Abstract
Background
Circulating tumor cells (CTCs) can be identified in approximately 25% of non-metastatic breast cancer patients (BC), and data are emerging regarding their prognostic significance. We hypothesized that CTCs identified prior to resection of the primary tumor would predict worse outcome in non-metastatic BC patients.
Study Design
We performed CTC enumerations on 509 patients with non-metastatic BC as part of an IRB approved study. CTCs (per 7.5 ml blood) were identified using the Cell Search® System (Janssen). The presence of ≥ 1 CTC meeting morphological criteria for malignancy was considered a positive result. Log-rank test and Cox regression analysis were applied to establish the association of CTCs with relapse-free and overall survival.
Results
Median follow-up was 48 months and mean age was 53 years. Fifty-nine percent of patients (299/509) had tumors >2cm, and 46% (234/509) had positive lymph nodes. One hundred sixty-six patients received neoadjuvant chemotherapy (NACT) prior to CTC assessment, and 343 patients were chemonaïve. One or more CTC was identified in 43/166 (26%) of NACT treated patients, and in 81/343 (24%) of chemonaïve patients. CTCs were not associated with tumor size, grade, or lymph node status (P= NS). Detection of one or more CTCs predicted decreased relapse-free (log-rank P<0.001, HR = 2.72, 95% CI, 1.57 to 4.72; P<0.001) and overall survival (log-rank P=0.02, HR = 2.29, 95% CI, 1.12 to 4.67; P = 0.03) at 48 months of follow-up.
Conclusions
One or more CTCs identified prior to resection of the primary breast tumor predicted worse relapse-free and overall survival, irrespective of primary tumor size, grade, or lymph node positivity.
Introduction
Distant metastasis is the primary cause of death for breast cancer patients. Metastasis is a complex, multi-step process orchestrated by a subpopulation of cells within a heterogeneous tumor that acquire the ability to disseminate from the primary tumor and enter the bloodstream and/or lymph nodes. Currently, lymph node metastasis is considered to be the most powerful prognostic predictor for breast cancer, and forms the basis of the current pN category of the American Joint Commission on Cancer (AJCC) Tumor Node Metastasis (TNM) staging system.(1) However, even lower grade, lymph node negative patients have relapse rates of 20% over 10 years, and the relapse rate increases to 30% for node negative patients with high grade tumors.(2) Conversely, many patients with lymph node metastases will not relapse following treatment.(2–4) These data suggest that: 1) occult dissemination of cancer cells mediates disease progression in a significant number of operable breast cancer patients, irrespective of lymph node involvement and 2) current staging procedures are not sensitive enough to reliably detect and predict disease progression in all patients.
Circulating tumor cells (CTCs) are rare cells (≥1CTC/106 hematopoietic cells)(5) within the peripheral blood that usually remain undetected by high-resolution imaging technologies.(6) For more than a decade clinical researchers have demonstrated the prognostic significance of CTCs in metastatic breast cancer patients using the FDA-approved CellSearch® System (Janssen, Raritan, NJ). Circulating tumor cell counts of ≥5 CTCs/7.5mL blood prior to administration of systemic treatment independently predict shortened progression-free (2.7 months versus 7.0 months in patients with less than 5 CTCs/7.5mL blood) and overall survival (10.1 months versus >18 months) in metastatic patients.(7) In addition, CTC monitoring throughout therapy predicted treatment response better than standard radiologic imaging in metastatic patients.(8, 9) Since metastatic patients account for only 5–8% of newly diagnosed breast cancer cases,(10) many clinical research groups have more recently focused on the prognostic significance of CTCs in non-metastatic patients. In 2010, our group published one of the first studies demonstrating that CTCs can be identified in early-stage breast cancer patients. Thirty percent of the T1/T2 patients in our study had ≥1 CTC/7.5mL blood, indicative of the early dissemination of these cells (11), and these data have been validated by several European studies.(12–16) However, despite the recent studies documenting that CTCs can be detected in a significant number (19–31%) of non-metastatic patients (11, 13–19), data regarding their prognostic significance in these patients has been lacking.
We hypothesized that CTC identification prior to removal of the primary tumor would predict worse progression-free and overall survival in non-metastatic breast cancer patients, irrespective of primary tumor characteristics, axillary lymph node status, or whether they had received neoadjuvant chemotherapy or not. If CTC presence were to contribute to the currently available prognostic information, it would be beneficial in identifying non-metastatic patients at high risk for relapse who could benefit from additional adjuvant therapies or inclusion in clinical trials of novel agents.
Methods
Patients
This study included 509 stage I-III BC patients undergoing surgery for their primary tumor between February 2005 and February 2014. All eligible patients with non-metastatic breast cancer were offered enrollment by the participating surgeons (from 2005–2010: Dr. Lucci, and from 2010 – 2014: Drs. Lucci, Kuerer, and DeSnyder) at The University of Texas MD Anderson Cancer Center. The institutional review board at The University at Texas MD Anderson Cancer Center approved this prospective study (04-0698; PI: A.L.), which included CTC assessment on samples taken prior to initial surgery for the primary breast cancer. We obtained informed written consent from all patients prior to collecting blood. Enrollment was strictly voluntary, and patient results were blinded from investigators by use of a random number system as the unique patient identifier. Patients with bilateral breast cancer, or any other malignancy within five years of diagnosis of the current cancer, were ineligible.
Staging and classification
The primary TNM staging [primary tumor (T), regional nodes (N), distant metastases (M)] and tumor grade was designated according to the criteria set by the American Joint Commission on Cancer (AJCC)(1) and Black’s nuclear grading system,(20) respectively. Clinical stage was defined as TNM stage determined at the time of first diagnostic procedure confirming the invasive component of the tumor. Tumor sections were immunostained for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) using previously published procedures.(11) Immunostaining results for HER2 were scored as positive when >10% of the tumor cells had membranous staining, or when fluorescence in situ hybridization (FISH) for HER2 gene amplification using the Abbott PathVysion HER2 DNA probe kit (Abbott Laboratories, Abbott Park, Ill) HER2/CEP17 ratio was >2.2. TNBC was defined by absence of primary tumor ER, PR expression and HER-2 immunostaining and/or gene amplification.
