Abstract
In this prospective survey of referring physicians, we investigated whether and how 68Ga-labeled prostate-specific membrane antigen 11 (68Ga-PSMA-11) PET/CT affects the implemented management of prostate cancer patients with biochemical recurrence (BCR). Methods: We conducted a prospective survey of physicians (NCT02940262) who referred 161 patients with prostate cancer BCR (median prostate-specific antigen value, 1.7 ng/mL; range, 0.05–202 ng/mL). Referring physicians completed one questionnaire before the scan to indicate the treatment plan without 68Ga-PSMA-11 PET/CT information (Q1; n = 101), one immediately after the scan to denote intended management changes (Q2; n = 101), and one 3–6 mo later to document the final implemented management (Q3; n = 56). The implemented management was also obtained via electronic chart review or patient contact (n = 45). Results: A complete documented management strategy (Q1 + Q2 + implemented management) was available for 101 of 161 patients (63%). Seventy-six of these (75%) had a positive 68Ga-PSMA-11 PET/CT result. The implemented management differed from the prescan intended management (Q1) in 54 of 101 patients (53%). The postscan intended management (Q2) differed from the prescan intended management (Q1) in 62 of 101 patients (61%); however, these intended changes were not implemented in 29 of 62 patients (47%). Pelvic nodal and extrapelvic metastatic disease on 68Ga-PSMA-11 PET/CT (PSMA T0N1M0 and PSMA T0N1M1 patterns) was significantly associated with implemented management changes (P = 0.001 and 0.05). Conclusion: Information from 68Ga-PSMA-11 PET/CT brings about management changes in more than 50% of prostate cancer patients with BCR (54/101; 53%). However, intended management changes early after 68Ga-PSMA-11 PET/CT frequently differ from implemented management changes.
Keywords: prostate cancer, biochemical recurrence, PET/CT, 68Ga-PSMA, impact on implemented management
See an invited perspective on this article on page 418.
Compared with conventional imaging, 68Ga-PSMA-11 PET/CT is superior for detecting sites of prostate cancer biochemical recurrence (BCR) (1–4), is sensitive for detecting regional and distant metastatic disease (3,5), is highly specific (4,) and is associated with a low interreader variability (6).
Health-care providers and government agencies frequently judge the value of novel diagnostic tests by measuring their impact on patient management. Often, this impact has been estimated from survey information after the index test information becomes available to treating physicians (7). However, intended management early after imaging results become available does not necessarily translate into implemented management (8–11). To our knowledge, the rate of implemented management changes related to 68Ga-PSMA-11 PET/CT has not been determined prospectively in patients with BCR. Two retrospective studies attempted to determine rates of implemented management changes (12,13), and 3 prospective studies evaluated the impact of 68Ga-PSMA-11 PET/CT on intended management changes (1,14,15).
Here, we investigated prospectively the impact of 68Ga-PSMA-11 PET/CT on the implemented management of prostate cancer patients with BCR.
MATERIALS AND METHODS
Patients, Registration, and Authorization
The Food and Drug Administration granted our investigational new drug application (NCT02940262) for a prospective study to evaluate the diagnostic performance of 68Ga-PSMA-11 PET/CT for localization of BCR. The primary endpoint of this study is the accuracy of 68Ga-PSMA-11 PET/CT for identifying lesions. Here, we report on a secondary endpoint in a consecutively recruited subgroup of patients: the impact of 68Ga-PSMA-11 PET/CT on patient management. The University of California, Los Angeles (UCLA), Institutional Review Board approved the protocol, the informed consent forms, the participant information forms, and the prospective referring physician questionnaires (approval #16-001095). From October 2016 to June 2017, we enrolled 161 patients with proven prostate adenocarcinoma and BCR after prostatectomy (prostate-specific antigen [PSA] level, >0.2 ng/mL >6 wk after surgery) or definitive radiotherapy (PSA rise, ≥2 ng/mL above the nadir). All patients provided written informed consent.
Survey Design
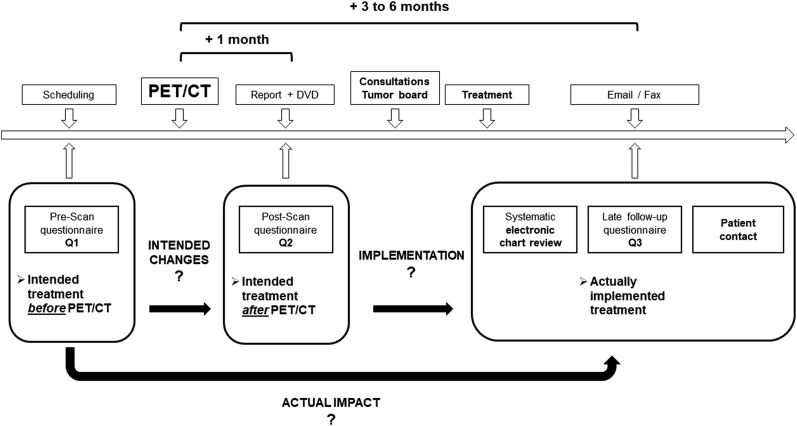
The survey design is depicted in Figure 1. We asked referring physicians to complete and return 3 questionnaires by email or fax. One questionnaire before the scan was required to indicate the treatment plan without 68Ga-PSMA-11 PET/CT information (Q1). A second questionnaire inquired about intended management immediately after receipt of the written clinical report and the images (Q2). A final questionnaire emailed 3–6 mo later verified whether intended management changes were in fact implemented (Q3). Up to 3 email reminders were sent to referring physicians if questionnaires were not returned. To further document the implemented management strategy, we reviewed the electronic charts and followed up with patients.
