Abstract
To determine the usefulness of urine urea (UU) as an index of dietary protein intake 10 postmenopausal women were enrolled and completed a randomized, double-blind, cross-over feeding trial, from September 2008 to May 2010, comparing ten days of a 45g whey supplement to ten days of a 45 g maltodextrin control. Urine nitrogen (UN), calcium (UCa), UU and bone turnover markers were measured at days 0, 7, and 10. Paired sample t tests, Pearson’s correlation statistic, and simple linear regression were used to assess differences between treatments, and associations among urinary metabolites. UN/urinary creatinine (UCreat) rose from 12.3 ± 1.7 g/g (99.6 ± 13.8 mmol/mmol) to 16.8 ± 2.2 g/g (135.5 ± 17.8 mmol/mmol) with whey supplementation but did not change with maltodextrin. Whey supplementation caused UCa to rise by 4.76 ± 1.84 mg (1.19 ± 0.46 mmol) without a change in bone turnover markers. Since our goal was to estimate protein intake from UN/UCreat, we used our data to develop the following equation: protein intake (g/d) = 71.221 + 1.719×(UN, g)/Creat, g) (R = 0.46, R2 = 0.21). As a more rapid and less costly alternative to UN/UCreat, we next determined if urinary urea (UU) could predict protein intake and found that protein intake (g/d) = 63.844 + 1.11×(UU, g/Creat, g) (R = 0.58, R2 = 0.34). These data indicate that UU/UCreat is at least as good a marker of dietary protein intake as is urinary nitrogen and easier to quantitate in nutrition intervention trials.
Keywords: Urinary nitrogen, Urinary urea, Whey Protein
Introduction
The effects of dietary protein on bone health are not well characterized. An ongoing 18-month, double-blind nutrition intervention trial (clinicaltrials.gov identifier: NCT00421408) will examine the effect of a 45 g whey protein isolate or maltodextrin control supplement on bone mineral density in older men and women who have low-normal protein intakes. Assessing compliance in long-term clinical trials can be challenging, but significantly affects study outcomes1, particularly in nutrition intervention trials. The increment in twenty-four hour urine urea is the pre-planned measure of adherence for study subjects in the above mentioned ongoing clinical trial that are randomized to the whey supplement. In an effort to precisely quantify the expected increment in urine urea, we conducted a short-term feeding study. Volunteers for this study met the same entry criteria as those recruited in our 18 month trial and were fed identical supplements using the same study protocol. Complete adherence was assured by feeding subjects all their meals, which were prepared in Yale New Haven Hospital Research Unit’s metabolic kitchen. The primary objective of this study was to determine how reproducibly urinary urea (UU) and urinary nitrogen (UN) change with a fixed increment in dietary protein and how well they correlated with each other. A secondary objective was to evaluate the effect of the whey protein supplement on novel and known markers of bone turnover. Using bone turnover markers to assess elevated bone remodeling in response to a wide variety of therapies is of increasing interest, as this methodology allows for rapid, noninvasive evaluation of skeletal homeostasis2.
Methods
Subjects and Design
We conducted a randomized, placebo-controlled, double-blind, cross-over study consisting of two, 10-day dietary interventions of a moderate protein diet to which was added either a whey protein isolate or maltodextrin control. During both 10-d experimental periods, subjects received all food from the Yale New Haven Hospital Research Unit’s metabolic kitchen. These diets contained controlled levels of calcium, sodium and phosphorus. After the first 10-day experimental diet, subjects followed a 2 week washout period prior to participating in the second intervention. As previously noted, the whey protein and maltodextrin supplements are identical to those used in the larger clinical trial.
Three timed 24-hour urines were obtained between days 0–1, 6–7 and 9–10 of each experimental period for measurement of creatinine (Creat), nitrogen (N), calcium (Ca), and urea (U). Fasting blood and urine samples were collected on days 1, 7 and 10 of each experimental period to measure serum parathyroid hormone (PTH), insulin-like growth factor 1 (IGF-1), osteocalcin and amino-terminal propeptide of type I collagen (P1NP) and urine creatinine (UCreat) and urinary markers of bone turnover.
