, and 1% without flunixin meglumine provided surgical anesthesia for 38.0 ± 11.0, 63.0 ± 17.0, and 72.5 ± 10.2 min, respectively. With the addition of 25 mg/kg flunixin meglumine to 0.1% benzocaine anesthesia, surgical anesthesia was 23.7 ± 9.5 min. The average time for mock oocyte collection was approximately 10 min (Figure 2).
Figure 2.
Duration of surgical anesthesia (that is, righting and withdrawal reflexes remained absent), when an abdominal incision was created to perform a mock oocyte harvest. The upper line represents the upper 95% confidence limit for MS222 (34 min), whereas the lower line represents the 10-min minimum needed to perform a surgical oocyte harvest. Doses of fluixin meglumine (flunixin) are given in mg/kg; doses of etomidate (Et) are given in mg/L; doses of benzocaine (B) are given in percentage concentration; and the dose for MS222 was 2 g/L.
Comparing the duration of surgical-plane anesthesia from MS222 with other anesthetics in the absence of analgesia revealed statistically significant (P < 0.05) differences for etomidate at 30 mg/L and benzocaine at 0.5% and 1.0%. Adding flunixin meglumine after each anesthetic (MS222, etomidate 22.5 mg/L, and benzocaine 0.1%) did not significantly change the duration of surgical anesthesia for etomidate 22.5 mg/L but prolonged it in the groups that received MS222 or benzocaine (P = 0.0305 and P = 0.0361, respectively) compared with the groups anesthetized without analgesia.
Recovery.
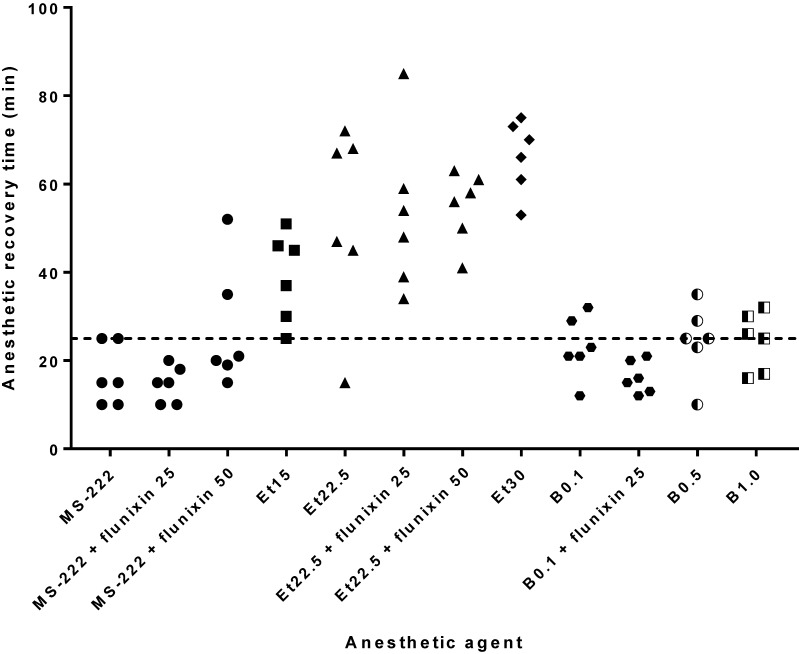
Recovery from surgical-plane anesthesia began when a score of 1 was obtained for either the righting or withdrawal reflex; complete recovery was achieved when frogs scored 2 for the withdrawal reflex and 3 for the righting reflex. The recovery time for frogs anesthetized with MS222 was 15.8 ± 16.8 min (95% confidence interval, 9.5 to 24 min). With the addition of flunixin meglumine, the duration of recovery at the low dose (25 mg/kg) was 14.7 ± 6.7 min and at the high dose (50 mg/kg) was 27.0 ± 14.0 min. Etomidate at 15, 22.5, and 30 mg/L doses produced recovery times of 39.0 ± 10.1, 52.3 ± 21.5, and 66.3 ± 8.2 min, respectively. The addition of flunixin meglumine at 25 mg/kg to etomidate at 22.5 mg/L gave a recovery time of 53.2 ± 18.1 min, and adding 50 mg/kg flunixin meglumine to the same concentration of etomidate yielded a recovery time of 54.8 ± 8.7 min. Benzocaine at 0.1%, 0.5%, and 1.0% had average recovery times of 23.0 ± 7.0, 24.5 ± 8.3, and 24.3 ± 6.6 min, respectively. Addition of flunixin meglumine at 25 mg/kg to benzocaine 0.1% anesthesia provided a recovery time of 16.2 ± 3.7 min (Figure 3).
