Abstract
Background and Objectives
Many US children use complementary health approaches (CHA), including some modalities that may be ineffective, unsafe, and/or costly. Yet little is known about the prevalence and correlates of CHA use among children with developmental disabilities (DD), as well as parent nondisclosure of CHA used for children with DD to healthcare providers. We, therefore, aimed to profile the use and nondisclosure of CHA among US children with DD.
Methods
We analyzed data from the 2012 National Health Interview Survey, which included the most recent Child Complementary and Alternative Medicine Supplement. The study sample was comprised of 2141 children with DD aged 4–17 years.
Results
Nearly one-quarter (23%) of US children with DD used CHA. Among those with a personal health provider, 42% of parents did not disclose some or all CHA used to the child’s provider. The adjusted odds of using CHA were greater among those with female gender, higher household income, residences not in the South, difficulty accessing care, or comorbid conditions. CHA was most commonly used because “it is natural.” Nondisclosure was associated with female gender, older age, no functional limitations, less conventional services use, or use of fewer CHA. The most common reason for nondisclosure was that the child’s provider did not ask.
Conclusions
CHA use is prevalent among US children with DD, and nondisclosure is likely among those who use CHA. Future intervention targeting education and communication about CHA for parents of children with DD and their healthcare providers may promote disclosure.
INTRODUCTION
Developmental disabilities (DD), such as attention deficit/hyperactivity disorder (ADHD) autism spectrum disorder (ASD), and cerebral palsy (CP), affect an estimated 1 in 6 children nationwide.1 DD include a group of chronic conditions characterized by cognitive, behavioral, and/or physical impairments.2 DD lack curative treatments,2 have adverse health impacts for children and their families,3 and incur substantial costs for families and the health system.4,5
Evidence-based treatments to manage DD symptoms exist and are widely recommended (e.g., early intensive behavioral intervention for ASD).6 These treatments may, however, vary in efficacy, take time to show noticeable positive effects, or may not alleviate all concerning associated symptoms (e.g., gastrointestinal problems for children with ASD). Together with other factors, these circumstances may influence a family’s decision to use complementary health approaches (CHA) for their child with DD.
CHA (also referred to as complementary and alternative medicine) encompass a wide array of modalities developed outside of or parallel to mainstream medicine.7 The National Center for Complementary and Integrative Health currently categorizes CHA as natural products (e.g., specific vitamins, herbal supplements), mind and body practices (e.g., qigong, yoga), and other approaches (e.g., special diets, homeopathy).7 CHA are typically used with conventional health services.7 At present, limited evidence exists regarding the efficacy and/or safety for many CHA used to treat DD symptoms in children.8–13
The types of CHA most commonly used has been previously assessed among certain condition-based subgroups of children with DD. For example, children with ASD are commonly reported to use special diets (e.g., gluten- and/or casein-free),14–18 certain natural products (e.g., specific vitamins/minerals, non-vitamin supplements such as probiotics), and/or mind and body practices (e.g., chiropractic or osteopathic manipulation).14–18 Children with ADHD are reported to frequently use mind and body practices (e.g., chiropractic or osteopathic manipulation, massage therapy), special diets, and/or some natural products (e.g., specific vitamins/minerals, non-vitamin supplements).19,20 Similarly, children with CP are most commonly reported to use certain mind and body practices (e.g., massage therapy, aquatherapy, hippotherapy).21,22 Within these subgroups, a minority of children are reported to use unsafe and/or inefficacious CHA, such as chelation or hyperbaric oxygen therapy, in relationship to their DD symptoms. Still, little is known about the types of CHA most commonly used by children with DD overall.
Past research also suggests that the prevalence of CHA use is higher among specific DD subgroups (e.g., ASD, ADHD) than it is among other children.14,17–20,23–25 That is, the use of CHA in the larger US child population was estimated to be 12.0% in 2007 and 11.6% in 2012.26 Recent studies using large databases estimate 17.3 percent23 to 28 percent14 of children with ASD use CHA and approximately 25 percent of US youth (aged 7–17 years) with ADHD use CHA.20 We are, however, unaware of previous studies that have estimated the prevalence of CHA use among US children with DD more broadly.
Greater knowledge of what factors are related to CHA use among children with DD is additionally needed. Prior research has identified some correlates of CHA use (e.g., age, symptomatology, parent education), again though, only among certain condition-based subgroups of children with DD.14,17,18,20,21,23,27 For instance, younger versus older children with ASD or CP have been found to be more likely to use CHA.18,21 Across these three DD subgroups (i.e., ADHD, ASD, CP) severity of symptoms or comorbidity have also been associated with the use of CHA.14,17,20,21 Higher parent education versus lower parent education has additionally been associated with CHA use in studies of children with ADHD, ASD, or CP.17,18,20,21,27 Some research also suggests that the use of certain conventional health services, particularly prescription medication, may be associated with the use of CHA among children with ASD or ADHD.17,18,20,27 Yet the extent to which a more comprehensive set of factors are associated with the use of CHA among US children with DD has not been determined.
Because CHA are most commonly used along with conventional health services for children, pediatric healthcare providers are recommended to routinely communicate with families about and monitor CHA used for children.28,29 A critical piece of this communication is a parent’s disclosure of CHA used for their child to the child’s healthcare provider(s). Limited research has, however, examined how often parent disclosure of CHA used for children occurs and what factors are related to disclosure.
Findings from several studies suggest that nondisclosure of CHA used in pediatric populations, including children with ADHD or asthma, occurs frequently.19,30,31 Younger child age (< six years old),30 use of mind and body practices,30 parent non-CHA use,30 or high parent-provider relationship quality31 have been shown to be associated with parent disclosure of CHA used for children. The most frequently reported reason for nondisclosure in one study of children with asthma was that the parent did not think the child’s provider needed to know.31 Disclosure of CHA used for children with ASD has also been explored in two recent qualitative studies. One of these studies identified barriers and facilitators to disclosure of CHA used for children with ASD from parents’ perspectives32 and the other study described the use of CHA as an influence on shared decision-making from the perspectives of parents of children with ASD and primary care pediatricians.33 Findings from these qualitative studies suggest that disclosure and subsequent discussion of CHA used for children with ASD can be challenging due to a number of factors such as provider attitudes and/or knowledge regarding CHA, as well as parent-provider relationship quality. Taken together, past research provides some indication of the frequency with which parent nondisclosure of CHA used occurs in pediatric healthcare settings; however, further research is needed to better understand parent nondisclosure of CHA used for children including those with DD.