Ten healthy women (average age 64.2 ± 7 y) who were at least five years postmenopausal volunteered for this study. Exclusion criteria included known skeletal or active inflammatory bowel diseases, untreated hyperparathyroidism, diabetes, history of renal disease or kidney stones, and chronic liver disease. Additional exclusion criteria included any cancer within the past 18 months or long-term use of chemotherapeutic drugs, aromatase inhibitors or tamoxifen, current use of methotrexate, phenytoin, phenobarbital, inhaled corticosteroids (greater than 800 ug/day), active treatment for leukemia or multiple myeloma, a change in thyroid medications, use of herbal supplements with estrogenic activity, medications known to affect calcium metabolism, and the use of proton pump inhibitors twice daily. Women with dietary protein intakes less than 0.6 g/kg or greater than 1.0 g/kg, or a BMI over 32 or under 20 were also excluded. The study was approved by Investigational Review Boards at Yale University (New Haven, CT) and the University of Connecticut (Storrs, CT). All participants gave their written informed consent.
Diets
Subjects completed a 4-day food record prior to starting the study to assess their usual nutrient intake which was used to guide the design of the experimental diets. The experimental diets contained between 0.6 g protein/kg and 1 g protein/kg, and matched the subject’s usual intake of protein. The protein sources used for the experimental diets were a typical mix of animal and vegetable sources. All subjects received a multivitamin daily (One-A-Day 50 plus, Bayer Nutritionals, Morristown, NJ). Dietary calcium was kept in a range of 1200 – 1500 mg (30 – 37.5 mmol) through diet alone or when necessary, the addition of a calcium supplement (Tums ® GlaxoSmithKline, Pittsburg, PA). Other nutrients known to affect calcium metabolism (sodium and phosphorus) and energy were matched to each subject’s usual intake. Forty- five grams of whey protein, Proven 290, (Glanbia Nutritionals Inc, Twin Falls, ID) or maltodextrin, Maltrin® M100, powder (Grain Processing Corporation, Muscatine, IA) was added directly to the subject’s foods/beverages each day throughout the experimental diet. Adding 45 g of whey protein isolate to the experimental diet provided subjects with an additional 40 g of protein. The whey protein isolate and maltodextrin (placebo) supplements were formulated to achieve equivalent caloric density (160 kcal/45g) as well as equivalent content of sodium (73–81mg, 3.2–3.5 mmol), potassium (182 mg, 4.7 mmol), phosphorus (112 mg, 3.6 mmol), and calcium (236–239 mg, 5.9–6.0 mmol) expressed as per 45g powder. Distilled water was provided ad lib. Subjects were also allowed to consume 1 serving of wine or beer/d.
Biochemical Assessment and Assays
Twenty-four hour UN was determined using a micro-Kjeldahl apparatus (Tecator Kjeltec System, Hoganus, Sweden). N balance was calculated using the following equation: N Balance (g) = N Input (g) − N Output (g) + 2 g (an estimation of fecal, dermal, and miscellaneous N losses). UU was analyzed using QuantiChrom™ Urea Assay (BioAssay Systems, Hayward, CA) at an optical density of 515nm. Urinary calcium (UCa) and UCreat were measured on an AlfaWasserman ACE® analyzer (Alfa Wassermann Diagnostic Technologies, LLC, West Caldwell, NJ). Serum intact P1NP was measured using a competitive RIA (Uniq™ P1NP RIA Orion Diagnostica Oy, Espoo, Finland). Serum IGF-1 and urinary alpha and beta urine crosslaps (α-CTX and β-CTX) were measured using commercially available ELISAs (Immunodiagnostic Systems Inc., Scottsdale, AZ). Osteocalcin was measured in the laboratory of Dr. Caren Gunberg (Department of Orthopaedics, Yale School of Medicine) using a double antibody RIA3.
To control for variability in the completeness of 24-h and 2-h urine collections, urinary excretion N, Ca, U and α-CTX and β-CTX were expressed as a ratio with Creat excretion.
Data Analysis
Analyses were performed using SPSS (version 12.0 for Windows, 2003, SPSS Inc., Chicago, IL). Graphical summaries were generated using Prism (version 4.0, GraphPad Software, La Jolla, CA). The baseline and intervention data are presented as mean ± SEM. An initial paired t-test showed no significant difference in any metabolites between day 7 and 10 (with the exception for IGF-1). Thus, data from day 7 and 10 were averaged and these mean data used when analyzing the effects of the two dietary interventions. Paired sample t tests, Pearson’s correlation statistic, and simple linear regression were used to assess differences between treatments, and associations among urinary metabolites. A probability level of P < 0.05 was statistically significant and 0.05–0.10 was suggestive of a trend.