FIGURE 1.
Study design.
The management options and changes are categorized in Table 1. We did not consider the addition or removal of androgen deprivation therapy to the treatment strategy to be a significant major management change except when active surveillance was intended or implemented.
TABLE 1.
Treatment Options and Management Changes
| Parameter | Description |
| Treatment option* | Salvage surgery |
| Salvage radiation therapy | |
| Metastasis directed ablative radiation therapy (stereotactic body radiation therapy) | |
| Androgen deprivation therapy | |
| Chemotherapy | |
| Bone radionuclide therapy | |
| PSMA radionuclide therapy | |
| Other systemic treatment (vaccine therapy, immunotherapy) | |
| Active surveillance | |
| Management change | Conversion to focal treatment/new focal treatment (for either prostate bed, lymph node, or metastasis ablation) |
| Conversion to systemic treatment | |
| Change in systemic treatment (adding new systemic treatment or removing systemic treatment) | |
| Conversion to active surveillance |
Multiple treatment options were possible.
Intended management changes between Q1 and Q2 represent the initial impact of imaging findings resulting in intended management changes, which may, however, not necessarily represent the implemented management change. Changes between Q2 and Q3/chart review/patient contact represent the difference between the intended postscan and implemented management plan. Changes between Q1 and Q3/chart review/patient contact represent the changes from prescan to the implemented management plan.
68Ga-PSMA-11 PET/CT Protocol
68Ga-PSMA-11 PET/CT imaging was performed according to recent guidelines (16) with a 64-detector PET/CT device (Biograph True Point 64 or Biograph mCT; Siemens). 68Ga-PSMA-11 (Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)]) was used as the PSMA ligand (17). The median injected dose was 196 MBq (range, 93–241 MBq). To reduce bladder activity, patients received 20 mg of furosemide at the time of tracer injection if there was no contraindication. The median uptake period was 62 min (range, 52–96 min). A diagnostic CT scan (200–240 mAs, 120 kV) was performed after intravenous injection of contrast agent (if no contraindication), followed by the whole-body PET image acquisition (2–4 min/bed position). Standard image reconstruction parameters were used (16,18).
68Ga-PSMA-11 PET/CT Image Analysis
68Ga-PSMA-11 PET/CT images were analyzed according to recent guidelines during clinical readouts by an experienced nuclear medicine physician who had unlimited access to all medical records (16,18,19): any focal uptake of 68Ga-PSMA-11 PET/CT above the background level and not associated with physiologic uptake or known pitfalls (6,18) was considered PSMA-positive.
We routinely adopt an image-based TNM staging system and analyze the following regions for recurrence: prostate, prostate bed, and seminal vesicle remnants (T), pelvic lymph nodes (N) (internal iliac, obturator, external iliac, perirectal, presacral, common iliac, other), extrapelvic lymph nodes (M1a) (retroperitoneal, inguinal, chest, other), bone (M1b), and visceral organs (M1c).
Statistics
All variables were summarized by descriptive statistics (median and range). The comparisons for management change rates between PSMA-positive and -negative patients were conducted using the χ2 test. Postscan intended management change (Q1 to Q2), nonimplementation of postscan intended management (Q2 to implemented), and implemented management change (Q1 to implemented) were considered 3 primary binary outcome variables in this study. Four potential predictor parameters were studied: National Comprehensive Cancer Network risk group, serum PSA level before 68Ga-PSMA-11 PET/CT, prior primary treatment (surgery or radiotherapy), and 68Ga-PSMA-11 PET/CT TNM pattern. Multiple logistic regression analysis was performed to investigate the potential association between these 4 predictors and the above 3 primary outcome variables. All statistical analyses were conducted in R (20).
RESULTS
Referring Physicians and Questionnaires
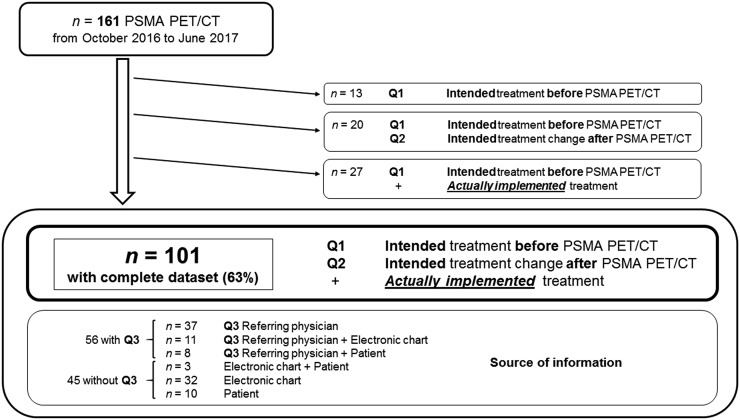
The flowchart is depicted in Figure 2. Fifty-seven physicians referred 161 patients for 68Ga-PSMA-11 PET/CT imaging. The complete documented management strategy (Q1 + Q2 + actual implemented management) was available for 101 of 161 patients (63%). Forty-two different physicians (10 from UCLA, 32 from other institutions) referred the 101 patients (38 from UCLA, 63 from other institutions).