Figure 3.
Recovery time. Surgical-plane anesthesia ended (and the recovery phase began) when the score for either the righting or withdrawal reflex was 1 or greater; recovery was complete when frogs scored 2 for the withdrawal reflex and 3 for the righting reflex. The dotted line represents the upper 95% confidence limit for MS222 (24 min). Doses of fluixin meglumine (flunixin) are given in mg/kg; doses of etomidate (Et) are given in mg/L; doses of benzocaine (B) are given in percentage concentration; and the dose for MS222 was 2 g/L.
When we compared the duration of recovery after MS222 in the absence of analgesia with those of other study groups, all doses of etomidate showed significantly (P < 0.05) prolonged recovery, whereas recovery time did not differ between MS222 and all doses of benzocaine without analgesia. The addition of flunixin meglumine to each anesthetic group (MS222, etomidate 22.5 mg/L, and benzocaine 0.1%) had no significant effect on recovery time compared with each respective anesthetic regimen without analgesia.
The creation of the abdominal incision had an unexpected adverse consequence during the postoperative period, when the frogs reacted negatively to daily handling to monitor body weight and the incision site. At approximately 1 wk after surgery, 8 frogs total (1 of the 8 from the MS222 group, 2 of the 8 given MS222 and 25 mg/kg flunixin meglumine, 2 of the 8 that received etomidate at 15 mg/L, 1 of the 8 in the 22.5-mg/L etomidate group, and 2 of the 8 from the 30-mg/L etomidate group) tore out their sutures when excessively kicking to avoid capture in their group-housing tank. These 8 frogs were euthanized, and the remaining frogs were housed individually until the incisions had healed (day 10 to 14). Sutures were not removed from the rest of the animals, because they shed them from the skin before the end of the study.
Flunixin meglumine administration.
The 25-mg/kg dose of flunixin meglumine was well tolerated and showed no adverse effects during initial recovery. However, 50 mg/kg caused unexpected death within 24 h of administration in 5 of the 12 frogs, despite an uneventful recovery when anesthetized with MS222 or 22.5 mg/L etomidate. Consequently, we did not administer the 50-mg/kg dose of flunixin meglumine to frogs anesthetized by using 0.1% benzocaine. Pathology from the affected frogs was inconclusive regarding a cause of death; one frog was diagnosed with lymphosarcoma, which was considered an incidental finding unrelated to experimental manipulation. Because of these complications, we excluded the data for the 50-mg/kg dose of flunixin meglumine from further analysis.
For AAT, a negative response after the application of acetic acid at 10% or greater was indicative of effective pain relief for the purposes of this experiment. One frog anesthetized with MS222 and given 25 mg/kg flunixin meglumine was euthanized due to complications of acid burns from the AAT despite no pain response to the maximal dose of acetic acid. Administration of flunixin meglumine was ineffective at providing pain relief when combined with MS222, but MS222 alone provided analgesic relief at the 6-h time point. At all of the time points assessed, the combination of 0.1% benzocaine and 25 mg/kg of flunixin meglumine provided effective pain relief. In addition, the combination of 22.5 mg/L etomidate and 25 mg/kg flunixin meglumine appeared to provide pain relief at the 24-h time point (Table 1).
Table 1.