Isolation, staining, and enumeration of circulating tumor cells
Peripheral blood (7·5 mL) was collected at the time of primary tumor surgery (but prior to any surgical manipulation of the primary tumor). CTC status was determined using the CellSearch System® (Janssen Diagnostics, LLC) within 72 hours of blood collection. This semi-automated technology enriches blood samples for cells expressing the epithelial-cell-adhesion molecule with antibody-coated magnetic beads, labels the nuclei of these enriched cells with fluorescent dye 4,2-diamidino-2-phenylindole dihydrochloride, and stains enriched cells using a combination of CK 8,18,19 and CD45 fluorescent antibodies. A semi-automated fluorescence-based microscope system was used to identify CTCs: nucleated cells positive for CK and negative for CD45, as described previously.(7) A qualified laboratory technician blinded to patient data reviewed all results.
Statistical Analyses
REMARK biomarker guidelines for reporting were utilized.(21) Chi-square or Fisher exact tests were employed to test associations between presence of CTCs and primary tumor characteristics. Fisher’s exact test was applied when expected value in one or more cells was less than 5. Endpoints were characterized using STEEP criteria with RFS and OS as the primary endpoints.(22) Relapse-free and overall survival were defined as time elapsed between date of diagnosis and either the date of clinical disease progression, death, or the last follow-up. Log-rank test was used to detect significant differences between groups. The Cox proportional hazards regression model was used to determine hazard ratios for RFS and OS. P values were two-tailed, and values < 0.05 were considered statistically significant. Kaplan-Meier curves were derived using STATA/IC 11.2 (StataCorp, College Station, TX) for comparison of groups defined by different counts of CTCs.
Results
Patient Characteristics
A total of 509 patients were enrolled for this study, and their demographic data are reported in Table 1. The mean age was 53 years, and median follow-up was 48 months. Two hundred eight of 509 (41%) patients had tumors <2cm, 227 patients (45%) were T2/T3, and 72 patients (14%) had T4 tumors. Two hundred seventy-three patients (54%) had node negative disease, 152 patients (30%) had 1–3 positive lymph nodes, and 82 patients (16%) had more than 3 positive lymph nodes on surgical pathologic assessment. Fifty-six of 509 (11%) had grade 1 tumors, 232 patients (46%) had grade 2 tumors, and 202 patients (40%) had grade 3 tumors. Three hundred fifty-eight of 509 (71%) patients had estrogen receptor positive tumors, 295 of (58%) had progesterone receptor positive tumors, 72 (14%) had tumors with Her2/neu amplification, and 93 (19%) had triple negative tumors.
Table 1.
Patient Demographics
| Variables | Overall cohort | 1 or more circulating tumor cells | |||
|---|---|---|---|---|---|
| n | % | Patients positive (%) | Patients negative (%) | p Value | |
| Total patients | 509 | 124 | 385 | ||
| Age, y, mean (range) | 53 (21 – 91) | 53 (25 – 84) | 53 (21 – 91) | ||
| Median follow-up, mo | 48 | 48 | 48 | ||
| Clinical tumor size | 0.47 | ||||
| <2 cm (T1) | 208/509 | 41 | 50/124 (40) | 158/385 (41) | |
| 2 cm to 5 cm (T2) | 176/509 | 35 | 38/124 (31) | 138/385 (36) | |
| >5 cm (T3) | 51/509 | 10 | 14/124 (11) | 37/385 (10) | |
| Skin/chest wall infiltration (T4) | 72/509 | 14 | 22/124 (18) | 50/385 (13) | |
| Missing | 2/509 | <1 | 2/385 (<1) | ||
| Pathologic nodal status | 0.24 | ||||
| Node negative (N0) | 273/509 | 54 | 64/124 (52) | 209/385 (54) | |
| 1–3 Lymph nodes | 152/509 | 30 | 34/124 (27) | 118/385 (31) | |
| >3 Lymph nodes | 82/509 | 16 | 26/124 (21) | 56/385 (15) | |
| Missing | 2/509 | <1 | 2/385 (<1) | ||
| Histologic tumor grade | 0.27 | ||||
| Low grade (grade 1) | 56/509 | 11 | 14/124 (11) | 42/385 (11) | |
| Intermediate grade (grade 2) | 232/509 | 46 | 50/124 (40) | 182/385 (47) | |
| High grade (grade 3) | 202/509 | 40 | 57/124 (46) | 145/385 (38) | |
| Missing | 19/509 | 4 | 3/124 (3) | 16/385 (4) | |
| Receptors | |||||
| Estrogen receptor positive | 358/509 | 71 | 88/124 (71) | 270/385 (70) | 0.95 |
| Progesterone receptor positive | 295/509 | 58 | 70/124 (56) | 225/385 (58) | 0.72 |
| Missing | 3/509 | 1 | 3/385 (1) | ||
| HER2 receptor positive | 72/509 | 14 | 14/124 (11) | 58/385 (15) | 0.33 |
| Missing or equivocal | 12/509 | 5/124 (4) | 7/385 (2) | ||
| Tumor phenotype | 0.63 | ||||
| Luminal A | 301/489 | 62 | 72/118 (61) | 229/371 (62) | |
| Luminal B | 55/489 | 11 | 13/118 (11) | 42/371 (11) | |
| HER2 | 40/489 | 8 | 7/118 (6) | 33/371 (9) | |
| Triple negative | 93/489 | 19 | 26/118 (22) | 67/371 (18) | |
| Type of surgery | 0.95 | ||||
| Segmental mastectomy | 256/509 | 50 | 63/124 (51) | 193/385 (50) | |
| Total mastectomy | 134/509 | 26 | 31/124 (25) | 103/385 (27) | |
| Modified radical mastectomy | 112/509 | 22 | 28/124 (23) | 84/385 (22) | |
| Radical mastectomy | 7/509 | 1 | 2/124 (2) | 5/385 (1) | |
| Post-menopausal women | 330/505 | 65 | 86/124 (69) | 244/385 (63) | 0.22 |
| Unknown | 4/509 | 1 | 1/124 (1) | 3/385 (1) | |
| NeoadjuvantcChemotherapy | 166/509 | 33 | 43/124 (35) | 123/385 (32) | 0.53 |
| Chemonaïve | 343/509 | 67 | 81/124 (65) | 262/385 (68) | |
| Relapses | 51/509 | 10 | 23/124 (19) | 28/385 (7) | <0.001 |
Circulating Tumor Cell Identification
One or more CTCs were identified in 124 (24%) of patients, two or more CTCs were identified in 39 patients (8%), and 24 patients (5%) had three or more CTCs (Table 1).