FIGURE 2.
Flowchart.
Q1 and Q2 were completed in all 101 patients, and Q3 was completed in 56 of 101 patients (55%). In 19 of these patients, electronic chart verification (n = 11) and patient contact (n = 8) were used to further verify the accuracy of Q3 information. In the remaining 45 of 101 patients (45%), Q3 was not completed, but electronic chart review (n = 32), patient contact (n = 10), or both (n = 3) was used to document the implemented management strategy (Fig. 2).
Referring physicians completed Q1 within a median of 18 d before the scan (range, 0–93 d). Q2 was completed within a median of 9 d after the scan (range, 1–89 d). We obtained information about the implemented treatment within medians of 105 d after the scan (range, 30–259 d) and 94 d after Q2 completion (range, 26–246 d).
Patient Population
Patient demographics are presented in Table 2. Briefly, in the 101 patients with a complete documented management strategy the median serum PSA value before 68Ga-PSMA-11 PET/CT was 1.7 ng/mL (range, 0.05–140 ng/mL). Eighty-seven of 101 patients (86%) had prior prostatectomy, and 14 of 101 (14%) had prior definitive radiotherapy. The median time between primary treatment and 68Ga-PSMA-11 PET/CT was 4.2 y (range, 0.12–18 y), and 21 of 101 patients (21%) had androgen deprivation therapy within 6 mo before 68Ga-PSMA-11 PET/CT.
TABLE 2.
Patient Characteristics (n = 101)
| Parameter | Data |
| Initial characteristics | |
| Initial PSA at diagnosis, median (ng/mL) | 6.8 (0.25–33.3) |
| 10 | 52 (52) |
| ≥10 to <20 | 10 (10) |
| ≥20 | 9 (9) |
| Unknown | 30 (30) |
| Gleason score | |
| ≤7 | 32 (32) |
| ≥8 | 66 (66) |
| Unknown | 3 (3) |
| Primary tumor stage | |
| T1–T2 | 35 (35) |
| T3–T4 | 35 (35) |
| Unknown | 31 (31) |
| Initial NCCN risk group | |
| Low | 5 (5) |
| Intermediate | 42 (42) |
| High | 44 (44) |
| N1 | 8 (8) |
| Unknown | 2 (2) |
| Prior treatment | |
| Primary surgery | 87 (86) |
| Surgery only | 47 (47) |
| Surgery + ADT | 10 (10) |
| Surgery + SRT ± ADT | 27 (27) |
| Surgery + SBRT ± ADT | 1 (1) |
| Surgery + chemotherapy ± ADT | 2 (2) |
| Primary RT | 14 (14) |
| RT only | 5 (5) |
| RT + ADT | 6 (6) |
| RT + SBRT ± ADT | 2 (2) |
| RT + chemotherapy ± ADT | 1 (1) |
| PET/CT | |
| Age at PET/CT, median (y) | 69 (43–88) |
| Time between primary treatment and PET/CT, median (y) | 4.2 (0.12–18) |
| ADT within 6 mo before imaging | 21 (21) |
| Serum PSA before PET/CT, median (ng/mL) | 1.7 (0.05–140) |
NCCN = National Comprehensive Cancer Network; ADT = androgen deprivation therapy; SRT = salvage radiation therapy; RT = radiation therapy; SBRT = stereotactic body radiation therapy.
Qualitative data are expressed as numbers followed by percentages in parentheses; continuous data are expressed as median followed by range in parentheses.
In the 60 patients without a complete documented management strategy, the median serum PSA value before PET/CT was 2.25 ng/mL (range, 0.2–202 ng/mL).
68Ga-PSMA-11 PET/CT Findings
68Ga-PSMA-11 PET/CT findings are detailed in Table 3. In brief, 76 of 101 patients (75%) had a positive PET/CT study: 64 of 101 (64%) had PSMA-positive intrapelvic lesions, and 37 of 101 (37%) had PSMA-positive extrapelvic lesions. Thus far, histopathologic verification is available for 18 of the 76 PSMA-positive patients (24%). PSMA-positive lesions corresponded to prostate adenocarcinoma in 14 of 18 patients (78%): in 4 of 7 (57%) with local recurrence, in 7 of 7 (100%) with pelvic LN recurrence, in 1 of 1 with retroperitoneal LN recurrence, and in 2 of 3 (66%) with lung metastases reported by PET. The remaining 4 of 18 cases (22%) may reflect 68Ga-PSMA-11 PET/CT false-positive or biopsy false-negative findings.
TABLE 3.
