Abstract
Rabbits are one of the most used experimental animals for biomedical research, particularly as a bioreactor for the production of antibodies. However, many unique features of the rabbit have also made it as an excellent species for examining a number of aspects of human diseases such as atherosclerosis. Rabbits are phylogenetically closer to humans than rodents, in addition to their relatively proper size, tame disposition, and ease of use and maintenance in the laboratory facility. Due to their short life spans, short gestation periods, high numbers of progeny, low cost (compared with other large animals) and availability of genomics and proteomics, rabbits usually serve to bridge the gap between smaller rodents (mice and rats) and larger animals, such as dogs, pigs and monkeys, and play an important role in many translational research activities such as pre-clinical testing of drugs and diagnostic methods for patients. The principle of using rabbits rather than other animals as an experimental model is very simple: rabbits should be used for research, such as translational research, that is difficult to accomplish with other species. Recently, rabbit genome sequencing and transcriptomic profiling of atherosclerosis have been successfully completed, which has paved a new way for researchers to use this model in the future. In this review, we provide an overview of the recent progress using rabbits with specific reference to their usefulness for studying human atherosclerosis.
Keywords: Animal models, Hypercholesterolemia, Atherosclerosis, Rabbits, Transgenic
Introduction
Experimental animal models play an important role in studying human atherosclerosis. Until now, many animals have been used in this field, including nonhuman primates, pigs, rabbits, and mice1, 2). Nowadays, genetically modified mice have become the most popular animal model for atherosclerosis research3). Which animals researchers use depends on the experimental purposes; however, there are general essential principles that need to be considered when choosing animals: (1) animals should be easy to acquire and maintain at a reasonable cost, easy to handle, and the proper size to allow for all anticipated experimental manipulations, (2) the animal should reproduce in a laboratory setting and have a well-defined genetic background, and (3) the animal model should share with humans the most important aspects of lipid metabolism and cardiovascular pathophysiology4). Although no animal model meets all of these requirements, rabbits are still valuable models for studying atherosclerosis. Indeed, rabbits were the first used animal model for the study of human atherosclerosis. Pioneer studies to establish rabbit atherosclerosis models were performed by two Russian scientists more than a century ago. In 1908, a Russian physician, Ignatowski, fed rabbits with a diet enriched in animal proteins (milk, meat and eggs) and observed intimal lesions with large clear cell (now referred to as foam cell) accumulation in the aorta5). Later, a Russian experimental pathologist, Anitschkow, used a cholesterol diet dissolved in vegetable oil to produce aortic atherosclerosis in rabbits similar with that seen in humans and proposed a causal role of cholesterol in atherosclerosis6). Their studies established the basis of atherosclerosis theory that in both humans and experimental animals, dietary cholesterol can induce atherosclerosis. Rabbit models were widely used and disclosed most of the pathophysiological significance between human atherosclerosis and lipid metabolism such as the discovery of LDL receptors and development of statins, the most prescribed lipid-lowering drugs in the world7). Compared with mice, rabbits have many features similar with humans, which facilitates the studies of lipid metabolism and atherosclerosis (Table 1). For example, like humans but unlike mice, rabbits have abundant plasma cholesteryl ester transfer protein (CETP), which facilitates studying the relationship between CETP and atherosclerosis8). For a long time, it has been controversial whether inhibition of CETP is beneficial for the treatment of atherosclerosis. Concurrently, human clinical trials of CETP inhibitors were generally unsuccessful due to off-target side effects (torcetrapib) or lack of efficacy (dalcetrapib and evacetrapib)9–12). Merck's anacetrapib was reported to be effective in reducing cardiovascular events in a Phase III clinical trial13). Recently, we successfully generated CETP KO rabbits using ZFN methods and demonstrated that genetic deletion of the CETP gene in rabbits protects against diet-induced atherosclerosis14).
Table 1. Comparison of lipid and lipoprotein metabolism features.
| Human | Rabbit | Mouse | |
|---|---|---|---|
| Major plasma lipoproteins | LDL | LDL | HDL |
| CETP | Abundant | Abundant | None |
| Hepatic apoB mRNA editing | No | No | Yes |
| apoB-48 | Chylomicrons | Chylomicrons | VLDLs/LDLs and Chylomicrons |
| apoB-100 | Can be bound to apo(a) | Can be bound to apo(a) | Cannot be bound to apo(a) |
| HDL | Heterogeneous | Heterogeneous | Homogeneous |
| apoAII | Dimer | Absent | Monomer |
| Hepatic LDL receptor activity | Down-regulated | Down-regulated | Usually high |
| Hepatic lipase | High, liver-bound | Low, liver-bound | High, 70% in circulation |
| Cholesterol pool | Mainly from hepatic synthesis | Mainly from hepatic synthesis | Mainly from dietary origin |
| Excretion of bile acid | Low | Low | High |
| Response to a high cholesterol diet | Sensitive | Sensitive | Resistant |
Although the rabbit model has brought about many breakthroughs in the history of atherosclerosis research7), apolipoprotein E (apo E) and LDL receptor gene knock-out (KO) mice became the major models to study atherosclerosis from 20007). In spite of this, rabbits are the second most-used animal models for the study of atherosclerosis based on research papers published in high-profile journals. The importance of using rabbits has been extensively reviewed in previous articles4, 7, 15–17), and in this short review, we will focus on how to use rabbits for cardiovascular research.