Knowledge regarding the use and nondisclosure of CHA among children with DD is currently limited. Greater understanding regarding the prevalence and correlates of CHA use and nondisclosure for children with DD is needed to improve healthcare delivery and quality, specifically in terms of how information about CHA is transferred between families of children with DD and healthcare providers. Increased knowledge of CHA use and nondisclosure among children with DD may also inform the development of future studies about pediatric health services utilization and outcomes, as well as intervention studies intended to promote family and provider communication about use of CHA and shared decision making more broadly. For these reasons, this study aimed to determine the prevalence and correlates of CHA use and nondisclosure among US children with DD. This study is novel because it applied the Complementary and Alternative Medicine (CAM) Healthcare Model to comprehensively examine both the use and nondisclosure of CHA among a large and nationally-representative sample of US children with DD, using one of the most extensive data sources presently available on the use and nondisclosure of CHA in the United States.
METHODS
Study Design and Data Source
We analyzed parent-reported data from the 2012 National Health Interview Survey (NHIS). The 2012 NHIS is the most recent NHIS to include the Child Complementary and Alternative Medicine (CAM) Supplement Questionnaire. The supplement, sponsored by the National Center for Complementary and Integrative Health, was intended to provide nationally representative information on CAM use among children aged 4–17 years.34 The final response rate was 69.7%.35 The Institutional Review Board at the affiliated university determined that this study did not require review.
Sample
Of children aged 4–17 years (n = 10,185) for whom the 2012 NHIS Child CAM Supplement Questionnaire was completed, 2141 were determined to have one or more DD. To determine DD status, we used the Centers for Disease Control and Prevention’s current DD definition2 and relevant past research.1,3 Specifically, DD status was based on affirmative responses by the child’s parent to a series of questions in the 2012 NHIS Sample Child Core asking if the child had ADHD, ASD, cerebral palsy, Down syndrome, intellectual disability, seizures, stammering or stuttering, moderate to profound hearing loss, visual impairment including blindness, learning disorders, or other developmental delays.
Predisposing, Enabling, and Healthcare Need Factors
We used the CAM Healthcare Model,36 a modification to the Behavioral Model of Health Services Utilization (the Behavioral Model),37 as the theoretical basis for determining factors potentially correlated with the use and nondisclosure of CHA. The CAM Healthcare model includes factors adapted from the Behavioral Model that are theorized to influence the use of CHA with or without conventional health services, and in turn, healthcare outcomes (e.g., quality of life). Factors potentially influencing the use of CHA more specifically include those that predispose an individual to use CHA (e.g., gender, age, education), enable an individual to use CHA (e.g., income, health insurance coverage), or influence their need for CHA (e.g., condition symptoms, perceived health status).
To operationalize predisposing, enabling, and healthcare need factors for this study, we used these definitions to identify relevant variables available in the data source. Predisposing factors included child age, gender, race and ethnicity, parent education, and family structure. Enabling factors included household income relative to the federal poverty level (FPL), health insurance coverage, household employment, and census region. Healthcare need factors were any problem or delay accessing conventional healthcare (e.g., prescription medication); current use of three or more conventional healthcare services (i.e., office visits, specialty care, emergency department visit, mental healthcare, therapy services, prescription medication, special education, or early intervention); and comorbidity per the 38 chronic conditions asked about in the 2012 NHIS. Additionally, healthcare need factors included functional limitations status based on if the child had any impairment limiting his or her physical abilities (e.g., walking); used special equipment (e.g., wheelchair); or had difficulties with emotions, concentration, behavior, or getting along with others that limited his or her daily activities. To account for confounding when examining nondisclosure correlates, the number of CHA used in the past year dichotomized at the median of one was included.
Use and Nondisclosure of CHA
Children were determined to have used any CHA if they had at least one affirmative response to items asking, “During the past 12-months, did [child] use…?” They were asked about: chelation, Ayurveda, acupuncture, qigong, hypnosis, biofeedback, energy healing, craniosacral therapy, naturopathy, Tai chi, traditional healers (e.g., Native American Healer), movement therapy (e.g., Pilates), guided imagery, meditation, special diets (i.e., vegetarian or vegan), homeopathy, massage therapy, yoga, chiropractic or osteopathic manipulation, herbal or non-vitamin supplements (e.g., fish oil), specific minerals (i.e., calcium, magnesium, iron, chromium, zinc, selenium, potassium), and specific vitamins (i.e., A, B, C, D, E, H, K). To have been counted as using acupuncture, Ayurveda, biofeedback, chelation, chiropractic or osteopathic manipulation, craniosacral therapy, energy healing therapy, hypnosis, massage, movement and exercise techniques, or naturopathy, the child had to have seen a practitioner for the modality in the past 12-months or the child used the given modality in the past 12-months. Per past research, we did not consider children who were only indicated to have used multi-vitamins or multi-minerals in the past 12-months as having used any CHA.26
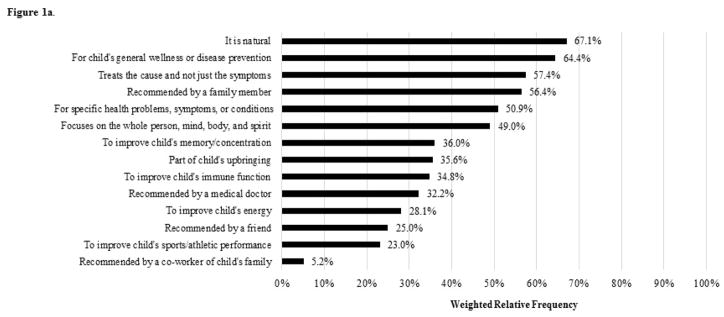
To further characterize CHA use, we additionally examined the reasons for use of CHA that were asked about. In the survey, parents were asked to select up to three modalities that children were reported to have used in the past 12-months that the parent considered to be most important for the child’s health. Parents were not able to select multivitamins/minerals, specific vitamins, specific minerals, or chelation in this survey section. Then for each modality selected as being most important for the child’s health, 14 reasons for use were subsequently asked about (Figure 1a).