68Ga-PSMA-11 PET/CT Findings (n = 101)
| Parameter | Data |
| 68Ga-PSMA-11 PET/CT+ | 77 (76) |
| Prostate/prostate bed (T+) | 23 (23) |
| Pelvic LN (N1) | 47 (47) |
| Extrapelvic LN (M1a) | 21 (21) |
| Bone (M1b) | 19 (19) |
| Visceral (M1c) | 7 (7) |
| 68Ga-PSMA-11 TNM pattern | |
| PSMA T+ N0 M0 | 12 (12) |
| PSMA T0 N1 M0 | 25 (25) |
| PSMA T+ N1 M0 | 2 (2) |
| PSMA T+ N0 M1 | 6 (6) |
| PSMA T0 N0 M1 | 12 (12) |
| PSMA T0 N1 M1 | 17 (17) |
| PSMA T+ N1 M1 | 3 (3) |
LN = lymph node.
Data are numbers followed by percentages in parentheses.
Forty-seven of 60 patients (78%) without a complete documented management strategy had a positive 68Ga-PSMA-11 PET/CT study.
Impact on Patient Management
Management changes are detailed in Table 4. Implemented management changes (Q1 to implemented) were recorded for 54 of 101 patients (53%). These consisted of conversion to focal treatment/new focal treatment in 29 of 101 (29%), conversion to systemic treatment in 13 of 101 (13%), change of systemic treatment approach in 5 of 101 (5%), and conversion to active surveillance in 7 of 101 (7%).
TABLE 4.
Individual Management Changes
| Management change | Q1 to Q2 | Q2 to implemented | Q1 to implemented |
| Conversion to focal treatment/new focal treatment | 40 (40) | 14 (14) | 29 (29) |
| Active surveillance to surgery ± ADT | 5 (5) | 0 (0) | 5 (5) |
| Active surveillance to SRT ± ADT | 9 (9) | 2 (2) | 3 (3) |
| Active surveillance to SBRT ± ADT | 0 (0) | 0 (0) | 1 (1) |
| ADT to surgery ± ADT | 3 (3) | 0 (0) | 1 (1) |
| ADT to surgery + SRT + ADT | 0 (0) | 0 (0) | 1 (1) |
| ADT to SRT ± ADT | 4 (4) | 1 (1) | 2 (2) |
| ADT to SRT + CTx + ADT | 1 (1) | 0 (0) | 0 (0) |
| ADT to SBRT ± ADT | 4 (4) | 0 (0) | 4 (4) |
| ADT to SBRT + CTx + ADT | 0 (0) | 0 (0) | 1 (1) |
| CTx + ADT to surgery | 1 (1) | 0 (0) | 0 (0) |
| CTx + ADT to SRT ± ADT | 1 (1) | 1 (1) | 1 (1) |
| CTx + ADT to SBRT ± ADT | 1 (1) | 0 (0) | 2 (2) |
| PSMA-RNT to SRT + ADT | 0 (0) | 1 (1) | 0 (0) |
| SRT ± ADT to surgery | 5 (5) | 2 (2) | 2 (2) |
| SRT ± ADT to SBRT ± ADT | 5 (5) | 1 (1) | 5 (5) |
| SRT ± ADT to SBRT + SRT ± ADT | 0 (0) | 1 (1) | 1 (1) |
| SRT ± ADT to SBRT + CTx + ADT | 0 (0) | 1 (1) | 0 (0) |
| SRT + CTx + ADT to SBRT + ADT | 0 (0) | 1 (1) | 0 (0) |
| SBRT + ADT to surgery | 1 (1) | 0 (0) | 0 (0) |
| Surgery + ADT to SRT + ADT | 0 (0) | 2 (2) | 0 (0) |
| Surgery + ADT to surgery + SRT + ADT | 0 (0) | 1 (1) | 0 (0) |
| Conversion to systemic treatment | 12 (12) | 7 (7) | 13 (13) |
| Active surveillance to ADT | 4 (4) | 1 (1) | 4 (4) |
| Active surveillance to other systemic treatment + ADT | 0 (0) | 0 (0) | 3 (3) |
| Surgery ± ADT to ADT | 0 (0) | 3 (3) | 0 (0) |
| SRT + CTx ± ADT to ADT | 1 (1) | 0 (0) | 1 (1) |
| SRT ± ADT to ADT | 5 (5) | 2 (2) | 5 (5) |
| SRT ± ADT to CTx + ADT | 1 (1) | 0 (0) | 0 (0) |
| SBRT + ADT to other systemic treatment + ADT | 1 (1) | 1 (1) | 0 (0) |
| Unknown to ADT | 0 (0) | 0 (0) | 0 (0) |
| Conversion to active surveillance | 5 (5) | 7 (7) | 7 (7) |
| Surgery ± ADT to active surveillance | 0 (0) | 3 (3) | 0 (0) |
| SRT ± ADT to active surveillance | 4 (4) | 2 (2) | 4 (4) |
| SRT + CTx to active surveillance | 0 (0) | 0 (0) | 0 (0) |
| ADT to active surveillance | 1 (1) | 2 (2) | 3 (3) |
| Change in systemic treatment | 5 (5) | 7 (7) | 5 (5) |
| ADT to CTx ± ADT | 3 (3) | 0 (0) | 0 (0) |
| ADT to other systemic treatment ± ADT | 0 (0) | 4 (4) | 2 (2) |
| CTx ± ADT to ADT | 0 (0) | 3 (3) | 1 (1) |
| CTx + bone RNT + ADT to ADT + CTx | 0 (0) | 0 (0) | 0 (0) |
| CTx + bone RNT + ADT to CTx + PSMA-RNT + ADT | 0 (0) | 0 (0) | 0 (0) |
| CTx + bone RNT + ADT to PSMA-RNT | 1 (1) | 0 (0) | 1 (1) |
| Bone RNT + ADT to CTx + ADT | 1 (1) | 0 (0) | 1 (1) |
| Bone RNT + ADT to PSMA-RNT | 0 (0) | 0 (0) | 0 (0) |
| Total | 62 (61) | 35 (35) | 54 (53) |
ADT = androgen deprivation therapy; SRT = salvage radiation therapy; SBRT = stereotactic body radiation therapy; CTx = chemotherapy; RNT = radionuclide therapy.