Methods to Induce Atherosclerosis in Rabbits
In general, aortic atherosclerotic lesions in rabbits can be easily induced by feeding a high cholesterol diet alone or in combination with arterial balloon injury. Compared with other animals, especially rodents, laboratory rabbits are sensitive to a high cholesterol diet and rapidly develop hypercholesterolemia7). Therefore, it is much easier to induce hypercholesterolemia in rabbits than in other animals by feeding a high cholesterol diet. Dietary cholesterol can range from 0.3%∼1% depending on the research purposes. We recommend the use of 0.3∼0.5% cholesterol in the diet for most experiments because it produces less health problems such as liver toxicity. If cholesterol content is too high (> 1%), rabbits develop massive hypercholesterolemia (plasma cholesterol levels > 1200 mg/dl), which is never observed in humans. Plasma cholesterol levels that are too high often lead to systemic lipid accumulation and liver dysfunction in rabbits, which is often criticized as “not physiological” by many researchers. Therefore, it is not recommended to feed rabbits with a diet containing cholesterol higher than 1% for more than 4 weeks. In addition to cholesterol, oil, such as soybean or corn oil, is essential in the diet. This is because oil in the diet helps in absorption of intestinal cholesterol. There are many commercially available dietary oils that may be used for rabbit experiments. However, coconut oil, as an exception, is rich in saturated fatty acids, unlike other vegetable dietary oils that are rich in polyunsaturated fatty acids. Coconut oil can be used for insulin resistance studies18, 19). It should be noted that cholesterol diets without the addition of oil lead to mobilization of internal fat tissue for absorption of exogenous cholesterol4); therefore, rabbits become extremely thin and sometimes develop cachexia. On a high cholesterol diet, rabbits develop aortic atherosclerosis by 12∼16 weeks. For more advanced lesions, the length of cholesterol diet feeding can be extended for more than 28 weeks20). As rabbits are out-bred animals, it is not unusual for some rabbits to exhibit inert responses to a high cholesterol diet, and are referred to as hypo-responders21). Watanabe heritable hyperlipidemic (WHHL) rabbits are genetically deficient in LDL receptor functions, and develop hypercholesterolemia and atherosclerosis spontaneously on a chow diet22). Although it is difficult to transport WHHL rabbits to many institutions, it is now possible to produce LDL receptor knock-out rabbits by CRISPR-Cas9 methods. Recently, we successfully generated LDL receptor KO rabbits that show a similar phenotype with WHHL rabbits (Liang et al. unpublished data).
Methods to Analyze Atherosclerosis
The rabbit aorta is mainly used for the analysis of atherosclerosis because it is easy to isolate and both gross and microscopic evaluation of aortic lesion size and quality can be made. The lesions usually start to form from the aortic arch followed by the intercostal orifice area and thoracic aorta. Abdominal aortic lesions are less frequently seen, which is different from human atherosclerosis. As the aortic lesions are large, it is also possible to collect a part of the lesions to analyze protein and mRNA expression using Western blotting and RT-PCR methods. If these analyses are required, these samples should be immediately frozen in nitrogen liquid and stocked at −80°C.
Gross analysis usually requires Sudan IV staining to visualize atherosclerotic lesions followed by image analysis to calculate the lesion percentage of each aortic segment23) (Fig. 1). Based on the gross observations, one can decide which parts to select to make paraffin-embedded sections for microscopic observations. Usually, the whole aortic arch and thoracic aorta will be cut into 10–12 segments (1-mm thick) for paraffin embedding23). Serial sections can be routinely used for hematoxylin-eosin (HE) staining and elastic van Gieson (EVG). HE staining is essential to evaluate the quality of the lesions such as cellular and extracellular matrix component features and plaque vulnerability. To quantitate the intimal lesion area on the sections, EVG staining is useful because it can visualize the internal elastic lamina (IEL), making it easy to measure intimal lesions and medial lesions separately. Paraffin sections can be used for direct immunohistochemical staining in most cases using most commercial antibodies. Routinely, it is essential to evaluate macrophages and smooth muscle cells using RAM-1 and smooth muscle α-actin antibodies (Fig. 1).
Fig. 1.