Circulating tumor cells and primary tumor characteristics
We identified no significant correlation between CTC presence and tumor size (≥2 cm vs <2 cm) (P=0.47), primary tumor grade (grade 3 vs grade 1/2) (P=0.27), estrogen receptor status (P=0.95), progesterone receptor status (P=0.72), HER2/neu status (P=0.34), or triple negative status (P=0.37) (Table 1).
Circulating tumor cells and axillary lymph node status
We identified no significant correlation between CTC presence and pathologic axillary lymph node status (LN negative versus positive P=0.57). This lack of significant association persisted after stratifying lymph node positive patients into one to three, or more than three positive lymph nodes (P=0.24) (Table 1).
Circulating tumor cells and neoadjuvant therapy
One hundred sixty-six patients (33%) received neoadjuvant chemotherapy (NACT) prior to CTC assessment, and 343 patients (67%) were chemonaïve. One or more CTC was identified in 43 of 166 (26%) of NACT treated patients, and in 81 of 343 (24%) of chemonaïve patients (P=0.53) (Table 1).
Circulating tumor cells and relapse-free survival
Fifty-one patients (11%) relapsed during the 48 month follow up period. Distant metastasis was observed in 46/51 patients. We analyzed association between presence of circulating tumor cells and the site of distant metastasis (lymph nodes, lung, liver, bone, brain, other). None of the sites were significantly associated with presence of circulating tumor cells.
Univariate analysis demonstrated one or more CTC predicted decreased RFS compared to patients with no CTCs (log-rank P<0.001; hazard ratio = 2.72; 95% confidence interval [CI], 1.57 to 4.72; P<0.001) (Figure 1A). These findings were confirmed with multivariate analysis (hazard ratio = 2.68; 95% confidence interval [CI], 1.49 to 4.80; P=0.001) (Table 2). Twenty-three of 124 patients (19%) with one or more CTC relapsed, compared to 28 of 385 patients (7%) with no CTCs. The relapse-free survival rate at 4 years was much lower (80%) in this group, than in patients who had no circulating tumor cells (91%) (Table 3 and 4).
Figure 1.
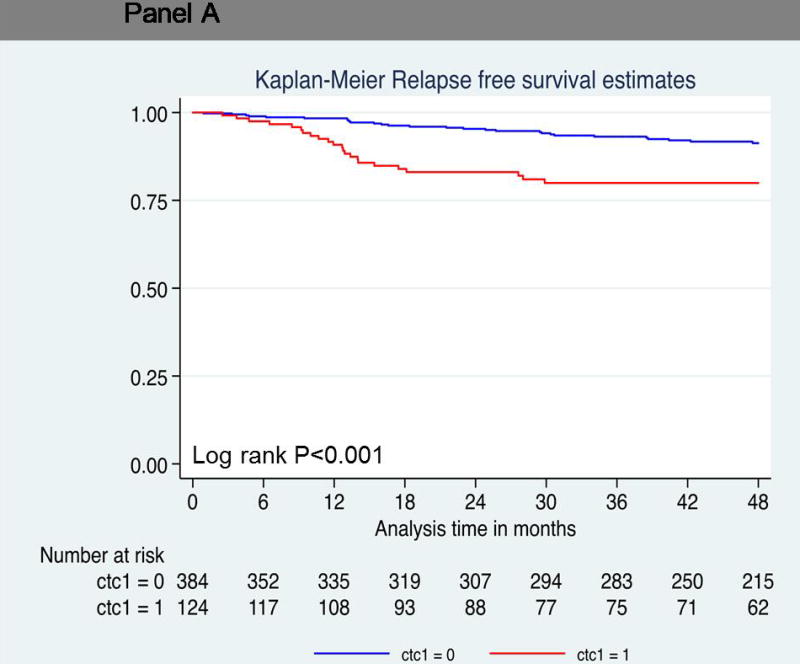
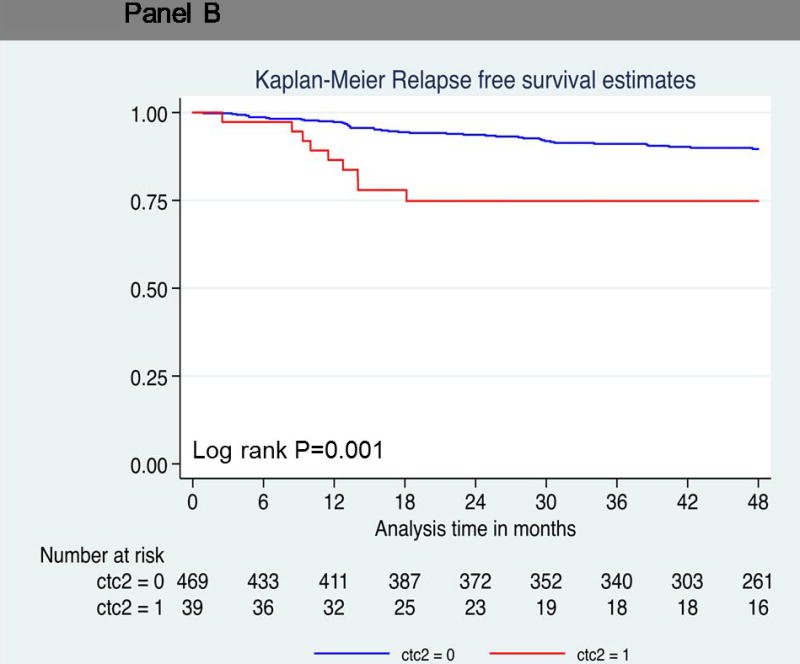
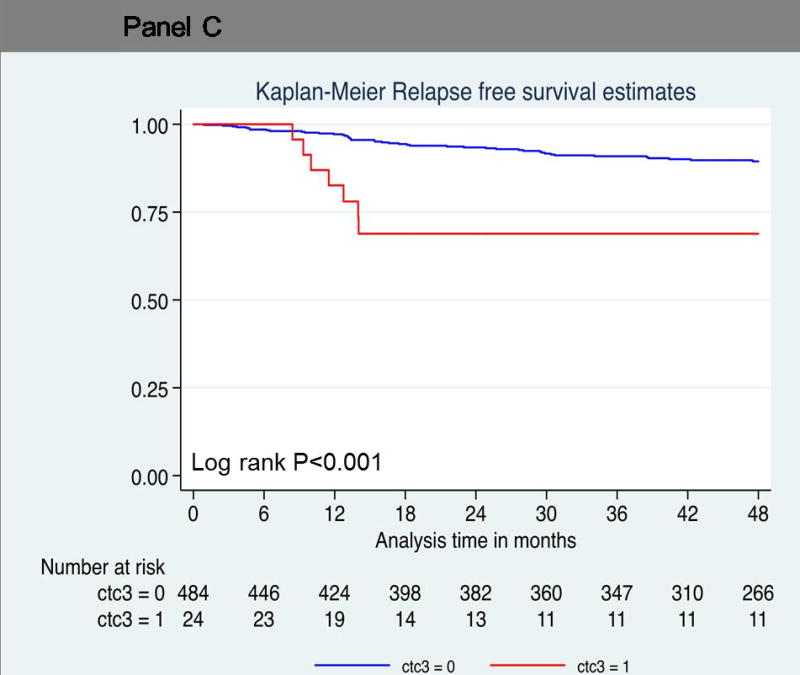
