Abstract
Background
In 2012, the National Institute for Health and Care Excellence (NICE) assessed guidance (DG7) on the use of tauroselcholic (75selenium) acid (also known as SeHCAT) for the investigation of diarrhoea due to bile acid malabsorption (BAM) in patients with IBS-D and in patients with Crohn’s disease who have not had an ileal resection. NICE concluded that tauroselcholic (75selenium) acid was recommended for use in research only. NICE will be reviewing the decision to update the guidance for tauroselcholic (75selenium) acid, for these populations, in March 2017.
Aim
Our aim is to summarise advances in BAM, also known as bile acid diarrhoea (BAD), and encourage clinicians to re-evaluate their understanding of this disorder.
Approach
We review the prevalence, diagnosis and treatment of BAD/BAM. We describe the new evidence available since the original NICE review in 2012, and discuss the economic issues associated with failure to diagnose or to treat BAD/BAM accurately.
Evidence update
There is new and compelling evidence available since DG7, which shows that tauroselcholic (75selenium) acid scanning is a powerful tool in the diagnosis of BAD/BAM. We summarise published prevalence data (approximately 1% prevalence in the UK, as suggested by clinical practice diagnosis rates), and highlight that the true prevalence of BAD/BAM could be far greater than this.
Conclusion
We present evidence that challenges current opinion about this disorder, and we commend both clinicians and health technology assessment (HTA) agencies for being open to arguments and new evidence in any future HTAs.
Keywords: diarrhoea, bile, malabsorption, cost-effectiveness
Introduction
Bile acid diarrhoea (BAD) and bile acid malabsorption (BAM) misdiagnosed as diarrhoea-predominant irritable bowel syndrome (IBS-D) affect approximately 1% of the UK population; there is also likely an additional large, but as yet unquantified, number of other people with BAM and BAD from secondary causes.1
Both BAD and BAM cause significant morbidity and are related to the production of bile acids, which are secreted into the gastrointestinal (GI) tract in response to the dietary intake of fat. If bile reaches the colon in more than small amounts, because of either true malabsorption in the terminal ileum (BAM) or excess secretion by the liver, and this overwhelms terminal ileal bile reabsorption mechanisms (BAD); the patient will consequently develop symptoms.
BAD/BAM symptoms can severely impact a patient’s quality of life. The symptom most commonly described as pathognomonic of BAD/BAM is ‘chronic watery diarrhoea’2; however, if patients eat minimal fat, there will be little secreted luminal bile, and the extent of the diarrhoea can vary according to this. In particular, triglycerides containing long-chain fatty acids, long-chain free fatty acids, aromatic–aliphatic amino acids and intact proteins in food —but not carbohydrates—trigger cholecystokinin secretion which stimulates secretion of bile acids into the duodenum.3 Patients with BAD/BAM also frequently describe bowel urgency and frequency, abdominal pain, cramps, excessive flatulence and unpredictable bowel habit and, less frequently, steatorrhoea as symptoms. One study found that 20% of patients with these conditions say that constipation following diarrhoea episodes is their worst symptom.4 If patients with such symptoms are investigated systematically, studies show a very high frequency of BAD/BAM diagnosis.4–6 Importantly, a recent (2017) review by Bannaga et al used patient-reported outcomes to highlight that BAD requires more recognition by clinicians to address the current delays in diagnosis, and that treatment improves physical and mental symptoms in the majority of participants.7