Data are numbers followed by percentages in parentheses.
68Ga-PSMA-11 PET/CT (Q1 to Q2) resulted in intended management changes in 62 of 101 patients (61%): these included conversion to focal treatment/new focal treatment in 40 of 101 (40%), conversion to systemic treatment in 12 of 101 (12%), change in systemic treatment in 5 of 101 (5%), and conversion to active surveillance in 5 of 101 (5%).
Intended treatment as indicated in Q2 was implemented in 66 of 101 patients (67.5%): implementation of the intended strategy occurred in 33 of 62 patients with (53%) and 33 of 39 patients without (85%) intended management changes. Nonimplementation of intended management changes after the 68Ga-PSMA-11 PET/CT study (Q2) occurred in 35 of 101 (35%) patients. Tumor board or other medical decisions (13/35; 37%), patient choice (11/35; 31%), and second opinions at other institutions (5/35; 14%) accounted for nonimplementation. Reasons remained unknown in 6 of 35 (17%). Figures 3 and 4 depict patients in whom subsequent decisions led to nonimplementation of intended management.
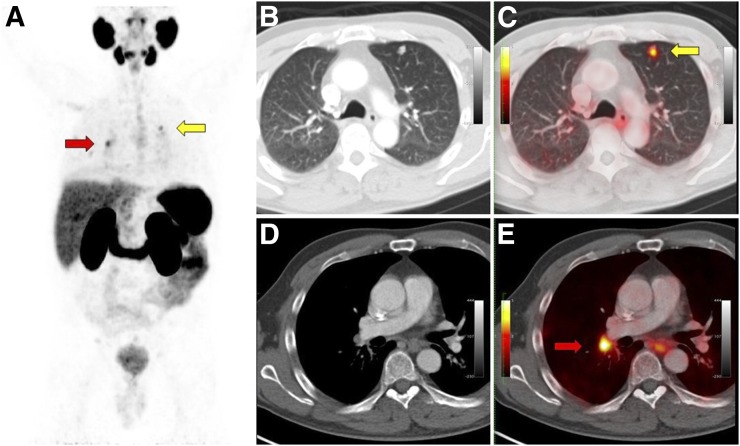
FIGURE 3.
A 67-y-old man with BCR (PSA level, 10.7 ng/mL; doubling time, 9.3 mo) of initially high-risk prostate cancer (Gleason 9; pT3a) 8 y after primary radical prostatectomy and adjuvant prostate bed irradiation. Intended prescan treatment was androgen deprivation therapy. 68Ga-PSMA-11 PET/CT showed intense 68Ga-PSMA-11 uptake (SUVmax, 7) in multiple lung nodules (yellow arrows) and thoracic lymph nodes (red arrows). Intended postscan treatment (Q2) was chemotherapy plus androgen deprivation therapy. CT-guided biopsy of upper left lung nodule confirmed metastatic prostatic adenocarcinoma. Patient elected to forgo chemotherapy because of potential side effects, and thus, intended postscan management (Q2) was not implemented. Actual management was androgen deprivation therapy alone, and thus, there was no change from prescan intended management as recorded on Q1. (A) 68Ga-PSMA-11 PET maximum-intensity projection. (B) Axial CT, lung window. (C) Axial 68Ga-PSMA-11 PET/CT, lung window. (D) Axial CT, mediastinal window. (E) Axial 68Ga-PSMA-11 PET/CT, mediastinal window.
FIGURE 4.
68Ga-PSMA-11 PET/CT in 75-y-old man with BCR (PSA level, 2.88 ng/mL; doubling time, 4.5 mo) of initially high-risk prostate cancer (Gleason score, 8; pT3) 4 y after primary radiotherapy without androgen deprivation therapy. Q1 listed androgen deprivation therapy as planned treatment. 68Ga-PSMA-11 PET/CT showed focal 68Ga-PSMA-11 uptake (SUVmax, 3.9) in right prostate lobe (yellow arrows). Intended treatment after 68Ga-PSMA-11 PET/CT (Q2) was surgery, which patient refused because of potential side effects. Actual management was thus androgen deprivation therapy as indicated on Q1 (no management change). (A) Coronal 68Ga-PSMA-11 PET/CT. (B) Coronal CT. (C) Sagittal 68Ga-PSMA-11 PET/CT. (D) Sagittal CT. (E) Axial 68Ga-PSMA-11 PET/CT. (F) 68Ga-PSMA-11 PET maximum-intensity projection. Yellow arrows denote focal tracer uptake consistent with intraprostatic recurrence.
