Abstract
The rationale for this study was to evaluate the feasibility of within-suite 89Zr-labeled radiotracer PET/CT-guided biopsy performed without reinjection. Methods: From 2013 to 2016, 12 patients (7 men, 5 women; mean age, 61 y; range, 40–75 y) with metastatic prostate or breast carcinoma suspected either on imaging or because of biochemical progression underwent 14 percutaneous biopsies after diagnostic PET/CT using 89Zr-labeled radiotracers (mean dose, 180 MBq; range, 126–189 MBq) targeting prostate-specific membrane antigen (n = 7) or human epidermal growth factor receptor 2 (n = 5). Biopsy was performed within a PET/CT suite without radiotracer reinjection. Results: There were no complications in any biopsies, which were performed a mean of 6.2 d (range, 0–13 d) after injection of the radiotracer. The biopsy sites were bone (n = 7), pleura (n = 3), lymph nodes (n = 2), and liver (n = 2). On pathologic examination of the biopsy samples, all were positive for malignancy. The initial diagnostic imaging findings were concordant with the biopsy results. The additional radiation (mean dose–length product) due to the CT procedures was 1,581 mGy/cm (range, 379–2,686 mGy/cm). Conclusion: PET/CT-guided biopsy using 89Zr-labeled radiotracers is safe and effective without tracer reinjection.
Keywords: biopsy, PET/CT, interventional radiology, 89Zr, metastasis
PET/CT-guided interventions are promising for radionuclide-avid lesions not well visualized with CT (1–3). However, when 18F-labeled radiotracers are used, such as 18F-FDG, a new injection is required before the intervention because of the short physical half-life of 18F (109.8 min). 89Zr may be more attractive because of its longer physical half-life of 78.4 h (4). After 89Zr diagnostic scans are obtained and interpreted, avid lesions retain activity for several additional days, enabling PET-guided biopsy later using the original injection of 89Zr-labeled agent (5).
In patients with metastatic cancer, 18F-FDG PET may fail to detect sites of disease because of a small lesion size and subsequent partial-volume effect, a low or heterogeneous utilization of glucose, or the nonspecificity of 18F-FDG (6). Conventional imaging, such as CT, may also fail to detect metastases or may insufficiently differentiate between aggressive and indolent lesions (7). Therefore, the potential of using other PET tracers with unique biologic specificities has been evaluated (8–10). Agents capable of specific binding, such as monoclonal antibodies, have shown promise for clinical use (9,10). Recently, we reported preliminary findings involving a human epidermal growth factor receptor 2 (HER2)–targeted PET tracer (trastuzumab) for HER2–positive metastases in patients with primary breast cancer and humanized third-generation prostate-specific membrane antigen (PSMA)–specific antibodies for soft tissue and bone metastases (9–11).
The purpose of this study was to evaluate the feasibility of performing delayed molecular imaging–guided biopsy using dedicated within-suite PET/CT guidance without radiotracer reinjection in patients presenting with metastatic prostate or breast cancer.
MATERIALS AND METHODS
Patients
All patients were enrolled in an Institutional Review Board–approved protocol. This protocol was compliant with the Health Insurance Portability and Accountability Act. The prospective protocols for molecular imaging were performed under an investigational-new-drug process (9–11).
From 2013 to 2016, 12 patients (mean age, 61 y; range, 40–75 y) with positive findings on PET/CT imaging with 89Zr-labeled anti-PSMA (J591 antibody or minibody, 7 men) or trastuzumab (5 women) were referred to interventional radiology for biopsy to confirm metastasis and obtain optimal molecular profiling of the tumor. The 7 men had a history of prostate cancer and presented with a rising level of prostatic-specific antigen despite treatment with testosterone-lowering hormonal therapy. The 5 women had confirmed HER2-negative primary breast cancer and underwent biopsy to assess 89Zr-trastuzumab foci suggestive of HER2-positive disease.
The exclusion criteria included a previous anaphylactic reaction to PET imaging or radiotracers, treatment with any new anticancer therapy (gonadotropin-releasing hormone analog allowed) while on the study, abnormal hepatic laboratory values (bilirubin > 1.5 times the upper limit of normal [ULN], aspartate aminotransferase/alanine aminotransferase > 2.5 ULN, albumin < 2 g/dL, γ-glutamyl transferase > 2.5 ULN if alkaline phosphatase > 2.5 ULN), abnormal renal laboratory values (creatinine > 1.5 ULN), or any other severe acute or chronic medical condition that might increase the risk associated with study participation or investigational product administration. The patient characteristics are summarized in Table 1.
TABLE 1.
