Abstract
Objective
Free-living adaptive responses to short term overfeeding were explored as predictors of longitudinal weight change in adults recruited as obesity-resistant (OR) or obesity-prone (OP) based on self-identification and personal/family weight history.
Methods
Adults identified as OP (n=21; BMI: 23.8±2.5 kg/m2) and OR (n=20; BMI: 20.2±2.1 kg/m2) completed 3 days of eucaloric (EU, 100% of energy needs) and 3 days of overfeeding (OF, 140% of energy needs). Following each condition, adaptive responses in physical activity (PA), total daily energy expenditure (TDEE), ad libitum energy intake (EI), and energy balance were objectively measured for 3 days in a free-living environment. Body mass and composition (DXA) were measured annually for 5 years. Adaptive responses to overfeeding were correlated with 5-year changes in body mass and composition.
Results
Increases in sedentary time correlated with longitudinally measured changes in fat mass (r=0.34, p=0.04) in the cohort taken as a whole. OP reduced their levels of PA following overfeeding while OR maintained or increased their PA. No other variables were found to correlate with weight gain.
Conclusion
Failure to decrease sedentary behavior following short-term overfeeding is one mechanism that may be contributing to fat mass gain.
Keywords: physical activity, energy intake, energy expenditure, Obesity Phenotypes
INTRODUCTION
Energy balance, which is necessary for weight maintenance, is achieved when energy intake (EI) matches energy expenditure (EE). Energy balance is a dynamic process and its complexities have been recognized previously.(1) Studying energy balance has proved to be difficult because it is likely that the dynamic processes responsible for achieving energy balance occur over a period of days not hours.(2, 3, 4, 5) The components of energy balance (EI and EE) are influenced by many environmental, behavioral, and biological factors. In addition, EI and EE may be interdependent.(6, 7, 8, 9) Thus, to study the system responsible for maintaining energy balance, scientists have traditionally perturbed the system by under or overfeeding animals and humans then assessing the subsequent changes in EI, EE, and body weight. Because the current environment promotes a state of positive energy balance and global rates of obesity continue to climb,(10) it may be useful to understand how humans respond to imposed overfeeding. Understanding the differential responses (physiological and behavioral) and inter-individual variability in the responses to brief periods of overfeeding may inform on future obesity prevention strategies.
Some individuals seem more susceptible to weight gain (i.e., obesity-prone, OP) than others (i.e., obesity-resistant, OR). These OP and OR phenotypes have been well studied in rodent models;(11, 12, 13, 14, 15) however, less work has been done in human subjects. Our Energy Adaptations over Time Study (EATS) was designed to measure the adaptive responses to short-term (3 days) overfeeding that may protect from, or promote weight gain in adults self-identified as OP and OR based on personal and family history.(5, 16) Our previous investigations have primarily focused on identifying differences between OP and OR in fuel utilization and energy expenditure during a period of imposed overfeeding. We found that both OP and OR exhibit similar responses in 24h EE and macronutrient oxidation; however, OP tend to downregulate nocturnal fat oxidation, which may drive weight gain.(16, 17) In a separate set of experiments, OP reported higher hunger, restraint and disinhibition compared to OR.(18) In addition, OP had altered regional brain activation in response to palatable food stimuli in brain areas known to be important for the regulation of EI.(19) Taken together, these altered responses may represent mechanisms contributing to obesity risk on both the energy input and energy output sides of the energy balance equation.
In the present study, our goal was to study the time-course of compensation following overfeeding in OP and OR adults. Participants were provided an ad libitum diet for 3 days following 3 days of controlled overfeeding or an energy-balanced condition in a randomized crossover design. We objectively measured daily changes in free-living EI, PA, total daily energy expenditure (TDEE), and energy balance during 3 days following the two conditions. Furthermore, we sought to determine whether changes in PA, EI, and energy balance were associated with changes in body weight and composition assessed annually over 5 years of follow-up. We hypothesized that OP would have lower TDEE and higher EI compared to OR following overfeeding, placing these individuals in positive energy balance and that these changes would be associated with greater rates of weight gain in OP.
METHODS
Participants
Participants were a subset of males and females (25–35 years of age) who participated in EATS examining the effects of overfeeding on nutrient oxidation.(16) Participants were empirically classified as either OP or OR based on family weight history and self-report information. Complete criteria for the OP/OR designation and other pertinent inclusion/exclusion criteria has been published previously.(5, 16) Briefly, OP had a BMI of 19.6–30.6 kg/m2, had one first-degree relative with a BMI of >30 kg/m2, and reported exerting conscious effort to maintain their body weight. In contrast, OR had a BMI of 16.9–25.5 kg/m2, had no first-degree relatives with a BMI >30 kg/m2, and reported difficulty gaining weight. All subjects were weight stable for at least 3 months prior to studies being conducted. Prior to enrollment, all participants provided written informed consent. This study was approved by the Colorado Multiple Institutional Review Board.