Despite being simple to diagnose and treat, BAD/BAM are not frequently considered as diagnoses. Tauroselcholic (75selenium) acid (also known as SeHCAT) is a radiopharmaceutical capsule that is licensed for use in measuring bile acid pool loss and investigating BAD/BAM. It is used to test bowel function by measuring how much the compound is retained or lost from the body into faeces.8 In 2012, the National Institute for Health and Care Excellence (NICE) assessed the use of tauroselcholic (75selenium) acid for the investigation of diarrhoea due to BAM in diagnostic guidance DG7.8 The guidance focused on two populations: patients with chronic diarrhoea considered likely to have IBS-D and patients with chronic diarrhoea diagnosed with Crohn’s disease who have not had an ileal resection. Several chronic diarrhoea populations were noted but excluded from the evaluation, including people with Crohn’s disease who have had an ileal resection, cholecystectomy or radiation-induced bowel damage. Other at-risk populations were not considered at all, such as those undergoing GI or pancreatic surgery, cancer chemotherapy and pelvic radiotherapy and those with diabetes or microscopic colitis. The committee concluded that, while tauroselcholic (75selenium) acid was a potentially clinically important diagnostic test, there was insufficient evidence to determine whether tauroselcholic (75selenium) acid was a cost-effective option for diagnosing BAM in England and Wales; tauroselcholic (75selenium) acid was recommended for use in research only.8
As part of the ongoing review of its guidance, in 2016, NICE advised that the decision on whether to review and update DG7 would be made in March 2017; NICE DG7 only focused on BAD/BAM in a population with IBS-D and Crohn’s disease without an ileal resection. Therefore, this paper aims to summarise our current understanding of the prevalence, diagnosis and treatment of BAD/BAM and to highlight the evidence available since the original NICE review in 2012. To support this aim, we used a targeted research approach to identify any key updates to this evidence base.
Prevalence of BAD/BAM
Chronic diarrhoea may account for up to 5% of GI clinical referrals; estimates of the prevalence of chronic diarrhoea in western populations range between 4% and 5%.8 BAD/BAM are one of several causes of chronic diarrhoea, but their diagnosis may be overlooked due to a lack of clinician awareness and access to appropriate investigations. Consequently, patients with BAD/BAM can be misdiagnosed with conditions such as IBS-D.9–13
The NICE review of tauroselcholic (75selenium) acid, DG7, estimated BAM prevalence to be up to 33% of people with a IBS-D diagnosis and 54% of people with Crohn’s disease who have not had an ileal resection in clinical remission.8 12 Since the NICE review, two studies have been published that further support these data for IBS-D.14 15 At the population level, the prevalence of BAD/BAM in IBS-D has been estimated by Walters and Pattni to be approximately 500 000 people in the UK, approximately 1% of the overall population.1 Based on experience in clinical practice and the evidence outlined in the review, we believe this is likely to be an underestimate; this prevalence exceeds that of better-known conditions, such as Crohn’s disease or ulcerative colitis, and is similar to the prevalence of coeliac disease.1
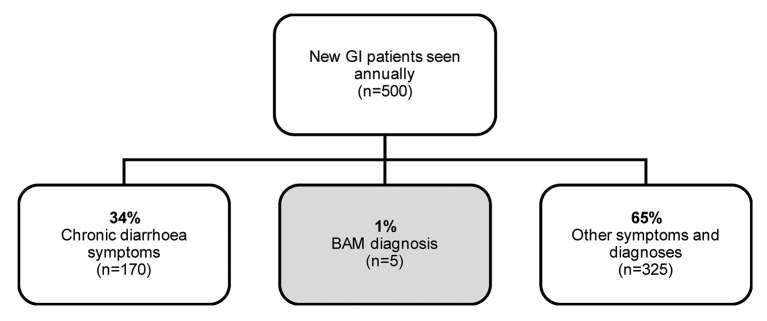
Given these data, there appears to be a clear disconnect between actual BAD/BAM prevalence in the population and the rate of BAD/BAM diagnosis in clinical practice. Khalid et al surveyed practising gastroenterologists who see, on average, 500 new patients annually. Of these patients, 34% have chronic diarrhoea, and only 1% are diagnosed with BAM, as shown in figure 1; this means that only approximately five patients are diagnosed with BAM each year per gastroenterologist. This echoes the recent findings from Bannaga et al, who reported that symptoms had been experienced for more than 5 years before diagnosis in 44% of BAD patient respondents when reporting their disease outcomes.7
Figure 1.