Probability of relapse-free survival. Kaplan-Meier survival estimates of probabilities of relpase-free survival according to circulating tumor cells in operable breast cancer. (A) Probability of relapse-free survival in patients with CTC count ≧1 (Hazard ratio 2.72; 95% CI 1.57 – 4.72; logrank P < 0.001). (B) Probability of relapse-free survival in patients with CTC count ≧2 (Hazard ratio 3.12; 95% CI 1.52 – 6.41; logrank P = 0.001). (C) Probability of relapse-free survival in patients with CTC count ≥ 3 (Hazard ratio 3.97; 95% CI 1.79 – 8.84; logrank P < 0.001).
Table 2.
COX Regression Analyses of Survival Associated with Presence of Circulating Tumor Cells
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | Cox p value | Hazard Ratio | 95% CI | Cox p value | |
| Relapse-free survival | ||||||
| Primary tumor > 2 cm | 4.79 | 2.15–10.66 | <0.001 | 1.94 | 0.81–4.62 | 0.13 |
| Pathologic node negative vs | ||||||
| 1 to 3 lymph nodes | 3.26 | 1.43–7.45 | 0.005 | 2.42 | 1.02–5.71 | 0.04 |
| >3 Lymph nodes | 12.97 | 6.09–27.6 | <0.001 | 7.64 | 3.37–17.33 | <0.001 |
| Histologic grade 3 | 4.91 | 2.51–9.64 | <0.001 | 2.56 | 1.18–5.55 | 0.02 |
| Estrogen receptor positive | 0.29 | 0.16–0.50 | <0.001 | 0.92 | 0.43–1.97 | 0.82 |
| Progesterone receptor positive | 0.22 | 0.12–0.42 | <0.001 | 0.53 | 0.22–1.30 | 0.17 |
| HER2 positive | 1.52 | 0.76–3.05 | 0.25 | 0.53 | 0.24–1.17 | 0.11 |
| CTC≧1 | 2.72 | 1.57–4.72 | <0.001 | 5.25 | 1.34–20.56 | 0.02 |
| CTC≥2 | 3.12 | 1.52–6.41 | 0.001 | – | – | – |
| CTC≥3 | 3.97 | 1.79–8.84 | <0.001 | – | – | – |
| Overall survival | ||||||
| Primary tumor > 2 cm | 3.94 | 1.51–10.25 | 0.001 | 1.32 | 0.45–3.79 | 0.61 |
| Pathologic node negative vs | ||||||
| 1 to 3 lymph nodes | 3.49 | 1.17–10.42 | 0.03 | 2.58 | 0.83–7.97 | 0.10 |
| >3 Lymph nodes | 13.36 | 4.92–36.24 | <0.001 | 7.33 | 2.48–21.69 | <0.001 |
| Histologic grade 3 | 6.76 | 2.58–17.71 | <0.001 | 3.27 | 1.11–9.63 | 0.03 |
| Estrogen receptor positive | 0.24 | 0.12–0.50 | <0.001 | 0.67 | 0.24–1.84 | 0.44 |
| Progesterone receptor positive | 0.20 | 0.08–0.45 | <0.001 | 0.57 | 0.17–1.91 | 0.37 |
| HER2 positive | 1.81 | 0.78–4.22 | 0.19 | 0.54 | 0.20–1.46 | 0.23 |
| CTC≧1 | 2.29 | 1.12–4.67 | 0.02 | 1.62 | 0.75–3.48 | 0.22 |
| CTC≥2 | 2.76 | 1.07–7.25 | 0.03 | – | – | – |
| CTC≥3 | 4.88 | 1.87–12.73 | <0.001 | – | – | – |
Table 3.