Results of acetic acid testing in frogs (n = 6 per group)
| MS222 | MS222 + FM | Etomidate | Etomidate + FM | Benzocaine | Benzocaine + FM | |||
| 1 h | 1 | 2 | 0 | 0 | 0 | 4 | ||
| 3 h | 2 | 2 | 1 | 0 | 1 | 4 | ||
| 6 h | 4 | 2 | 0 | 1 | 0 | 4 | ||
| 24 h | 2 | 2 | 1 | 4 | 1 | 5 |
Frogs were anesthetized with either MS222, etomidate (22.5 mg/L), or benzocaine (0.1%) and subsequently did or did not receive flunixin meglumine (FM; 25 mg/kg) in the right dorsal lymph sac. Data are reported as the number of animals that did not react when ≥10% acetic acid was applied to the back legs at the indicated time points.
Histology.
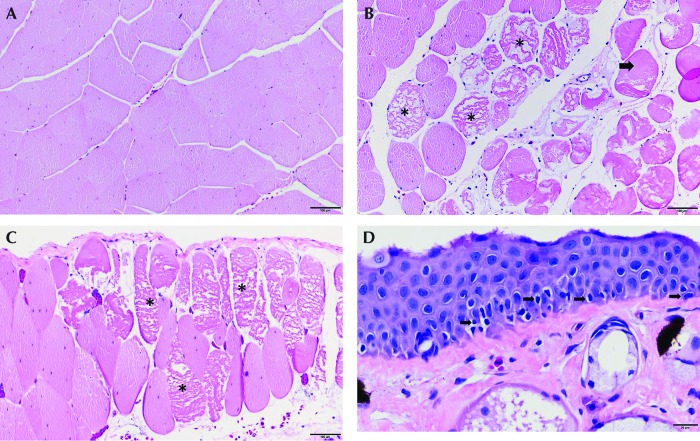
In all study groups, liver samples showed mild to moderate diffuse hepatocellular vacuolation, and kidneys had mild, multifocal tubular luminal mineral. The lungs of all specimens were unremarkable, independent of the anesthetic or analgesic dose. Control groups that did not receive flunixin meglumine had no lesions in the skin or skeletal muscle around the dorsal lymph sac. At each time point, the benzocaine group that received 25 mg/kg flunixin meglumin had myocyte degeneration and necrosis around the dorsal lymph sac (Table 2). In the frogs anesthetized with MS222 or etomidate, 25 mg/kg flunixin meglumine did not increase the incidence of myocyte degeneration and necrosis, except in the day-3 sample from the group that received etomidate 22.5 mg/L, which showed moderate degeneration and necrosis similar to those of the frog that received benzocaine 0.1% anesthesia at day 3. The myocytic degeneration and tissue necrosis appeared to be transient and resolved over time (Figure 4).
Table 2.
Histology of the dorsal lymph sac of frogs
| Flunixin meglumine (mg/kg) | Postsurgical day | MS222 (2 g/L) | Etomidate 22.5 mg/L | Benzocaine 0.1% |
| 0 mg/kg | 3 | NSL | NSL | NSL |
| 0 mg/kg | 14 | NSL | NSL | NSL |
| 0 mg/kg | 28 | NSL | NSL | NSL |
| 25 mg/kg | 3 | NSL | Moderate, locally extensive, monophasic myocyte degeneration and necrosis | Moderate, locally extensive myocyte degeneration and necrosis; mild, multifocal individual epithelial cell necrosis |
| 25 mg/kg | 14 | NSL | NSL | Minimal, chronic, locally extensive degeneration and regeneration |
| 25 mg/kg | 28 | NSL | NSL | Mild, subacute, locally extensive myocyte degeneration and regeneration |
NSL, no significant lesions
Figure 4.
Postoperative day 3. Skeletal muscle deep to the right dorsal lymph sac. (A) Normal myocytes adjacent the right dorsal lymph sac in a frog given MS222 and 25 mg/kg flunixin meglumine. (B) Degenerate and necrotic myocytes (*) in frog that received etomidate at 22.5 mg/L and 25 mg/kg flunixin meglumine. Myocytes are swollen and fragmented, with loss of cross striations. (C) Frog anesthetized with benzocaine at 0.1% and 25 mg/kg flunixin meglumine exhibits hypereosinophilic, degenerate (arrow) and necrotic, fragmented (*) myocytes. (D) Skin over the right dorsal lymph sac. Frog anesthetized with benzocaine 0.1% and 25 mg/kg flunixin meglumine. Basal keratinocytes in the skin over the dorsal lymph sac are necrotic with pyknotic nuclei (arrow). The basement membrane is intact. Hematoxylin and eosin staining; magnification, 10× (A–C); 40× (D).