Gastroenterologists survey results—BAM diagnosis (adapted from Khalid et al)14 BAM, bile acid malabsorption; GI, gastrointestinal.
In those with chronic diarrhoea, only 6% of gastroenterologists investigated for BAM in the first treatment line setting, while 61% considered the diagnosis only in selected patients or not at all. We believe that this highlights the underdiagnosis of BAM in clinical practice,16 and note that the investigation of BAD/BAM may be limited by the availability of tauroselcholic (75selenium) acid testing in secondary centres in Europe (and the absence of the test in the USA).17
We suggest that undiagnosed (and therefore untreated) BAD/BAM will result in reduced patient quality of life because of persisting symptoms and unnecessary costs to the health system due to repeated patient visits to primary/secondary care for further tests/treatments. It is therefore important to diagnose BAD/BAM accurately for patients to be appropriately treated.
Tauroselcholic (75selenium) acid use in BAD/BAM diagnosis
At the time of the NICE tauroselcholic (75selenium) acid review in 2012, BAD/BAM diagnosis in UK clinical practice was reported through a combined analysis of personal history, investigations to exclude ’red flag' symptoms and various other diagnostic tests.8 Tauroselcholic (75selenium) acid was recognised as a specific diagnostic for BAM; however, it was noted that the retention rate results of tauroselcholic (75selenium) acid required some interpretation. The committee assessing tauroselcholic (75selenium) acid observed that there was no definitive cut-off between normal and abnormal test results; although it was noted that, in usual practice, retention values of less than 15% may be considered abnormal and indicative of BAM.8 Importantly, the committee did not consider the impact of dietary fat intake on symptom burden, which is known to affect the rate of luminal bile secretion, and therefore the level of diarrhoea.
Since 2012, further evidence has been published. A King’s Technology Evaluation Centre (KiTEC) study collecting data from 38 centres and over 1000 tauroselcholic (75selenium) acid tests reported that, for centres using the test (n=32/38), the majority (69%, n=22/32) reported a retention cut-off point between a normal and an abnormal result to be 15%.18 Some centres further disaggregated this to account for mild, moderate and severe BAD/BAM; however, these severity grades are not standardised. Other recent studies provide evidence on both the reliability of tauroselcholic (75selenium) acid as a diagnostic tool and the increasing use of the test in clinical practice.6 9 19 Valentin et al reported results from a large systematic review and meta-analysis carried out to identify a biomarker for idiopathic BAD in patients with functional bowel disorder with diarrhoea.19 They identified 36 studies that enrolled >5000 patients and analysed data for four different BAD tests. These tests were assessed for their diagnostic yield; that is, the likelihood that a test or procedure will provide the information needed for diagnosis. Of these four tests, the authors concluded that tauroselcholic (75selenium) acid had the highest diagnostic yield (table 1).19
Table 1.
BAD diagnostic test yields (adapted from Valentin et al)19
| Diagnostic test | Average diagnostic yield | 95% CI |
| Tauroselcholic (75selenium) acid | 0.308 | 0.247 to 0.377 |
| Total faecal bile acid secretion | 0.255 | 0.071 to 0.606 |
| Serum FGF19 | 0.248 | 0.147 to 0.385 |
| Serum C4 | 0.171 | 0.134 to 0.217 |
FGF19, fibroblast growth factor 19.