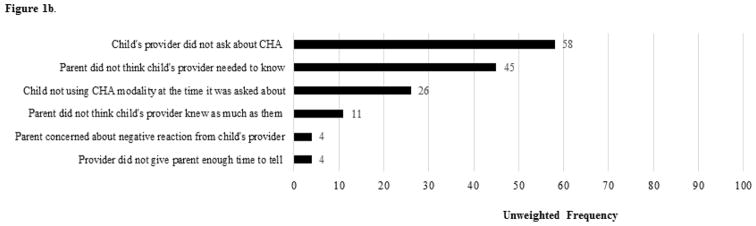
Figure 1.
Figure 1a. Weighted Relative Frequencies of Reasons for Use of CHA among US Children with DD aged 4–17 years (n = 255)
Figure 1b. Unweighted Frequencies of Reasons for Nondisclosure of CHA used for US Children with DD aged 4–17 years (n = 95)
CHA nondisclosure was only assessed among children with a usual source of healthcare and a personal health provider, defined as a health professional who knew the child well and was familiar with the child’s health history (e.g., general doctor). To have been asked about CHA nondisclosure, the child additionally needed to have used one or more CHA from the subset of modalities that reasons for use were asked about. The following item was asked for up to three of these modalities, which were reported as being most important for the child’s health, “During the past 12-months, did you let [child]’s personal health provider know about [his/her] use of [modality]?” Nondisclosure of any CHA was defined when the parent said “No” for any of the modalities asked about. Because correlates of nondisclosure of any versus all CHA asked about may differ among children with DD, we also included a nondisclosure of all CHA measure (i.e., nondisclosure was reported for all modalities asked about). When any nondisclosure was reported by parents, eight reasons for nondisclosure were subsequently asked about (Figure 1b). We examined six reasons for nondisclosure, collapsing three reasons into a single variable about negative provider reaction, due to low response frequency and content similarity.
Analysis
To describe the sample and estimate the prevalence of CHA use and nondisclosure among US children with DD, descriptive statistics were first computed for all variables of interest. We also computed CHA use by DD status to further contextualize the prevalence of CHA use for children with DD. Bivariate statistics were then computed to determine differences in the distributions of predisposing, enabling, and healthcare need variables by use and nondisclosure of CHA. Bivariate analysis results informed three multivariable logistic regression models fit to determine adjusted associations of predisposing, enabling, and healthcare need factors with (1) any CHA use, (2) nondisclosure of any CHA, and (3) nondisclosure of all CHA. Factors that had an unadjusted association with CHA use and/or nondisclosure significant at a P < .10 level were initially included in the respective multivariable model but were only retained if their association remained statistically significant at the P < .05 level. Given sample size constraints, we descriptively examined reasons for CHA use and nondisclosure. We weighted analyses per NCHS guidance35 and performed all analyses in Stata 14.2.
RESULTS
Population Characteristics
The sample represented an estimated 12,382,376 US children with DD aged 4–17 years. The most common DD was ADHD (47.4%) followed by learning disabilities (39.5%), other DD (22.9%), visual impairment (15%), moderate or profound hearing loss (11.9%), stuttering or stammering (8.6%), ASD (6.4%), ID (5.9%), seizures (3.4%), and CP (1.4%). The mean child age was 11 years (SD = 3.8 years). Many children with DD were male, non-Hispanic white, had at least one parent with more than a high school education, had a household income level above the FPL, had one or more working adult in their household, had no problems or delays accessing conventional care, and had comorbid conditions (Table 1).
Table 1.
Characteristics of US Children with DD aged 4–17 years (n = 2,141)
| n | % | |
|---|---|---|
| Predisposing characteristics | ||
| Age, years | ||
| 4–9 | 757 | 36.50 |
| 10–12 | 498 | 24.70 |
| 13–17 | 886 | 38.80 |
| Gender | ||
| Female | 798 | 36.70 |
| Male | 1,343 | 63.30 |
| Race/ethnicity | ||
| White, non-Hispanic | 1,107 | 59.12 |
| Hispanic | 504 | 18.89 |
| Black, non-Hispanic | 342 | 14.15 |
| Other race, non-Hispanic | 188 | 7.83 |
| Highest parent education level | ||
| ≤ High school diploma or GED | 753 | 32.32 |
| More than high school | 1,385 | 67.68 |
| Family structure | ||
| 2 parent biological/adoptive | 683 | 35.62 |
| 2 parent with step parent | 160 | 9.14 |
| Single mother | 413 | 16.83 |
| Other family structure | 884 | 38.40 |
| Enabling resources | ||
| Household income level | ||
| 0–99% FPL | 590 | 27.72 |
| 100–199% FPL | 554 | 24.63 |
| 200–399% FPL | 551 | 25.59 |
| ≥ 400% FPL | 446 | 22.06 |
| Health insurance coverage | ||
| Public insurance or uninsured | 1,217 | 54.90 |
| Any private insurance | 914 | 45.10 |
| Household employment | ||
| ≥ 1 adult working | 1,771 | 84.81 |
| No adults working | 370 | 15.19 |
| Census region | ||
| Midwest | 436 | 22.58 |
| Northeast | 359 | 15.78 |
| South | 816 | 40.05 |
| West | 530 | 21.59 |
| Healthcare need factors | ||
| Problems or delays getting care | ||
| No problems or delays | 1,729 | 80.57 |
| ≥ 1 problem or delay | 412 | 19.43 |
| Conventional care use | ||
| ≤ 2 services | 1,132 | 52.05 |
| 3 or more services | 1,009 | 47.95 |
| Chronic condition comorbidity | ||
| 1 chronic condition | 512 | 23.61 |
| ≥ 2 of 38 chronic conditions | 1,629 | 76.39 |
| Functional limitations status | ||
| No functional limitations | 1,430 | 66.73 |
| Functional limitations | 707 | 33.27 |
| Overall health status | ||
| Excellent or very good | 1,466 | 69.60 |
| Good, fair, or poor | 675 | 30.40 |
Note. Percentages were computed using weighted analyses.