Discussion
For the first portion of the experiment, the central question addressed was whether any of the alternative anesthetic regimens produced anesthesia similar to that of MS222 in Xenopus frogs. To do this, the length of induction, duration of surgical plane anesthesia, and time to recovery were assessed and compared with MS222 parameters for each anesthetic regimen.
Body weight influenced induction time, according to the results of the multivariable linear regression analysis. This factor is likely related to the absorption of the anesthetic as an effect of surface area. It is important to note that although specific differences between sexes were not considered, weight was a factor in the induction of anesthesia, and given that female frogs typically are larger than male, this sexually dimorphic characteristic could result in the prolonged induction time observed. Because surgical incisions are created in both male and female Xenopus, we evaluated both sexes to determine whether pain relief could be achieved, but due to small sample numbers, specific differences in pain relief between the sexes were not evaluated.
Rapid methods of induction generally are preferred over slower regimens, and we determined that 6 min was the maximal time necessary for MS222 to induce a surgical plane of anesthesia. To be considered as generating a similar anesthetic event as MS222, alternative anesthetics should achieve an average induction time of 6 min or less. All doses of etomidate took longer than 6 min to induce anesthesia in frogs, whereas all doses of benzocaine took less than 6 min; however, the average induction time for 30 mg/L etomidate did not differ significantly from MS222. Therefore, in terms of a rapid method of induction, benzocaine produces a statistically similar induction time as MS222.
Regarding the duration of surgical-plane anesthesia, the mean time for MS222 was 27.8 min, with a 95% confidence interval of 21.5 to 34.2 min. However, 10 min was required on average to complete the surgical oocyte harvest procedure, given the skill level and experience of the surgeon. Therefore, we chose 10 min as the minimal duration of surgical anesthesia necessary. All doses of benzocaine and etomidate—in the absence of flunixin meglumine—met this criterion. In addition, with increasing doses of etomidate and benzocaine, the average duration of surgical-plane anesthesia increased as well. However, when compared with the duration of surgical-plane anesthesia from MS222, after using 30 mg/L etomidate or 0.5% or 1% benzocaine, it was significantly longer and therefore potentially beneficial for procedures requiring an extended surgical time. Consequently, depending on the type of experiment and the duration of surgical-plane anesthesia required, various alternative anesthetic regimens could be used to achieve longer or shorter periods of surgical-plane anesthesia.
Extended recovery time is not usually desirable when anesthetizing animals, both for the health of the animal and for reasons of managerial efficiency. When calculating the length of recovery, the upper 95% confidence interval for frogs to recover from MS222 anesthesia was 24 min, which we used as the criterion for comparison. The average time for recovery from 22.5 mg/L etomidate was almost double the 24-min limit, whereas recovery after 0.1% benzocaine was shorter than the 24-min limit. All doses of etomidate produced significantly longer recovery periods than that from MS222, whereas recovery time after all benzocaine did not differ significantly from that for MS222. Therefore, when choosing an anesthetic according to recovery time, benzocaine is similar to MS222.
To determine if the alternative anesthetics tested produced an overall anesthetic event similar or superior to MS222, the anesthetic had to qualify as having all 3 parameters consisting of rapid induction time, duration of surgical plane anesthesia, and recovery time within their respective optimal ranges. Benzocaine 0.1% was the only anesthetic to not have any statistically significant differences within these parameters. Both of the other doses of benzocaine, 0.5% and 1%, produced a similar quick induction and recovery time, but the surgical plane time was significantly longer as compared with MS222, which could be considered for experiments requiring an extended duration of surgical plane anesthesia.