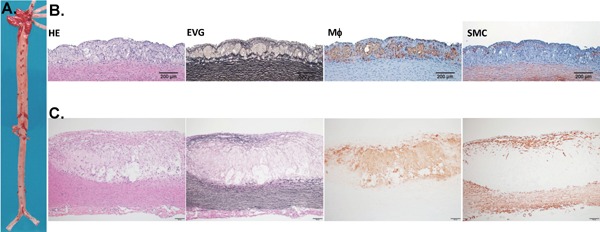
Representative aortic lesions in cholesterol-fed rabbits. A. Gross lesions of the aorta stained by Sudan IV (visualized as red area). B. Representative micrographs of early stage lesions (fatty streaks). C. A typical fibrous plaque with a lipid core covered by a fibrous cap. Serial paraffin sections of the aortic arch were stained with hematoxylin and eosin (HE) and elastica van Gieson (EVG), or immunohistochemically stained with monoclonal antibodies (mAbs) against either macrophages (M?) or α-smooth muscle actin for smooth muscle cells (SMC).
If analysis of protein and mRNA expression is required, a piece of fresh tissue proceeded in liquid nitrogen at the time of aorta collection should be collected. In hypercholesterolemic rabbits, iliac arteries, carotid arteries and subclavian arteries seldom exhibit atherosclerosis; however, coronary lesions of rabbits can be observed at the large branches such as the left and right coronary arteries. The method for rabbit coronary lesion analysis has been described in detail in the previous review7). Balloon injury of aortas or wire injury of iliac arteries in rabbits is sometimes used to produce intimal lesion models. However, these lesions induced by injuries are mainly composed of proliferating smooth muscle cells forming so-called “neo-intima”, which is different from fatty streaks induced by cholesterol diet feeding. However, balloon injury may also be performed in cholesterol-fed rabbits, which will accelerate the formation of the lesions24).
Transgenic Rabbits
In addition to cholesterol-fed rabbits, transgenic (Tg) rabbits have also been used for the study of human cardiovascular disease and lipoprotein metabolism during the last two decades. However, in contrast to Tg mice, Tg rabbits were only used by a few institutions to study atherosclerosis. Elucidation of gene functions in terms of lipoprotein metabolism and atherosclerosis using Tg rabbits was essentially based on the premise that rabbits bear features more similar with those of humans such as metabolism and susceptibility to atherosclerosis (Table 1). Transgenes are usually expressed in the liver or macrophages to study their functions (Table 2). To date, more than 20 genes have been introduced into Tg rabbits, and have provided considerable insight into the molecular mechanisms associated with their functions in lipoprotein metabolism and atherosclerosis25).
Table 2. Transgenic rabbits for the study of human cardiovascular diseases.
| Genes | Expressing cells | Major phenotypes | References |
|---|---|---|---|
| Apolipoproteins: | |||
| Apo(a) | Liver | Atherogenic | 33–37 |
| ApoA-I | Liver | Athero-protective | 38, 39 |
| ApoA-II | Liver | Athero-protective | 40, 41 |
| ApoB-100 | Liver | LD ↑, HDL ↓ | 42 |
| ApoCIII | Liver | VLDL ↑ | 43 |
| ApoE2 | Liver | Atherogenic | 44 |
| ApoE3 | Liver | Atherogenic | 45, 46 |
| Enzymes: | |||
| Hepatic lipase | Liver | Athero-protective | 47 |
| apoB mRNA editing protein | Liver | LDL ↓ | 48 |
| LCAT | Liver | Athero-protective | 49, 50 |
| Lipoprotein lipase | Universal | Athero-protective | 51 |
| PLTP | Universal | Atherogenic | 52 |
| Endothelial lipase | Liver | Athero-protective | 53 |
| Vascular factors: | |||
| Lipoprotein lipase | Macrophage | Atherogenic | 54 |
| MMP-12 | Macrophage | Atherogenic | 20, 55 |
| C-reactive protein | Liver | Thrombogenic | 24, 56 |
| 15-lypooxygenase | Macrophage | Athero-protective | 57 |
| VEGF | Liver | hemangiomas and impaired glomerular functions | 58, 59 |
ND: not done. LCAT, lecithin: cholesterol acyltransferase; PLTP, phospholipid transfer protein; MMP, matrix metalloproteinase; VEGF, vascular endothelial cell growth factor.