Preliminary Assessments
At baseline the following assessments were completed; 1) height; 2) weight assessed on a digital scale; 3) body composition assessed using dual-energy x-ray absorptiometry (DXA, Hologic Discover W, Bedford, Massachusetts); 4) resting EE measured by hood indirect calorimetry (Parvo Medics TrueOne 2400, Sandy, Utah); 5) 24-hour EE measured by whole-room calorimetry and; 6) free-living PA assessed for 1 week using a pedometer (Digi-Walker, New Lifestyles, Inc., Lee’s Summit, Missouri).
Study Design
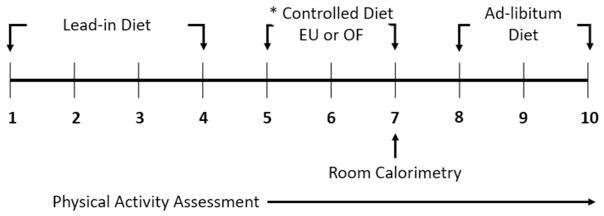
All subjects completed two study conditions (Controlled Eucaloric, EU vs. Controlled Overfeeding, OF) completed in randomized order separated by at least 1 month. Each condition included a total observation period of 10 days (Figure 1). All meals were prepared by the metabolic kitchen of the Colorado Clinical Translational Sciences Institute (CCTSI). During both controlled feeding conditions, individuals were provided an energy-balanced “lead-in” diet during days 1–4. Energy needs were individually estimated based on the average of resting metabolic rate as measured by hood indirect calorimetry and the following equation: (23.9 x fat-free mass (kg) + 372), where fat-free mass was determined by DXA.(20) The average of these two methods was multiplied by an activity factor (1.4–1.65) which was determined by average number of steps taken per day during the 7 day baseline PA assessment. On days 5–7, individuals were provided either the EU diet or the OF diet. During EU, participants were provided the same diet as the lead-in period; whereas, during OF, participants were provided the same dietary composition with 140% of their estimated energy needs. Participants were instructed to eat all of the food provided for days 5–7. Days 5 and 6 were spent in a free-living condition followed by a 23h stay in a whole-room calorimeter on day 7. For the next 3 days following the room calorimeter stay, participants were studied under free-living conditions (i.e., allowing them to engage in normal activities) and were given meals and snacks that provided 125% of their estimated energy needs. Participants were told that they did not need to eat everything that was offered; however, they did not know that the uneaten food would be weighed to calculate consumed calories and macronutrients. Participants were also told in advance that they could request more of any food. This design was aimed to neither restrict intake, nor encourage marked overconsumption. All food, wrappers, and containers were weighed before and after consumption by the metabolic kitchen to determine daily EI.
Figure 1. Schematic overview of the study design.
* Study conditions were conducted in a randomized order separated by a period of at least 1 month.
Physical Activity Assessment
PA was assessed using a physical activity monitoring system (PAMS) designed by Dr. James Levine and colleagues.(5) This system used paired inclinometers and accelerometers positioned on the upper and lower body to assess changes in body position and movement. As previously reported, we collected pilot data to validate this approach.(5). Data from these devices (worn for all waking hours from days 5–10) were used to divide PA into 6 categories; time spent 1) sitting/lying 2) standing still; 3) walking at a slow pace; 4) walking at a normal pace; 5) walking at a quick pace and; 6) vigorous activity.(5) We subsequently collapsed these categories into 3 domains for the analysis reported here; 1) sedentary time (sitting/lying and standing still); 2) light PA (LPA, walking at a slow pace and walking at a normal pace), and moderate-to-vigorous PA (MVPA, walking at a quick pace and vigorous activity). PAMS data was considered valid if the subject wore the device for ≥10 hours/day.(21)
To calculate TDEE, we converted resting EE (kilocalories, kcals/day) as measured using indirect calorimetry to kcal/min. Next, time spent in each domain was multiplied by an appropriate metabolic equivalent (MET) (sedentary behavior= 1.3 METs, LPA= 2.5 METs, MVPA= 4.0 METs) and by the individual’s resting energy expenditure (REE). All of these assigned MET values are within published ranges for the various domains of PA.(22, 23) Because the PAMS was worn during the whole-room calorimeter visit, we were able to calibrate our assigned MET values with measures of EE obtained by indirect calorimetry. Non-wear time was assumed to be time spent sleeping or resting and assigned 1.0 METs. The thermic effect of food (TEF) was assumed to be 10% of EI. Thus the calculation for TDEE was:
In addition to TDEE, we also calculated PA energy expenditure (PAEE) as follows:
Follow-up Assessments
Body weight (digital scale) and body composition (DXA) were assessed at the University of Colorado Hospital annually for 5 years. Rate of weight change (RoWC) and rate of fat mass change (RoFMC) were used to assess longitudinal changes in weight and body composition because follow-up duration varied among individuals (minimum: 1 year, maximum: 5 years). RoWC and RoFMC were calculated as Δ weight (kg)/number of years of follow-up and Δ FM (kg)/number of years of follow-up, respectively.