Etomidate at 15 and 22.5 mg/L had significantly longer induction times but similar durations of surgical-plane anesthesia compared with MS222, whereas 30 mg/L etomidate had a similar induction time to MS222 but a prolonged period of surgical plane anesthesia. At all 3 doses of etomidate, recovery time was significantly prolonged compared with MS222. In the context of experiments in which multiple frogs require oocyte harvesting within a short time period (for example, a single day), etomidate is not a preferable method of anesthesia, because its prolonged recovery period is undesirable.
We considered all of these parameters when determining the doses of etomidate and benzocaine to use in the second portion of the experiment. We chose 0.1% benzocaine because all anesthetic parameters did not differ significantly when compared with MS222. When determining the etomidate dose, we chose 22.5 mg/L because despite having a prolonged induction time compared with MS222, it was quicker than that for the 15 mg/L dose, and 30 mg/L etomidate produced a significantly longer duration of surgical-plane anesthesia than that for MS222.
We then wanted to determine whether postoperative NSAID administration provided prolonged anesthesia and whether the type of anesthesia interacted with the analgesic effects. We restricted the test to include only those agents with similar durations of surgical-plane anesthesia, induction, and recovery as MS222. The lower NSAID dose significantly decreased the duration of surgical-plane anesthesia due to MS222, whereas the higher dose prolonged it. However, the addition of flunixin meglumine at either dose did not significantly affect the average duration of surgical plane anesthesia while using etomidate. As noted with MS222, low-dose flunixin meglumine decreased the duration of surgical-plane anesthesia for frogs anesthetized with 0.1% benzocaine as compared with those that did not receive analgesia. The exact mechanism of this analgesia-associated alteration in the duration of surgical-plane anesthesia is unknown and warrants additional studies.
We were concerned that, due to synergistic effects between analgesia and anesthesia, recovery from anesthesia might be prolonged with analgesia or the frogs might not recover from anesthesia at all.13 All frogs recovered initially, regardless of the type of anesthesia or the dose of flunixin meglumine used.
The effect of flunixin meglamine is interesting in that it appeared to be affected by the type of anesthetic agent used. With MS222 alone, the only negative response during AAT in the absence of analgesia occurred at the 6-h time point, and the addition of low-dose (25 mg/kg) flunixin meglumine did not appear to provide appropriate pain relief. When etomidate was used for anesthesia, the analgesic effect of flunixin meglamine at the 25-mg/kg dose was delayed until the 24-h time point, likely because of residual effects of etomidate.7 However, with the use of benzocaine for anesthesia, the addition of flunixin meglumine at 25 mg/kg provided analgesia at all time points, but in only 4 of the 6 frogs at each of the 1-, 3-, and 6-h time points and in 5 of the 6 animals at the 24-h time point. These variable effects may be due to synergism (or lack thereof) with the anesthetic agent used, individual animal metabolism, or variability in absorption from the lymph sac. Given the observed variation in response to the administration of flunixin meglumine, we do not consider it an effective postoperative analgesic for African clawed frogs without further studies, such as pharmacokinetic analyses.
Because of unexpected mortality after the administration of 50 mg/kg flunixin meglumine, all data from the frogs that received that dose were excluded from further calculations. Although all of the frogs in these groups recovered from anesthesia uneventfully, 5 of the 12 were found dead in less than 24 h, and some in as little as 6 h. In addition, many of these frogs received the maximal concentration of acetic acid (50% solution) during AAT, and some of these animals showed no response to this dose, thus indicating that flunixin meglumine may have provided adequate pain relief. Therefore, toxicity due to the increased acetic acid levels or high flunixin meglumine concentration may have been factors contributing to the deaths of these frogs. However, pathology results from these frogs were inconclusive in regard to the exact causes of death after flunixin meglumine administration.
In consideration of the histologic findings, the administration of flunixin meglumine was relatively safe, with mild effects only at the site of administration and no adverse effects in kidney or liver samples from any study group. The hepatocellular vacuolation and tubular luminal material in kidney specimens were not clinically significant and are an incidental finding in African clawed frogs. In addition, myocyte degeneration and necrosis of tissues surrounding the dorsal lymph sac seemed more likely when the NSAID was administered to frogs that received 0.1% benzocaine compared with MS222 or etomidate. However, these lesions were minimal and resolved over time.