Data source: 2012 National Health Interview Survey
Use of CHA
Among US children with DD aged 4–17 years, 23.3% (95% CI: 21.0%–25.8%) or an estimated 2,841,436 children reported using any CHA in the past year. By comparison, we found that 19.8% (95% CI: 18.5%–21.1%) of US children without DD aged 4–17 years reported using any CHA use in the past year. The proportion of children with DD reported to use CHA was significantly higher than the proportion of children without DD reported to use CHA (P = .005).
For children with DD, specific vitamins (14.7%), herbal supplements (8.1%), and specific minerals (7.7%) were the most commonly used modalities followed by chiropractic or osteopathic manipulation (3.2%). Certain modalities that can be unsafe, such as special diets (~1%), were infrequently reported to be used by children with DD, and no children with DD were reported to have used chelation. Although bivariate analysis results showed statistically significant associations of CHA use with child age, race and ethnicity, parent education, health insurance, household employment, conventional care use, or functional limitations status, these associations did not remain statistically significant in the multivariable analysis. Multivariable regression results showed higher adjusted odds of CHA use for children with DD who were female, had higher household income, did not live in the South, experienced any problem or delay accessing conventional care, or had one or more comorbid chronic condition (Table 2). Among children with DD who used one or more of the select CHA modalities, the most frequently reported reasons for use were because it is natural, for the child’s general wellness or disease prevention, and because it treats the cause not just the symptoms (Figure 1a).
Table 2.
Bivariatea and Multivariable Associationsb of CHA Use with Predisposing, Enabling, and Healthcare Need Factors among US Children with DD aged 4–17 years (n = 2,103)
| % | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|
| Predisposing characteristics | |||
| Age, years | |||
| 4–9 | 19.40 | 1.00 | — |
| 10–12 | 23.83 | 1.30 (0.91–1.86) | — |
| 13–17 | 26.68 | 1.51 (1.12–2.05) | — |
| Gender | |||
| Female | 26.85 | 1.36 (1.04–1.77) | 1.38 (1.06–1.81)* |
| Male | 21.27 | 1.00 | 1.00 |
| Race/ethnicity | |||
| White, non-Hispanic | 25.11 | 1.00 | — |
| Hispanic | 19.39 | 0.72 (0.52–0.98) | — |
| Black, non-Hispanic | 15.77 | 0.56 (0.37–0.85) | — |
| Other race, non-Hispanic | 33.13 | 1.48 (0.96–2.27) | — |
| Highest parent education level | |||
| ≤ High school diploma or GED | 16.55 | 1.00 | — |
| More than high school | 26.49 | 1.82 (1.37–2.40) | — |
| Family structure | |||
| 2 parent biological or adoptive | 25.60 | 1.00 | — |
| 2 parent with step parent | 26.58 | 1.05 (0.62–1.79) | — |
| Single mother | 20.29 | 0.74 (0.50–1.09) | — |
| Other family structure | 21.72 | 0.81 (0.60–1.09) | — |
| Enabling resources | |||
| Household income | |||
| 0–99% FPL | 16.09 | 1.00 | 1.00 |
| 100–199% FPL | 17.76 | 1.13 (0.77–1.66) | 1.23 (0.83–1.83) |
| 200–399% FPL | 25.85 | 1.82 (1.28–2.57) | 2.09 (1.44–3.03)*** |
| ≥ 400% FPL | 35.60 | 2.88 (1.99–4.17) | 3.50 (2.35–5.21)*** |
| Health insurance coverage | |||
| Public insurance only/uninsured | 17.79 | 1.00 | — |
| Any private insurance | 29.89 | 1.97 (1.52–2.56) | — |
| Household employment | |||
| ≥ 1 adult working | 24.52 | 1.00 | — |
| No adults working | 16.50 | 0.61 (0.43–0.86) | — |
| Census region | |||
| South | 16.66 | 1.00 | — |
| West | 31.54 | 2.30 (1.60–3.33) | 2.24 (1.56–3.22)*** |
| Northeast | 25.75 | 1.73 (1.18–2.55) | 1.52 (1.02–2.26)* |
| Midwest | 25.66 | 1.73 (1.23–2.43) | 1.69 (1.20–2.39)** |
| Healthcare need factors | |||
| Problems or delays getting conventional care | |||
| No problems or delays | 21.83 | 1.00 | 1.00 |
| ≥ 1 problem and/or delay | 29.50 | 1.50 (1.10–2.04) | 1.64 (1.23–2.20)** |
| Conventional care utilization | |||
| ≤ 2 conventional care services | 20.63 | 1.00 | — |
| 3 or more conventional care services | 26.29 | 1.37 (1.04–1.82) | — |
| Chronic condition comorbidity | |||
| 1 chronic condition | 17.09 | 1.00 | — |
| ≥ 2 of 38 chronic conditions | 25.27 | 1.64 (1.16–2.32) | 1.71 (1.18–2.48)** |
| Functional limitations | |||
| No functional limitations | 21.54 | 1.00 | — |
| Functional limitations | 26.91 | 1.34 (1.02–1.77) | — |
| Overall health status | |||
| Excellent or very good | 23.13 | 1.00 | — |
| Poor, fair, or good | 23.75 | 1.04 (0.78–1.37) | — |
Bolded values indicate the unadjusted association was significant at a P < .10 level and was initially included in the multivariable model. For the multivariable model results:
P < .001,
P < .01,
P < .05
Only the final multivariable model results are shown. Dashes indicate variables were not included or were removed.
Note. All analyses were weighted.
Data source: 2012 National Health Interview Survey
Nondisclosure
Among US children aged 4–17 years with DD who used any CHA in the past year and had a personal health provider, nondisclosure of any CHA used was reported for an estimated 533,333 or 41.9% (95% CI: 34.2%–50.0%) of children. Nondisclosure of all CHA used was reported for an estimated 461,750 or 36.2% (95% CI: 28.3%–45.0%) of children with DD. For children with DD, the most frequently nondisclosed modalities were herbal supplements (i.e., fish oil, melatonin, probiotics), certain mind and body practices (i.e., yoga, tai chi, qigong, chiropractic or osteopathic manipulation), and homeopathy (data not shown). Multivariable regression results demonstrated higher adjusted odds of nondisclosure of any CHA used for children with DD who were female or had no functional limitations (Table 3). Higher adjusted odds of nondisclosure of all CHA used were found for children with DD who were older in age, used two or fewer conventional healthcare services, or only used one modality of CHA (Table 3). The most frequently reported nondisclosure reason was that the child’s health provider did not ask (Figure 1b).