Statistical Analyses
The primary goal of this analysis was to determine whether adaptive responses to 3 days of overfeeding (e.g., change in PA, EI, TDEE, and energy balance) differ between OP and OR. In addition, we sought to examine whether these responses were correlated with the rate of change in body weight and FM measured over a 5-year follow-up period. All variables are reported as mean ± standard deviation (SD). Statistical significance was set at P ≤0.05. Differences between OP and OR in baseline characteristics, EI, and PA during the lead-in period were explored using t tests. To determine whether OP and OR had different responses in energy balance to overfeeding, data were analyzed using repeated measures ANOVA. We also calculated delta values for each variable of interest (Δ response = OF condition – EU condition) to aid in interpretation of the data. Paired t tests with Bonferroni adjustment were used to examine within group changes. Changes in sedentary time, LPA, MVPA, EI, TDEE, and energy balance averaged over 3 days following OF were explored as potential predictor variables for 5-year weight gain. Pearson correlations were conducted to examine the correlation between changes in these adaptive responses and 5-year rate of bodyweight and FM change. Data were analyzed using SPSS Statistics version 24.0 (IBM Corp., Armonk, New York).
RESULTS
EATS enrolled a total of 55 subjects.(16) For the present analysis, individuals without longitudinal weight change were excluded (n=6; 3M/3F) and individuals without valid PA data on both the EUC and OF conditions were excluded (n=8; 3M/5F). Thus, forty-one subjects (OP; n=21, OR; n=20) were included in the final analysis. OP had a significantly higher age, BMI, and percent body fat compared to OR (p<0.05); however, there were no other differences between the two groups (Table 1). In addition, there were no differences in EE, EI, or PA between OP and OR during the controlled feeding periods (Days 5–7, Table 2). Subjects who completed both EU and OF were prospectively followed providing changes in weight and body composition for 5 years.
Table 1.
Subject characteristics
| OP | OR | p-value | |
|---|---|---|---|
| Female, n, % | 13, 59% | 10, 50% | 0.76 |
| Age (years) | 28.9 ± 2.6 | 27.4 ± 2.2 | 0.05* |
| Weight (kg) | 68.4 ± 10.0 | 61.3 ± 10.9 | 0.05* |
| BMI (kg/m2) | 23.8 ± 2.5 | 20.2 ± 2.1 | <0.001* |
| Body Fat % | 25.6 ± 8.5 | 19.0 ± 5.7 | 0.01* |
| Fat Free Mass (kg) | 50.5 ± 10.3 | 49.6 ± 10.8 | 0.79 |
| REE (kcal/day) | 1509 ± 259 | 1510 ± 247 | 0.98 |
Significant difference between OP and OR (p<0.05)
Table 2.
Energy balance and physical activity during days 5–7 of controlled EU and OF
| OP | OR | p-value | |
|---|---|---|---|
| Calorie Intake (EU) (kcal/day) | 2325 ± 351 | 2274 ± 409 | 0.67 |
| Calorie Intake (OF) (kcal/day) | 3251 ± 513* | 3139 ± 531* | 0.49 |
| Energy Expenditure (EU) (kcal/day) | 2367 ± 378 | 2367± 408 | 0.99 |
| Energy Expenditure (OF) (kcal/day) | 2439 ± 407 | 2441 ± 438 | 0.99 |
| Energy Balance (EU) (kcal/day) | −44 ± 293 | −94 ± 220 | 0.54 |
| Energy Balance (OF) (kcal/day) | 819 ± 361* | 697 ± 260* | 0.22 |
| SED (EU) (min/day) | 616.0 ± 75.0 | 631.2 ± 74.2 | 0.51 |
| SED (OF) (min/day) | 585.9 ± 95.2 | 605.6 ± 56.6 | 0.43 |
| Light Activity (EU) (min/day) | 140.2 ± 32.4 | 148.6 ± 37.2 | 0.44 |
| Light Activity (OF) (min/day) | 141.4 ± 44.8 | 157.1 ± 46.8 | 0.28 |
| MVPA (EU) (min/day) | 60.7 ± 20.1 | 63.7 ± 23.6 | 0.65 |
| MVPA (OF) (min/day) | 59.8 ± 34.5 | 56.9 ± 19.9 | 0.75 |
Significant difference between the OF and EU condition (p<0.05).
Accuracy of the calculated TDEE
The average TDEE generated by our equation was 2285 ± 385 kcals/day compared to whole-room indirect calorimetry on day 7 of EU (2280 ± 452 kcals/day) with no differences between the measurements (p>0.05) and a Pearson correlation of 0.71 (r2=0.51). A Bland-Altman analysis showed that the mean bias between measurements was −5 kcals/day (95% CI; −109 – 99 kcals/day). This method of estimating TDEE has similar accuracy compared to using other types of PA monitoring devices.(24, 25)
Changes in Physical Activity and PAEE
The PAMS was worn for an average of 13.4 ± 1.2 h/day across all study visits with no differences between OP and OR. Wear time for the PAMS was not different between the OF and EU conditions; thus, within subject changes in PA derived from the PAMS were not confounded by wear time. There were no significant differences between OP and OR in any measurement of PA during the controlled EU and OF study periods (Days 5–7, Table 2).