Knock-Out Rabbits
For a long time, generation of knock-out rabbits has been a dream for researchers because rabbit embryonic stem (ES) cells are not available and genomic information for rabbits is lacking. Rabbit genome sequencing was initially completed by a European group26), and two years later, we along with Chinese and US groups, successfully sequenced three rabbit genomes (New Zealand White, Japanese White and Watanabe heritable hyperlipidemic rabbits) and completed transcriptomic profiling of atherosclerotic lesions and the liver for these rabbits27). These genomic and RNA seq data will provide many insights into understanding the pathogenesis of atherosclerosis in rabbits. Another advancement in this field is the emergence of novel gene-editing technologies (ZFN, TALENs, CRISPR-Cas9) in succession, which has made it possible to make KO rabbits just as KO mice. The first KO rabbits were generated by the ZFN method to target the IgM gene28), and later, we along with others, successfully generated apoCIII29) and CETP KO rabbits14) using the same method. Although TALENs were still used to create KO rabbits30), CRISPR-Cas9 has now been become the main method for generation of KO rabbits in this field, such as apoE and LDL receptor KO rabbits31, 32) (Table 3). Therefore, one can predict that in the next few years, through these novel technologies, KO rabbits will be generated and used for translational studies of cardiovascular diseases.
Table 3. KO rabbits recently reported.
| Genes | Methods | Research models | References |
|---|---|---|---|
| ApoCIII | ZFN | Atherosclerosis | 29 |
| ApoE | ZFN/CRISPR/Cas9 | Atherosclerosis | 32, 60 |
| CETP | ZFN | Atherosclerosis | 14 |
| αA-Crystallin/GJA8 | CRISPR/Cas9 | Congenital Cataracts | 61, 62 |
| Sex-determining region Y | CRISPR/Cas9 | Male-to-female sex reversal syndrome | 63 |
| Fumarylacetoacetate hydroxylase | TALENs | Tyrosinemia | 30 |
| PHEX | CRISPR/Cas9 | Inheritable rickets | 64 |
| Myostatin | CRISPR/Cas9 | Muscle hypertrophy | 65 |
Advantages and Disadvantages
The rationale of using rabbits rather than mice is dependent upon the research purpose, as described above. As shown in Table 1, like humans but unlike mice, rabbits have a unique lipid metabolism system which facilitates the study of the human lipid metabolism (Table 1). Second, rabbit atherosclerosis lesions resemble those of humans, from early to advanced lesions. Third, adult rabbit body weights (2000∼3500 g) are ∼100-fold larger than mice (20∼30 g), thus the large size of rabbits (including their arteries) enables preclinical studies for surgical experiments such as studies on stents and catheters. The large amount of tissue also makes it possible to perform many analyses using a single animal. Finally, it is important to use rabbits to perform translational research such as development of new lipid-lowering therapeutics. Rabbits should not be considered as a simple substitute for rodents because compared with mice, rabbits may have disadvantages. The individual price of adult rabbits (150∼200 US$ in Japan) is 10-fold higher than for mice (15∼20 US$). Second, the breeding expense of rabbits (0.6∼1$ /rabbit/day) is 10-fold more expensive than for mice (0.06$ per day). Rabbits are kept in individual cages, whereas mice are kept in a cage with a group of 8∼16 mice; therefore, a large space is required for rabbits compared with mice. Being aware of these disadvantages is very important for one to make a decision regarding whether to use rabbits. The most critical point is whether experiments are designed to solve clinical problems such as for translational research.
In conclusion, rabbits are still a powerful model for the study of human atherosclerosis. With the advances in nuclease-editing technology, KO rabbits will provide a new means for translational research in this field. These new models will help develop novel therapeutics and diagnostics for cardiovascular disease in the future.
Sources of Funding
This work was supported in part by a research grant from the National Key Research and Development Program of China (No.2016YFE0126000), Ono Medical Foundation, Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, and Technology, Japan (22390068, 25670190, and 15H04718), and the National Natural Science Foundation of China (No.81570392 and81770457).
Conflicts of Interest
None.