Table 3.
Bivariatea and Multivariable Associationsb of Nondisclosure with Predisposing, Enabling, and Healthcare Need Factors for US Children with DD aged 4–17 Years (n = 223)
| % | Nondisclosure of Any CHA | % | Nondisclosure of All CHA | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | |||
| Predisposing characteristics | ||||||
| Age, years | ||||||
| 4–9 | 30.85 | 1.00 | — | 24.53 | 1.00 | 1.00 |
| 10–12 | 51.34 | 2.36 (0.98–5.73) | — | 39.39 | 2.00 (0.81–4.94) | 2.32 (0.91–5.92) |
| 13–17 | 44.75 | 1.82 (0.89–3.72) | — | 44.11 | 2.42 (1.17–5.04) | 2.40 (1.16–4.97)* |
| Gender | ||||||
| Female | 55.67 | 2.76 (1.41–5.40) | 2.74 (1.41–5.32)** | 43.35 | 1.72 (0.85–3.47) | — |
| Male | 31.26 | 1.00 | 1.00 | 30.78 | 1.00 | — |
| Race/ethnicity | ||||||
| White, non-Hispanic | 42.27 | 1.00 | — | 37.30 | 1.00 | — |
| Hispanic | 41.66 | 0.98 (0.36–2.63) | — | 26.23 | 0.60 (0.23–1.58) | — |
| Black, non-Hispanic | 44.52 | 1.10 (0.32–3.80) | — | 44.52 | 1.35 (0.38–4.80) | — |
| Other race, non-Hispanic | 38.62 | 0.86 (0.31–2.37) | — | 35.51 | 0.93 (0.34–2.51) | — |
| Highest parent education level | ||||||
| ≤ High school diploma or GED | 38.67 | 1.00 | — | 38.67 | 1.00 | — |
| More than high school | 42.39 | 1.17 (0.44–3.12) | — | 35.83 | 0.89 (0.33–2.39) | — |
| Family structure | ||||||
| 2 parent biological or adoptive | 39.60 | 1.00 | — | 35.04 | 1.00 | — |
| 2 parent with step parent | 54.03 | 1.79 (0.50–6.61) | — | 45.22 | 1.53 (0.43–5.44) | — |
| Single mother | 32.96 | 0.75 (0.22–2.60) | — | 29.50 | 0.78 (0.21–2.92) | — |
| Other family structure | 44.66 | 1.23 (0.63–2.40) | — | 37.69 | 1.12 (0.55–2.29) | — |
| Enabling resources | ||||||
| Household income level | ||||||
| 0–99% FPL | 43.03 | 1.00 | — | 37.21 | 1.00 | — |
| 100–199% FPL | 52.72 | 1.47 (0.48–4.58) | — | 39.56 | 1.10 (0.33–3.70) | — |
| 200–399% FPL | 38.85 | 0.84 (0.33–2.18) | — | 42.42 | 1.24 (0.46–3.35) | — |
| ≥ 400% FPL | 38.68 | 0.84 (0.33–2.12) | — | 30.10 | 0.73 (0.27–1.93) | — |
| Health insurance coverage | ||||||
| Public insurance or uninsured | 48.44 | 1.00 | — | 44.53 | 1.00 | — |
| Any private insurance | 38.27 | 0.66 (0.34–1.27) | — | 31.49 | 0.57 (0.29–1.14) | — |
| Household employment | ||||||
| ≥ 1 adult working | 41.87 | 1.00 | — | 36.33 | 1.00 | — |
| No adults working | 41.71 | 0.99 (0.41–2.43) | — | 35.41 | 0.96 (0.38–2.42) | — |
| Census region | ||||||
| South | 46.56 | 1.00 | — | 42.58 | 1.00 | — |
| West | 39.28 | 0.74 (0.33–1.66) | — | 31.98 | 0.63 (0.26–1.57) | — |
| Northeast | 43.23 | 0.87 (0.26–2.95) | — | 37.07 | 0.79 (0.22–2.82) | — |
| Midwest | 39.23 | 0.74 (0.31–1.76) | — | 34.29 | 0.70 (0.29–1.73) | — |
| Healthcare need factors | ||||||
| Problems or delays getting care | ||||||
| No problems or delays | 41.73 | 1.00 | — | 36.56 | 1.00 | — |
| ≥ 1 problem and/or delay | 42.33 | 1.03 (0.44–2.36) | — | 35.03 | 0.94 (0.40–2.19) | — |
| Conventional care use | ||||||
| ≤ 2 services | 50.81 | 1.83 (0.91–3.69) | — | 49.15 | 2.50 (1.22–5.12) | 3.08 (1.40–6.79)** |
| 3 or more services | 36.07 | 1.00 | — | 27.89 | 1.00 | 1.00 |
| Chronic condition comorbidity | ||||||
| 1 chronic condition | 46.12 | 1.00 | — | 50.67 | 1.00 | — |
| ≥ 2 of 38 chronic conditions | 41.21 | 0.82 (0.28–2.42) | — | 34.05 | 0.50 (0.17–1.48) | — |
| Functional limitations status | ||||||
| No functional limitations | 48.49 | 2.13 (1.08–4.22) | 2.11 (1.05–4.22)* | 43.18 | 2.34 (1.15–4.78) | — |
| Functional limitations | 30.63 | 1.00 | 1.00 | 24.50 | 1.00 | — |
| Overall health status | ||||||
| Excellent or very good | 44.08 | 1.00 | — | 40.29 | 1.00 | — |
| Poor, fair, or good | 36.51 | 0.73 (0.35–1.52) | — | 26.51 | 0.53 (0.25–1.16) | — |
| Number of CHA usedc | ||||||
| 1 modality | 43.01 | 1.12 (0.59–2.13) | — | 45.71 | 2.91 (1.43–5.94) | 3.64 (1.70–7.81)** |
| 2 or more modalities | 40.17 | 1.00 | — | 22.43 | 1.00 | 1.00 |
Bolded values indicate the unadjusted association was significant at a P < .10 level and was initially included in the multivariable model. For the multivariable model results:
P < .01,
P < .05
Only the final multivariable model results are shown. Dashes indicate variables were not included or were removed.