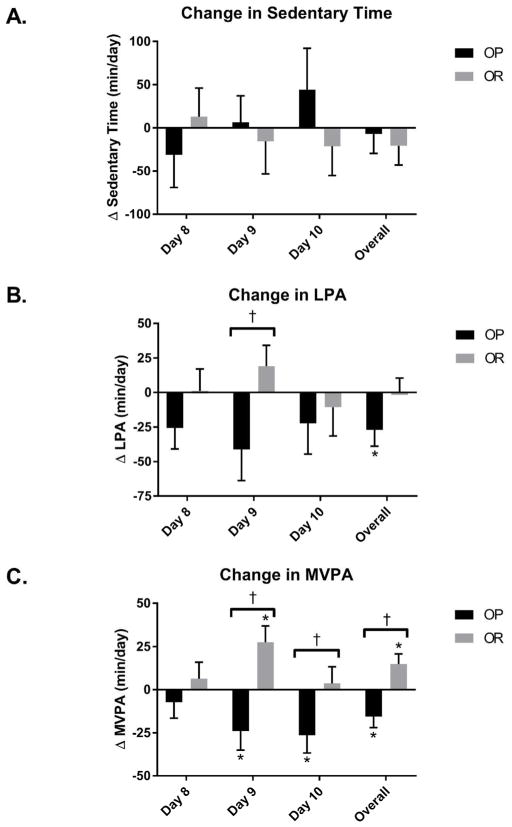
Changes in PA during days 8–10 (free-living conditions with ad libitum feeding) are shown in Figure 2. There was a significant group X feeding condition interaction for overall MVPA across days 8–10 (p<0.001, Figure 2C). OP decreased overall MVPA across the days 8–10 following OF compared to EU by −15.6 ± 26.1 min/day while OR significantly increased overall MVPA during days 8–10 (14.7 ± 25.5 min/day). In addition, there were significant group X feeding condition interactions for MVPA on day 9 (p<0.001) and day 10 (p=0.03). OP had significant decreases in MVPA on day 9 (−24.0 ± 42.5 min/day) and day 10 (−26.4 ± 34.2 min/day), while OR increased MVPA on day 9 (27.5 ± 39.5 min/day) and maintained MVPA on day 10 (3.6 ± 34.5 min/day). There was a significant group X feeding condition interaction for LPA on day 9 (p=0.02, Figure 2B) with OP decreasing LPA (−41.1 ± 88.2 min/day) and OR increasing LPA (19.1 ± 64.1 min/day). OP also had an overall decrease in LPA across days 8–10 following OF (p=0.02). There were no significant differences in sedentary time between OP and OR following OF (Figure 2A).
Figure 2. Changes in physical activity and sedentary time following overfeeding.
Δ calculated at OF-EU on protocol days 8, 9, 10 and the average of these 3 days. † Significant interaction effect with differences between OP and OR (p<0.05). * Significant within group change (p<0.05).
There was a significant group X feeding condition interaction for PAEE across day 8–10 (p<0.01). OP decreased overall PAEE by −117 ± 156 kcal/day across days 8–10 following OF compared to EU, while OR maintained PAEE (43 ± 118 kcal/day) across days 8–10. Similarly, there was a group X feeding condition interaction for PAEE on day 9 (p<0.001). OP decreased PAEE on day 9 (−139 ± 239 kcal/day,) and while OR increased PAEE on day 9 by 120 ± 162 kcal/day. OP also significantly decreased PAEE on day 10 (p=0.02).
Changes in EI, TDEE and Energy Balance
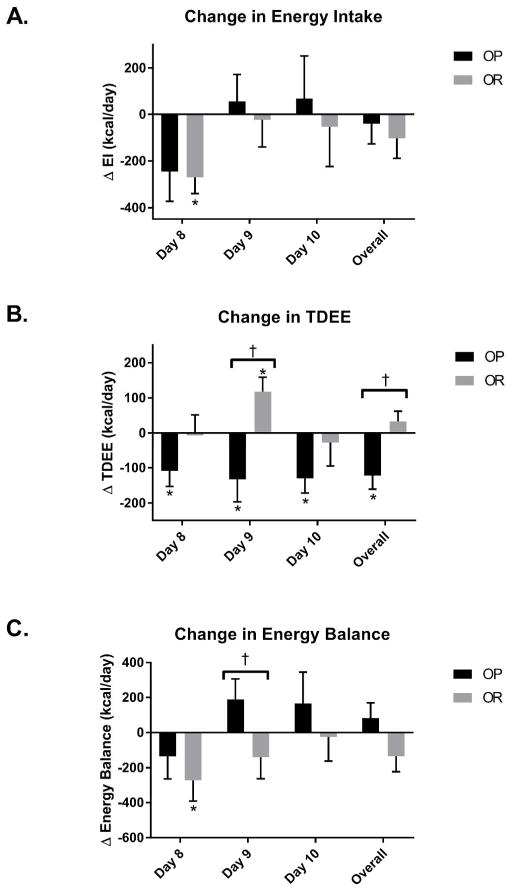
There were no differences in TDEE, EI, or energy balance (calculated as EI-TDEE) between OP and OR during the controlled EU and OF study periods (Days 5–7, Table 2). There was no significant interaction for EI for days 8–10 (Figure 3A). However, OR had a statistically significant reduction in EI during day 8 of OF compared to EU (−270 ± 477 kcal/day, p=0.03). There was a significant group X feeding condition interaction for TDEE across days 8–10 (p=0.001, Figure 3B). Following the controlled feeding conditions, OP decreased overall TDEE (−122 ± 160 kcal/day) during days 8–10 of OF compared to EU while OR maintained EE (33 ± 118 kcal/day). Similarly there was a group X feeding condition interaction for TDEE on day 9 (p=0.001) with OP decreasing TDEE (−133 ± 247 kcal/day) during OF compared to EU and OR increasing TDEE (118 ± 157 kcal/day). There was no significant interaction for overall energy balance across days 8–10 (Figure 3C) However, there was a significant group X feeding condition interaction on day 9 (p=0.03). OP increased energy balance (190 ± 440 kcal/day) during day 9 following OF while OR decreased energy balance (−140 ± 459 kcal/day).
