Abstract
Pituitary adenylate cyclase activating polypeptide (PACAP) is a multifunctional neuropeptide with widespread occurrence throughout the body including the gastrointestinal system. In the small and large intestine, effects of PACAP on cell proliferation, secretion, motility, gut immunology and blood flow, as well as its importance in bowel inflammatory reactions and cancer development have been shown and reviewed earlier. However, no current review is available on the actions of PACAP in the stomach in spite of numerous data published on the gastric presence and actions of the peptide. Therefore, the aim of the present review is to summarize currently available data on the distribution and effects of PACAP in the stomach. We review data on the localization of PACAP and its receptors in the stomach wall of various mammalian and non-mammalian species, we then give an overview on PACAP’s effects on secretion of gastric acid and various hormones. Effects on cell proliferation, differentiation, blood flow and gastric motility are also reviewed. Finally, we outline PACAP’s involvement and changes in various human pathological conditions.
Keywords: PACAP, stomach, secretion, motility, cancer, ulcer, gastritis
Introduction
PACAP was isolated as a neuroendocrine regulator of the hypothalamo-hypophyseal system in two forms, PACAP1–27 and PACAP1–38, with 27 and 38 amino acid residues, respectively. PACAP and VIP belong to the same peptide family (VIP-secretin-glucagon), based on the structural similarity between the shorter form of PACAP and VIP. Later PACAP was shown throughout the body with a diverse array of functions, including the gastrointestinal system. VIP has long been known as a gastrointestinal peptide, but shortly after the isolation of its related peptide many, partially overlapping, gastrointestinal effects of PACAP have also been described. PACAP, similarly to VIP, occurs along the entire digestive tract, and both peptides are abundantly expressed in the enteric nervous system (ENS) and enteroendocrine cells, influencing secretion, motility and reflexes. PACAP acts through its specific PAC1 receptor and the VPAC1 and 2 receptors, which also bind VIP with similar affinity (1).
In the small and large intestine, PACAP is involved in several biological processes. Its effects on cell proliferation, secretion, motility, gut immunology, and blood flow have been shown (1–3). Under pathological circumstances, it has been shown that PACAP decreases inflammatory reactions both in the small and in the large intestine, while lack of PACAP is associated with increased inflammatory reactions and colon cancer development (4–6). The intestinal effects of PACAP have been reviewed several times by different authors (7–10). However, no current review is available on the actions of PACAP in the stomach in spite of dozens of articles published on the gastric presence and actions of the peptide. Therefore, the aim of the present review is to summarize currently available data on the distribution and effects of PACAP in the stomach.
Presence and Distribution of PACAP and Its Receptors in the Stomach
The presence and distribution of PACAP have been studied in several species with different methods, and all have found PACAP expression in the stomach wall, with some qualitative and quantitative differences between species and methods used (11). Soon after its discovery, a radioimmunoassay (RIA) study described the distribution of PACAP in various tissues of the rat and found that PACAP1–38 was the dominant type in mammalian tissues. They could detect PACAP also in the gastrointestinal tract, with stomach, duodenum and jejunum showing higher levels compared to other parts of the intestinal system (12). In another study in rats, Hannibal et al. found high levels of PACAP1–38 in the stomach by RIA and lower levels of PACAP1–27 and PACAP-related protein. There was no difference between levels measured in the esophagus and antrum/fundus parts of the stomach, but higher levels were measured in the small intestine (11). The dominance of PACAP1–38 was also confirmed in the antrum part of the porcine stomach by another RIA study, with PACAP1–27 just reaching detection limit (13). The authors measured mucosal and muscularis extracts separately and found slightly higher levels in the muscularis part.
The first comparative immunohistochemical description of PACAP1–27-like immunoreactivity in the alimentary canal of several species came from Sundler et al. (14), who found immunopositivity in nerve fibers in the wall of the gastrointestinal tract of all mammalian species examined, namely mouse, rat, hamster, guinea pig, ferret, cat, pig, sheep and man. In the gastric mucosa, they observed delicate PACAP-immunoreactive fibers in the mouse, rat, hamster and human, but not in the other species examined. Fine varicose fibers were found in the mucosa (both oxynthic and pyloric parts) and also in the muscularis layer in the rat stomach. Moderate number of PACAP-containing fibers was seen in the submucous ganglia, while numerous nerve fibers as well as immunopositive nerve cell bodies in the myenteric ganglia (11). PACAP is frequently colocalized with the sensory neuropeptide CGRP and also with VIP. In pigs, PACAP-immunoreactivity was described in beaded nerve fibers in all layers of the antrum, with higher number in the muscular and submucosal layers than in the mucosa. Furthermore, immunoreactivity was observed in nerve cell bodies of the myenteric ganglia, but not in the submucosal plexus (13). Colocalization with VIP was observed both in the fibers and in the ganglionic cells and some fibers costored CGRP, especially those innervating submucosal blood vessels (13). In the mucosa, only few PACAP positive nerve fibers could be found, mainly around blood vessels and some associated with basal glandular cells (13).
Other studies have also confirmed these findings. Kantor et al. (15) could detect PACAP-specific mRNA in the oxynthic mucosa of the rat stomach with RT-PCR. PACAP mRNA was also demonstrated by in situ hybridization in a few nerve cell bodies in the myenteric ganglia indicating some intrinsic synthesis of the peptide (11). Studying further the origin of PACAP-ergic nerves Hannibal et al. performed capsaicin-induced denervation as well as surgical denervation. They found that neonatal capsaicin treatment reduced the concentration of PACAP in the stomach by about one-third. This was mainly confined to the oxynthic part of the submucosa, where a reduced number of immunopositive nerve fibers was observed, while fibers remained unaffected in the mucosa, muscularis and myenteric ganglia. After surgical extrinsic denervation, a modest decrease was observed. These data proved that the origin of PACAP in the stomach wall is dual: both intrinsic and extrinsic. The extrinsic innervation is most probably sensory, also supported by the observations of PACAP in the jugular-nodose ganglion of the vagus nerve and dorsal root ganglia (16–18).
PACAP immunoreactivity was also studied in the sheep digestive tract (19). Fibers were mainly detected in the muscular layer of the stomach, including cardia, corpus, antrum, and pylorus, with pyloric sphincter showing very strong PACAP-ergic innervation (19). Scarse immunolabeled fibers were detected in the mucosa, mainly in the lamina muscularis mucosae. Fibers and few perikarya were detected in myenteric ganglia (19). Presence of PACAP in the stomach wall has also been confirmed in cats (20). In the guinea pig, myenteric fibers showed weak immunoreactivity, together with lamina propria around glands and submucosal blood vessels, with weaker expression than in other mammalian species (21). PACAP immunoreactivity showed similar pattern in another rodent, Mastomys stomach, where PACAP was found in the oxynthic mucosa between the glands and in the submucosa (22).
Based on the very limited available data, PACAP occurs also in the human stomach, similarly to the distribution in other mammals. As mentioned earlier, Sundler et al. (14) observed delicate PACAP1–27-like immunoreactive fibers in the stomach. Vincze et al. have described first the presence of PACAP in the normal human stomach (20). In addition to the few PACAP-positive fibers in the mucosa, numerous cells contain PACAP in the glands of the fundus and corpus, and less in the cardia and pylorus. Electron microscopical observations showed that mainly the parietal cells contained perinuclear PACAP immunoreactivity (20). During fetal development, PACAP immunoreactivity appears in the human gastric glands, of both corpus and pylorus, around the 18th to 20th intrauterine weeks (23).