References
- 1). Daugherty A, Tall AR, Daemen M, Falk E, Fisher EA, Garcia-Cardena G, Lusis AJ, Owens AP, 3rd, Rosenfeld ME, Virmani R: Recommendation on Design, Execution, and Reporting of Animal Atherosclerosis Studies: A Scientific Statement From the American Heart Association. Circ Res, 2017; [DOI] [PubMed] [Google Scholar]
- 2). Moghadasian MH: Experimental atherosclerosis: a historical overview. Life Sci, 2002; 70: 855-865 [DOI] [PubMed] [Google Scholar]
- 3). Getz GS, Reardon CA: Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol, 2012; 32: 1104-1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Fan J, Watanabe T: Cholesterol-fed and transgenic rabbit models for the study of atherosclerosis. J Atheroscler Thromb, 2000; 7: 26-32 [DOI] [PubMed] [Google Scholar]
- 5). Ignatowski AC: Influence of animal food on the organism of rabbits. S. Peterb. Izviest. Imp. Voyenno-Med. Akad., 1908; 16: 154-173 [Google Scholar]
- 6). Steinberg D: Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part I. J Lipid Res, 2004; 45: 1583-1593 [DOI] [PubMed] [Google Scholar]
- 7). Fan J, Kitajima S, Watanabe T, Xu J, Zhang J, Liu E, Chen YE: Rabbit models for the study of human atherosclerosis: from pathophysiological mechanisms to translational medicine. Pharmacol Ther, 2015; 146: 104-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Tall AR: Plasma cholesteryl ester transfer protein. J. Lipid. Res., 1986; 27: 361-367 [PubMed] [Google Scholar]
- 9). Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, Investigators I : Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med, 2007; 357: 2109-2122 [DOI] [PubMed] [Google Scholar]
- 10). Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM: Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med, 2007; 356: 1304-1316 [DOI] [PubMed] [Google Scholar]
- 11). Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS, dal OI: Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med, 2012; 367: 2089-2099 [DOI] [PubMed] [Google Scholar]
- 12). McLain JH, Alsterda AJ, Arora RR: Cholesteryl Ester Transfer Protein Inhibitors: Trials and Tribulations. J Cardiovasc Pharmacol Ther, 2016; [DOI] [PubMed] [Google Scholar]
- 13). Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N Engl J Med, 2017; [DOI] [PubMed] [Google Scholar]
- 14). Zhang J, Niimi M, Yang D, Liang J, Xu J, Kimura T, Mathew AV, Guo Y, Fan Y, Zhu T, Song J, Ackermann R, Koike Y, Schwendeman A, Lai L, Pennathur S, Garcia-Barrio M, Fan J, Chen YE: Deficiency of Cholesteryl Ester Transfer Protein Protects Against Atherosclerosis in Rabbits. Arterioscler Thromb Vasc Biol, 2017; 37: 1068-1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Taylor JM, Fan J: Transgenic rabbit models for the study of atherosclerosis. Front Biosci, 1997; 2: d298-308 [DOI] [PubMed] [Google Scholar]
- 16). Fan J, Challah M, Watanabe T: Transgenic rabbit models for biomedical research: current status, basic methods and future perspectives. Pathol Int, 1999; 49: 583-594 [DOI] [PubMed] [Google Scholar]
- 17). Fan J, Watanabe T: Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther, 2003; 99: 261-282 [DOI] [PubMed] [Google Scholar]
- 18). Waqar AB, Koike T, Yu Y, Inoue T, Aoki T, Liu E, Fan J: High-fat diet without excess calories induces metabolic disorders and enhances atherosclerosis in rabbits. Atherosclerosis, 2010; 213: 148-155 [DOI] [PubMed] [Google Scholar]
- 19). Ning B, Wang X, Yu Y, Waqar AB, Yu Q, Koike T, Shiomi M, Liu E, Wang Y, Fan J: High-fructose and high-fat diet-induced insulin resistance enhances atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Nutr Metab (Lond), 2015; 12: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Liang J, Liu E, Yu Y, Kitajima S, Koike T, Jin Y, Morimoto M, Hatakeyama K, Asada Y, Watanabe T, Sasaguri Y, Watanabe S, Fan J: Macrophage metalloelastase accelerates the progression of atherosclerosis in transgenic rabbits. Circulation, 2006; 113: 1993-2001 [DOI] [PubMed] [Google Scholar]
- 21). Poorman JA, Buck RA, Smith SA, Overturf ML, Loose-Mitchell DS: Bile acid excretion and cholesterol 7 alpha-hydroxylase expression in hypercholesterolemia-resistant rabbits. J Lipid Res, 1993; 34: 1675-1685 [PubMed] [Google Scholar]