Figure 3. Changes in energy intake, TDEE, and energy balance following overfeeding.
Δ calculated at OF-EU on protocol days 8, 9, 10 and the average of these 3 days. † Significant interaction effect with differences between OP and OR (p<0.05). * Significant within group change (p<0.05).
Associations with Weight Change over 5-years
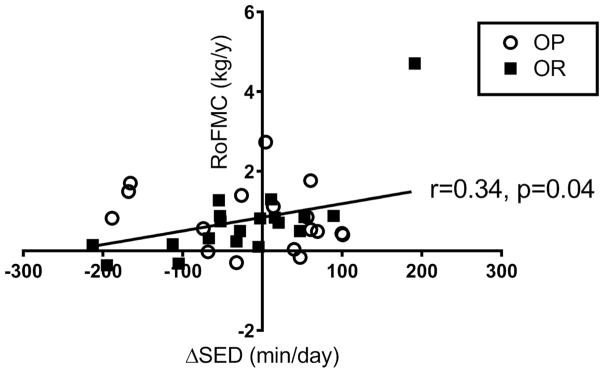
OP and OR gained 4.2 ± 3.1 kg and 3.0 ± 3.2 kg respectively with no significant differences between groups, during 4.1 ± 1.4 years of follow up. These changes in bodyweight correlated to a change in fat mass of 3.0 ± 3.0 kg and 2.5 ± 2.3 kg for OP and OR, respectively. The 3-day mean changes in sedentary time, LPA, MVPA, EI, TDEE, and energy balance were explored as predictors of 5-year changes in body weight and composition. When examining predictor variables for the entire cohort (OP and OR), none of the adaptive responses predicted longitudinal weight gain. However, changes in sedentary time were correlated with longitudinal changes in the rate of fat mass change (r=0.34, p=0.04, Figure 4). After visual inspection there was one notable outlier. Thus, analyses were performed without this subject and the correlation was no longer significant (r=0.10, p=0.58). When performing the analysis separately for each phenotype, we found that this relationship was primarily driven by OR (r=0.73, p<0.001). In addition, change in sedentary time for OR was associated with rate of weight change (r=0.68, p<0.01). When removing the outlier again, these relationships remained significant. There were no associations between changes in any of the other variables and changes in body weight or body composition for OP and OR.
Figure 4. Correlations between Δ sedentary time and 5-year changes in fat mass.
Δ calculated as OF-EU on the 3-day mean of days 8, 9, 10. RoFMC: rate of fat mass change.
DISCUSSION
We correlated adaptive responses to short-term overfeeding with measured changes in weight and body composition over 5 years in a sample of individuals enriched in those with a propensity for and those at lower risk for weight gain. Based upon previous observations (5, 16), we hypothesized that individuals who were able to compensate for the overfeeding (by either decreasing EI or increasing TDEE through PA) would be protected against weight gain over time. We found that an increase in sedentary time following 3 days of overfeeding correlated with longitudinally measured changes in fat mass in the entire cohort. This finding was largely driven by the OR group that had a variable response to overfeeding and somewhat less weight gain over time. However, these adaptive responses were not strong predictors of weight gain. It is possible that examining responses to one overfeeding episode does not correlate well with weight change because significant weight gain only occurs after numerous overeating episodes summed over time. These findings while not dramatic, support the idea that engaging in more sedentary behavior following overfeeding may be one mechanism which is contributing to weight gain, specifically fat gain, for some individuals.