Results and Discussion
Participants
Ten subjects completed and tolerated the study without difficulty. Two subjects did not complete the study, one due to distaste for study food and one because of newly diagnosed type 2 diabetes. Body weight (71.3 ± 8.4 kg) remained constant in all subjects throughout the study. The average nutrient content of the experimental diets (exclusive of the study supplements) were as follows: protein: 80.4 ± 19.4 g, calcium: 1156 ± 124 mg (28.9 ± 3.1 mmol), phosphorus: 1206 ± 240 mg (38 ± 8 mmol), sodium: 2528 ± 628 mg (110 ± 27 mmol), magnesium: 281 ± 48 mg (12 ± 2 mmol), potassium: 2800 ± 632 mg (72 ± 16 mmol) and fiber: 19.6 ± 5.5 g. Within each subject there were no significant differences in the nutrient content of the experimental food sources between the two interventions. The small sample size, which can be considered a study limitation, allowed the feasibility of the tightly controlled dietary intervention, a primary strength of the study.
Urinary Markers of Protein Intake
Baseline and intervention measures of urine metabolites, markers of bone turnover and N balance are presented in the Table. As expected baseline urinary metabolites did not differ between interventions. UN/Creat rose with whey supplementation in comparison to baseline (from 12.3 ± 1.7 g/g (99.6 ± 13.8 mmol/mmol) to 16.8 ± 2.2 g/g (135.5 ± 17.8 mmol/mmol), P = 0.004), but did not change with maltodextrin. A similar trend was observed for UU/Creat excretion during the protein intervention (from 24.9 ± 3.5 g/g (100.73 ± 14.09 mmol/mmol) to 34.0 ± 4.0 g/g (137.2 ± 16.1 mmol/mmol), P = 0 .069). UN/Creat (16.8 ± 2.2 g/g (135.5 ± 17.8 mmol/mmol)) and UU/Creat (34.0 ± 4.0 g/g (137.2 ± 16.1 mmol/mmol)) were higher during the protein supplemented diet compared to the maltodextrin control (10.6 ± 1.1g/g (85.5 ± 9.1 mmol/mmol), P = 0.008, 21.0 ± 2.2 g/g (84.8 ± 8.9 mmol/mmol), P = 0 .007, respectively). Our finding that 24-hr UN increased with increased dietary protein is consistent with several previously published reports4–8. As noted, we measured 24-hr UU to determine if it could serve as a convenient measure of adherence in our larger intervention. In women UN faithfully tracks with UU, with UU representing a constant fraction of UN except at very low levels of protein intake9. The relationship between total N and urea nitrogen has also been examined9–11. For individuals consuming greater than adequate amounts of dietary protein, 82 ± 2% of total UN is in the form of urea9. However, if individuals consume a lower protein diet, the other N sources, such as Creat, contribute relatively more N, and urea may not accurately depict total UN to the same extent11. Currently, urea is regularly measured by clinicians because it is simpler and faster than the Kjeldahl method of measuring total UN11.
Table.
Baseline and intervention measures of urine and serum markers of bone turnover and protein status of 10 postmenopausal women participating in a cross-over study consisting of two 10-day interventions where dietary protein was manipulated
| Control | Protein | |||
|---|---|---|---|---|
|
|
|
|||
| Baseline | Intervention | Baseline | Intervention | |
| Urine ratios |
|
|||
| 24-h UNa/urinary creatinine (g/g) | 11.7±2.4 | 10.6±1.1 | 12.3±1.7 | 16.8±2.2bc |
| 24-h UCad/urinary creatinine (mg/mg) | 0.21±0.04 | 0.22±0.05 | 0.29±0.06 | 0.35±0.06c |
| 24-h UUe/urinary creatinine (g/g) | 26.8±6.1 | 21.0±2.2 | 24.9±3.5 | 34.0±4.0c |
| 2-h α-CTX/urinary creatinine (µg/mmol) | 0.69±0.12 | 0.64±0.09 | 0.7±0.11 | 0.77±0.11 |
| 2-h β-CTX/urinary creatinine (µg/mmol) | 2.61±0.70 | 2.27±0.41 | 2.34±0.53 | 2.59±0.48 |
| Serum | ||||
| Intact parathyroid hormone (pg/mL) | 39.3±4.5 | 38.7±3.3 | 41.8±3.2 | 38.3±3.2 |
| Propeptide of type I collagen (ng/mL) | 55.9±4.5 | 55.9±5.2 | 56.7±4.3 | 54.9±4.0 |
| Insulin-like growth factor 1 (ng/mL) | 86.34±7.40 | 86.41±7.56 | 85.04±7.71 | 89.69±7.94 |
| Osteocalcin (ng/mL) | 7.4±0.9 | 7.7±1.0 | 7.4±0.8 | 7.3±0.8 |
| Nitrogen balance (g) | 4.1±1.4 | 2.3±1.1 | 3.6±1.4 | 6.4±1.8cf |
UN=urinary nitrogen.