The presence of PACAP has also been investigated in the alimentary tract, including the stomach, of several non-mammalian species. For example, PACAP/GHRH-like mRNA could be detected in the stomach of catfish (24). PACAP and receptor transcripts have been found in another fish [tilapia (25)]. In zebrafish, immunohistochemistry established the presence of gut neurons expressing PACAP in the proximal part of the developing gut from the first stage investigated (2 days postfertilization) and before regular motility was observed. At 5 days postfertilization, PACAP reduced the regular propagating wave frequency of gut motility. This suggests that both excitatory and inhibitory pathways develop at an early stage in the gut, independent of exogenous feeding. This supports physiological results that gut motility is under neuronal control during the period when regular motility patterns develop (26). PACAP mRNA has also been identified in the olive flounder pylorus (27). Among reptiles, Valiante et al. (28) found mRNA for PACAP in gastric glands with in situ hybridization and PACAP peptide with immunohistochemistry as well as immunoreactivity with antisera against all three PACAP receptors in the lizard stomach. In frog species, Olsson showed the presence of PACAP in the entire gastrointestinal tract, including the stomach, of the African clawed frog (29). He showed immunopositivity in all layers of the stomach, in the endocrine cells of the mucosa, and in nerve fiber bundles and ganglionic cells of the myenteric plexus. PACAP and VIP colocalization was observed in most places.
In birds, several studies have shown the presence of PACAP in the proventriculus (glandular part of avian stomach) and gizzard. Sundler et al. (14) studied PACAP1–27-like immunoreactivity in the digestive tract of chicken in addition to several mammalian species (see above). In the proventriculus, numerous PACAP-immunoreactive endocrine cells could be observed which were identical to the serotonin-containing cells storing gastrin-releasing peptide (14). Simon et al. (30) described PACAP gene expression increase in the glandular stomach in case of food restriction. In addition, nerve elements in other layers also contained immunoreactivity. Studying the ontogeny of PACAP-containing elements, Salvi et al. (31) found the first PACAP-immunoreactive elements at embryonic days 4.5–5 in the mesenchymal bud of the proventriculus/gizzard. After the pharyngeal appearance at E4, PACAP elements formed a weak network in the marginal mesenchymal zone of the stomach bud, followed by gradual appearance in myenteric and submucous plexuses (31). PACAP immunoreactivity has also been studied in another avian species by Mirabella et al. (32–34). The presence of both PACAP1–38 and PACAP1–27 was demonstrated, the former being the predominant form, in the gastrointestinal tract of the duck (32). They found PACAP immunoreactivity in neurons and fibers of the ENS, in endocrine cells and in the gut-associated lymphoid tissue, suggesting multiple roles of the peptide in the duck gastrointestinal system. The majority of mucosal ganglion cells in the proventriculus were shown to contain PACAP (33). In pigeons, the coexistence of PACAP/VIP was revealed in the stomach in NADPH-positive myenteric neurons, implying that the nitrergic nerve population of the pigeon gastrointestinal tract takes part in regulation of muscle motility as an inhibitory descending nerve pathway (34).
Even invertebrates contain PACAP-like immunoreactivity in their alimentary canal, including the areas corresponding to mammalian foregut/stomach regions. PACAP1–27 has been shown to be the dominant form of the peptide according to a RIA study, but immunoreactivity to both peptides could be shown in the foregut/gizzard regions of the earthworm Lumbricus polyphemus (35). PACAP-immunoreactive elements have been identified mainly in the ganglia supplying the alimentary canal in three annelid species: Lumbricus terrestris, Eisenia fetida, and Lumbricus polyphemus (36, 37). This has also been shown during earthworm development, along with the appearance of PAC1 receptor-like immunoreactivity in the subesophageal and other ventral cord ganglia (38, 39). Interestingly, and in concert with the well-known regeneration-promoting effect of PACAP (40, 41), significant increases in the concentration of PACAP-like compounds were found in the body wall, alimentary canal, and in coelomocytes during regeneration. The most characteristic morphological feature was the accumulation of immunolabeled neoblasts in the injured tissues, especially in the ventral nerve cord ganglion that initiates and mediates regeneration processes, including that of the digestive tract (42). Although no functional data are available, taken together, these morphological observations indicate that PACAP (or a PACAP-like peptide) occurs early during phylogeny, and is present not only in vertebrates, but also in the invertebrate alimentary tract.
Early receptor binding studies already detected PACAP binding in the stomach (43). Receptors for both PACAP and VIP have been identified in the stomach wall. In porcine antrum, mRNA for PAC1 and VPAC1 and 2 was identified (13), PAC1 and VPAC2 were mainly expressed in the muscular, but not in the submucosal/mucosal layers, while VPAC1 was found in all layers (13). In the gastric smooth muscle of guinea pigs VPAC2 receptor was found (44). Other studies have also shown PACAP receptors in different layers of the stomach. For example, VPAC2 receptors have been detected in the smooth muscle cells isolated from rabbit and guinea pig stomach (45), and others also detected VPAC2 receptors in the muscularis mucosae and the main muscularis layers of the mouse stomach, while no VPAC2 was found in other layers (46). PACAP binding has been shown in various pharmacological studies investigating motility (47–49). Enterochromaffin-like (ECL) cells express PAC1 receptor, and it is believed that PACAP from the myenteric neurons acts on these cells (50–52). It is well supported by some studies that ECL cells express PAC1 receptor, but not the VPAC receptors in rats and mice (53, 54), although all three receptors were found by others in Mastomys (22). All splice variants of the PAC1 receptors have been described on ECL cells (55).
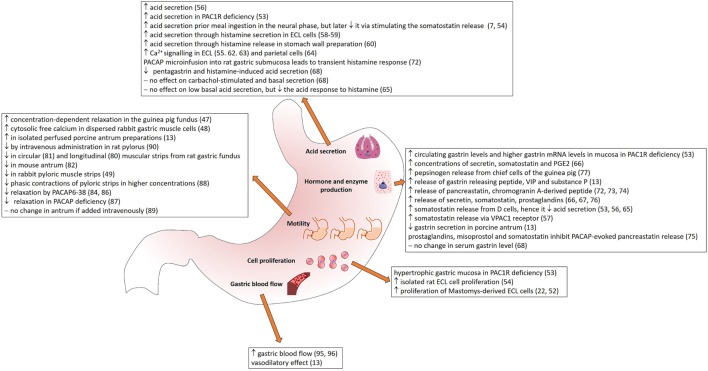
The wide distribution of PACAP and its receptors in secretory cells, in the nerves innervating different gastric layers and around blood vessels supplying the stomach (summarized in Table 1) suggests that PACAP plays various roles in gastric secretion, motility, and blood flow. In the following sections, we summarize currently available data on the different effects of PACAP in the stomach (Figure 1).
Table 1.
Presence and main distribution sites of PACAP and its receptors in the stomach of various species.