Over the 3 days following overfeeding, OP tended to decrease PA leading to decreases in TDEE and PAEE. OR tended to increase PA, resulting in increases in PAEE and TDEE, in a manner that partially compensated for the overfeeding. During and following overfeeding, there is an inherent increase in both sleeping and resting EE despite little or no change in PA.(4, 16, 26) We previously reported a 4–5% increase in 24h EE during 140% overfeeding,(16) while Thearle et al. and He et al. reported an increase of ~10% during 200% and 150% overfeeding respectively.(26) Nonetheless, these immediate increases in 24h EE are insufficient to completely offset the overfeeding stimulus. Consequently, individuals may need to increase non-exercise activity thermogenesis (NEAT) or energy expended in PA to restore energy balance. Immediate compensation for a substantial overfeeding episode (~1000 kcal/day), such as in the current study, would require an extreme amount of PA to be performed the following day (15–20 km of walking). Thus, it is more likely that the individual would attain energy balance by increasing PA over a period of several days. In the current study, OP decreased TDEE by an average ~125 kcal/day over the 3 days following overfeeding while OR increased TDEE by an average of ~50 kcal/day. This suggests adaptive responses in PA likely occur over a period of several days following a period of energy imbalance. Moreover, it is likely that increasing EE alone is not sufficient to restore energy balance and individuals would need to reduce EI following overfeeding to adequately compensate for the extra ingested calories and achieve energy balance.
Both OP and OR demonstrated non-significant decreases in EI following 3 days of overfeeding when compared to the energy-balanced condition with large inter-individual variations in both groups. Rodents have demonstrated an ability to downregulate food intake following overfeeding;(27, 28, 29) however, previous studies in humans have failed to observe sufficient, immediate downregulation of EI following overfeeding.(3, 4, 30) It has been suggested that the time-course for adaptive responses in EI to overfeeding in humans may take 3–4 days.(2) We observed an immediate downregulation of EI on the first day following overfeeding, however, we failed to see complete compensation for the overfeeding condition even after 3 days of observation. Jebb et al. observed large inter-individual variations in ad libitum EI following three different overfeeding conditions(3) suggesting that some individuals either innately or cognitively have the ability to compensate for periods of overfeeding. Other studies have suggested that changes in hormones such as insulin, leptin, peptide tyrosine-tyrosine (PYY), ghrelin, and glucagon-like peptide-1 (GLP-1) may help regulate food intake following periods of energy imbalance; however, the overall impact of these changes on EI have been questioned.(3, 4, 31) Moreover, other studies suggest that PA may help to regulate EI. However, we failed to observe any consistent interactions between PA an EI following OF. Based on our study and others, it appears that following short-term periods of positive energy balance, humans do not immediately downregulate food intake to an extent necessary to restore energy balance. This inability to compensate for periods of overeating may increase humans’ propensity for weight gain.
In the current study, OR increased both LPA and MVPA following overfeeding, while OP tended to decrease both LPA and MVPA. Neither OP or OR had significant changes in sedentary behavior. Previous studies have proposed that increasing PA and NEAT, and decreasing sedentary behavior may help compensate following overfeeding; thus, helping to avoid weight gain.(4, 9, 32) It is well established that MVPA is important for long-term weight loss and weight maintenance;(33, 34, 35) however, the mechanisms through which PA acts to regulate bodyweight are unclear. PA may help prevent weight gain by increasing EE,(35) regulating EI,(8) increasing metabolic flexibility,(36) or through other mechanisms that are not fully understood. Future studies should focus on delineating the pathways through which increases in PA promote weight maintenance. In addition, the regulating mechanisms through which overfeeding would elicit changes in PA are unclear. These changes may be due to hormonal, behavioral, or cognitive mechanisms.
A unique aspect of this study is that we prospectively assessed changes in bodyweight and body composition for 5 years after the acute study period. We found that changes in free-living sedentary behavior following overfeeding was correlated with prospective changes in fat mass. This relationship was primarily driven by individuals self-identifying as OR. It is unclear why the adaptive responses between OR and OP would contribute to weight gain differently. It is possible that individuals with different phenotypes may gain weight through different mechanisms. However, more research in this area is needed. Regardless, our findings align with prior literature stating that sedentary behavior and NEAT may play a fundamental role in body weight regulation.(4, 9)
This study has a number of limitations. First, we estimated PAEE and TDEE using a PA monitor (accelerometry) that was calibrated to the measurement of 24h EE from whole-room indirect calorimetry and individuals’ resting metabolic rate. Estimates of TDEE based on PA and movement are not as accurate as measures such as doubly labeled water. Although diet order was randomized, participants were not blinded to the diet. Therefore, we do not know whether individuals altered ad libitum food intake and PA as a conscious effort to compensate for the period of overfeeding or whether these responses were based on physiological differences between individuals. Finally, we had a limited sample for investigating predictors of long-term weight change and as a result may not have had adequate power to find relationships that may have been present.
Taken together, this study demonstrates that the dynamic process of energy balance likely occurs over several days. Both OP and OR have different regulatory responses to a brief episode of overfeeding. OP tended to decrease PA and TDEE following a period of overfeeding while OR individuals increased or maintained levels of PA and TDEE. In general, both OP and OR had minimal decreases or no change in EI following the overfeeding episode. These differences in adaptive responses to overfeeding may affect body weight regulation differently depending on the OP/OR phenotype. Finally, for some individuals time spent sedentary during the days following overfeeding was related to prospective changes in body weight and body composition. Future investigations should focus on the mechanisms through which small changes sedentary behavior, NEAT, and PA may contribute to weight gain.