Relapse-Free Survival in Patients with Circulating Tumor Cells
| Variable name, status, by no. of circulating tumor cells | n | Relapses, n/N (%) | Disease-free survival rate at 3 y | 95% CI | p Value, log-rank test |
|---|---|---|---|---|---|
| One or more circulating tumor cells | <0.001 | ||||
| + | 124 | 23/124 (19) | 80 | 0.71–0.86 | |
| − | 385 | 28/385 (7) | 91 | 0.87–0.93 | |
| Two or more circulating tumor cells | 0.001 | ||||
| + | 39 | 9/39 (23) | 75 | 0.57–0.86 | |
| − | 470 | 42/470 (9) | 75 | 0.57–0.86 | |
| Three or more circulating tumor cells | <0.001 | ||||
| + | 24 | 7/24 (29) | 69 | 0.45–0.83 | |
| − | 485 | 44/485 (9) | 89 | 0.86–0.92 |
Table 4.
Overall Survival in Patients with Circulating Tumor Cells
| Variable name, status, by no. of circulating tumor cells | n | Relapses, n/N (%) | Overall survival rate at 3 y | 95% CI | p Value, log-rank test |
|---|---|---|---|---|---|
| One or more circulating tumor cells | 0.02 | ||||
| + | 124 | 13/124 (10) | 87 | 0.79–0.93 | |
| − | 385 | 18/385 (94) | 94 | 0.91–0.96 | |
| Two or more circulating tumor cells | 0.03 | ||||
| + | 39 | 5/39 (12) | 83 | 0.63–0.93 | |
| − | 470 | 26/470 (6) | 93 | 0.90–0.95 | |
| Three or more circulating tumor cells | <0.001 | ||||
| + | 24 | 5/24 (21) | 73 | 0.45–0.88 | |
| − | 485 | 26/485 (5) | 94 | 0.91–0.96 |
The hazard ratios associated with relapse increased with increasing CTC number. Patients with two or more circulating cells showed significantly decreased relapse-free survival compared to patients with no CTCs (log-rank P=0.001; hazard ratio = 3.12; 95% CI, 1.52 to 6.41) (Figure 1B). Nine of 39 patients (23%) with two or more CTCs relapsed, compared to 42 of 470 patients (9%) with no CTCs. The relapse-free survival rate at 4 years was much lower (75%) in this group than in patients who had no circulating tumor cells (90%) (Table 3 and 4). Seven patients with three or more circulating tumor cells demonstrated a hazard ratio of 3.97 for disease progression at 4 years (log-rank P<0.001; 95% CI, 1.79 to 8.84) (Figure 1C). The relapse-free survival rate was 69% in patients with three or more circulating tumor cells compared to patients with no circulating tumor cells (89%) (Table 3).
Circulating tumor cells and overall survival
Thirty-one patients died during the follow up period. As shown in Table 2, univariate (log-rank P=0.02; hazard ratio = 2.29; 95% confidence interval [CI], 1.12 to 4.67; P = 0.03) (Figure 2A) demonstrated that one or more CTC predicted decreased OS compared to patients with no CTCs. However, one or more CTC did not reach statistical significance using multivariate analysis (hazard ratio = 1.62; 95% confidence interval [CI], 0.75 to 3.48; P=0.22). Thirteen of 124 patients (10%) with one or more CTC died compared to 18 of 385 patients (5%) with no CTCs. Patients with two or more circulating cells showed significantly decreased overall survival compared to patients with no CTCs (log-rank P=0.03; hazard ratio = 2.78; 95% CI, 1.07 to 7.25) (Figure 2B). Five of 39 patients (12%) with two or more CTCs died, compared to 26 of 470 patients (6%) with no CTCs. The overall survival rate at 4 years was much lower (83%) in this group than in patients who had no circulating tumor cells (93%) (Table 3 and 4). Five patients with three or more circulating tumor cells demonstrated a hazard ratio of 4.88 for death at 4 years (log-rank P<0.001; 95% CI, 1.87 to 12.73) (Figure 2C). The overall survival rate was 73% in patients with three or more circulating tumor cells compared to patients with no circulating tumor cells (94%) (Table 4). Larger numbers of events are needed to perform multivariate analyses on patients with ≥2CTCs and ≥3CTCs.
Figure 2.