| Species | Localization of PACAP | Localization of PACAP receptor(s) | Reference |
|---|---|---|---|
| VERTEBRATE | Dominant form: 1–38 | ||
| Mammalian | |||
| Cat | Nerve fibers of myenteric ganglia and muscularis | (14, 20) | |
| Ferret | Nerve fibers in gastrointestinal wall | (14) | |
| Guinea pig | Myenteric fibers, lamina propria, submucosal blood vessels, nerve fibers of muscularis | Gastric smooth muscle: VPAC2 | (14, 21, 44, 45) |
| Hamster | Nerve fibers in mucosa, myenteric ganglia and muscularis | (14) | |
| Human | Gastric glands, mainly parietal cells, nerve fibers of mucosa, myenteric ganglia, and muscularis | (14, 20, 23) | |
| Mastomys | Oxynthic mucosa and submucosa | PAC1, VPAC1 and 2 on enterochromaffin-like cells | (22) |
| Mouse | Nerve fibers of mucosa, myenteric ganglia, and muscularis | Muscularis mucosae, muscularis: VPAC2 PAC1 on enterochromaffin-like cells |
(14, 46, 53) |
| Pig | Nerve fibers in all layers of antrum, nerve cell bodies of myenteric ganglia, muscularis | Muscularis of antrum: PAC1, VPAC2 All layers of antrum: VPAC1 |
(13, 14) |
| Rabbit | Gastric smooth muscle: VPAC2 | (45) | |
| Rat | Submucous and myenteric ganglia, mucosa, submucosa, muscularis | PAC1 on enterochromaffin-like cells | (11, 14, 15, 50–52, 54) |
| Sheep | Muscularis of cardia, corpus, antrum and pylorus, muscularis mucosae, fibers, and perikarya of myenteric ganglia | (14, 19) | |
| Non-mammalian | |||
| Catfish | Stomach wall | (24) | |
| Chicken | Endocrine cells in proventriculus, proventriculus, mesenchymal bud of proventriculus/gizzard, myenteric, and submucous plexus | PAC1 in proventriculus | (14, 30, 31) |
| Duck | Neurons and fibers of enteric nervous system, mucosal ganglionic cells in proventriculus | (32, 33) | |
| Frog | All layers of gastric wall, endocrine cells of mucosa, myenteric plexus | (29) | |
| Lizard | Gastric glands | PAC1, VPAC1, VPAC2 | (28) |
| Olive flounder | Pylorus | (27) | |
| Pigeon | Myenteric neurons | (34) | |
| Tilapia | PAC1 | (25) | |
| Zebrafish | Gut neurons in proximal part of developing gut | (26) | |
| INVERTEBRATE | Dominant form: 1–27 | ||
| Lumbricus polyphemus | Foregut, ganglia of alimentary canal | (35–37) | |
| Lumbricus terrestris Eisenia fetida |
Ganglia of alimentary canal | (36, 37) | |
Figure 1.
Summary of the effects of PACAP in stomach.
Effects of PACAP in the Stomach
Effects on Gastric Secretion
As one of its main actions, PACAP leads to increases in cAMP levels also in the stomach (56). Interestingly, this action could not be shown in isolated stomach wall preparation under unchallenged conditions but was markedly potentiated in the presence of phosphodiesterase inhibitors, and it was four times greater than in the presence of the inhibitor alone, without PACAP addition (56).
A lot of work has been done in order to clarify the role of PACAP on acid secretion (Figure 1). In the beginning, several contradictory studies were published, some reporting stimulatory effects on acid secretion, while others inhibitory. The primary site for acid secretion is the parietal cells situated in the gastric corpus. In the cephalic phase, vagal afferents stimulate acid secretion through the release of acetylcholine and PACAP. The gastric phase of acid secretion is regulated by gastrin released from G cells, by histamine from ECL cells and somatostatin from D cells (7). The ECL cells control parietal cells by releasing histamine in their immediate vicinity. According to a number of studies, gastrin and PACAP stimulate histamine secretion from isolated ECL cells, while somatostatin and galanin inhibit stimulated secretion (57).
Plenty of studies show stimulatory actions of PACAP on acid secretion. PACAP increases acid secretion directly and also through stimulating histamine release (56). The histamine-releasing effect of PACAP in ECL cells has been shown in vitro (55, 58) and in stomach wall preparations (59). It has also been demonstrated that the PACAP-evoked secretion of histamine depends on Ca2+ entry (60). Several other studies have proven that through its receptor, PACAP activates calcium signaling and histamine release from ECL cells (55, 61, 62). Not only ECL cells respond to PACAP with elevated calcium levels, but also adjacent parietals cells (63). The PACAP-induced increase in gastric acid secretion can be blocked by the phosphodiesterase 4 inhibitor rolipram, which inhibits the degradation of cAMP (56).
The action of PACAP on gastric acid secretion has some controversial aspects. Although many studies describe that PACAP stimulates gastric acid secretion, the peptide is also known to stimulate somatostatin release from D cells (53, 56, 64), thus, it decreases gastric acid secretion. Li et al. described that the inhibition on gastric acid secretion in rat stomach by PACAP is mediated by secretin, somatostatin, and prostaglandin E2 (PGE2) (65, 66). Similarly, Mungan et al. found that intravenous PACAP inhibits pentagastrin and histamine-induced acid secretion in conscious pylorus-ligated rats and in gastric fistula rats, while no effect on carbachol-stimulated or basal secretion in pylorus ligated rats (67). Others have confirmed this observation: in wild-type urethane-anesthetized mice, PACAP1–38 did not affect the low basal acid secretion, but inhibited the acid response to pentagastrin, histamine, and bethanechol (64). Also, the involvement of somatostatin has been confirmed in this action, as in conscious wild-type mice, but not in somatostatin receptor 2 knockout mice, PACAP1–38 inhibited gastric acid secretion induced by 2-h pylorus ligation (64).
The contradictions have been partially resolved by studies showing that ECL cells possess PAC1 receptors, through which PACAP increases acid secretion, but also stimulates somatostatin release from D cells via VPAC1 receptor inhibiting gastrin and acid secretion through a less robust pathway (7, 54, 68–70). Pisegna’s group hypothesized that in the neural phase, PACAP increases acid secretion prior to meal ingestion, but later it inhibits it via stimulating the gastrin-inhibitory somatostatin release (7, 54). This theory is supported by the observations in PAC1-deficient mice: knockout mice develop gastric hypersecretion accompanied by elevated circulating gastrin levels and higher gastrin mRNA levels in the mucosa (53) in contrast to the findings of Mungan et al. (67), who detected no elevation in serum gastrin levels after intravenous PACAP administration. PAC1 receptor-deficient mice have elevated basal gastric output (nearly threefold increase compared to wild-type mice), have higher threshold to the effects of exogenous gastrin, but have an intact histamine stimulatory pathway (53). Another study has shown that microinfusion of PACAP into rat gastric submucosa produces a transient histamine response, in contrast to gastrin that shows a sustained action (71). The authors suggest that this transient response reflects receptor desensitization and/or depletion of secretory products (72). These observations can have translational value, as for example, PAC1 receptor knockout mice have been suggested to be a model for the human Zollinger-Ellison syndrome (65, 68). Also, the PACAP-driven regulatory mechanisms could play a role in several other clinical conditions related to disturbed gastric acid secretion (68).
As already mentioned earlier, PACAP affects release of several other factors. Stimulatory effects on somatostatin have been shown in porcine antrum where PACAP decreases gastrin secretion (13). ECL cells also produce pancreastatin in addition to histamine (71, 72). PACAP has been shown to evoke release of pancreastatin, a chromogranin A-derived peptide, with actions like inhibition of insulin secretion, pancreatic enzyme release, and gastric acid secretion (73). The PACAP-evoked release of pancreastatin can be inhibited by prostaglandins (72), galanin, misoprostol, and somatostatin (74). The stimulatory effects of PACAP on other substances have also been revealed. For example, PACAP stimulates the release of gastrin releasing peptide, VIP, and substance P (13). The release of somatostation, GRP, VIP and SP was not inhibited by PACAP6–38, suggesting the involvement of VPAC1 receptors, on which PACAP6–38 has no effect (13). PACAP also stimulates secretin release along with somatostatin and prostaglandins (65, 66, 75). PACAP is able to induce pepsinogen release from chief cells of the guinea pig (76). In isolated chief cells, PACAP and VIP binding sites were identified and PACAP1–38 induced biphasic pepsinogen release with the same potency as PACAP1–27 and VIP did. Li et al. (65) examined the inhibitory effect of PACAP1–27 on gastric acid secretion and its mechanism. It dose-dependently hindered both basal and pentagastrin-induced acid secretion. This inhibitory effect could be reversed using antisecretin, antisomatostatin serum, and indomethacin indicating that PACAP’s effect involves local release of secretin, somatostatin, and PGE2.