The 8 frogs that were removed from the study postoperatively due to self-induced trauma at the incision site resulted in individual housing of all other frogs until incision sites had healed (approximately 14 d postoperatively). When the frogs tore their incision sites, the ligatures remained intact, indicating that the lesions were not due to dehiscence but to excessive force exerted by the frogs. The change in housing and decreased handling effectively prevented the removal of additional frogs from the study.
Because AAT is commonly used in amphibians, especially frogs, we anticipated no skin irritation in our animals, providing the test was performed correctly; in testing the pain response, acetic acid should cause only minor annoyance to encourage the frog (if conscious) to wipe its leg. In this study, the high doses of acetic acid disrupted the mucous layer, such that skin irritation and lesions occurred in multiple frogs. An alternative testing method for pain response involves the use of the Hargreaves apparatus, which involves placing a hindleg on a plate that heats up to elicit a response to a noxious stimulus.19 This apparatus was unavailable for the current study.
In consideration of the 3Rs—reduction, replacement, and refinement—the creation of the incision during the determination of adequate anesthetic levels served as a control for the characterization of the effectiveness of flunixin meglumine in frogs. This strategy thus reduced the number of animals needed for the experiment.20
In conclusion, etomidate and benzocaine offer MS222-comparable alternative anesthetic regimens for Xenopus frogs, depending on the specific anesthetic criteria required for a given experiment. Benzocaine at 0.1% was the only anesthetic regimen that did not induce significant differences in induction time, duration of surgical-plane anesthesia, and recovery time when compared with MS222. However, when 25 mg/kg flunixin meglumine was added to the 0.1% benzocaine anesthetic regimen, myocyte degeneration and necrosis of tissues surrounding the dorsal lymph sac was increased as compared with the same analgesic dose combined with MS222. Low-dose flunixin meglumine (25 mg/kg) appears relatively safe in light of the lack of negative clinical signs and only minor histologic changes. However further studies examining additional doses between 25 and 50 mg/kg, the specific time points at which therapeutic levels are achieved, and testing at time points greater than 24 h are warranted to determine the long-term efficacy of flunixin meglumine as an effective analgesic in African clawed frogs. High doses of flunixin meglumine (50 mg/kg or greater) do not appear safe in Xenopus frogs, in light of the associated high fatality rates in our experiment.
Acknowledgments
We thank Drs Courtney Baetge, Vincent Gresham, and John Stallone for their support and guidance in completion of the project. We also thank Dr Michael Criscitiello for donation of the animals and the Small Animal Department of Clinical Sciences for helping to fund the project.
References
- 1.Bartlett HL, Escalera RB, 2nd, Patel SS, Wedemeyer EW, Volk KA, Lohr JL, Reinking BE. 2010. Echocardiographic assessment of cardiac morphology and function in Xenopus. Comp Med 60:107–113. [PMC free article] [PubMed] [Google Scholar]
- 2.Beattie MS, Bresnahan JC, Lopate G. 1990. Metamorphosis alters the response to spinal cord transection in Xenopus laevis frogs. J Neurobiol 21:1108–1122. [DOI] [PubMed] [Google Scholar]
- 3.Boston University. [Internet] 2015. Xenopus surgical oocyte harvest. [Cited 29 September 2017]. Available at: https://bu.edu/researchsupport/compliance/animal-care/getting-animals/xenopus/xenopus-surgical-oocyte-harvest/