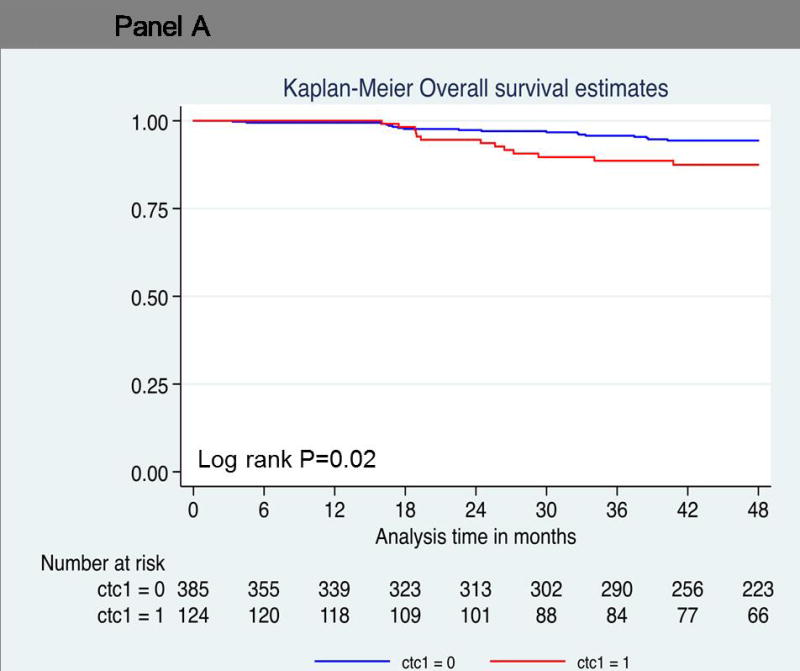
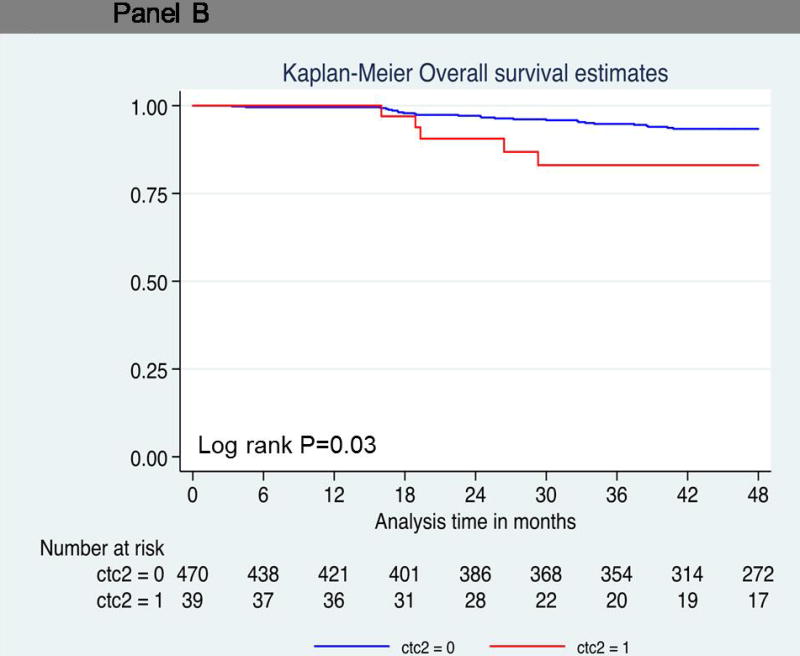
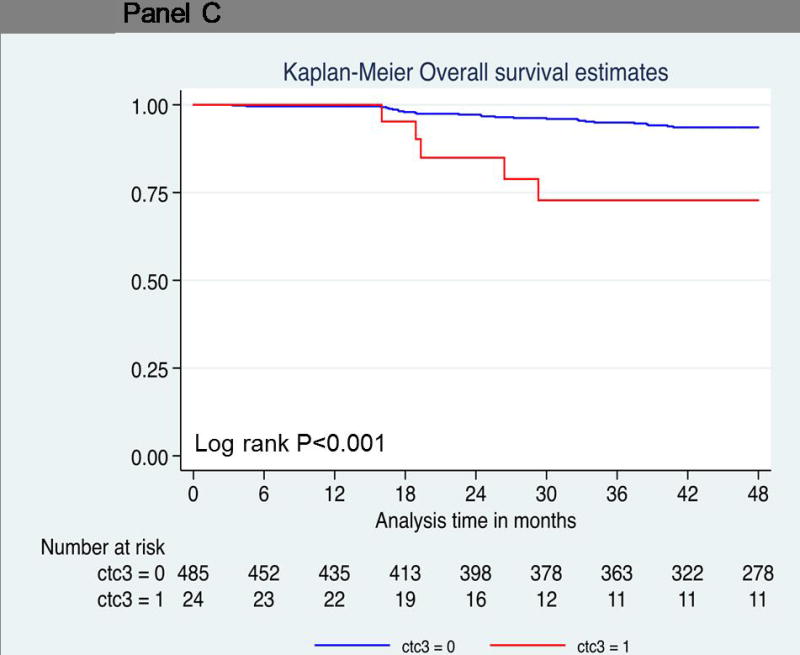
Probability of overall survival. Kaplan-Meier survival estimates of probabilities of overall survival according to circulating tumor cells in operable breast cancer. (A) Probability of overall survival in patients with CTC count ≧ 1 (Hazard ratio 2.29; 95% CI 1.12 – 4.67; logrank P = 0.02). (B) Probability of overall survival in patients with CTC count ≧ 1 (Hazard ratio 2.78; 95% CI 1.07 – 7.25; logrank P = 0.03). (C) Probability of overall survival in patients with CTC count ≧ 1 (Hazard ratio 4.88; 95% CI 1.87 – 12.73; logrank P < 0.001).
Discussion
To our knowledge, this is the first US-based study demonstrating that CTC detection prior to primary breast surgery predicted worse relapse-free and overall survival in non-metastatic breast cancer patients, regardless of whether they had received neoadjuvant chemotherapy or were chemonaïve. Circulating tumor cells were identified in 124 of 509 (24%) patients within our cohort, which is in agreement with the positivity rates reported in previously published European studies (13, 14, 16, 18, 19), and previous reports from our group.(11, 17) Interestingly, CTC presence was not associated with primary tumor characteristics such as tumor size, grade, or hormone receptor status in any of these studies. Fifty-four percent of the patients included within the study cohort were axillary lymph node negative, 30% of patients had 1–3 positive lymph nodes, and only 16% had more than three positive lymph nodes upon pathologic assessment. We found no significant difference in CTC detection rates between lymph node negative and lymph node positive patients. This lack of significant association persisted after stratifying lymph node positive patients into one to three, or more than three positive lymph nodes (P=0.24). Despite the lack of association between CTC presence and lymph node status, both univariate (HR 2.72) and multivariate analyses (HR 2.63) demonstrated that CTC detection predicted relapse-free survival. These data suggest that CTC assessments may provide additional information that would be useful in identifying patients at risk for disease progression. This is especially important because many patients will undergo only limited lymph node removal (such as sentinel nodes alone) with the widespread implementation of the ACOSOG Z0011 trial results, (23) which demonstrated no reduced local relapse or overall survival for patients who had sentinel node biopsy alone with limited axillary nodal disease. We are prospectively comparing CTC results to the OncotypeDX test, which uses a primary tumor gene signature algorithm to predict response to therapy and relapse risk in early stage patients. It would be of interest to determine whether these tests provide information independent of each other in the early-stage, node negative, ER-positive population of patients.