Significantly different from protein baseline; P<0.01.
Significantly different from control intervention; P<0.05.
UCa=urinary calcium.
UU=urinary urea.
Significantly different from protein baseline; P<0.05.
In our study, we observed a statically significant positive relationship between UN and UU (P < 0.0001, r = 0.73). To determine if UN and/or UU could be used as indices of dietary protein intake, we pooled baseline and intervention data to develop the following equations relating protein intake to these two urine parameters. First, using UN, we developed the following equation: protein intake (g/d) = 71.221 + 1.719 × (UN, g)/Creat, g) (R = 0.46, R2 = 0.21). For UU, the formula was: protein intake (g/d) = 63.844 + 1.11 × (UU, g/Creat, g) (R = 0.58, R2 = 0.34). As can be seen from these two equations UU proved to correlate at least as well if not better than UN with dietary protein. Measuring UN is cumbersome, time consuming and requires considerable operator experience to ensure reasonable interassy coefficients of variation. In contrast measuring UU is rapid and simple and uses a highly reliable ELISA methodology. Because our larger clinical trial employs dietary protein intakes that are considered moderate, along with the ease and greater precision of UU measurement, our findings support the conclusion that UU is a reliable index for protein intakes in this range. Consequently, UU is an appropriate measure of compliance for the ongoing larger clinical trial.
Dietary Assessment Methods
A number of current dietary assessment techniques rely on various methods of dietary recall, tracking and recording which depend heavily on an individual’s memory and level of motivation12, 13. Commonly used assessment methods tend to require prior knowledge of nutrition and portion sizes, literacy and/or time, thus burdening the patient or subject and decreasing the likelihood of obtaining reliable dietary intake data13–15. Our method relies on a quantifiable bioindex of dietary protein intake thus circumventing limitations seen with a number of current more subjective dietary assessment tools12–15.
Urinary Calcium and Dietary Protein
As summarized in the Table, UCa/Creat tended to increase during the protein intervention (0.35 ± 0.06 mg/mg (1.00 ± 0.18 mmol/mmol)) compared to protein baseline (0.29 ± 0.06 mg/mg (0.83 ± 0.18 mmol/mmol), P = 0 .064). There were also strong, significant, positive correlations between UCa/Creat and UN/Creat (P < 0.0001, r = 0.66) and UCa/Creat and UU/Creat (P < 0.0001, r = 0.58; Figure). These findings are consistent with the well-established observation that increasing dietary protein results in an increase in UCa. A review of over 20 clinical trials found a strong positive linear association between protein intake and UCa16. On average, for every 50 g increase in dietary protein, there is approximately a 6.4 mg (1.6 mmol) increase in 24-h UCa16. In this current study, a modest increase of only 40 g of protein resulted in a 4.76 ± 1.84 mg (1.19 ± 0.46 mmol) average increase in UCa. The magnitude of change in UCa between the two studies is remarkably close, particularly given the fact that the Kerstetter review included many studies of varying lengths, interventions and subjects. These findings underscore the fact that dietary protein has an important and measurable impact on UCa17, 18. Consistent with our previous work, there were no short-term changes in bone turnover with protein supplementation (Table), indicating that the increase in UCa we observed, is more than likely primarily due to improved intestinal Ca efficiency19, 20.
Figure 1.