Predictors of Management Changes
Among 4 tested parameters (National Comprehensive Cancer Network risk group, PSA level before PET/CT, prior primary treatment, and 68Ga-PSMA-11 TNM pattern), the 68Ga-PSMA-11 PET/CT TNM pattern was the only significant predictor of intended (Q1 to Q2) and implemented (Q1 to implemented) management changes.
Specifically, the probability of having an intended management change was higher in patients with pelvic nodal disease only (PSMA T0N1M0) than in patients with negative scans (P = 0.02). Furthermore, intended management changes occurred more frequently in patients with positive 68Ga-PSMA-11 PET/CT scans (52/76; 68%) than in those with negative scans (10/25; 40%) (P = 0.02). Figure 5 illustrates a PSMA T0N1M0 pattern.
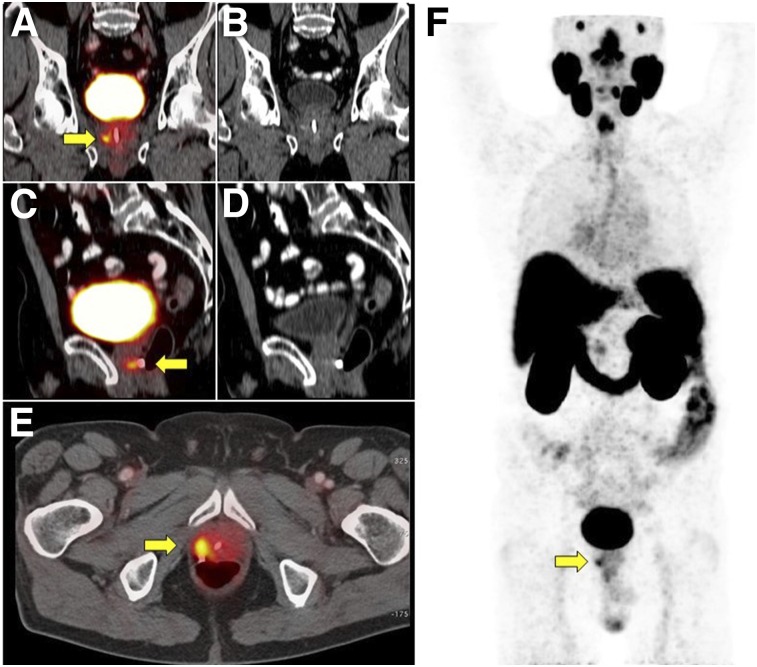
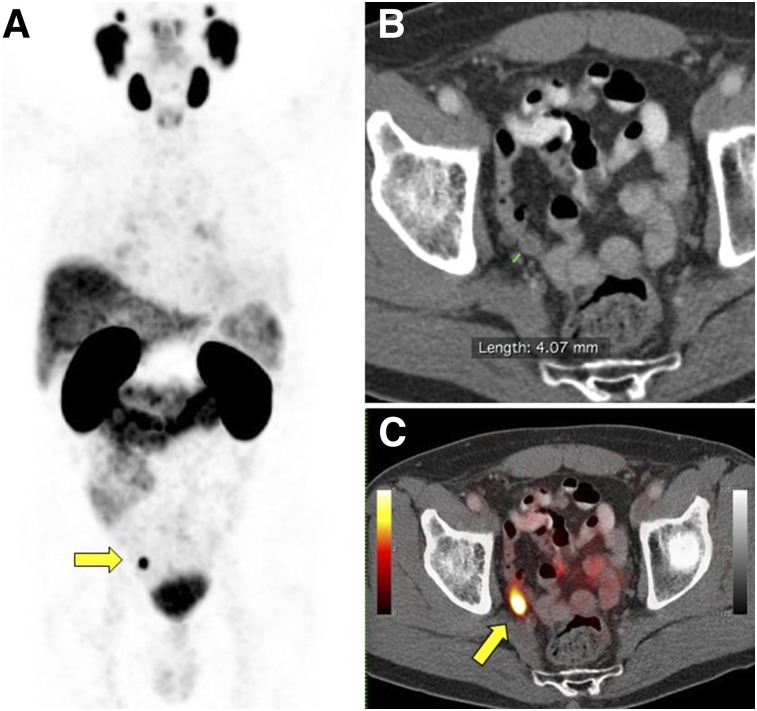
FIGURE 5.