Effects on Cell Proliferation and Differentiation
In isolated rat gastric ECL cells, Oh et al. (54) demonstrated a dose-dependent stimulation of proliferation, with 100 nM PACAP causing a maximum, 30% increase. Another study has also observed that PACAP stimulates proliferation of Mastomys-derived ECL cells, with 100 times more potency than VIP (22, 52). This could be inhibited by PACAP antagonist. In rabbit smooth muscle cells isolated from the antrum, no effect has been shown on the cell number in cultures, in contrast to colonic muscle cells, where PACAP inhibited the serum induced increase in cell number (77). This shows region-specific effects of PACAP on cell proliferation in the gastrointestinal tract. In PAC1 receptor deficient mice, hypertrophic gastric mucosa was observed with greater mucosal thickness resulting from greater gland height, but no difference in the pit sizes (53). This was accompanied by an increased cell mass, especially with increased parietal cell number, while total neuroendocrine cell number and D cell number was unaltered (53) (Figure 1).
Effects on Motility
PACAP is known to have actions on smooth muscle of inner organs and blood vessels (78). In the stomach fundus, effects on motility resemble those in the esophagus (10, 47, 48). PACAP exerts similar, relaxant activity in the body and fundus of the stomach. In rat longitudinal gastric fundus muscle strips, a relaxant effect was produced by VIP, PACAP1–27, and secretin (79). Similarly, PACAP exerts relaxant activity on circular muscle strips from rat gastric fundus (80) and in the mouse antrum (81). In guinea pig gastric fundus, VIP, PACAP1-27 and PACAP1-38 induced concentration-dependent relaxation that was partly inhibited by the antagonists VIP10–28 and PACAP6–38 and the NO synthase inhibitor NG-nitro-l-arginine (l-NNA). Only relaxation induced by PACAP1–27 and PACAP1–38 was partly inhibited by apamin (47). Furthermore, electrical-field stimulation induced PACAP release. The authors conclude that the inhibitory transmission in guinea pig gastric fundus represents the combined actions of VIP, PACAP and NO released from nerve terminals and NO generated in muscle cells, which possess VPAC receptors, but no PAC1 receptor (47). PACAP binding was shown in dispersed rabbit gastric muscle cells, where PACAP, like VIP, stimulated cytosolic-free calcium and the formation of l-[3H]-citrulline, NO-3/NO-2, cGMP, and cAMP and induced relaxation (48). According to this latter study, the action of PACAP is mediated via the common VPAC receptors (48). PACAP also inhibits relaxation in the porcine lower esophageal sphincter (82). A recent study has confirmed the relaxant activity of PACAP in a dose dependent manner in mouse gastric fundus, while PACAP6–38 suppresses gastric relaxation (83). Evidence has also been published for the involvement of VIP/PACAP receptors in the afferent pathway mediating surgery-induced fundic relaxation in the rat (84) (Figure 1).
The role of endogenous PACAP in the regulation of motility is supported by the inhibition of the sustained relaxation by a PACAP receptor antagonist, PACAP6–38 (85). Furthermore, muscle strips prepared from PACAP knockout mice showed decreased level of sustained relaxation, which was about one-half of that observed in wild-type mice (85). PACAP6–38 inhibited electrical field stimulation-induced sustained relaxation (33.5% of control) in PACAP knockout mice. These findings were subsequently confirmed by others showing that mice deficient in PACAP have decreased relaxation in the stomach (86).
Pyloric sphincter muscle function is of utmost importance in gastric emptying, and its regulation is very complex, including regulation through nitric oxide, ATP in concert with nonadrenergic and noncholinergic transmission (87). Effects of PACAP on pyloric motility are somewhat contradictory. PACAP has been shown to increase motility of isolated perfused porcine antrum preparations (13). This could be blocked by PACAP6–38, which had no effect when applied alone. The relaxation in other parts of the stomach is not contradictory to the constriction in the pyloric part, since sphincter muscles in the GI tract are often antagonistically innervated or regulated. However, contradictory findings have also been reported. In dogs, intravenous administration of PACAP did not cause a change in antral motility using chronically implanted antral force transducers (88). Similarly, Ishiguchi et al. (87) found that lower concentrations of VIP and PACAP (nM) had no effect on isolated pyloric strips, only higher concentrations inhibited phasic contractions. Parkman et al. (49) also reported that VIP, PACAP1–38 and PACAP1–27 inhibited pyloric muscle in rabbit pylorus muscle strips. They also found an inhibitory effect of PACAP6–27 on both PACAP and VIP-induced relaxation, suggesting that PACAP and VIP act on the same receptor (49). In vivo, intravenous administration of PACAP (0.3–3 nmol/kg) caused significant relaxations of the rat pylorus suggesting that PACAP acts as inhibitory neurotransmitter in the rat pylorus and regulates gastric emptying (89). These discrepancies may be due to differences in species or experimental paradigms (13).
Effects of PACAP on Gastric Blood Flow
PACAP-immunoreactive fibers are often observed around and in the walls of blood vessels. The general vasodilatory action of PACAP is well established in several species and experimental models (78, 90–93). This was also described for the stomach wall (13). Especially rich PACAP-ergic innervation could be observed in the submucosa, but the few PACAP-ergic nerve fibers observed in the mucosa were associated mainly with blood vessels, indicating a potential role in the blood supply of the stomach (13). Indeed, it has been confirmed that both PACAP1–27 and PACAP1–38 increase gastric blood flow, shown in the left gastric artery of dogs (94, 95), where PACAP resulted to be a potent vasodilator, stronger than VIP, in the gastric arterial bed in vivo. These studies applied the peptides intravenously in conscious dogs, previously implanted with flowmeters. The authors conducted similar experiments with PACAP fragments. They showed that C-terminal deletions or changing single amino acids in the N-terminal did not cause a change in the vasodilator capacity, but substituting amino acids 4 and 5 significantly changed the biological activity. Responses of the left gastric artery to Ala4, Val5-PACAP1–27 and VIP were similar, demonstrating that positions 4 and 5 are the key N-terminal residues for PACAP1–27 (94). These data indicate a physiological role of the peptide in the regulation of gastric blood flow.
Changes of PACAP Expression Under Physiological and Pathological Conditions
Increased PACAP1–38 release has been observed in the porcine antrum upon vagus nerve stimulation and capsaicin treatment (13). As neonatal capsaicin treatment decreased PACAP content in rat stomach, PACAP is probably released from sensory nerve terminals (11). These observations are consistent with other studies in visceral organs, for example, similar results were obtained in the trachea: both capsaicin and electrical-field stimulation increased PACAP-release (96). PACAP or receptor upregulation has been observed in a few other conditions in the stomach: for example, CCK knockout mice show upregulated PACAP receptor expression in the mucosa, possibly indicating a compensatory process (97).
PACAP in the Dorsal Vagal Nucleus in Gastric Pathological Conditions
PACAP can be observed under normal conditions in approximately 30% of the neurons in the dorsal vagal nucleus projecting to the prepyloric region in pigs (98). According to several studies, PACAP is one of the main neuropeptides in this nucleus, as its levels are higher under normal conditions than those of other examined neuropeptides (98, 99). In acetylsalicylic acid-induced gastritis, the number of PACAP-expressing neurons in the dorsal vagal nucleus increases by almost 50% in addition to de novo appearance of numerous other peptides, including VIP and galanin. These data suggest that neuronal PACAP is included in the mediation of the neural response to stomach inflammation (99). Similar results were obtained after partial gastric resection, a model of traumatic neuronal injury of the stomach: the number of PACAP-expressing neurons increased by 45%, along with increases of other neuropeptides (98). The authors suggest that the upregulation of PACAP implies its trophic and protective role in these gastric pathological conditions (98). These actions seem to be generally present in the digestive tract, as increases in PACAP-expressing neurons in the intestinal ganglia were reported both after axotomy and inflammation in the descending colon, in contrast to VIP, the levels of which did not change in these models (100, 101).