Patient Characteristics
| Characteristic | Data |
| Biopsies | 14 |
| Patients | 12 |
| Sex | |
| Male | 7 |
| Female | 5 |
| Age (y) | 61.1 (40–75) |
| Prostatic-specific antigen level (ng/mL) | 88.8 (2–323) |
| Dose (MBq) | 179.5 (125.8–189.8) |
| Delay after injection (d) | 6.2 (0–13) |
| Location of biopsy | |
| Bone | 7 |
| Pleura | 3 |
| Lymph nodes | 2 |
| Liver | 2 |
Qualitative data are expressed as numbers; continuous data are expressed as mean followed by range in parentheses.
Preprocedural Imaging
Patients received radiolabeled antibody with a mean administered activity of 180 MBq (range, 126–190 MBq). A single dose of 10 mg of anti-PSMA antibody (J591) (11) or miniature antibody (df-IAB2M) (9) or 50 mg of HER2-targeted PET tracer (trastuzumab) (10) labeled with 185 MBq of 89Zr was administrated intravenously over 5–10 min.
All imaging was performed on the same PET/CT scanner (Discovery DSTE; GE Healthcare). A CT protocol designed for attenuation correction with iterative reconstruction was used for anatomic localization of PET abnormalities. Thus, each patient underwent a single 80-mA CT scan followed by a 10-mA CT scan on each of the remaining days of imaging.
Molecular Imaging–Guided Interventional Procedures
Targeted lesions were determined on the basis of 89Zr-labeled radiotracer PET/CT positivity and accessibility (target location, target size, needle path, and skin-to-target distance) by consensus at a multidisciplinary conference comprising oncologists, interventional radiologists, and nuclear medicine physicians.
The procedures were performed in an interventional radiology suite equipped with a PET/CT scanner (Discovery 690; GE Healthcare) without radiotracer reinjection. The patients had limited PET/CT imaging (1–2 bed positions) over the biopsy region of interest. A CT scan for attenuation correction and anatomic coregistration was obtained without oral or intravenous contrast material in every case (imaging parameters: 120 kVp, 115 mA, 1.25-mm collimation, 3.75-mm reconstructed slice thickness, and 512 × 512 matrix). Immediately after CT, PET was performed in 3-dimensional mode (128 × 128 matrix and 4.24 × 4.25 × 3.27 mm voxels) at 7 min per field of view. Biopsy needles were inserted under local anesthesia and conventional CT or CT–fluoroscopy guidance, but the CT images were intermittently fused with the PET dataset.
A coaxial 11-gauge system (Madison; Laurane Medical) was used for biopsy of bone, whereas other types of biopsy used needle diameters ranging from 18- to 20-gauge (Temno Evolution; Carefusion). Following an institutional protocol, 2 tissue samples were requested in bone biopsies and 3 in soft-tissue biopsies, but additional samples could be obtained. Pathologic evaluation of the tissue samples was performed at the time of biopsy.
Procedure-related complications were noted and classified on the basis of the Society of Interventional Radiology’s proposed criteria and the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0) (12).
Statistical Analysis
Diagnostic accuracy was reported in terms of sensitivity and positive predictive value. The positive predictive value was defined as the proportion of malignant lesions and was calculated with 95% confidence intervals using the Wilson method.
RESULTS
Targeting of Lesions
Biopsy was performed a mean of 6.2 d (range, 0–13 d) after the diagnostic injection of 89Zr. All lesions were successfully visualized (Figs. 1–3). The 89Zr tracer–positive lesions were targeted for biopsy, which was without complications in all cases. The mean number of needle passes was 3 (range, 2–5). The additional radiation (mean dose–length product) due to the CT procedures was 1,581 (range, 379–2,686 mGy/cm).
FIGURE 1.
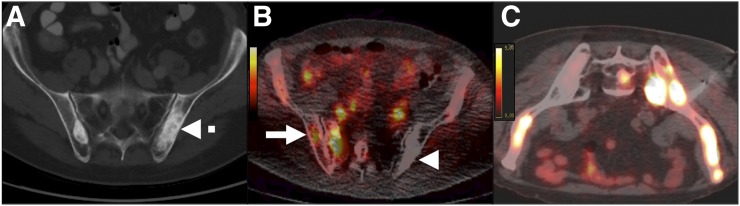
Delayed PET/CT-guided biopsy of bone metastasis from prostate carcinoma in 64-y-old man with strongly suspected metastatic prostate carcinoma (prostatic-specific antigen level, 91 ng/mL), without history of bone radiotherapy. (A) Axial CT scan shows suggestive increase in density (dashed arrow) on iliac bones. (B) Axial 89Zr anti-PSMA–targeted PET/CT image shows new lesions on right iliac wing and sacrum (solid arrow). Interestingly, lesion identified on CT shows no uptake on PET (arrowhead). (C) Axial 89Zr anti-PSMA–targeted PET/CT image 7 d after injection shows lesions, including the one that was biopsied. Pathologic examination confirmed metastasis from prostate carcinoma.