Circulating tumor cells appear to add important information regarding an individual patient’s risk for relapse. CTCs were independently predictive of relapse on multivariate analysis, as were positive lymph nodes and high-grade primary tumors. Yet not all patients with CTCs experienced relapse in our study. In fact, 19% of the patients with one or more CTCs relapsed, compared to 7% of the CTC-negative group, and this was a significant difference. Not all CTC-positive patients experience recurrence, just as not all lymph node-positive patients recur.(3) Identification of CTCs or positive lymph nodes adds relative risk but neither is an absolute indicator of relapse. We also know that 20% of lymph node negative patients experience relapse.(2) This data underscores the fact that not all tumors spread by the lymphatic route. Therefore, in order to fully elucidate the risk for relapse, addition of evaluation of the hematogenous compartment will in the future likely be a routine component of staging to optimally identify all patients at risk for relapse. However, routine measurement of CTCs is not currently recommended in clinical practice in the management of non-metastatic breast cancer patients.(24)
In this study, one or more CTC predicted overall survival using a univariate analysis, however, one or more CTC failed to independently predict overall survival upon multivariate analysis. More than seventy percent of the patients included in the present study had ER positive tumors. Since breast cancer specific death in estrogen receptor positive patients has been reported to occur ≥10 years following diagnosis,(25) the lack of significance for overall survival on multivariate analysis might be due to the relatively short follow up period (median 4 years) in this study.
We measured CTCs in 343 patients who were chemonaïve at the time of CTC assessment, as well as in 166 patients who received neoadjuvant therapy prior to CTC assessment. We identified no significant difference in CTC positivity rates between chemonaïve (24%) and neoadjuvant treated (26%) patients, irrespective of the systemic chemotherapy treatments administered. These results are in agreement with previously published European reports demonstrating that CTCs can be identified using (immunocytochemical methods) in 18–49% of patients following neoadjuvant and adjuvant treatments.(14, 18, 26) In these studies, CTC identification following neoadjuvant or adjuvant therapies predicted early relapse. Circulating tumor cells have been described as having low proliferation rates (27), as well as high levels of multidrug resistance proteins,(28) which might explain why CTCs persist following standard cytotoxic neoadjuvant therapies. Targeted therapies directed against primary tumor characteristics, such as estrogen receptor (ER) expression and HER2/neu amplification, may also be ineffective at CTC eradication. Discordant ER (lack of) expression and HER2/neu (acquired) expression have been reported between primary tumor and recurrent disease,(25) lymph nodes,(29) and metastatic sites (30) in a significant number of patients. Similarly, circulating tumor cell ER expression is reported to be absent in up to 70% of patients with primary tumors that express ER,(31) and circulating tumor cell HER2/neu amplification is observed in up to 48% of patients with HER2/neu negative primary tumors.(31–33)
To date, most clinical trials utilizing CTC assessments have been in the metastatic setting. The recently completed SWOG0500 trial was the first randomized trial to test the clinical utility of CTCs. In this study, metastatic patients without a decrease in CTCs after one cycle of first-line chemotherapy were randomized to continue the first-line therapy or to change to second-line chemotherapy. While switching to a second-line therapy did not improve outcomes for these patients, this study did validate earlier findings demonstrating the negative impact of CTCs that persist during treatment in the metastatic setting.(34) The high reported HER2 discordance rate reported between CTCs and primary tumor, lymph nodes, and metastatic sites has prompted new clinical investigations to determine if anti-HER2 therapy (trastuzumab) in HER2 negative patients provides survival benefit. Two ongoing interventional trials, the DETECT III for CTC-HER2 positive metastatic patients with HER2-negative tumors and the TREAT-CTC trial (NCT01548677) for CTC positive operable patients with HER2-negative tumors, will provide valuable information with respect to the biology of the metastatic process as well as the clinical utility of CTC measurements.
Current clinical practice guidelines, including those of the ASCO and the NCCN, do not recommend routine use of CTC information to make treatment decisions or to stage non-metastatic breast cancer patients.(24, 35) Future clinical trials are needed that demonstrate clinical utility of CTC information for monitoring response to therapy, in addition to providing prognostic information. Another issue is the utility of serial CTC assessment in clinical practice. Our study utilized a single time point (at the time of primary breast surgery) to determine the presence of CTCs in this study. Since blood draws are minimally invasive, future trials should employ longitudinal CTC monitoring throughout treatment and during follow-up visits, since CTC enumeration has the potential of providing “real-time” assessments of occult micrometastatic dissemination and recurrence risk at the time of diagnosis, throughout treatment, and during follow up.
Currently, most of the prognostic data for CTCs in non-metastatic breast cancer patients is based upon enumeration of cells expressing epithelial markers such as epithelial cell adhesion molecule (EpCAM).(36) New technologies are emerging that utilize multiple antibody capture in order to isolate cells that may have undergone epithelial mesenchymal transition (EMT) and have low or absent epithelial marker expression.(6) Other methodologies isolate rare tumor cells based on physical attributes such as size or rigidity.(6) These capture techniques, combined with recently developed molecular technologies, will allow for characterization of these cells. Characterization of CTCs is important since it has been shown that therapeutic targets such as HER2neu may be found on CTCs, even when the primary tumor is HER2 negative.(37) Such findings would have clear significance for future clinical trials designed at targeted eradication of CTCs. The concept of “liquid biopsy”, including gene mutation analysis in cell-free DNA, holds promise of providing real-time snapshots of the microscopic tumor burden throughout treatment.
Acknowledgments
Support: This work was supported in part by a Society of Surgical Oncology Clinical Investigator Award (2008–2010), “Detection and characterization of disseminated tumor cells in stage I–III breast cancer”, and by philanthropic funds, for which we thank our many generous donors.