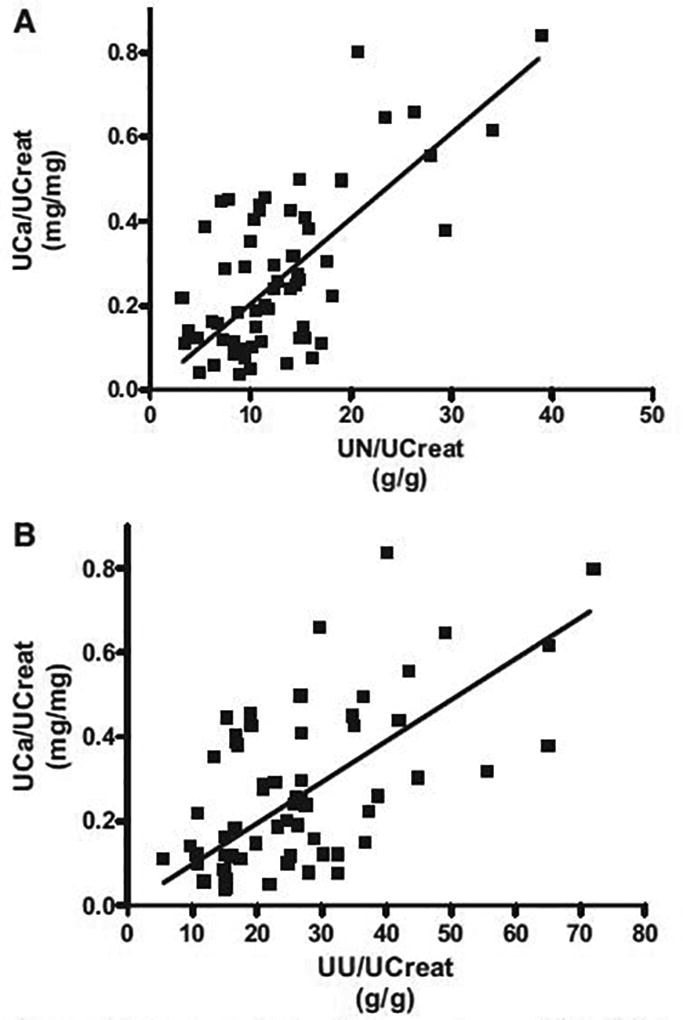
Significant correlation between urinary calcium (UCa) and (A) urinary nitrogen (UN) (P<0.0001; r=0.66) and (B) urinary urea (UU) (P<0.0001, r=0.58) from 10 postmenopausal women participating in a cross-over study consisting of two 10-day interventions where dietary protein was manipulated (pooled data).
Conclusion
In summary urinary urea holds promise as a more convenient and equally reliable index of dietary protein consumption as compared to urinary nitrogen. Larger and longer clinical trials should provide more robust estimates of this relationship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jessica D. Bihuniak, The University of Connecticut, Department of Nutritional Sciences, Roy E. Jones Building, Unit 4017, Storrs, Connecticut, USA. ph: 203-737-1656, fax: 203-785-6462..
Christine A. Simpson, Yale University, Department of Internal Medicine, Section of Endocrinology, P.O. Box 208020, New Haven, Connecticut, USA. ph: 203-737-1656, fax: 203-785-6462..
Rebecca R. Sullivan, Yale University, Department of Internal Medicine, Section of Endocrinology, P.O. Box 208020, New Haven, Connecticut, USA. ph: 203-737-1656, fax: 203-785-6462..
Donna M. Caseria, The Yale Center for Clinical Investigation and Food and Nutritional Services, Yale University School of Medicine and Yale New Haven Hospital, 2 Church St. South, New Haven, CT, USA. ph: 203-688-4731, fax: 203-737-2480..
Jane E. Kerstetter, The University of Connecticut, Department of Allied Health Sciences, 358 Mansfield Road, Unit 1101, Storrs, Connecticut, USA. ph: 860-486-1996, fax: 860-486-5375..
Karl L. Insogna, Yale University, Department of Internal Medicine, Section of Endocrinology, P.O. Box 208020, New Haven, Connecticut, USA. ph: 203-737-1656, fax: 203-785-6462..