FIGURE 3.
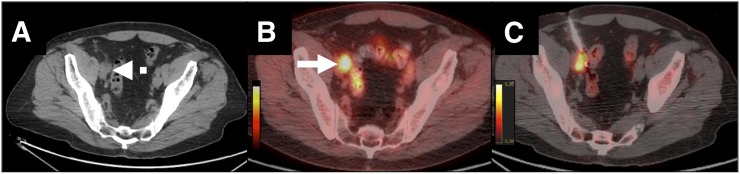
Delayed PET/CT-guided biopsy of lymph node metastasis from prostate carcinoma in 75-y-old man with strongly suspected metastatic prostate carcinoma (prostatic-specific antigen level, 10 ng/mL). (A) Axial CT scan shows 15-mm right iliac lymph node (dashed arrow). (B) Axial 89Zr anti-PSMA–targeted PET/CT image shows lymph node (arrow). (C) Corresponding images from within-suite PET/CT biopsy 6 d after injection show residual uptake. Pathologic examination confirmed metastasis from prostate carcinoma.
FIGURE 2.
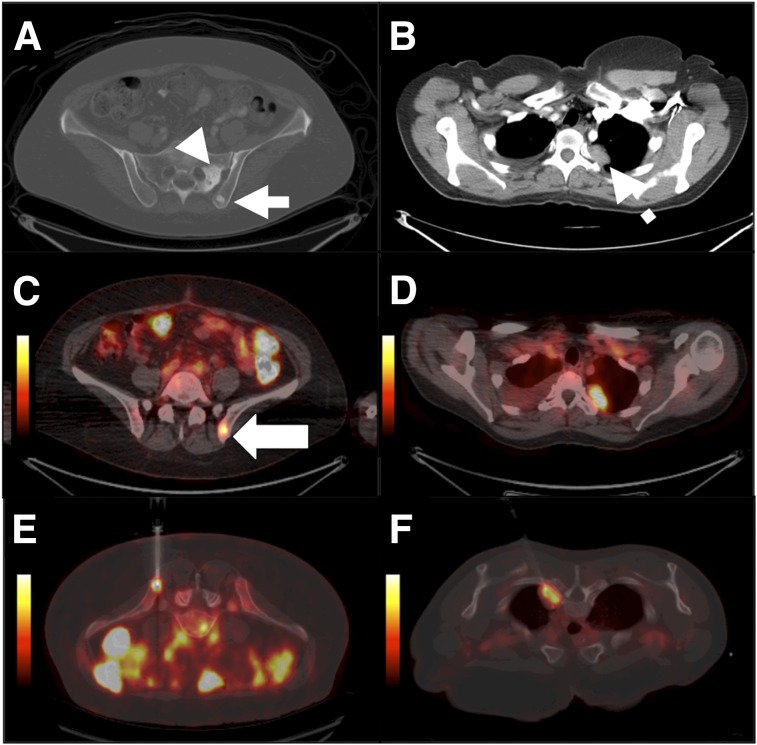
Delayed PET/CT-guided biopsy of bone and lung metastases in 51-y-old woman with breast carcinoma. (A and B) Axial contrast-enhanced CT scans show bone lesions in left iliac wing (arrowhead) and sacrum (solid arrow), as well as a thoracic mass (dashed arrow). (C and D) Axial 89Zr antiHER2–targeted PET/CT images show metastases in left iliac crest (arrow) and lung but no lesion in sacrum. (E and F) Corresponding images from within-suite PET/CT biopsy 2 d after injection show residual uptake. Pathologic examination confirmed metastasis from breast carcinoma.
Pathologic Results
For all positive biopsies, specimens adequate for pathologic and molecular profiling were obtained. A diagnosis of prostate or breast carcinoma metastasis was confirmed for all lesions biopsied (n = 14). The initial diagnostic imaging findings were concordant with the biopsy results. Biopsy performance is summarized in Table 2.
TABLE 2.
Results and Performance of Biopsy
| Parameter | Data |
| Total biopsies (n) | 14 |
| Biopsies positive for malignancy (n) | 14 |
| Biopsies negative for malignancy (n) | 0 |
| Sensitivity (%) | 100% |
| Positive predictive value (%) | 100% (52%–100%*) |
| Accuracy (%) | 100% |
95% confidence interval.