PACAP in Gastritis and Ulcer
As PAC1 receptor deficient mice develop higher gastric acid production accompanied by gastric mucosal hypertrophy, it is suggested that the PACAP/PAC1 system plays an important role in gastric acid secretion not only under normal, but also under diseased conditions (53). Indeed, an earlier study found altered PACAP tissue expression during ulcer healing in rats (102). In a model of experimental ulcer, induced by local injection of acetic acid, PACAP and VIP immunohistochemistry was performed during the healing process. Starting on day 1, a marked depletion of PACAP immunoreactivity in nerve fibers at the margin of the ulcer was observed, again observed on days 10 and 15 (102). This was in contrast to VIP immunoreactivity, which did not show any alterations during the ulcer healing process. Immunoreactivity was also studied in the smooth muscle underlying the ulcer, where an upregulation of PACAP and VIP could be observed from day 10, along with an upregulation of PACAP and VIP mRNA in the myenteric ganglia in the ulcer’s neighborhood (102). This shows that neuronal PACAP depletion was transient and fully reversible. The authors argue that the selective decrease of PACAP at the ulcer margin might be due to either excessive release or a decrease in synthesis. Duodenal ulcers are linked to gastric acid-induced lesions and PACAP has been shown to have protective effects also in a duodenal ulcer model (103). The peptide is a known stimulant of duodenal bicarbonate secretion (104–107) and thus, can protect against the irritant effects of gastric acid. Indeed, intravenous bolus injection or infusion of PACAP1–27 increased duodenal bicarbonate secretion even in the presence of mepirizole, without an effect on acid secretion, and significantly reduced the severity of duodenal lesions (103).
PACAP and Stomach Cancer
PACAP has been shown to play a role in cell proliferation and differentiation in numerous normal and tumorous cells and expression of PACAP or its receptors is suggested to correlate with tumor growth and differentiation (108–110). It has also been shown that expression of the peptide and/or its receptors show marked changes in certain tumor types either in the tumor itself or in peritumoral areas (111–115). Little is known about the connection between gastric tumors and PACAP, but some data indicate that PACAP may play a regulatory role in gastric tumor biology. Studying human tumors, it has been revealed that PACAP receptors are expressed in gastric cancer. Overexpression of PACAP receptors has been reported in various types of cancer, including stomach cancer in about half of the examined cases (114–117). Schulz et al. could detect VPAC1 and VPAC2 receptors in gastric cancer (118, 119), while they could not detect PAC1 expression in gastric tumors.
PACAP is linked to proliferative signaling pathways and tumor growth (120). It was proposed that, similarly to solid tumors, PAC1 receptors are expressed on neuroendocrine tumor cells and may mediate biological effects induced by PACAP, such as secretion and growth (120). In Mastomys ECL cells, transformation was induced by histamine 2 receptor blockade (22). During the process of mucosal hyperplasia induced by endogenous hypergastrinemia, PACAP-LI increased significantly and was identified primarily in a linear-punctuated pattern. In the stage of carcinoid tumor formation, PACAP-LI was present in striking abundance and mostly presented in the basket-like pattern. Indeed, most of the basket-like IR was identified within neoplastic lesions. In the fundus, PACAP immunoreactivity significantly increased in the tumor mucosa compared to controls (22). Investigation of the receptor subtypes revealed that the expression levels of PAC1 and VPAC2 were modestly upregulated in tumor ECL cells compared with naive cells, but that of VPAC1 receptor subtype appeared to be downregulated (22). PACAP induced the proliferation of transformed ECL cells, and this effect was stronger than that of TGFα or gastrin (22). These effects could be completely antagonized by PACAP6–38 and to a lesser extent by a VPAC1 antagonist (22). Although it is not known whether this process plays a role in human gastric tumor formation, these results indicate that PACAP potently modulates ECL cell proliferation and is involved in ECL cell transformation. The growth of the neuroendocrine-derived ECL cells in the stomach has been shown to be influenced by PACAP (121). Lieu et al. (120) have demonstrated that PAC1 receptors are expressed in the well-established neuroendocrine cell-derive BON cell line. The authors propose that PACAP may regulate the biological release of peptides and serotonin from BON cells and that, like in solid tumors, PACAP could potentially stimulate the growth of BON cells (120). These few data indicate that PACAP may play a role in the growth of different types of stomach cancer.
Motility Disorders
In spite of the large amount of data regarding actions of PACAP on motility, very little is known about motility disorders associated with PACAP signaling. The role of endogenous PACAP in gastric motility is supported by observations in PACAP KO mice: relaxation effect of PACAP is reduced by about 50% in muscle strips prepared from PACAP knockout mice (85). These findings were subsequently confirmed by others: mice deficient in PACAP have decreased relaxation in the stomach (86). Although no in vivo data are available for PACAP, VIP knockout mice have been shown to develop intestinal motility dysfunction, similar to that observed in human Hirschprung’s disease (122). Furthermore, dystrophic mice have been reported to develop gastrointestinal motor disorders, where PACAP6–38 abolished off-relaxations and also caused a reduction in amplitude of the contractile responses and efficacy of PACAP 6–38 on the excitatory responses was lower in strips from dystrophic mice than in wild types (123). The observed stronger peptidergic modulatory action can contribute to the altered gastric contractile responses in this motility disorder (123). Whether these alterations are also present in human conditions, awaits further investigations.
Author Contributions
DR, AI, BO, ES, AT, and GH have collected data in the literature in order to give an accurate review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. EFOP-3.6.2-16-2017-00008 “The role of neuro-inflammation in neurodegeneration: from molecules to clinics”/Centre for neuroscience, PTE AOK Research Grant KA-2016-03, KA-2017-17, NKFIH119759, 2017-1.2.1-NKP-2017-00002, Bolyai Scholarship, GINOP-2.3.2-15-2016-00050 “PEPSYS,” MTA-TKI 14016, Kiválósági Centrum Program.