Moreover, tauroselcholic (75selenium) acid use in clinical practice is increasing. Smith and Perkins reported responses from 129 UK centres regarding tauroselcholic (75selenium) acid use in clinical practice; 57% of centres used tauroselcholic (75selenium) acid, with 70% reporting an increase in tauroselcholic (75selenium) acid workload over the previous 3 years.6 Gracie et al reported results for 373 patients with chronic diarrhoea who underwent tauroselcholic (75selenium) acid scanning in two UK hospitals between January 2005 and December 2011.9 The number of tauroselcholic (75selenium])acid scans requested each year increased (statistically) significantly from 26 in 2005 to 111 in 2011.9
We consider that the growing use of the tauroselcholic (75selenium) acid test in clinical practice is a consequence of a recognition of its value. Many patients with BAD/BAM have spent long symptomatic periods awaiting correct treatment; Pattni et al reported that the average duration of diarrhoea prior to diagnosis was 24–33 months, depending on the BAD subtype and ranged from 12 to 114 months.2 Growing tauroselcholic (75selenium) acid use may reflect the value of reducing this waiting time on patient quality of life and cost. Furthermore, tauroselcholic (75selenium) acid is also now used to diagnose other GI indications, such as pelvic radiation disease in cancer.20 21
Some studies have suggested that tauroselcholic (75selenium) acid should be considered at an early stage in the diagnostic pathway in younger patients.22 The KiTEC study observed that many patients underwent this test without a prior colonoscopy,18 and suggested that the placement of tauroselcholic (75selenium) acid in the pathway laid out by the British Society of Gastroenterology (BSG) guidelines for chronic diarrhoea may need to be reconsidered; as we believe the BSG guidelines to be somewhat outdated.
Treatment for patients with BAD/BAM
In the UK, patients with a confirmed or suspected BAD/BAM clinical diagnosis are generally offered treatment with a bile acid sequestrant (BAS) such as colestyramine, colesevelam or colestipol.11 The KiTEC study reported that a BAS was prescribed to 73% (n=117/161) of patients with an abnormal tauroselcholic (75selenium) acid result.18 Other less commonly used BAM treatments may include dietary fat manipulation; whereby reduced dietary fat intake may reduce the volume of bile secreted and thus may reduce patient symptoms.20 Furthermore, dietary fat reduction is likely to have additional health benefits other than just improving GI symptoms, as well as being an extremely cheap intervention.
Of the available BAS treatments, colestyramine is the most frequently prescribed. The KiTEC study reported that, of those patients receiving a BAS, 70% received colestyramine.18 Colestyramine has previously been associated with poor compliance due to its unpleasant taste.8 Colesevelam, a newer BAS that is available in tablet form, may lead to higher compliance; however, colesevelam is not licensed for BAM treatment.23 Furthermore, the off-label use of colesevelam has been critically reviewed by NICE, however the evidence summary does not include recommendations on its use.23
A definitive BAD/BAM diagnosis is likely to improve colestyramine compliance because there will be clear and expected (and actual, in most cases) benefit to offset treatment unpleasantness. Orekoya et al reported that offering colestyramine without a diagnostic justification is unlikely to prove effective, as patients have no motivating diagnosis to warrant colestyramine continuation.10 They also reported that patients with BAM who are not diagnosed and subsequently discontinue colestyramine are unlikely to be prescribed colesevelam because it is assumed that they do not have BAM.10
Evidence suggests that response to BAS treatment is directly related to the retention cut-off point established from the tauroselcholic (75selenium) acid diagnostic test.18 Wedlake et al presented data showing that response to colestyramine occurred in 96% of patients with <5% retention, 80% at <10% retention and 70% at <15% retention.13 Moreover, Kumar et al reported data on the impact of the diagnostic test on treatment decisions: in a retrospective audit of 88 patients who had a tauroselcholic (75selenium) acid test, the result changed treatment in 84% of patients with an abnormal scan and 33% of patients with a normal scan.24 Kumar et al therefore demonstrates how pivotal the tauroselcholic (75selenium) acid test is in treating BAD/BAM in clinical practice.
For all BAD/BAM treatment options, a clear diagnosis using the best available test is essential to target treatment successfully.25 Future implications of this could mean that it will be possible to stratify BAD/BAM treatment for individual patients by their tauroselcholic (75selenium) acid retention. This is further strengthened by Walters et al, who suggest that the continuous distribution of tauroselcholic (75selenium) acid retention can act as a biomarker for predicting a response to a BAS.25
Tauroselcholic (75selenium) acid cost effectiveness
The 2012 NICE DG7 review included a cost-effectiveness evaluation. Two economic models were developed to evaluate tauroselcholic (75selenium) acid in BAM diagnosis in patients with IBS-D and in patients with Crohn’s disease who have not had an ileal resection. The models were developed with the best available data regarding BAM and tauroselcholic (75selenium) acid.