The number of CHA modalities reported by children with DD ranged from 1–7, with the median number of modalities used = 1.
Note. All analyses were weighted.
Data source: 2012 National Health Interview Survey
DISCUSSION
This study is one of the first to examine the prevalence and correlates of CHA use and nondisclosure among US children with DD. Our study found higher CHA use prevalence for US children with DD (23.3%) compared to those without DD (19.8%). Prior research utilizing data from other databases among subgroups of US children with DD has shown prevalence of CHA use close to this study, ranging from 17.3%23 to 28.0%14 for children with ASD and 10.2%25 to 24.7%20 for children with ADHD. Variability in prevalence estimates of CHA use may be due in part to differences in how CHA use is measured (e.g., the exclusion of specific vitamins and/or specific minerals from the definition of CHA), as well as how children are sampled. Consistent measurement and sampling are, therefore, needed in future research intended to determine time trends in CHA use among children with DD.
Being female, higher household income, not living in the South, difficulty accessing healthcare, and comorbidity were each associated with greater odds of CHA use. These findings are consistent with past research suggesting female versus male children with mental health conditions including ADHD20 are more likely to use CHA. Past research similarly shows that children with ADHD who have higher household income are more likely to use CHA.20,25 Our study findings regarding regional variation in CHA use among children with DD are also consistent with past research demonstrating children with ADHD25 or ASD23 in the South are less likely to use CHA. Past research additionally shows that various markers of elevated healthcare need (e.g., problems accessing care, service use, comorbidity, symptom severity) are associated with greater CHA use in DD subgroups (e.g., ASD, ADHD),14,20,25 and our study also showed that problems accessing healthcare and comorbidity were associated with greater odds of CHA use among children with DD. Future research is needed to determine how different aspects of healthcare need (e.g., changes in the need for certain conventional DD treatments) may interact over time in relationship to CHA use for children with DD.
The most frequently reported reason for use (i.e., it is natural) among children with DD who used any CHA aligns with some prior research demonstrating parents use CHA because they believe CHA is safer than other treatments (e.g., medication),24 even though this may not be true. We also found the use of CHA to treat the cause not just the symptoms was another frequently reported reason. A potential rationale for CHA use by children with ASD and other neurodevelopmental disorders—particularly the use of biomedical modalities—is that they are believed to address underlying disease mechanisms (e.g., oxidative stress, immune abnormalities).13 Our study suggests this belief may contribute to CHA use in children with DD more broadly. Still, how these and other beliefs, together, drive patterns of conventional healthcare and CHA use in children with DD remains largely unstudied and an area for future research.
We found nondisclosure was relatively common for some or all modalities reported to be most important for the child’s health. Past studies19,30,31 have found higher nondisclosure rates for other specific populations (e.g., children with asthma). Methodological differences (e.g., nonprobability versus probability sampling) may account for some of this variation or it may be that children with DD are relatively well-connected to the health system and are more likely to disclose CHA use. We also found discrepancies in the factors significantly associated with nondisclosure of any versus all CHA modalities used by children with DD. Specifically, though nondisclosure of any CHA may be more likely for female children or those without functional limitations, nondisclosure of all modalities may most commonly occur among older children, those receiving fewer conventional care services, or those using few CHA modalities. Overall, our study’s findings align with past research showing younger age, more severe symptomatology, and routine conventional health services use may prompt disclosure. Gender has not, however, been a characteristic previously identified as potentially influencing nondisclosure among children with DD, suggesting that greater exploration of gender-based differences in family-provider communication about CHA use in children with DD is needed.
Like past research,30,31,38 the most frequently reported reasons for nondisclosure in this study were that the child’s health provider did not ask or that the parent felt the child’s provider did not need to know, pointing to a need for improved family-provider communication about CHA and greater education of families and providers about DD treatment options including CHA. Past research shows that some pediatric providers encourage the use of certain CHA (e.g., fatty acids, melatonin) for children with ASD, and that most of these providers inquire about CHA use and would like to receive more training about CHA.39 Relatedly, limited provider knowledge and/or negative attitudes about CHA may contribute to nondisclosure and ultimately greater discord in shared decision-making between families of children with ASD and their healthcare providers.32,33 Additional research is needed to determine how family-provider communication and other aspects of pediatric healthcare visits (e.g., visit questionnaires asking about CHA use, provider prompts to ask about CHA in the electronic health record system) could be used to reduce nondisclosure and potentially improve pediatric healthcare quality more broadly (e.g., receipt of routine immunizations, use of antibiotics).
Our findings should be interpreted with several limitations in mind. Foremost, the cross-sectional design precludes causal inferences from being drawn. Descriptive results on reasons for CHA use and nondisclosure do, however, provide some insights into the motivations for these behaviors. Future longitudinal and mixed methods research is needed to better understand why families use CHA for children with DD and do not disclose it to their child’s health provider. Though the definitions used to construct measures of DD status, as well as CHA use and nondisclosure, are congruent with past research, measurement variability across the entire body of relevant research may contribute to differences in findings. For example, the 2012 NHIS CAM Supplement did not ask about all potential modalities used (e.g., hyperbaric oxygen therapy, essential oils), and this may contribute to differences in estimates of CHA use prevalence across studies. In addition, nondisclosure of CHA used was only assessed for a subset of children in the 2012 NHIS (i.e., those with a usual source of care and personal health provider who used the CHA asked about). Further research is, therefore, needed to better understand nondisclosure among the broader population of children with DD who may not have a health provider and/or use modalities that were not asked about. Although the timeframe of all NHIS items used to assess CHA use and nondisclosure was the past 12-months, the potential for recall bias still exists in terms of under- or over-reporting. Other data sources such as information available from children’s electronic health records and/or patient portals may be considered in future research insofar as they may be able to provide additional and potentially more recent insights into CHA use and disclosure among children with DD.