What is already known about the subject
Humans exhibit large inter-individual metabolic adaptive responses to overfeeding. These adaptive responses to overfeeding likely occur over a period of several days. As the body attempts to maintain energy balance, these adaptive responses happen on both the energy intake and energy expenditure sides of the energy balance equation.
Some individuals increase spontaneous physical activity or decrease sedentary behavior in response to overfeeding. In addition, some humans are able to downregulate energy intake following overfeeding, while others are not.
What does your study add
Few studies have measured metabolic adaptive responses in a free-living environment following short-term overfeeding. This study objectively measured changes in energy intake, energy expenditure, and physical activity for 3 days following overfeeding.
It is unclear whether changes in energy intake, energy expenditure, or energy balance following overfeeding can predict longitudinal changes in weight and body composition. This study shows adaptive responses to overfeeding are highly variable. Increased sedentary behavior in the days following a period of overfeeding may be one mechanism that contributes to weight gain, specifically a gain in fat mass.
Acknowledgments
Funding: This research was supported by: NIH/NIDDK Colorado Nutrition Obesity Research Center 5 P30 DK048520-21, K24 DK02935, RO1 DK62874 (DH Bessesen); NIH/NCRR Colorado CTSI Grant: UL 1 RR025780; NIH/NIDDK K01 DK113063 (CA Rynders); NIH T32 HL116276 (SA Creasy, CA Rynders); NIH/NIDDK K99DK100465 (A Bergouignan).
We wish to thank the Nursing, Clinical Lab, Bionutrition Staffs of the University of Colorado CTRC. We also thank the volunteers who participated in this study.
Footnotes
Clinical Trial Registration: clinicaltrials.gov: NCT00072917
Disclosures: The authors declare no conflict of interest. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 2.Bray GA, Flatt J-P, Volaufova J, DeLany JP, Champagne CM. Corrective responses in human food intake identified from an analysis of 7-d food-intake records. Am J Clin Nutr. 2008;88:1504–1510. doi: 10.3945/ajcn.2008.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jebb SA, Siervo M, Fruhbeck G, Goldberg GR, Murgatroyd PR, Prentice AM. Variability of appetite control mechanisms in response to 9 weeks of progressive overfeeding in humans. Int J Obes. 2006;30:1160–1162. doi: 10.1038/sj.ijo.0803194. [DOI] [PubMed] [Google Scholar]
- 4.He J, Votruba S, Pomeroy J, Bonfiglio S, Krakoff J. Measurement of ad libitum food intake, physical activity, and sedentary time in response to overfeeding. PLoS One. 2012;7:e36225. doi: 10.1371/journal.pone.0036225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt SL, Harmon KA, Sharp TA, Kealey EH, Bessesen DH. The effects of overfeeding on spontaneous physical activity in obesity prone and obesity resistant humans. Obesity. 2012;20:2186–2193. doi: 10.1038/oby.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stubbs RJ, Hughes DA, Johnstone AM, Whybrow S, Horgan GW, King N, et al. Rate and extent of compensatory changes in energy intake and expenditure in response to altered exercise and diet composition in humans. Am J Physiol Regul Integr Comp Physiol. 2004;286:R350–358. doi: 10.1152/ajpregu.00196.2003. [DOI] [PubMed] [Google Scholar]
- 7.Whybrow S, Hughes DA, Ritz P, Johnstone AM, Horgan GW, King N, et al. The effect of an incremental increase in exercise on appetite, eating behaviour and energy balance in lean men and women feeding ad libitum. Br J Nutr. 2008;100:1109–1115. doi: 10.1017/S0007114508968240. [DOI] [PubMed] [Google Scholar]
- 8.Mayer J, Roy P, Mitra KP. Relation between caloric intake, body weight, and physical work: studies in an industrial male population in West Bengal. Am J Clin Nutr. 1956;4:169–175. doi: 10.1093/ajcn/4.2.169. [DOI] [PubMed] [Google Scholar]
- 9.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science (New York, NY) 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 10.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dake BL, Oltman CL. Cardiovascular, metabolic, and coronary dysfunction in high-fat-fed obesity-resistant/prone rats. Obesity. 2015;23:623–629. doi: 10.1002/oby.21009. [DOI] [PubMed] [Google Scholar]