However, in DG7, key data responsible for driving the model results were based on assumptions that had no supporting evidence or the opinion of a small number of clinicians.8 Particularly after initial diagnosis and treatment either using or not using tauroselcholic (75selenium) acid, patients within the model could move between health states of ‘diarrhoea’ and ‘no diarrhoea’ over the long term. The probability of moving between these health states was based on assumed values, with no clinical input or evidence.
Given the uncertainty in the model parameters, a range of scenarios were analysed. For both the population with IBS-D and Crohn’s disease without ileal resection, the analysis found that in some scenarios, the use of tauroselcholic (75selenium) acid was cost effective, while in others it was not. A key driver was the probability of moving between health states. The results of the economic evaluations therefore showed considerable uncertainty regarding the cost effectiveness of tauroselcholic (75selenium) acid in these populations.
Should a health technology assessment (HTA) agency reassess the cost effectiveness of tauroselcholic (75selenium) acid in the future, we note several areas where the analysis could be strengthened. First, given that the key data driving the models developed for DG7 were based on assumptions, we recommend that these data gaps be identified and populated with more reliable data prior to any DG7 review. Second, we suggest that the model used to frame the analysis may not fully capture the potential cost and quality of life savings associated with tauroselcholic (75selenium)acid use. The key rationale for this assertion is that outcomes for patients with undiagnosed BAM were not explicitly captured in the original structure. Instead, these patients were assumed to experience the same costs and quality of life impact as patients with IBS-D or Crohn’s disease without ileal resection. In fact, patients with undiagnosed BAM are likely to have considerable additional ongoing costs such as repeat appointments or additional tests or, in the case of Crohn’s disease, potentially expensive treatment for a relapse where the cause of symptoms may be BAD/BAM. Moreover, patients with undiagnosed BAM have a significant quality of life burden because of their ongoing symptoms.
Discussion
Our review highlights that, since the NICE DG7 guidance, there has been a substantial update to the evidence, and we believe this supports the view that tauroselcholic (75selenium) acid could be considered as a ‘gold standard’ for BAD/BAM assessment. We also emphasise the use of tauroselcholic (75selenium) acid retention as continuum rather than one point cut-off to tailor the patient treatment and predict/assess response to the treatment.
BAD/BAM prevalence may have previously been undervalued. It may be justifiable to consider a condition unimportant when it is rare, has little impact on patients, is difficult to diagnose, treatment options are few or non-existent and there are minimal health economic consequences. However, BAD/BAM fulfil none of these criteria and frequently cause severe symptoms. Patient symptoms respond favourably to dietary fat intake reduction, which is an important health intervention with many positive benefits over and above improvement in bowel health; for others, there is a range of effective medications.
BAD/BAM are also likely being underdiagnosed in the clinical setting. Failure to diagnose this condition means that many patients undergo unnecessary and expensive tests (eg, colonoscopy), often repeatedly, and are offered ineffective and frequently costly medications. In addition, BAD/BAM are being increasingly described in various patients during and after cancer treatment: evidence shows that cancer treatment is a common cause of diarrhoea-with chemotherapy, biological treatments after many forms of GI surgery and pelvic radiotherapy (during and after) compromising these peoples’ opportunity to receive curative therapy and enormously increasing the cost of cancer care delivery.
This review highlights the importance to HTA agencies and the gastroenterology clinical community of providing updated evidence for a high-quality appraisal when DG7 is reviewed in March 2017 through both additional clinical data and an update of the economic analysis supporting any future HTA application. Based on the updated evidence we have presented, it is tempting to speculate that true BAD/BAM prevalence, misdiagnosis and burden of disease constitute a substantial demand on healthcare services. Additionally, the lack of informed guidance and full evaluation of proper diagnosis is further contributing to this demand. However, if BAD/BAM are diagnosed using tauroselcholic (75selenium) acid and therefore treated effectively, we believe that this would reduce costs and improve patient care by avoiding unnecessary care resource use. Furthermore, this would substantially improve symptoms and quality of life for the large affected population.