- 22). Watanabe Y: Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). Atherosclerosis, 1980; 36: 261-268 [DOI] [PubMed] [Google Scholar]
- 23). Koike T, Liang J, Wang X, Ichikawa T, Shiomi M, Sun H, Watanabe T, Liu G, Fan J: Enhanced aortic atherosclerosis in transgenic Watanabe heritable hyperlipidemic rabbits expressing lipoprotein lipase. Cardiovasc Res, 2005; 65: 524-534 [DOI] [PubMed] [Google Scholar]
- 24). Matsuda S, Yamashita A, Sato Y, Kitajima S, Koike T, Sugita C, Moriguchi-Goto S, Hatakeyama K, Takahashi M, Koshimoto C, Matsuura Y, Iwakiri T, Chen YE, Fan J, Asada Y: Human C-reactive protein enhances thrombus formation after neointimal balloon injury in transgenic rabbits. J Thromb Haemost, 2011; 9: 201-208 [DOI] [PubMed] [Google Scholar]
- 25). Peng X: Transgenic rabbit models for studying human cardiovascular diseases. Comp Med, 2012; 62: 472-479 [PMC free article] [PubMed] [Google Scholar]
- 26). Carneiro M, Rubin CJ, Di Palma F, Albert FW, Alfoldi J, Martinez Barrio A, Pielberg G, Rafati N, Sayyab S, Turner-Maier J, Younis S, Afonso S, Aken B, Alves JM, Barrell D, Bolet G, Boucher S, Burbano HA, Campos R, Chang JL, Duranthon V, Fontanesi L, Garreau H, Heiman D, Johnson J, Mage RG, Peng Z, Queney G, Rogel-Gaillard C, Ruffier M, Searle S, Villafuerte R, Xiong A, Young S, Forsberg-Nilsson K, Good JM, Lander ES, Ferrand N, Lindblad-Toh K, Andersson L: Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication. Science, 2014; 345: 1074-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Wang Z, Zhang J, Li H, Li J, Niimi M, Ding G, Chen H, Xu J, Zhang H, Xu Z, Dai Y, Gui T, Li S, Liu Z, Wu S, Cao M, Zhou L, Lu X, Wang J, Yang J, Fu Y, Yang D, Song J, Zhu T, Ning B, Koike T, Shiomi M, Liu E, Chen L, Fan J, Chen YE, Li Y: Hyperlipidemia-associated gene variations and expression patterns revealed by whole-genome and transcriptome sequencing of rabbit models. Sci Rep, 2016; 6: 26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Flisikowska T, Thorey IS, Offner S, Ros F, Lifke V, Zeitler B, Rottmann O, Vincent A, Zhang L, Jenkins S, Niersbach H, Kind AJ, Gregory PD, Schnieke AE, Platzer J: Efficient Immunoglobulin Gene Disruption and Targeted Replacement in Rabbit Using Zinc Finger Nucleases. PLoS One, 2011; 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Yang D, Zhang J, Xu J, Zhu T, Fan Y, Fan J, Chen YE: Production of Apolipoprotein C-III Knockout Rabbits using Zinc Finger Nucleases. J Vis Exp, 2013; 10.3791/50957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Li L, Zhang Q, Yang H, Zou Q, Lai C, Jiang F, Zhao P, Luo Z, Yang J, Chen Q, Wang Y, Newsome PN, Frampton J, Maxwell PH, Li W, Chen S, Wang D, Siu TS, Tam S, Tse HF, Qin B, Bao X, Esteban MA, Lai L: Fumarylacetoacetate Hydrolase Knock-out Rabbit Model for Hereditary Tyrosinemia Type 1. J Biol Chem, 2017; 292: 4755-4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Yang D, Xu J, Zhu T, Fan J, Lai L, Zhang J, Chen YE: Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. J Mol Cell Biol, 2014; 6: 97-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Niimi M, Yang D, Kitajima S, Ning B, Wang C, Li S, Liu E, Zhang J, Eugene Chen Y, Fan J: ApoE knockout rabbits: A novel model for the study of human hyperlipidemia. Atherosclerosis, 2016; 245: 187-193 [DOI] [PubMed] [Google Scholar]
- 33). Rouy D, Duverger N, Lin SD, Emmanuel F, Houdebine LM, Denefle P, Viglietta C, Gong E, Rubin EM, Hughes SD: Apolipoprotein(a) yeast artificial chromosome transgenic rabbits. Lipoprotein(a) assembly with human and rabbit apolipoprotein B. J Biol Chem, 1998; 273: 1247-1251 [DOI] [PubMed] [Google Scholar]
- 34). Fan J, Shimoyamada H, Sun H, Marcovina S, Honda K, Watanabe T: Transgenic rabbits expressing human apolipoprotein(a) develop more extensive atherosclerotic lesions in response to a cholesterol-rich diet. Arterioscler Thromb Vasc Biol, 2001; 21: 88-94 [DOI] [PubMed] [Google Scholar]
- 35). Ichikawa T, Unoki H, Sun H, Shimoyamada H, Marcovina S, Shikama H, Watanabe T, Fan J: Lipoprotein(a) promotes smooth muscle cell proliferation and dedifferentiation in atherosclerotic lesions of human apo(a) transgenic rabbits. Am J Pathol, 2002; 160: 227-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Sun H, Unoki H, Wang X, Liang J, Ichikawa T, Arai Y, Shiomi M, Marcovina SM, Watanabe T, Fan J: Lipoprotein( a) enhances advanced atherosclerosis and vascular calcification in WHHL transgenic rabbits expressing human apolipoprotein(a). J Biol Chem, 2002; 277: 47486-47492 [DOI] [PubMed] [Google Scholar]