CONCLUSION
CHA use is prevalent among children with DD nationwide. The most common reason for CHA use (“it is natural”) reflects parents’ perception that CHA may be safe. This perception may be consistent with current scientific evidence for some CHA used to treat DD, while for other CHA it may not be. Families of children with DD may view healthcare providers as information resources or brokers regarding DD treatment options, including CHA. Healthcare providers may, however, possess limited knowledge of CHA.39 Increased education for healthcare providers about CHA is, therefore, needed. Greater knowledge of CHA among healthcare providers may, in turn, help families to better understand the safety and efficacy of CHA along with other DD treatment options. Disclosure of CHA is necessary for shared treatment decision-making to occur. Our study revealed nondisclosure may be common among children with DD. Common reasons for nondisclosure highlight a need for improved family-provider communication about CHA, specifically in terms of providers initiating dialogue about CHA. Provider prompts in electronic health record systems to ask families about CHA reported on intake forms, as well as brief training to enhance providers’ family-centered communication in discussing health risks40 may be used to increase disclosure. Interventions informed by this study’s findings may help improve healthcare for children with DD, particularly related to the use and nondisclosure of CHA.
Acknowledgments
Sources of Financial Support: Dr. Lindly’s effort was support in part by the Oregon State University Ruth E. Warnke Graduate Fellowship and grant number T32HS000063 from the Agency for Healthcare Research and Quality.
We also acknowledge the guidance provided by Dr. Adam Branscum, Dr. Joseph Catania, Dr. Emily Ho, and Dr. Kari-Lyn Sakuma on the research reported. We also acknowledge the constructive guidance provided through the 2015 Society for Developmental and Behavioral Pediatrics Research Scholars Symposium related to the research reported.
Abbreviations
- ADHD
attention deficit/hyperactivity disorder
- ASD
autism spectrum disorder
- CAM
complementary and alternative medicine
- CHA
complementary health approaches
- CP
cerebral palsy
- DD
developmental disabilities
- FPL
federal poverty level
- NCHS
National Center for Health Statistics
- NHIS
National Health Interview Survey
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Boyle CA, Boulet SL, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127(6):1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 2.National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. [Accessed July 19, 2015];Facts About Developmental Disabilities. http://www.cdc.gov/ncbddd/developmentaldisabilities/facts.html. Published July 9, 2015.
- 3.Boulet SL, Boyle CA, Schieve LA. Health care use and health and functional impact of developmental disabilities among US children, 1997–2005. Arch Pediatr Adolesc Med. 2009;163(1):19–26. doi: 10.1001/archpediatrics.2008.506. [DOI] [PubMed] [Google Scholar]
- 4.Pelham WE, Foster EM, Robb JA. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. J Pediatr Psychol. 2007;32(6):711–727. doi: 10.1093/jpepsy/jsm022. [DOI] [PubMed] [Google Scholar]
- 5.Ganz M. The lifetime distribution of the incremental societal costs of autism. Arch Pediatr Adolesc Med. 2007;161(4):343–349. doi: 10.1001/archpedi.161.4.343. [DOI] [PubMed] [Google Scholar]
- 6.Weitlauf AS, McPheeters ML, Peters B, et al. Therapies for Children with Autism Spectrum Disorder: Behavioral Interventions Update. Vanderbilt Evidence-based Practice Center; 2014. www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
- 7.National Center for Complementary and Integrative Health, U.S. Department of Health and Human Services. [Accessed April 27, 2015];CAM Basics. 2015 Mar; https://nccih.nih.gov/sites/nccam.nih.gov/files/CAM_Basics_Whats_In_A_Name_03-26-2015.pdf.
- 8.Huffman LC, Sutcliffe TL, Tanner ISD, et al. Management of symptoms in children with autism spectrum disorders: a comprehensive review of pharmacologic and complementary-alternative medicine treatments. J Dev Behav Pediatr. 2011;32:56–68. doi: 10.1097/DBP.0b013e3182040acf. [DOI] [PubMed] [Google Scholar]
- 9.Akins CRS, Angkustsiri K, Hansen RL. Complementary and alternative medicine in autism: An evidence-based approach to negotiating safe and efficacious interventions with families. Neurotherapeutics. 2010;7(3):307–319. doi: 10.1016/j.nurt.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehouse AJ. Complementary and alternative medicine for autism spectrum disorders: Rationale, safety and efficacy. J Paediatr Child Health. 2013;49:E438–E442. doi: 10.1111/jpc.12242. [DOI] [PubMed] [Google Scholar]
- 11.Anagnostou E, Hansen R. Medical treatment overview: Traditional and novel psychopharmacological and complementary and alternative medications. Curr Opin Pediatr. 2011;23:621–627. doi: 10.1097/MOP.0b013e32834cba3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown KA, Patel DR. Complementary and alternative medicine in developmental disabilities. Indian J Pediatr. 2005;72(11):949–952. doi: 10.1007/BF02731671. [DOI] [PubMed] [Google Scholar]
- 13.Hendren RL. Autism: Biomedical complementary treatment approaches. Child Adolesc Psychiatr Clin N Am. 2013;22:443–456. doi: 10.1016/j.chc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Perrin JM, Coury DL, Hyman SL, et al. Complementary and alternative medicine use in a large pediatric autism sample. Pediatrics. 2012;130:S77–S82. doi: 10.1542/peds.2012-0900E. [DOI] [PubMed] [Google Scholar]
- 15.Hall SE, Riccio CA. Complementary and alternative treatment use for autism spectrum disorders. Complement Ther Clin Pract. 2012;18:159–163. doi: 10.1016/j.ctcp.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Valicenti-McDermott M, Burrows B, Bernstein L, et al. Use of complementary and alternative medicine in children with autism and other developmental disabilities: Associations with ethnicity, child comorbid Symptoms, and parental stress. J Child Neurol. 2013;29(3):360–367. doi: 10.1177/0883073812474489. [DOI] [PubMed] [Google Scholar]