Footnotes
Contributors: The contents of the manuscript are our original work and are not under consideration for publication elsewhere. We have adhered to the ICMJE criteria for authorship and have followed the journal guidelines for manuscript development. All authors have read and approved this version of the manuscript.
Funding: Financial support for this study was provided by GE Healthcare.
Competing interests: Financial support for this study was provided by General Electric (GE) Healthcare. The funding agreement ensured the authors’ independence in designing the study, interpreting the literature and writing the manuscript. The authors AD, ET and RA are employed by BresMed, which was reimbursed by GE Healthcare as a consultancy for its time developing the manuscript. JA reports personal fees and non-financial support from GE Healthcare, from Sanofi Aventis, during the conduct of the study. MK has no potential conflicts to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: This paper has been amended since it was published Online First. Owing to a scripting error, some of the publisher names in the references were replaced with ’BMJ Publishing Group'. This only affected the full text version, not the PDF. We have since corrected these errors and the correct publishers have been inserted into the references.
References
- 1. Walters JR, Pattni SS. Managing bile acid diarrhoea. Therap Adv Gastroenterol 2010;3:349–57. doi:10.1177/1756283X10377126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pattni SS, Brydon WG, Dew T, et al. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther 2013;38:967–76. doi:10.1111/apt.12466 [DOI] [PubMed] [Google Scholar]
- 3. Brand SJ SW. Textbook of Gastroenterology. Philadelphia: JB Lippincott Company, 1995:25–71. [Google Scholar]
- 4. Gupta A, Muls AC, Lalji A, et al. Outcomes from treating bile acid malabsorption using a multidisciplinary approach. Support Care Cancer 2015;23:2881–90. doi:10.1007/s00520-015-2653-5 [DOI] [PubMed] [Google Scholar]
- 5. Phillips F, Muls AC, Lalji A, et al. Are bile acid malabsorption and bile acid diarrhoea important causes of loose stool complicating Cancer therapy? Colorectal Dis 2015;17:730–4. doi:10.1111/codi.12932 [DOI] [PubMed] [Google Scholar]
- 6. Smith MJ, Perkins AC. A survey of the clinical use of SeHCAT in the UK. Nucl Med Commun 2013;34:306–13. doi:10.1097/MNM.0b013e32835e8989 [DOI] [PubMed] [Google Scholar]
- 7. Bannaga A, Kelman L, O’Connor M, et al. How bad is bile acid diarrhoea: an online survey of patient-reported symptoms and outcomes. BMJ Open Gastroenterol 2017;4:e000116 doi:10.1136/bmjgast-2016-000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence (NICE). DG7: sehcat (tauroselcholic [75 selenium] acid) for the investigation of diarrhoeadue to bile acid malabsorption in people with diarrhoea-predominant irritable bowel syndrome (IBS-D) or Crohn’s disease without ileal resection. 2012. https://www.nice.org.uk/guidance/dg7/resources/sehcat-tauroselcholic-75-selenium-acid-for-the-investigation-of-diarrhoea-due-to-bile-acid-malabsorption-in-people-with-diarrhoeapredominant-irritable-bowel-syndrome-ibsd-or-crohns-disease-without-il-29277341125 (accessed 12 Jul 2016).