- 37). Kitajima S, Jin Y, Koike T, Yu Y, Liu E, Shiomi M, Marcovina SM, Morimoto M, Watanabe T, Fan J: Lp(a) enhances coronary atherosclerosis in transgenic Watanabe heritable hyperlipidemic rabbits. Atherosclerosis, 2007; 193: 269-276 [DOI] [PubMed] [Google Scholar]
- 38). Duverger N, Kruth H, Emmanuel F, Caillaud JM, Viglietta C, Castro G, Tailleux A, Fievet C, Fruchart JC, Houdebine LM, Denefle P: Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein A-I- transgenic rabbits. Circulation, 1996; 94: 713-717 [DOI] [PubMed] [Google Scholar]
- 39). Duverger N, Viglietta C, Berthou L, Emmanuel F, Tailleux A, Parmentier-Nihoul L, Laine B, Fievet C, Castro G, Fruchart JC, Houbebine LM, Denefie P: Transgenic rabbits expressing human apolipoprotein A-I in the liver. Arterioscl Thromb Vasc Biol, 1996; 16: 1424-1429 [DOI] [PubMed] [Google Scholar]
- 40). Wang Y, Niimi M, Nishijima K, Yu Y, Koike T, Kitajima K, Inoue T, Waqar AB, Liu E, Kohashi M, Ketamura Y, Yoshikawa T, Zhang J, Ma L, Zha X, Watanabe T, Asada Y, Chen EY, Fan J: Human apolipoprotein AII protects against diet-induced atherosclerosis in transgenic rabbits. Arterioscler Thromb Vas Biol, 2013; 33: 224-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Koike T, Kitajima S, Yu Y, Li Y, Nishijima K, Liu E, Sun H, Waqar AB, Shibata N, Inoue T, Wang Y, Zhang B, Kobayashi J, Morimoto M, Saku K, Watanabe T, Fan J: Expression of human apoAII in transgenic rabbits leads to dyslipidemia: a new model for combined hyperlipidemia. Arterioscler Thromb Vasc Biol, 2009; 29: 2047-2053 [DOI] [PubMed] [Google Scholar]
- 42). Fan J, McCormick SP, Krauss RM, Taylor S, Quan R, Taylor JM, Young SG: Overexpression of human apolipoprotein B-100 in transgenic rabbits results in increased levels of LDL and decreased levels of HDL. Arterioscler Thromb Vasc Biol, 1995; 15: 1889-1899 [DOI] [PubMed] [Google Scholar]
- 43). Ding Y, Wang Y, Zhu H, Fan J, Yu L, Liu G, Liu E: Hypertriglyceridemia and delayed clearance of fat load in transgenic rabbits expressing human apolipoprotein CIII. Transgenic Res, 2011; 20: 867-875 [DOI] [PubMed] [Google Scholar]
- 44). Huang Y, Schwendner SW, Rall SCJ, Sanan DA, Mahley RW: Apolipoprotein E2 transgenic rabbits. J Biol Chem, 1997; 272: 22685-22694 [DOI] [PubMed] [Google Scholar]
- 45). Fan J, Ji Z-S, Huang Y, de Silva H, Sanan D, Mahley R, Innerarity T, Taylor J: Increased expression of apolioprotein E in transgenic rabbits results in reduced levels of very low density lipoproteins and an accumulation of low density lipoproteins in plasma. J Clin Invest, 1998; 101: 2151-2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Huang Y, Ji ZS, Brecht WJ, Rall SC, Jr., Taylor JM, Mahley RW: Overexpression of apolipoprotein E3 in transgenic rabbits causes combined hyperlipidemia by stimulating hepatic VLDL production and impairing VLDL lipolysis. Arterioscler Thromb Vasc Biol, 1999; 19: 2952-2959 [DOI] [PubMed] [Google Scholar]
- 47). Fan J, Wang J, Bensadoun A, Lauer SJ, Dang Q, Mahley RW, Taylor JM: Overexpression of hepatic lipase in transgenic rabbits leads to a marked reduction of plasma high density lipoproteins and intermediate density lipoproteins. Proc Natl Acad Sci U S A, 1994; 91: 8724-8728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Yamanaka S, Balestra ME, Ferrell LD, Fan J, Arnold KS, Taylor S, Taylor JM, Innerarity TL: Apolipoprotein B mRNA-editing protein induces hepatocellular carcinoma and dysplasia in transgenic animals. Proc Natl Acad Sci U S A, 1995; 92: 8483-8487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Hoeg JM, Santamarina-Fojo S, Berard AM, Cornhill JF, Herderick EE, Feldman SH, Haudenschild CC, Vaisman BL, Hoyt RF, Jr., Demosky SJ, Jr., Kauffman RD, Hazel CM, Marcovina SM, Brewer HB, Jr.: Overexpression of lecithin: cholesterol acyltransferase in transgenic rabbits prevents diet-induced atherosclerosis. Proc. Natl. Acad. Sci. USA, 1996; 93: 11448-11453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Hoeg JM, Kauffman RD, Herderick E, Demonsky SJ, Jr., Evans W, Brousseau M: Lecithin: cholesterol acyl transferase requires functional LDL receptors to prevent atherosclerosis. Circulation, 1998; 98 (Supl): I-464 (Abstract) [Google Scholar]