- 4.Brennan C, Henderson LP. 1995. Androgen regulation of neuromuscular junction structure and function in a sexually dimorphic muscle of the frog Xenopus laevis. J Neurobiol 27:172–188. [DOI] [PubMed] [Google Scholar]
- 5.Cecala KK, Price SJ, Dorcas ME. 2007. A comparison of the effectiveness of recommended doses of MS222 (tricaine methanesulfonate) and Orajel (benzocaine) for amphibian anesthesia. Herpetol Rev 38:63–66. [Google Scholar]
- 6.Chatigny F, Kamunde C, Creighton CM, Stevens ED. 2017. Uses and doses of local anesthetics in fish, amphibians, and reptiles. J Am Assoc Lab Anim Sci 56:244–253. [PMC free article] [PubMed] [Google Scholar]
- 7.Coble DJ, Taylor DK, Mook DM. 2011. Analgesic effects of meloxicam, morphine sulfate, flunixin meglumine, and xylazine hydrochloride in African clawed frogs (Xenopus laevis). J Am Assoc Lab Anim Sci 50:355–360. [PMC free article] [PubMed] [Google Scholar]
- 8.d'Ovidio D, Spadavecchia C, Angeli G, Adami C. 2015. Etomidate anaesthesia by immersion in oriental fire-bellied toads (Bombina orientalis). Lab Anim 49:319–326. [DOI] [PubMed] [Google Scholar]
- 9.Daabiss M. 2011. American Society of Anaesthesiologists physical status classification. Indian J Anaesth 55:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Department of Justice Drug Enforcement Agency. [Internet] 2014. Schedules of controlled substances: placement of alfaxalone into schedule IV. 21 CFR Part 1308. [Cited 29 September 2017]. Available at: https://deadiversion.usdoj.gov/fed_reds/rules/2014/fr0227_2.htm
- 11.Doss GA, Nevarez JG, Fowlkes N, da Cunha AF. 2014. Evaluation of metomidate hydrochloride as an anesthetic in leopard frogs (Rana pipiens). J Zoo Wildl Med 45:53–59. [DOI] [PubMed] [Google Scholar]
- 12.Goulet F, Helie P, Vachon P. 2010. Eugenol anesthesia in African clawed frogs (Xenopus laevis) of different body weights. J Am Assoc Lab Anim Sci 49:460–463. [PMC free article] [PubMed] [Google Scholar]
- 13.Green SL. 2003. Postoperative analgesics in South African clawed frogs (Xenopus laevis) after surgical harvest of oocytes. Comp Med 53:244–247. [PubMed] [Google Scholar]
- 14.Green SL. 2009. Veterinary care, p 110-112. In: The laboratory Xenopus spp. Boca Raton (FL): CRC Press Taylor and Francis Group. [Google Scholar]
- 15.Guénette SA, Beaudry F, Vachon P. 2008. Anesthetic properties of propofol in African clawed frogs (Xenopus laevis). J Am Assoc Lab Anim Sci 47:35–38. [PMC free article] [PubMed] [Google Scholar]
- 16.Guénette SA, Helie P, Beaudry F, Vachon P. 2007. Eugenol for anesthesia of African clawed frogs (Xenopus laevis). Vet Anaesth Analg 34:164–170. [DOI] [PubMed] [Google Scholar]
- 17.Guo R, Liu G, Du M, Shi Y, Jiang P, Liu X, Liu L, Liu J, Xu Y. 2016. Early ketamine exposure results in cardiac enlargement and heart dysfunction in Xenopus embryos. BMC Anesthesiol 16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton PW, Henry JJ. 2014. Prolonged in vivo imaging of Xenopus laevis. Dev Dyn 243:1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. 1988. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88. [DOI] [PubMed] [Google Scholar]
- 20.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 21.Lalonde-Robert V, Beaudry F, Vachon P. 2012. Pharmacologic parameters of MS222 and physiologic changes in frogs (Xenopus laevis) after immersion at anesthetic doses. J Am Assoc Lab Anim Sci 51:464–468. [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell MA. 2009. Anesthetic considerations for amphibians. J Exotic Pet Med 18:40–49. [Google Scholar]