References
- 1.Pullar T, Kumar S, Feely M. Compliance in clinical trials. Ann Rheum Dis. 1989;48(10):871–5. doi: 10.1136/ard.48.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnick SL, Shulman L. Monitoring osteoporosis therapy: bone mineral density, bone turnover markers, or both? Am J Med. 2006;119((4)(Suppl 1)):S25–31. doi: 10.1016/j.amjmed.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Gundberg CM, Looker AC, Nieman SD, Calvo MS. Patterns of osteocalcin and bone specific alkaline phosphatase by age, gender, and race or ethnicity. Bone. 2002;31(6):703–8. doi: 10.1016/s8756-3282(02)00902-x. [DOI] [PubMed] [Google Scholar]
- 4.Kerstetter JE, Caseria DM, Mitnick ME, et al. Increased circulating concentrations of parathyroid hormone in healthy, young women consuming a protein-restricted diet. Am J Clin Nutr. 1997;66(5):1188–96. doi: 10.1093/ajcn/66.5.1188. [DOI] [PubMed] [Google Scholar]
- 5.Bingham SA, Cummings JH. Urine nitrogen as an independent validatory measure of dietary intake: a study of nitrogen balance in individuals consuming their normal diet. Am J Clin Nutr. 1985;42(6):1276–89. doi: 10.1093/ajcn/42.6.1276. [DOI] [PubMed] [Google Scholar]
- 6.Campbell WW, Crim MC, Dallal GE, Young VR, Evans WJ. Increased protein requirements in elderly people: new data and retrospective reassessments. Am J Clin Nutr. 1994;60(4):501–9. doi: 10.1093/ajcn/60.4.501. [DOI] [PubMed] [Google Scholar]
- 7.Campbell WW, Fleet JC, Hall RT, Carnell NS. Short-term low-protein intake does not increase serum parathyroid hormone concentration in humans. J Nutr. 2004;134(8):1900–4. doi: 10.1093/jn/134.8.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uauy R, Scrimshaw NS, Young VR. Human protein requirements: nitrogen balance response to graded levels of egg protein in elderly men and women. Am J Clin Nutr. 1978;31(5):779–85. doi: 10.1093/ajcn/31.5.779. [DOI] [PubMed] [Google Scholar]
- 9.Bingham SA, Williams R, Cole TJ, Price CP, Cummings JH. Reference values for analytes of 24-h urine collections known to be complete. Ann Clin Biochem. 1988;25(Pt 6):610–9. doi: 10.1177/000456328802500603. [DOI] [PubMed] [Google Scholar]
- 10.Consolazio CF, Nelson RA, Matoush LO, Harding RS, Canham JE. Nitrogen excretion in sweat and its relation to nitrogen balance requirements. J Nutr. 1963;79:399–406. doi: 10.1093/jn/79.4.399. [DOI] [PubMed] [Google Scholar]
- 11.Bingham SA. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr. 2003;133(Suppl 3):921S–4S. doi: 10.1093/jn/133.3.921S. [DOI] [PubMed] [Google Scholar]
- 12.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient non-compliance with paper diaries. Bmj. 2002;324(7347):1193–4. doi: 10.1136/bmj.324.7347.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson FE, Byers T. Dietary assessment resource manual. J Nutr. 1994;124(suppl 11):S2245–S317. doi: 10.1093/jn/124.suppl_11.2245s. [DOI] [PubMed] [Google Scholar]
- 14.Burke LE, Warziski M, Starrett T, et al. Self-monitoring dietary intake: current and future practices. J Ren Nutr. 2005;15(3):281–90. doi: 10.1016/j.jrn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van't Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr. 2009;101(Suppl 2):S73–85. doi: 10.1017/S0007114509990602. [DOI] [PubMed] [Google Scholar]
- 16.Kerstetter JE, O'Brien KO, Insogna KL. Low protein intake: the impact on calcium and bone homeostasis in humans. J Nutr. 2003;133(3):S855–S61. doi: 10.1093/jn/133.3.855S. [DOI] [PubMed] [Google Scholar]
- 17.Hegsted M, Schuette SA, Zemel MB, Linkswiler HM. Urinary calcium and calcium balance in young men as affected by level of protein and phosphorus intake. J Nutr. 1981;111(3):553–62. doi: 10.1093/jn/111.3.553. [DOI] [PubMed] [Google Scholar]
- 18.Zemel MB. Calcium utilization: effect of varying level and source of dietary protein. Am J Clin Nutr. 1988;48(suppl 3):S880–S3. doi: 10.1093/ajcn/48.3.880. [DOI] [PubMed] [Google Scholar]
- 19.Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab. 2005;90(1):26–31. doi: 10.1210/jc.2004-0179. [DOI] [PubMed] [Google Scholar]
- 20.Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein affects intestinal calcium absorption. Am J Clin Nutr. 1998;68(4):859–65. doi: 10.1093/ajcn/68.4.859. [DOI] [PubMed] [Google Scholar]