68Ga-PSMA-11 PET/CT scan in 70-y-old man with BCR (PSA level, 1.06 ng/mL; doubling time, 7 mo) of initially high-risk prostate cancer (Gleason score, 8; pT2c) 1 y after primary radical prostatectomy. Intended treatment before 68Ga-PSMA-11 PET/CT was active surveillance (Q1). 68Ga-PSMA-11 PET/CT showed intense 68Ga-PSMA-11 uptake (SUVmax, 10.8) in 4-mm right internal iliac pelvic lymph node (yellow arrows). Intended treatment after 68Ga-PSMA-11 PET/CT (Q2) was surgery, which was implemented. Patient underwent pelvic lymph node dissection 2 mo later, which confirmed metastatic prostatic adenocarcinoma. (A) 68Ga-PSMA-11 PET maximum-intensity projection. (B) Axial CT. (C) Axial 68Ga-PSMA-11 PET/CT.
The probability of having implemented management changes (Q1 to implemented) was higher in patients with PSMA T0N1M0 (P = 0.001) and T0N1M1 patterns (P = 0.05) than in those with negative scans. Finally, implemented management changes (Q1 to implemented) occurred more frequently in patients with positive 68Ga-PSMA-11 PET/CT scans (48/76; 63%) than in those with negative scans (6/25; 24%) (P < 0.002).
None of the 4 parameters predicted nonimplementation of the intended management after the 68Ga-PSMA-11 PET/CT study (Q2 to implemented).
DISCUSSION
This prospective survey enabled a systematic assessment of how referring physicians respond to the diagnostic information provided by 68Ga-PSMA-11 PET/CT imaging. The implemented management differed from the prescan treatment plan (Q1) in 54 of 101 patients (53%). PSMA T0N1M0 and PSMA T0N1M1 patterns were significantly associated with a higher likelihood of implemented management changes (P = 0.001 and 0.05, respectively) (Fig. 5). These 2 patterns frequently lead to focal therapy with surgery or radiation therapy (especially pelvic node–only recurrence), a strategy that can be considered only after scan findings are available.
A significant impact of any diagnostic test on management suggests value for patients and is a prerequisite for widespread acceptance (7). However, one concern about studies using intended management changes as an endpoint is that these changes may not be implemented (8–11). The implemented management reflects the true impact of an index test (8,9). In fact, implemented management changes are the most reliable source for cost or cost-effectiveness analyses (8,21,22). Implemented management can be assessed retrospectively—for instance, from large databases (22). However, intended management before image information becomes available can be determined only prospectively. Thus, reliable information must arise from information that is prospectively recorded before and after the index test is performed.
We documented actual management changes in 53% of prostate cancer patients with BCR in response to 68Ga-PSMA-11 PET/CT imaging. These findings are in line with the mean pooled rate of 57.3% (range, 39%–76%) from retrospective studies (12,13). The current rate of intended management changes in 61% of patients is also consistent with the pooled rate of 55.8% (range, 51%–63%) from prospective studies (1,14,15). Other studies enrolled patients prospectively for other reasons, but management changes were not assessed prospectively (23).
We demonstrated that in 1 of 3 patients, the intended management changes (Q2) were not implemented, consistent with previous studies (8). Prostate cancer patients are offered multiple treatment options, including androgen deprivation therapy, surgery, radiotherapy, or combinations of these. Clinical decisions are often based on imaging information, tumor boards, expert opinions, and patient preference. The timing of surveys conducted early after the index test, in our case Q2, precludes consideration of other factors that can affect final decision making. Imaging is obviously not the only determinant of management decisions. These are often based on tumor board or expert opinions, second opinions, patient preference, and other factors. Thus, intended management changes, used as study endpoints in many studies, do not provide actual patient management information. In the current study, intended management changes were often either not implemented or changed to yet other management plans after more information became available to referring physicians. Interestingly, the rate of nonimplementation was much higher in patients with intended changes after the scan (47%) than in patients without intended changes (15%). The current findings underscore a severe limitation of surveys using intended management changes as an endpoint: surveys cannot detect changes induced by tumor board or expert opinions, patient preference, and other factors. To clearly define the implemented management, verification of the management plan using other sources such as electronic chart review, patient information, and clinician information is a prerequisite for appropriate assessment.
Complete information was available for only 101 of 161 patients (63%) (24–26). This less than 100% completion rate may have introduced a responder bias. However, because 96 of 161 patients (60%) were referred from different external institutions, we considered a 63% completion rate as satisfactory. In addition, the large number of participating physicians (n > 40) argues against a significant bias. Furthermore, imaging findings and clinical parameters were comparable between the 101 patients with a complete documented management strategy and the 60 patients without (detection rate of 75% vs. 78%; median PSA levels of 1.7 ng/mL [range, 0.05–140 ng/mL] vs. 2.25 ng/mL [range, 0.2–202 ng/mL]). The relatively low completion rate of Q3 is a negligible problem, because we verified implemented management strategies via other means (electronic chart review and contact with patients).
CONCLUSION
This prospective referring physician–based survey shows a significant impact (54/101; 53%) of 68Ga-PSMA-11 PET/CT on the actual management of prostate cancer patients with BCR. Importantly, intended management changes after 68Ga-PSMA-11 PET/CT were further modified in almost 50% of the patients, underlining the limitations of survey-based management assessment.