- 23.Mosley C. 2011. Pain and nociception in reptiles. Vet Clin North Am Exot Anim Pract 14:45–60. [DOI] [PubMed] [Google Scholar]
- 24.O'Rourke DP. 2007. Amphibians used in research and teaching. ILAR J 48:183–187. [DOI] [PubMed] [Google Scholar]
- 25.O'Rourke DP, Rosenbaum MD. 2015. Biology and diseases of amphibians, Chapter 18, p 931-965. In: Fox JG, Anderson LC, Otto G, Prichett-Corning KR, Whary MT.Laboratory animal medicine, 3rd ed San Diego (CA): Elsevier. [Google Scholar]
- 26.PHARMAQ. [Internet] 2010. MS 222 material safety data sheet. [Cited 29 September 2017]. Available at: https://www.pharmaq.no/sfiles/58/7/file/pharmaq-ms-222-eng-materialsafetydatasheet.pdf
- 27.Posner LP, Bailey KM, Richardson EY, Motsinger-Reif AA, Harms CA. 2013. Alfaxalone anesthesia in bullfrogs (Lithobates catesbeiana) by injection or immersion. J Zoo Wildl Med 44:965–971. [DOI] [PubMed] [Google Scholar]
- 28.Sanna E, Murgia A, Casula A, Biggio G. 1997. Differential subunit dependence of the actions of the general anesthetics alphaxalone and etomidate at γ-aminobutyric acid type A receptors expressed in Xenopus laevis oocytes. Mol Pharmacol 51:484–490. [PubMed] [Google Scholar]
- 29.Slater BJ, Liu KJ, Kwan MD, Quarto N, Longaker MT. 2009. Cranial osteogenesis and suture morphology in Xenopus laevis: a unique model system for studying craniofacial development. PLoS One 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith JM, Stump KC. 2000. Isoflurane anesthesia in the African clawed frog (Xenopus laevis). Contemp Top Lab Anim Sci 39:39–42. [PubMed] [Google Scholar]
- 31.Stevens CW. 1996. Relative analgesic potency of µ, δ and κ opioids after spinal administration in amphibians. J Pharmacol Exp Ther 276:440–448. [PubMed] [Google Scholar]
- 32.Stevens CW. 2004. Opioid research in amphibians: an alternative pain model yielding insights on the evolution of opioid receptors. Brain Res Brain Res Rev 46:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens CW. 2011. Analgesia in amphibians: preclinical studies and clinical applications. Vet Clin North Am Exot Anim Pract 14:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens CW, MacIver DN, Newman LC. 2001. Testing and comparison of nonopioid analgesics in amphibians. Contemp Top Lab Anim Sci 40:23–27. [PMC free article] [PubMed] [Google Scholar]
- 35.Torreilles SL, McClure DE, Green SL. 2009. Evaluation and refinement of euthanasia methods for Xenopus laevis. J Am Assoc Lab Anim Sci 48:512–516. [PMC free article] [PubMed] [Google Scholar]
- 36.United States Geological survey. [Internet] 2001. Anesthesia of amphibians in the field. Amphibian Research and Monitoring Initiative SOP no. 104. [Cited 29 September 2017]. Available at: https://mwhc.usgs.gov/publications/amphibian_research_procedures/field_amphibian_anesthesia.pdf
- 37.University of North Carolina. [Internet] 2014. Standard operating procedure for MS222. [Cited 29 September 2017]. Available at: https://ehs.unc.edu/files/2015/09/ms-222.pdf
- 38.University of Notre Dame. [Internet] 2016. Standard operating procedure for benzocaine immersion anesthesia for frogs. [Cited 29 September 2017]. Available at: http://freimann.nd.edu/ assets/238793/ap.x_benzocaine_anesthesia_sop16.pdf
- 39.Weber ES., 3rd 2011. Fish analgesia: pain, stress, fear aversion, or nociception? Vet Clin North Am Exot Anim Pract 14:21–32. [DOI] [PubMed] [Google Scholar]
- 40.West G, Heard D, Caulkett N. 2014. Zoo animal and wildlife immobilization and anesthesia, 2nd ed Ames (IA): Wiley-Blackwell. [Google Scholar]
- 41.Zornik E, Yamaguchi A. 2011. Vocal pathway degradation in gonadectomized Xenopus laevis adults. J Neurophysiol 105:601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]