References
- 1.Abad C, Gomariz RP, Waschek JA. Neuropeptide mimetics and antagonists in the treatment of inflammatory disease: focus on VIP and PACAP. Curr Top Med Chem (2006) 6:151–63. 10.2174/156802606775270288 [DOI] [PubMed] [Google Scholar]
- 2.Illes A, Opper B, Reglodi D, Kerenyi M, Czetany P, Boronkai A, et al. Effects of pituitary adenylate cyclase activating polypeptide on small intestinal INT 407 cells. Neuropeptides (2017) 65:106–13. 10.1016/j.npep.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 3.Wu MJ, Kee KH, Na J, Kim SW, Bae Y, Shin DH, et al. Pituitary adenylate cyclase-activating polypeptide inhibits pacemaker activity of colonic interstitial cells of Cajal. Korean J Physiol Pharmacol (2015) 19:435–40. 10.4196/kjpp.2015.19.5.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heimesaat MM, Dunay IR, Schulze S, Fischer A, Grundmann U, Alutis M, et al. Pituitary adenylate cyclase-activating polypeptide ameliorates experimental acute ileitis and extra-intestinal sequelae. PLoS One (2014) 9:e108389. 10.1371/journal.pone.0108389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azuma YT, Hagi K, Shintani N, Kuwamura M, Nakajima H, Hashimoto H, et al. PACAP provides colonic protection against dextran sodium sulfate induced colitis. J Cell Physiol (2008) 216:111–9. 10.1002/jcp.21381 [DOI] [PubMed] [Google Scholar]
- 6.Nemetz N, Abad C, Lawson G, Nobuta H, Chhith S, Duong L, et al. Induction of colitis and rapid development of colorectal tumors in mice deficient in the neuropeptide PACAP. Int J Cancer (2008) 122:1803–9. 10.1002/ijc.23308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vu JP, Benhammou JN, Goyal D, Luong L, Oh S, Germano P, et al. PACAP regulation of gastrointestinal function and obesity. In: Reglodi D, Tamas A, editors. Current Topics of Neurotoxicity 11 Pituitary Adenylate Cyclase Activating Polypeptide-PACAP. Switzerland: Springer International Publishing AG; (2016). p. 261–70. [Google Scholar]
- 8.Padua D, Vu JP, Germano PM, Pisegna JR. The role of neuropeptides in mouse models of colitis. J Mol Neurosci (2016) 59:203–10. 10.1007/s12031-015-0688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horvath G, Illes A, Heimesaat MM, Bardosi A, Bardosi S, Tamas A, et al. Protective intestinal effects of pituitary adenylate cyclase activating polypeptide. In: Reglodi D, Tamas A, editors. Current Topics of Neurotoxicity 11 Pituitary Adenylate Cyclase Activating Polypeptide-PACAP. Switzerland: Springer International Publishing AG; (2016). p. 271–88. [Google Scholar]
- 10.Lauffer JM, Modlin IM, Tang LH. Biological relevance of pituitary adenylate cyclase-activating polypeptide (PACAP) in the gastrointestinal tract. Regul Pept (1999) 84:1–12. 10.1016/S0167-0115(99)00024-5 [DOI] [PubMed] [Google Scholar]
- 11.Hannibal J, Ekblad E, Mulder H, Sundler F, Fahrenkrug J. Pituitary adenylate cyclase activating polypeptide (PACAP) in the gastrointestinal tract of the rat: distribution and effects of capsaicin or denervation. Cell Tissue Res (1998) 291:65–79. 10.1007/s004410050980 [DOI] [PubMed] [Google Scholar]
- 12.Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology (1991) 129:2787–9. 10.1210/endo-129-5-2787 [DOI] [PubMed] [Google Scholar]
- 13.Tornoe K, Hannibal J, Georg B, Schmidt PT, Hilsted L, Fahrenkrug J, et al. PACAP 1-38 as neurotransmitter in the porcine antrum. Regul Pept (2001) 101:109–21. 10.1016/S0167-0115(01)00276-2 [DOI] [PubMed] [Google Scholar]
- 14.Sundler F, Ekblad E, Absood A, Hakanson R, Koves K, Arimura A. Pituitary adenylate cyclase activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience (1992) 46:439–54. 10.1016/0306-4522(92)90064-9 [DOI] [PubMed] [Google Scholar]
- 15.Kantor O, Heinzlmann A, Suzuki N, Vincze E, Kocsis K, Koves K. Distribution of PACAP and its mRNA in several nonneural tissues of rats demonstrated by sandwich enzyme immunoassay and RT-PCR technique. Regul Pept (2002) 109:103–5. 10.1016/S0167-0115(02)00192-1 [DOI] [PubMed] [Google Scholar]
- 16.Mulder H, Uddman R, Moller K, Elsås T, Ekblad E, Alumets J, et al. Pituitary adenylate cyclase activating polypeptide is expressed in autonomic neurons. Regul Pept (1995) 59:121–8. 10.1016/0167-0115(95)00082-M [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Shi TJ, Ji RR, Zhang YZ, Sundler F, Hannibal J, et al. Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res (1995) 705:149–58. 10.1016/0006-8993(95)01150-1 [DOI] [PubMed] [Google Scholar]
- 18.Zhang YZ, Hannibal J, Zhao Q, Moller K, Danielsen N, Fahrenkrug J, et al. Pituitary adenylate cyclase activating peptide expression in the rat dorsal root ganglia: up-regulation after peripheral nerve injury. Neuroscience (1996) 74:1099–110. 10.1016/0306-4522(96)00168-6 [DOI] [PubMed] [Google Scholar]
- 19.Koves K, Arimura A, Vigh S, Somogyvari-Vigh A, Miller J. Immunohistochemical localization of PACAP in the ovine digestive system. Peptides (1993) 14:449–55. 10.1016/0196-9781(93)90131-Y [DOI] [PubMed] [Google Scholar]
- 20.Vincze E, Kantor O, Kiss A, Gonda G, Gombas P, Kiss J, et al. Pituitary adenylate cyclase activating polypeptide (PACAP) is present in human and cat gastric glands. Peptides (1999) 20:937–41. 10.1016/S0196-9781(99)00084-4 [DOI] [PubMed] [Google Scholar]
- 21.Portbury AL, McConalogue K, Furness JB, Young HM. Distribution of pituitary adenylyl cyclase activating peptide (PACAP) immunoreactivity in neurons of the guinea-pig digestive tract and their projections in the ileum and colon. Cell Tissue Res (1995) 279:385–92. 10.1007/BF00318496 [DOI] [PubMed] [Google Scholar]
- 22.Lauffer JM, Tang LH, Zhang T, Hinoue T, Rahbar S, Odo M, et al. PACAP mediates the neural proliferative pathway of Mastomys enterochromaffin-like cell transformation. Regul Pept (2001) 102:157–64. 10.1016/S0167-0115(01)00314-7 [DOI] [PubMed] [Google Scholar]
- 23.Vincze E, Kantor O, Kausz M, Nemeth J, Arimura A, Gonda P, et al. Comparative study on the appearance of various bioactive peptides in foregut derivates during the ontogenesis. J Physiol Paris (2001) 95:99–103. 10.1016/S0928-4257(01)00014-6 [DOI] [PubMed] [Google Scholar]
- 24.McRory JE, Parker DB, Ngamvongchon S, Sherwood NM. Sequence and expression of cDNA for pituitary adenylate cyclase activating polypeptide (PACAP) and growth hormone-releasing hormone (GHRH)-like peptide in catfish. Mol Cell Endocrinol (1995) 108:169–77. 10.1016/0303-7207(94)03467-8 [DOI] [PubMed] [Google Scholar]
- 25.Huang WT, Li CJ, Wu PJ, Chang YS, Lee TL, Weng CF. Expression and in vitro regulation of pituitary adenylate cyclase-activating polypeptide (pacap38) and its type I receptor (pac1-r) in the gonads of tilapia (Oreochromis mossambicus). Reproduction (2009) 137:449–67. 10.1530/REP-08-0422 [DOI] [PubMed] [Google Scholar]
- 26.Holmberg A, Schwerte T, Pelster B, Holmgren S. Ontogeny of the gut motility control system in zebrafish Danio rerio embryos and larvae. J Exp Biol (2004) 207:4085–94. 10.1242/jeb.01260 [DOI] [PubMed] [Google Scholar]