- 17.Salomone E, Charman T, McConachie H, et al. Prevalence and correlates of use of complementary and alternative medicine in children with autism spectrum disorder in Europe. Eur J Pediatr. 2015 doi: 10.1007/s00431-015-2531-7. [DOI] [PubMed] [Google Scholar]
- 18.Owen-Smith AA, Bent S, Lynch FL, et al. Prevalence and predictors of complementary and alternative medicine use in a large insured sample of children with autism spectrum disorders. Res Autism Spectr Disord. 2015;17:40–51. doi: 10.1016/j.rasd.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan E, Rappaport LA, Kemper KJ. Complementary and alternative therapies in childhood attention and hyperactivity problems. J Dev Behav Pediatr. 2003;24(1):4–8. doi: 10.1097/00004703-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Kemper KJ, Gardiner P, Birdee GS. Use of complementary and alternative medical therapies among youth with mental health concerns. Acad Pediatr. 2013;13(6):540–545. doi: 10.1016/j.acap.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurvitz EA, Leonard C, Ayyangar R, et al. Complementary and alternative medicine use in families of children with cerebral palsy. Dev Med Child Neurol. 2003;45(6):364–370. doi: 10.1017/s0012162203000707. [DOI] [PubMed] [Google Scholar]
- 22.Liptak GS. Complementary and alternative therapies for cerebral palsy. Ment Retard Dev Disabil. 2005;11:156–163. doi: 10.1002/mrdd.20066. [DOI] [PubMed] [Google Scholar]
- 23.Zuckerman KE, Lindly OJ, Sinche BK, et al. Parent health beliefs, child health services utilization, and child health care quality among US children with autism and other developmental conditions. J Dev Behav Pediatr. 2015;36(3):146–157. doi: 10.1097/DBP.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanson E, Kalish LA, Bunce E, et al. Use of complementary and alternative medicine among children diagnosed with autism spectrum disorder. J Autism Dev Disord. 2007;37:628–636. doi: 10.1007/s10803-006-0192-0. [DOI] [PubMed] [Google Scholar]
- 25.Visser SN, Bitsko RH, Danielson ML, et al. Treatment of Attention Deficit/Hyperactivity Disorder among Children with Special Health Care Needs. J Pediatr. 2015;166(6):1423–1430. doi: 10.1016/j.jpeds.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black LI, Clarke TC, Barnes PM, et al. Use of Complementary Health Approaches among Children Aged 4–17 Years in the United States: National Health Interview Survey, 2007–2012. Hyattsville, MD: National Center for Health Statistics; 2015. [PMC free article] [PubMed] [Google Scholar]
- 27.Akins CRS, Krakowiak P, Angkustsiri K, et al. Utilization patterns of conventional and complementary/alternative treatments in children with autism spectrum disorders and developmental disabilities in a population-based study. J Dev Behav Pediatr. 2014;35(1):1–10. doi: 10.1097/DBP.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agency for Healthcare Research and Quality. [Accessed November 13, 2016];The National Quality Strategy. http://www.ahrq.gov/workingforquality/index.html.
- 29.Kemper KJ, Vohra S Task Force on Complementary and Alternative Medicine, Provisional Section on Complementary, Holistic, and Integrative Medicine. The use of complementary and alternative medicine in pediatrics. Pediatrics. 2008;122(6):1374–1386. doi: 10.1542/peds.2008-2173. [DOI] [PubMed] [Google Scholar]
- 30.Sibinga EM, Ottolini MC, Duggan AK, et al. Parent-pediatrician communication about complementary and alternative medicine use for children. Clin Pediatr (Phila) 2004;43(4):367–373. doi: 10.1177/000992280404300408. [DOI] [PubMed] [Google Scholar]
- 31.Sidora-Arcoleo K, Yoos HL, Kitzman H, et al. Don’t ask, don’t tell: Parental nondisclosure of complementary and alternative medicine and over-the-counter medication use in children’s asthma management. J Pediatr Healthc. 2008;22(4):221–229. doi: 10.1016/j.pedhc.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindly O, Thorburn S, Heisler K, et al. Parent disclosure of complementary health approaches used for children with autism spectrum disorder: barriers and facilitators. Complement Ther Med. 2017;35:47–52. doi: 10.1016/j.ctim.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy S, Frasso R, Colantonio S, et al. Shared decision making and treatment decisions for young children with autism spectrum disorder. Acad Pediatr. 2016;16(6):571–578. doi: 10.1016/j.acap.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Stussman BJ, Bethell CD, Gray C, et al. Development of the adult and child complementary medicine questionnaires fielded on the National Health Interview Survey. BMC Complement Altern Med. 2013;13:238. doi: 10.1186/1472-6882-13-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Division of Health Interview Statistics, National Center for Health Statistics, Centers for Disease Control and Prevention. 2012 National Health Interview Survey (NHIS) Public Use Data Release: NHIS Survey Description. Hyattsville, MD: 2013. [Accessed September 9, 2015]. p. 135. http://ftp.cdc.gov/pub/health_statistics/nchs/Dataset_Documentation/NHIS/2012/srvydesc.pdf. [Google Scholar]
- 36.Fouladbakhsh JM, Stommel M. Using the behavioral model for complementary and alternative medicine: The CAM healthcare model. J Complement Integr Med. 2007;4(1) [Google Scholar]
- 37.Andersen R. National health surveys and the behavioral model of health services use. Med Care. 2008;46:647–653. doi: 10.1097/MLR.0b013e31817a835d. [DOI] [PubMed] [Google Scholar]
- 38.Wong H, Smith RG. Patterns of complementary and alternative medical therapy use in children diagnosed with autism spectrum disorder. J Autism Dev Disord. 2006;36(7):901–909. doi: 10.1007/s10803-006-0131-0. [DOI] [PubMed] [Google Scholar]
- 39.Golnik AE, Ireland M. Complementary alternative medicine for children with autism: A physician survey. J Autism Dev Disord. 2009;39(7):996–1005. doi: 10.1007/s10803-009-0714-7. [DOI] [PubMed] [Google Scholar]
- 40.Helitzer DL, LaNoue M, Wilson B, et al. A randomized controlled trial of communication training with primary care providers to improve patient-centeredness and health risk communication. Patient Educ Couns. 2011;82:21–29. doi: 10.1016/j.pec.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]