Footnotes
Disclosure Information: Dr. Lucci was paid in 2014 for developing educational materials for Janssen Diagnostics, LLC. Dr. Kuerer serves as a paid member on the scientific advisory board of Lightpoint Medical, Inc., is employed as an associate editor for the New England Journal of Medicine, and has received research funding from Genomic Health, Inc. All other authors have nothing to disclose.
Presented at the Western Surgical Association 123rd Scientific Session, Napa Valley, CA, November 2015.
References
- 1.AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 2.Harbeck N, Thomssen C. A new look at node-negative breast cancer. Oncologist. 2011;16:51–60. doi: 10.1634/theoncologist.2011-S1-51. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 4.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst. 2001:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 5.Naume B, Borgen E, Beiske K, et al. Immunomagnetic techniques for the enrichment and detection of isolated breast carcinoma cells in bone marrow and peripheral blood. J Hematother. 1997;6:103–114. doi: 10.1089/scd.1.1997.6.103. [DOI] [PubMed] [Google Scholar]
- 6.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339–351. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 7.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 8.Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 9.Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–4224. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. Cancer J. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 11.Krishnamurthy S, Cristofanilli M, Singh B, et al. Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer. 2010;116:3330–3337. doi: 10.1002/cncr.25145. [DOI] [PubMed] [Google Scholar]
- 12.Bidard FC, Mathiot C, Delaloge S, et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010;21:729–733. doi: 10.1093/annonc/mdp391. [DOI] [PubMed] [Google Scholar]
- 13.Franken B, de Groot MR, Mastboom WJ, et al. Circulating tumor cells, disease recurrence and survival in newly diagnosed breast cancer. Breast Cancer Res. 2012;14:R133. doi: 10.1186/bcr3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rack B, Schindlbeck C, Juckstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riethdorf S, Muller V, Zhang L, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16:2634–2645. doi: 10.1158/1078-0432.CCR-09-2042. [DOI] [PubMed] [Google Scholar]
- 16.van Dalum G, van der Stam GJ, Tibbe AG, et al. Circulating tumor cells before and during follow-up after breast cancer surgery. Int J Oncol. 2015;46:407–413. doi: 10.3892/ijo.2014.2694. [DOI] [PubMed] [Google Scholar]
- 17.Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13:688–695. doi: 10.1016/S1470-2045(12)70209-7. [DOI] [PubMed] [Google Scholar]
- 18.Pierga JY, Bidard FC, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14:7004–7010. doi: 10.1158/1078-0432.CCR-08-0030. [DOI] [PubMed] [Google Scholar]
- 19.Schindlbeck C, Andergassen U, Hofmann S, et al. Comparison of circulating tumor cells (CTC) in peripheral blood and disseminated tumor cells in the bone marrow (DTC-BM) of breast cancer patients. J Cancer Research Clin Oncol. 2013;139:1055–1062. doi: 10.1007/s00432-013-1418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105:97–102. [PubMed] [Google Scholar]
- 21.McShane LM, Altman DG, Sauerbrei WT, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 22.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 23.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016 Feb 8; doi: 10.1200/JOP.2016.010868. [DOI] [PubMed] [Google Scholar]
- 25.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008 Mar 10;26(8):1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 26.Wulfing P, Borchard J, Buerger H, et al. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res. 2006;12:1715–1720. doi: 10.1158/1078-0432.CCR-05-2087. [DOI] [PubMed] [Google Scholar]
- 27.Muller V, Stahmann N, Riethdorf S, et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res. 2005;11:3678–3685. doi: 10.1158/1078-0432.CCR-04-2469. [DOI] [PubMed] [Google Scholar]
- 28.Gradilone A, Naso G, Raimondi C, et al. Circulating tumor cells (CTCs) in metastatic breast cancer (MBC): prognosis, drug resistance and phenotypic characterization. Ann Oncol. 2011;22:86–92. doi: 10.1093/annonc/mdq323. [DOI] [PubMed] [Google Scholar]
- 29.Aitken SJ, Thomas JS, Langdon SP, et al. Quantitative analysis of changes in ER, PR and HER2 expression in primary breast cancer and paired nodal metastases. Ann Oncol. 2010;21:1254–1261. doi: 10.1093/annonc/mdp427. [DOI] [PubMed] [Google Scholar]
- 30.Arslan C, Sari E, Aksoy S, Altundag K. Variation in hormone receptor and HER-2 status between primary and metastatic breast cancer: review of the literature. Expert Opinion Therapeutic Targets. 2011;15:21–30. doi: 10.1517/14656566.2011.537260. [DOI] [PubMed] [Google Scholar]
- 31.Fehm T, Hoffmann O, Aktas B, et al. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 2009;11:R59. doi: 10.1186/bcr2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ignatiadis M, Rothe F, Chaboteaux C, et al. HER2-positive circulating tumor cells in breast cancer. PloS One. 2011;6:e15624. doi: 10.1371/journal.pone.0015624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnamurthy S, Bischoff FZ, Mayer JA, et al. Detection of discordant HER2 status by FISH in circulating tumor cells and disseminated tumor cells in early-stage breast cancer using a microfluidic-based cell enrichment and extraction platform (OncoCEE) ASCO Meeting. 2012:TPS10631. Abstracts. [Google Scholar]
- 34.Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32:3483–3489. doi: 10.1200/JCO.2014.56.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Comprehensive Care Network. NCCN Clinical Practice Guidelines in Oncology. Fort Washington, PA: Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 36.Bidard FC, Proudhon C, Pierga JY. Circulating tumor cells in breast cancer. Molecular Oncol. 2016 Jan;:12. doi: 10.1016/j.molonc.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnamurthy S, Bischoff F, Ann Mayer J, et al. Discordance in HER2 gene amplification in circulating and disseminated tumor cells in patients with operable breast cancer. Cancer Med. 2013;2:226–233. doi: 10.1002/cam4.70. [DOI] [PMC free article] [PubMed] [Google Scholar]