- 27.Nam BH, Moon JY, Kim YO, Kong HJ, Kim WJ, Kim DG, et al. Structural and functional characterization of pituitary adenylyl cyclase-activating polypeptide (PACAP)/PACAP-related peptide (PRP) and its receptor in olive flounder (Paralichthys olivaceus). Comp Biochem Physiol B Biochem Mol Biol (2013) 164:18–28. 10.1016/j.cbpb.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 28.Valiante S, Prisco M, De Falco M, Sellitti A, Zambrano I, Sciarrillo R, et al. Distribution and molecular evolution of the neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors in the lizard Podarcis sicula (Squamata, Lacertidae). J Mol Neurosci (2009) 39:144–56. 10.1007/s12031-009-9178-7 [DOI] [PubMed] [Google Scholar]
- 29.Olsson C. Distribution and effects of PACAP, VIP, nitric oxide and GABA in the gut of the African clawed frog Xenopus laevis. J Exp Biol (2002) 205:1123–34. [DOI] [PubMed] [Google Scholar]
- 30.Simon A, Olah J, Komlosi I, Javor A, Nemeth J, Szilvassy Z, et al. Changes in expression of neuropeptides and their receptors in the hypothalamus and gastrointestinal tract of calorie restricted hens. Acta Biol Hung (2017) 68:237–47. 10.1556/018.68.2017.3.1 [DOI] [PubMed] [Google Scholar]
- 31.Salvi EP, Vaccaro R, Renda TG. Ontogeny of PACAP immunoreactivity in extrinsic and intrinsic innervation of chicken gut. Peptides (2000) 21:1703–9. 10.1016/S0196-9781(00)00320-X [DOI] [PubMed] [Google Scholar]
- 32.Mirabella N, Squillacioti C, Colitti M, Germano G, Pelagalli A, Paino G. Pituitary adenylate cyclase activating peptide (PACAP) immunoreactivity and mRNA expression in the duck gastrointestinal tract. Cell Tissue Res (2002) 308:347–59. 10.1007/s00441-002-0555-6 [DOI] [PubMed] [Google Scholar]
- 33.Mirabella N, Squillacioti C, Genovese A, Germano G, Paino G. Topography and neurochemistry of the enteric ganglia in the proventriculus of the duck (Anas platyrhynchos). Anat Embryol (Berl) (2003) 207:101–8. 10.1007/s00429-003-0342-5 [DOI] [PubMed] [Google Scholar]
- 34.Mirabella N, Lamanna C, Assisi L, Botte V, Cecio A. The relationships of nicotinamide adenine dinucleotide phosphate-d to nitric oxide synthase, vasoactive intestinal polypeptide, galanin and pituitary adenylate activating polypeptide in pigeon gut neurons. Neurosci Lett (2000) 293:147–51. 10.1016/S0304-3940(00)01450-6 [DOI] [PubMed] [Google Scholar]
- 35.Somogyvari-Vigh A, Reglodi D, Li M, Lengvari I, Vigh S, Arimura A. Tissue distribution of PACAP27 and -38 in oligochaeta: PACAP27 is the predominant form in the nervous system of Lumbricus polyphemus. Peptides (2000) 21:1185–91. 10.1016/S0196-9781(00)00258-8 [DOI] [PubMed] [Google Scholar]
- 36.Molnar L, Pollak E, Boros A, Reglödi D, Tamas A, Lengvari I, et al. Comparative anatomy of PACAP-immunoreactive structures in the ventral nerve cord ganglia of lumbricid oligochaetes. Ann N Y Acad Sci (2006) 1070:427–30. 10.1196/annals.1317.056 [DOI] [PubMed] [Google Scholar]
- 37.Reglodi D, Lengvari I, Szelier M, Vigh S, Arimura A. Distribution of PACAP-like immunoreactivity in the nervous system of oligochaeta. Peptides (2000) 21:183–8. 10.1016/S0196-9781(99)00201-6 [DOI] [PubMed] [Google Scholar]
- 38.Boros A, Reglodi D, Herbert Z, Kiszler G, Nemeth J, Lubics A, et al. Changes in the expression of PACAP-like compounds during the embryonic development of the earthworm Eisenia fetida. J Mol Neurosci (2008) 36:157–65. 10.1007/s12031-008-9102-6 [DOI] [PubMed] [Google Scholar]
- 39.Boros A, Somogyi I, Engelmann P, Lubics A, Reglodi D, Pollak E, et al. Pituitary adenylate cyclase-activating polypeptide type 1 (PAC1) receptor is expressed during embryonic development of the earthworm. Cell Tissue Res (2010) 339:649–53. 10.1007/s00441-009-0909-4 [DOI] [PubMed] [Google Scholar]
- 40.Waschek JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev Neurosci (2002) 24:14–23. 10.1159/000064942 [DOI] [PubMed] [Google Scholar]
- 41.Somogyvari-Vigh A, Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr Pharm Des (2004) 10:2861–89. 10.2174/1381612043383548 [DOI] [PubMed] [Google Scholar]
- 42.Varhalmi E, Somogyi I, Kiszler G, Nemeth J, Reglodi D, Lubics A, et al. Expression of PACAP-like compounds during the caudal regeneration of the earthworm Eisenia fetida. J Mol Neurosci (2008) 36:166–74. 10.1007/s12031-008-9125-z [DOI] [PubMed] [Google Scholar]
- 43.Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology (1994) 135:2662–80. 10.1210/endo.135.6.7988457 [DOI] [PubMed] [Google Scholar]
- 44.Zhou H, Huang J, Murthy KS. Molecular cloning and functional expression of a VIP-specific receptor. Am J Physiol Gastrointest Liver Physiol (2006) 291:G728–34. 10.1152/ajpgi.00138.2006 [DOI] [PubMed] [Google Scholar]
- 45.Teng B, Murthy KS, Kuemmerle JF, Grider JR, Makhlouf GM. Selective expression of vasoactive intestinal peptide (VIP)2/pituitary adenylate cyclase-activating polypeptide (PACAP)3 receptors in rabbit and guinea pig gastric and tenia coli smooth muscle cells. Regul Pept (1998) 77:127–34. 10.1016/S0167-0115(98)00112-8 [DOI] [PubMed] [Google Scholar]
- 46.Harmar AJ, Sheward WJ, Morrison CF, Waser B, Gugger M, Reubi JC. Distribution of the VPAC2 receptor in peripheral tissues of the mouse. Endocrinology (2004) 145:1203–10. 10.1210/en.2003-1058 [DOI] [PubMed] [Google Scholar]
- 47.Katsoulis S, Schmidt WE, Schwarzhoff R, Folsch UR, Jin JG, Grider JR, et al. Inhibitory transmission in guinea pig stomach mediated by distinct receptors for pituitary adenylate cyclase-activating peptide. J Pharmacol Exp Ther (1996) 278:199–204. [PubMed] [Google Scholar]
- 48.Murthy KS, Jin JG, Grider JR, Makhlouf GM. Characterization of PACAP receptors and signaling pathways in rabbit gastric muscle cells. Am J Physiol (1997) 272:G1391–9. [DOI] [PubMed] [Google Scholar]
- 49.Parkman HP, Pagano AP, Ryan JP. PACAP and VIP inhibit pyloric muscle through VIP/PACAP-preferring receptors. Regul Pept (1997) 71:185–90. 10.1016/S0167-0115(97)01031-8 [DOI] [PubMed] [Google Scholar]
- 50.Miampamba M, Germano PM, Arli S, Wong HH, Scott D, Taché Y, et al. Expression of pituitary adenylate cyclase-activating polypeptide and PACAP type 1 receptor in the rat gastric and colonic myenteric neurons. Regul Pept (2002) 105:145–54. 10.1016/S0167-0115(02)00003-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng N, Kang T, Lyu RM, Wong H, Wen Y, Walsh JH, et al. The pituitary adenylate cyclase activating polypeptide type 1 receptor (PAC1-R) is expressed on gastric ECL cells: evidence by immunocytochemistry and RT-PCR. Ann N Y Acad Sci (1998) 865:147–56. 10.1111/j.1749-6632.1998.tb11173.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lauffer JM, Modlin IM, Hinoue T, Kidd M, Zhang T, Schmid SW, et al. Pituitary adenylate cyclase-activating polypeptide modulates gastric enterochromaffin-like cell proliferation in rats. Gastroenterology (1999) 116:623–35. 10.1016/S0016-5085(99)70184-8 [DOI] [PubMed] [Google Scholar]