- 51). Fan J, Unoki H, Kojima N, Sun H, Shimoyamada H, Deng H, Okazaki M, Shikama H, Yamada N, Watanabe T: Overexpression of lipoprotein lipase in transgenic rabbits inhibits diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem, 2001; 276: 40071-40079 [DOI] [PubMed] [Google Scholar]
- 52). Masson D, Deckert V, Gautier T, Klein A, Desrumaux C, Viglietta C, Pais de Barros JP, Le Guern N, Grober J, Labbe J, Menetrier F, Ripoll PJ, Leroux-Coyau M, Jolivet G, Houdebine LM, Lagrost L: Worsening of diet-induced atherosclerosis in a new model of transgenic rabbit expressing the human plasma phospholipid transfer protein. Arterioscler Thromb Vasc Biol, 2011; 31: 766-774 [DOI] [PubMed] [Google Scholar]
- 53). Wang C, Nishijima K, Kitajima S, Niimi M, Yan H, Chen Y, Ning B, Matsuhisa F, Liu E, Zhang J, Chen YE, Fan J: Increased Hepatic Expression of Endothelial Lipase Inhibits Cholesterol Diet-Induced Hypercholesterolemia and Atherosclerosis in Transgenic Rabbits. Arterioscler Thromb Vasc Biol, 2017; 37: 1282-1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Ichikawa T, Liang J, Kitajima S, Koike T, Wang X, Sun H, Morimoto M, Shikama H, Watanabe T, Yamada N, Fan J: Macrophage-derived lipoprotein lipase increases aortic atherosclerosis in cholesterol-fed Tg rabbits. Atherosclerosis, 2005; 179: 87-95 [DOI] [PubMed] [Google Scholar]
- 55). Yamada S, Wang KY, Tanimoto A, Fan J, Shimajiri S, Kitajima S, Morimoto M, Tsutsui M, Watanabe T, Yasumoto K, Sasaguri Y: Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol, 2008; 172: 1419-1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Kondo M, Sakai T, Komeima K, Kurimoto Y, Ueno S, Nishizawa Y, Usukura J, Fujikado T, Tano Y, Terasaki H: Generation of a transgenic rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci, 2009; 50: 1371-1377 [DOI] [PubMed] [Google Scholar]
- 57). Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kuhn H, Guevara NV, Chan L: Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest, 1996; 98: 2201-2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Liu E, Morimoto M, Kitajima S, Koike T, Yu Y, Shiiki H, Nagata M, Watanabe T, Fan J: Increased expression of vascular endothelial growth factor in kidney leads to progressive impairment of glomerular functions. J Am Soc Nephrol, 2007; 18: 2094-2104 [DOI] [PubMed] [Google Scholar]
- 59). Kitajima S, Liu E, Morimoto M, Koike T, Yu Y, Watanabe T, Imagawa S, Fan J: Transgenic rabbits with increased VEGF expression develop hemangiomas in the liver: a new model for Kasabach-Merritt syndrome. Lab Invest, 2005; 85: 1517-1527 [DOI] [PubMed] [Google Scholar]
- 60). Ji D, Zhao G, Songstad A, Cui X, Weinstein EJ: Efficient creation of an APOE knockout rabbit. Transgenic Res, 2015; 24: 227-235 [DOI] [PubMed] [Google Scholar]
- 61). Yuan L, Yao H, Xu Y, Chen M, Deng J, Song Y, Sui T, Wang Y, Huang Y, Li Z, Lai L: CRISPR/Cas9-Mediated Mutation of alphaA-Crystallin Gene Induces Congenital Cataracts in Rabbits. Invest Ophthalmol Vis Sci, 2017; 58: BIO34-BIO41 [DOI] [PubMed] [Google Scholar]
- 62). Yuan L, Sui T, Chen M, Deng J, Huang Y, Zeng J, Lv Q, Song Y, Li Z, Lai L: CRISPR/Cas9-mediated GJA8 knockout in rabbits recapitulates human congenital cataracts. Sci Rep, 2016; 6: 22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). Song Y, Liu T, Wang Y, Deng J, Chen M, Yuan L, Lu Y, Xu Y, Yao H, Li Z, Lai L: Mutation of the Sp1 binding site in the 5’ flanking region of SRY causes sex reversal in rabbits. Oncotarget, 2017; 8: 38176-38183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Sui T, Yuan L, Liu H, Chen M, Deng J, Wang Y, Li Z, Lai L: CRISPR/Cas9-mediated mutation of PHEX in rabbit recapitulates human X-linked hypophosphatemia (XLH). Hum Mol Genet, 2016; 25: 2661-2671 [DOI] [PubMed] [Google Scholar]
- 65). Lv Q, Yuan L, Deng J, Chen M, Wang Y, Zeng J, Li Z, Lai L: Efficient Generation of Myostatin Gene Mutated Rabbit by CRISPR/Cas9. Sci Rep, 2016; 6: 25029. [DOI] [PMC free article] [PubMed] [Google Scholar]