- 9. Gracie DJ, Kane JS, Mumtaz S, et al. Prevalence of, and predictors of, bile acid malabsorption in outpatients with chronic diarrhea. Neurogastroenterol Motil 2012;24:983–e538. doi:10.1111/j.1365-2982.2012.01953.x [DOI] [PubMed] [Google Scholar]
- 10. Orekoya O, McLaughlin J, Leitao E, et al. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin Med 2015;15:252–7. doi:10.7861/clinmedicine.15-3-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riemsma R, Al M, Corro Ramos I, et al. SeHCAT [tauroselcholic (selenium-75) acid] for the investigation of bile acid malabsorption and measurement of bile acid pool loss: a systematic review and cost-effectiveness analysis. Health Technol Assess 2013;17:1–236. doi:10.3310/hta17610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith MJ, Cherian P, Raju GS, et al. Bile acid malabsorption in persistent diarrhoea. J R Coll Physicians Lond 2000;34:448–51. [PMC free article] [PubMed] [Google Scholar]
- 13. Wedlake L, A’Hern R, Russell D, et al. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009;30:707–17. doi:10.1111/j.1365-2036.2009.04081.x [DOI] [PubMed] [Google Scholar]
- 14. Aziz I, Mumtaz S, Bholah H, et al. High prevalence of idiopathic bile acid diarrhea among patients with diarrhea-predominant Irritable bowel syndrome based on rome III criteria. Clin Gastroenterol Hepatol 2015;13:1650–5. doi:10.1016/j.cgh.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 15. Slattery SA, Niaz O, Aziz Q, et al. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther 2015;42:3–11. doi:10.1111/apt.13227 [DOI] [PubMed] [Google Scholar]
- 16. Khalid U, Lalji A, Stafferton R, et al. Bile acid malabsoption: a forgotten diagnosis? Clin Med 2010;10:124–6. doi:10.7861/clinmedicine.10-2-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mottacki N, Simrén M, Bajor A. Review article: bile acid diarrhoea - pathogenesis, diagnosis and management. Aliment Pharmacol Ther 2016;43:884–98. doi:10.1111/apt.13570 [DOI] [PubMed] [Google Scholar]
- 18. Summers JA, Peacock J, Coker B, et al. Multicentre prospective survey of SeHCAT provision and practice in the UK. BMJ Open Gastroenterol 2016;3:e000091 doi:10.1136/bmjgast-2016-000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valentin N, Camilleri M, Altayar O, et al. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: a systematic review and meta-analysis. Gut 2016;65 doi:10.1136/gutjnl-2015-309889 [DOI] [PubMed] [Google Scholar]
- 20. Andreyev HJ, Muls AC, Norton C, et al. Guidance: the practical management of the gastrointestinal symptoms of pelvic radiation disease. Frontline Gastroenterol 2015;6:53–72. doi:10.1136/flgastro-2014-100468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andreyev J, Ross P, Donnellan C, et al. Guidance on the management of diarrhoea during Cancer chemotherapy. Lancet Oncol 2014;15:e447–60. doi:10.1016/S1470-2045(14)70006-3 [DOI] [PubMed] [Google Scholar]
- 22. Kok B, Malhotra R, Mistry A, et al. PTU-184 Unload the Burden of Unnecessary Investigations and Reduce the Delay in Diagnosing Bile Acid Malabsorption (BAM). Gut 2013;62(Suppl 1):A124.1–A124. doi:10.1136/gutjnl-2013-304907.274 [Google Scholar]
- 23. National Institute for Health and Care Excellence (NICE). Bile acid malabsorption: colesevelam 2013. https://www.nice.org.uk/advice/esuom22/ifp/chapter/About-this-information (accessed 10 Jul 2016).
- 24. Kumar L, Jamshed B, Emmanuel A. PTH-179 An Audit of Clinical outcomes of Sehcat Study in Patients with Chronic Diarrhoea: Abstract PTH-179 Table 1. Gut 2013;62(Suppl 1):A284.1–A284. doi:10.1136/gutjnl-2013-304907.666 [Google Scholar]
- 25. Walters JR. Defining primary bile acid diarrhea: making the diagnosis and recognizing the disorder. Expert Rev Gastroenterol Hepatol 2010;4:561–7. doi:10.1586/egh.10.54 [DOI] [PubMed] [Google Scholar]