DISCUSSION
As shown in this preliminary study, within-suite delayed PET/CT-guided biopsy can be accurately performed using 89Zr-labeled radiotracers. In this study, 89Zr anti-PSMA or trastuzumab radiotracers were accurate for both early identification of metastases (9–11,13) and biopsy guidance. Moreover, unlike 18F-FDG, which would require an additional injection of isotope before the delayed PET/CT-guided procedure, 89Zr has a long half-life that allows for biopsy to be performed without reinjection after the diagnostic injection. A workflow that allows for diagnostic PET/CT followed by PET/CT-guided biopsy several days later after a multidisciplinary review meeting is feasible and efficient for patients.
This study was limited by the small size of our highly selected population. All patients had a known cancer diagnosis before biopsy. These results are therefore probably not translatable to other populations or other situations. Although not evaluated, the additional radiation exposure related to the use of 89Zr-labeled radiotracers has been estimated to be a 68-mSv effective dose per scan on average (11). This brief communication is presented as a proof of concept, but larger studies will be needed to further evaluate the value of these biopsies.
CONCLUSION
PET/CT-guided percutaneous biopsy can be safely and accurately performed within the PET/CT suite without reinjection of the 89Zr-labeled radiotracer after the initial injection for diagnostic imaging. 89Zr-labeled radiotracers targeting specific proteins may be useful for both detection and molecular profiling in high-risk patients presenting with widely disseminated disease. Studies such as this may add clinical justification for the use of molecular imaging in interventional oncology and for promoting future access to interventional PET imaging facilities.
DISCLOSURE
This research was funded in part through an NIH/NCI Cancer Center support grant (P30 CA008748). The 89Zr-J591 immuno-PET study was funded by Starr Cancer Consortium (the prostate cancer program of MSKCC), the Center for Targeted Radioimmunotherapy and Theranostics (of the Ludwig Center for Cancer Immunotherapy), and the David H. Koch Foundation. The 89Zr-Df-IAB2M anti-PSMA minibody study was funded by ImaginAb, Inc., and the DOD Clinical Consortium (PC071610). The 89Zr-trastuzumab study was supported by Breakthrough Award BC132676 from the Breast Cancer Research Program of the Department of Defense (Gary A. Ulaner), the Center for Targeted Radioimmunotherapy and Theranostics (of the Ludwig Center for Cancer Immunotherapy), and the Geoffrey Beene Cancer Center (of MSKCC). In addition, the Radiochemistry and Molecular Imaging Probes Core of MSKCC is supported in part by the Landy Research Fund and the Hascoe Charitable Foundation. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Klaeser B, Mueller MD, Schmid RA, Guevara C, Krause T, Wiskirchen J. PET-CT-guided interventions in the management of FDG-positive lesions in patients suffering from solid malignancies: initial experiences. Eur Radiol. 2009;19:1780–1785. [DOI] [PubMed] [Google Scholar]
- 2.Shyn PB, Tatli S, Sahni VA, et al. PET/CT-guided percutaneous liver mass biopsies and ablations: targeting accuracy of a single 20 s breath-hold PET acquisition. Clin Radiol. 2014;69:410–415. [DOI] [PubMed] [Google Scholar]
- 3.Cornelis F, Silk M, Schoder H, et al. Performance of intra-procedural 18-fluorodeoxyglucose PET/CT-guided biopsies for lesions suspected of malignancy but poorly visualized with other modalities. Eur J Nucl Med Mol Imaging. 2014;41:2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol. 2009;36:729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon SB, Cornelis F. Interventional molecular imaging. J Nucl Med. 2016;57:493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segall G, Delbeke D, Stabin MG, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51:1813–1820. [DOI] [PubMed] [Google Scholar]
- 7.Schiavina R, Ceci F, Borghesi M, et al. The dilemma of localizing disease relapse after radical treatment for prostate cancer: which is the value of the actual imaging techniques? Curr Radiopharm. 2013;6:92–95. [DOI] [PubMed] [Google Scholar]
- 8.Ulaner GA, Hyman DM, Ross DS, et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-trastuzumab PET/CT. J Nucl Med. 2016;57:1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandit-Taskar N, O’Donoghue JA, Ruan S, et al. First-in-human imaging with 89Zr-Df-IAB2M anti-PSMA minibody in patients with metastatic prostate cancer: pharmacokinetics, biodistribution, dosimetry, and lesion uptake. J Nucl Med. 2016;57:1858–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulaner GA, Castillo R, Goldman DA, et al. 18F-FDG-PET/CT for systemic staging of newly diagnosed triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2016;43:1937–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandit-Taskar N, O’Donoghue JA, Durack JC, et al. A phase I/II study for analytic validation of 89Zr-J591 immunoPET as a molecular imaging agent for metastatic prostate cancer. Clin Cancer Res. 2015;21:5277–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology. 2014;273:241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med. 2010;51:1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]