DISCLOSURE
Jeremie Calais is the recipient of a grant from the Fondation ARC pour la recherche sur le cancer (grant SAE20160604150). Wolfgang Fendler received a scholarship from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, grant 807122). Matthias Eiber was supported by the SFB 824 (DFG Sonderforschungsbereich 824, Project B11) from the Deutsche Forschungsgemeinschaft, Bonn, Germany. Nicholas Nickols is a Prostate Cancer Foundation Young Investigator and a recipient of a VA Career Development Award (5IK2BX002520), a UCLA Prostate SPORE (4P50CA092131) Career Enhancement Award, a STOP Cancer Foundation Career Development Award, and a UCLA JCCC Seed Grant. Johannes Czernin is the recipient of a grant from the Prostate Cancer Foundation (2017 Challenge award; 17CHAL02) and the Johnson Comprehensive Cancer Center NIH-NCI Cancer Center Support Grant (P30 CA016042). Johannes Czernin is a founder and board member and holds equity in Sofie Biosciences and Trethera Therapeutics. Intellectual property has been patented by the University of California and has been licensed to Sofie Biosciences and Trethera Therapeutics. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. [DOI] [PubMed] [Google Scholar]
- 2.Rauscher I, Maurer T, Beer AJ, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57:1713–1719. [DOI] [PubMed] [Google Scholar]
- 3.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. [DOI] [PubMed] [Google Scholar]
- 4.Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga–prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. [DOI] [PubMed] [Google Scholar]
- 5.Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fendler WP, Calais J, Allen-Auerbach M, et al. 68Ga-PSMA-11 PET/CT interobserver agreement for prostate cancer assessments: an international multicenter prospective study. J Nucl Med. 2017;58:1617–1623. [DOI] [PubMed] [Google Scholar]
- 7.Hillner BE, Siegel BA, Liu D, et al. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the national oncologic PET registry. J Clin Oncol. 2008;26:2155–2161. [DOI] [PubMed] [Google Scholar]
- 8.Hillner BE, Tosteson TD, Tosteson ANA, et al. Intended versus inferred management after PET for cancer restaging: analysis of Medicare claims linked to a coverage with evidence development registry. Med Care. 2013;51:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calais J, Czernin J, Eiber M, et al. Most intended management changes after 68Ga-DOTATATE PET/CT are implemented. J Nucl Med. 2017;58:1793–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine MN, Julian JA. Registries that show efficacy: good, but not good enough. J Clin Oncol. 2008;26:5316–5319. [DOI] [PubMed] [Google Scholar]
- 11.Larson SM. Practice-based evidence of the beneficial impact of positron emission tomography in clinical oncology. J Clin Oncol. 2008;26:2083–2084. [DOI] [PubMed] [Google Scholar]
- 12.Albisinni S, Artigas C, Aoun F, et al. Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU Int. 2017;120:197–203. [DOI] [PubMed] [Google Scholar]
- 13.Afaq A, Alahmed S, Chen SH, et al. Impact of 68Ga-prostate-specific membrane antigen PET/CT on prostate cancer management. J Nucl Med. 2018;59:89–92. [DOI] [PubMed] [Google Scholar]
- 14.Hope TA, Aggarwal R, Chee B, et al. Impact of Ga-68 PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med. 2017;58:1956–1961. [DOI] [PubMed] [Google Scholar]
- 15.Roach PJ, Francis R, Emmett L, et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. J Nucl Med. 2019;59:82–88. [DOI] [PubMed] [Google Scholar]
- 16.Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 17.Eder M, Schäfer M, Bauder-Wüst U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–697. [DOI] [PubMed] [Google Scholar]
- 18.Schwarzenboeck SM, Rauscher I, Bluemel C, et al. PSMA ligands for PET-imaging of prostate cancer. J Nucl Med. 2017;58:1545–1552. [DOI] [PubMed] [Google Scholar]
- 19.Rauscher I, Maurer T, Fendler WP, Sommer WH, Schwaiger M, Eiber M. 68Ga-PSMA ligand PET/CT in patients with prostate cancer: how we review and report. Cancer Imaging. 2016;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Team RCR. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 21.Yang Y, Czernin J. Contribution of imaging to cancer care costs. J Nucl Med. 2011;52(suppl 2):86S–92S. [DOI] [PubMed] [Google Scholar]
- 22.Dinan MA, Curtis LH, Hammill BG, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999-2006. JAMA. 2010;303:1625–1631. [DOI] [PubMed] [Google Scholar]
- 23.Mena E, Lindenberg ML, Shih JH, et al. Clinical impact of PSMA-based 18F-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur J Nucl Med Mol Imaging. 2018;45:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seltzer MA, Yap CS, Silverman DH, et al. The impact of PET on the management of lung cancer: the referring physician’s perspective. J Nucl Med. 2002;43:752–756. [PubMed] [Google Scholar]
- 25.Cartwright A. Professionals as responders: variations in and effects of response rates to questionnaires, 1961-77. Br Med J. 1978;2:1419–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donaldson GW, Moinpour CM, Bush NE, et al. Physician participation in research surveys: a randomized study of inducements to return mailed research questionnaires. Eval Health Prof. 1999;22:427–441. [DOI] [PubMed] [Google Scholar]