- 12.Jackman MR, MacLean PS, Bessesen DH. Energy expenditure in obesity-prone and obesity-resistant rats before and after the introduction of a high-fat diet. Am J Physiol Regul Integr Comp Physiol y. 2010;299:R1097–1105. doi: 10.1152/ajpregu.00549.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas Braymer H, Zachary H, Schreiber AL, Primeaux SD. Lingual CD36 and nutritional status differentially regulate fat preference in obesity-prone and obesity-resistant rats. Physiol Behav. 2017;174:120–127. doi: 10.1016/j.physbeh.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rupprecht LE, Smith TT, Donny EC, Sved AF. Self-administered nicotine differentially impacts body weight gain in obesity-prone and obesity-resistant rats. Physiol Behav. 2017 doi: 10.1016/j.physbeh.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackman MR, Kramer RE, MacLean PS, Bessesen DH. Trafficking of dietary fat in obesity-prone and obesity-resistant rats. Am J Physiol Endocrinol Metab. 2006;291:E1083–1091. doi: 10.1152/ajpendo.00159.2006. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt SL, Kealey EH, Horton TJ, VonKaenel S, Bessesen DH. The effects of short-term overfeeding on energy expenditure and nutrient oxidation in obesity-prone and obesity-resistant individuals. Int J Obes. 2013;37:1192–1197. doi: 10.1038/ijo.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rynders CA, Bergouignan A, Kealey E, Bessesen DH. Ability to adjust nocturnal fat oxidation in response to overfeeding predicts 5-year weight gain in adults. Obesity. 2017;25:873–880. doi: 10.1002/oby.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas EA, Bechtell JL, Vestal BE, Johnson SL, Bessesen DH, Tregellas JR, et al. Eating-related behaviors and appetite during energy imbalance in obese-prone and obese-resistant individuals. Appetite. 2013;65:96–102. doi: 10.1016/j.appet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornier MA, McFadden KL, Thomas EA, Bechtell JL, Eichman LS, Bessesen DH, et al. Differences in the neuronal response to food in obesity-resistant as compared to obesity-prone individuals. Physiol Behav. 2013;110–111:122–128. doi: 10.1016/j.physbeh.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunwald GK, Melanson EL, Forster JE, Seagle HM, Sharp TA, Hill JO. Comparison of Methods for Achieving 24-Hour Energy Balance in a Whole-Room Indirect Calorimeter. Obesity. 2003;11:752–759. doi: 10.1038/oby.2003.105. [DOI] [PubMed] [Google Scholar]
- 21.Miller GD, Jakicic JM, Rejeski WJ, Whit-Glover MC, Lang W, Walkup MP, et al. Effect of varying accelerometry criteria on physical activity: the look ahead study. Obesity. 2013;21:32–44. doi: 10.1038/oby.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakicic JM, Tate DF, Lang W, Davis KK, Polzien K, Neiberg RH, et al. Objective physical activity and weight loss in adults: The step-up randomized clinical trial. Obesity. 2014;22:2284–2292. doi: 10.1002/oby.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 24.Plasqui G, Bonomi A, Westerterp K. Daily physical activity assessment with accelerometers: new insights and validation studies. Obes Rev. 2013;14:451–462. doi: 10.1111/obr.12021. [DOI] [PubMed] [Google Scholar]
- 25.Chomistek AK, Yuan C, Matthews CE, Troiano RP, Bowles HR, Rood J, et al. Physical Activity Assessment with the ActiGraph GT3X and Doubly Labeled Water. Med Sci Sports Exerc. 2017 doi: 10.1249/MSS.0000000000001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. J Clin Endocrinol Metab. 2013;98:2791–2799. doi: 10.1210/jc.2013-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris RB, Kasser TR, Martin RJ. Dynamics of recovery of body composition after overfeeding, food restriction or starvation of mature female rats. J Nutr. 1986;116:2536–2546. doi: 10.1093/jn/116.12.2536. [DOI] [PubMed] [Google Scholar]
- 28.Harris RB, Martin RJ. Changes in lipogenesis and lipolysis associated with recovery from reversible obesity in mature female rats. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY) 1989;191:82–89. doi: 10.3181/00379727-191-42893. [DOI] [PubMed] [Google Scholar]
- 29.Seeley RJ, Matson CA, Chavez M, Woods SC, Dallman MF, Schwartz MW. Behavioral, endocrine, and hypothalamic responses to involuntary overfeeding. Am J Physiol. 1996;271:R819–823. doi: 10.1152/ajpregu.1996.271.3.R819. [DOI] [PubMed] [Google Scholar]
- 30.Levitsky DA, Obarzanek E, Mrdjenovic G, Strupp BJ. Imprecise control of energy intake: absence of a reduction in food intake following overfeeding in young adults. Physiol Behav. 2005;84:669–675. doi: 10.1016/j.physbeh.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Votruba SB, Kirchner H, Tschop M, Salbe AD, Krakoff J. Morning ghrelin concentrations are not affected by short-term overfeeding and do not predict ad libitum food intake in humans. Am J Clin Nutr. 2009;89:801–806. doi: 10.3945/ajcn.2008.27011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murgatroyd P, Goldberg G, Leahy F, Gilsenan M, Prentice A. Effects of inactivity and diet composition on human energy balance. Int J Obes. 1999;23:1269–1275. doi: 10.1038/sj.ijo.0801062. [DOI] [PubMed] [Google Scholar]
- 33.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 34.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66:239–246. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 36.Rynders CA, Blanc S, DeJong N, Bessesen DH, Bergouignan A. Sedentary behaviour is a key determinant of metabolic inflexibility. J Physiol. 2017 doi: 10.1113/JP273282. [DOI] [PMC free article] [PubMed] [Google Scholar]