- 53.Lu Y, Germano P, Ohning GV, Vu JP, Pisegna JR. PAC1 deficiency in a murine model induces gastric mucosa hypertrophy and higher basal gastric acid output. J Mol Neurosci (2011) 43:76–84. 10.1007/s12031-010-9440-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh DS, Lieu SN, Yamaguchi DJ, Tachiki K, Lambrecht N, Ohning GV, et al. PACAP regulation of secretion and proliferation of pure populations of gastric ECL cells. J Mol Neurosci (2005) 26:85–97. 10.1385/JMN:26:1:085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng N, Athmann C, Kang T, Lyu RM, Walsh JH, Ohning GV, et al. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest (1999) 104:1383–91. 10.1172/JCI7537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okuda S, Honda M, Ito Y, Aihara E, Kato S, Mitsufuji S, et al. Phosphodiesterase isozymes involved in regulating acid secretion in the isolated mouse stomach. J Physiol Pharmacol (2009) 60(Suppl 7):183–90. [PubMed] [Google Scholar]
- 57.Lindstrom E, Lerner UH, Hakanson R. Isolated rat stomach ECL cells generate prostaglandin E(2) in response to interleukin-1 beta, tumor necrosis factor-alpha and bradykinin. Eur J Pharmacol (2001) 416:255–63. 10.1016/S0014-2999(01)00881-0 [DOI] [PubMed] [Google Scholar]
- 58.Hakanson R, Chen D, Lindstrom E, Bernsand M, Norlén P. Control of secretion from rat stomach ECL cells in situ and in primary culture. Scand J Clin Lab Invest Suppl (2001) 234:53–60. 10.1080/003655101753352059 [DOI] [PubMed] [Google Scholar]
- 59.Sandvik AK, Cui G, Bakke I, Munkvold B, Waldum HL. PACAP stimulates gastric acid secretion in the rat by inducing histamine release. Am J Physiol Gastrointest Liver Physiol (2001) 281:G997–1003. 10.1152/ajpgi.2001.281.4.G997 [DOI] [PubMed] [Google Scholar]
- 60.Lindstrom E, Eliasson L, Bjorkqvist M, Hakanson R. Gastrin and the neuropeptide PACAP evoke secretion from rat stomach histamine-containing (ECL) cells by stimulating influx of Ca2+ through different Ca2+ channels. J Physiol (2001) 535:663–77. 10.1111/j.1469-7793.2001.00663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng N, Sachs G. Properties of isolated gastric enterochromaffin-like cells. Yale J Biol Med (1998) 71:233–46. [PMC free article] [PubMed] [Google Scholar]
- 62.Zeng N, Athmann C, Kang T, Walsh JH, Sachs G. Role of neuropeptide-sensitive L-type Ca(2+) channels in histamine release in gastric enterochromaffin-like cells. Am J Physiol (1999) 277:G1268–80. [DOI] [PubMed] [Google Scholar]
- 63.Athmann C, Zeng N, Scott DR, Sachs G. Regulation of parietal cell calcium signaling in gastric glands. Am J Physiol Gastrointest Liver Physiol (2000) 279:G1048–58. 10.1152/ajpgi.2000.279.5.G1048 [DOI] [PubMed] [Google Scholar]
- 64.Piqueras L, Tache Y, Martinez V. Peripheral PACAP inhibits gastric acid secretion through somatostatin release in mice. Br J Pharmacol (2004) 142:67–78. 10.1038/sj.bjp.0705739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li P, Chang TM, Coy D, Chey WY. Inhibition of gastric acid secretion in rat stomach by PACAP is mediated by secretin, somatostatin, and PGE(2). Am J Physiol Gastrointest Liver Physiol (2000) 278:G121–7. 10.1152/ajpgi.2000.278.1.G121 [DOI] [PubMed] [Google Scholar]
- 66.Chey WY, Chang CH, Pan HJ, Chang C, Kim BM, Park IS, et al. Evidence on the presence of secretin cells in the gastric antral and oxyntic mucosa. Regul Pept (2003) 111:183–90. 10.1016/S0167-0115(02)00286-0 [DOI] [PubMed] [Google Scholar]
- 67.Mungan Z, Hammer RA, Akarca US, Komaki G, Ertan A, Arimura A. Effect of PACAP on gastric acid secretion in rats. Peptides (1995) 16:1051–6. 10.1016/0196-9781(95)00083-V [DOI] [PubMed] [Google Scholar]
- 68.Phan J, Benhammou JN, Pisegna JR. Gastric hypersecretory states: investigation and management. Curr Treat Options Gastroenterol (2015) 13:386–97. 10.1007/s11938-015-0065-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wank SA. PACAP upsets stomach theory. J Clin Invest (1999) 104:1341–2. 10.1172/JCI8732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schubert ML. Gastric secretion. Curr Opin Gastroenterol (2002) 18:639–49. 10.1097/00001574-200211000-00002 [DOI] [PubMed] [Google Scholar]
- 71.Bernsand M, Hakanson R, Norlen P. Tachyphylaxis of the ECL-cell response to PACAP: receptor desensitization and/or depletion of secretory products. Br J Pharmacol (2007) 152:240–8. 10.1038/sj.bjp.0707385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindstrom E, Hakanson R. Prostaglandins inhibit secretion of histamine and pancreastatin from isolated rat stomach ECL cells. Br J Pharmacol (1998) 124:1307–13. 10.1038/sj.bjp.0701953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindstrom E, Bjorkqvist M, Boketoft A, Chen D, Zhao CM, Kimura K, et al. Neurohormonal regulation of histamine and pancreastatin secretion from isolated rat stomach ECL cells. Regul Pept (1997) 71:73–86. 10.1016/S0167-0115(97)01018-5 [DOI] [PubMed] [Google Scholar]
- 74.Bjorkqvist M, Bernsand M, Eliasson L, Hakanson R, Lindstrom E. Somatostatin, misoprostol and galanin inhibit gastrin- and PACAP-stimulated secretion of histamine and pancreastatin from ECL cells by blocking specific Ca2+ channels. Regul Pept (2005) 130:81–90. 10.1016/j.regpep.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 75.Chang CH, Chey WY, Erway B, Coy DH, Chang TM. Modulation of secretin release by neuropeptides in secretin-producing cells. Am J Physiol (1998) 275:G192–202. [DOI] [PubMed] [Google Scholar]
- 76.Felley CP, Qian JM, Mantey S, Pradhan T, Jensen RT. Chief cells possess a receptor with high affinity for PACAP and VIP that stimulates pepsinogen release. Am J Physiol (1992) 263:G901–7. [DOI] [PubMed] [Google Scholar]
- 77.van Assche G, Depoortere I, de Vos R, Geboes K, Janssens JJ, Collins SM, et al. Region-specific antiproliferative effect of VIP and PACAP-(1-38) on rabbit enteric smooth muscle. Am J Physiol (1999) 276:G303–10. [DOI] [PubMed] [Google Scholar]
- 78.Ivic I, Fulop BD, Juhasz T, Reglodi D, Toth G, Hashimoto H, et al. Backup mechanism maintains PACAP/VIP-induced arterial relaxations in PACAP-deficient mice. J Vasc Res (2017) 54:180–92. 10.1159/000457798 [DOI] [PubMed] [Google Scholar]
- 79.Robberecht P, De Neef P, Lefebvre RA. Influence of selective VIP receptor agonists in the rat gastric fundus. Eur J Pharmacol (1998) 359:77–80. 10.1016/S0014-2999(98)00662-1 [DOI] [PubMed] [Google Scholar]
- 80.Azuma YT, Hayashi S, Nishiyama K, Kita S, Mukai K, Nakajima H, et al. Na+/Ca2+ exchanger-heterozygote knockout mice display increased relaxation in gastric fundus and accelerated gastric transit in vivo. Neurogastroenterol Motil (2016) 28:827–36. 10.1111/nmo.12779 [DOI] [PubMed] [Google Scholar]
- 81.Takeuchi T, Toyoshima M, Mukai K, Hagi K, Matsui M, Nakajima H, et al. Involvement of M(2) muscarinic receptors in relaxant response of circular muscle of mouse gastric antrum. Neurogastroenterol Motil (2006) 18:226–33. 10.1111/j.1365-2982.2005.00733.x [DOI] [PubMed] [Google Scholar]
- 82.Farre R, Auli M, Lecea B, Martinez E, Clave P. Pharmacologic characterization of intrinsic mechanisms controlling tone and relaxation of porcine lower esophageal sphincter. J Pharmacol Exp Ther (2006) 316:1238–48. 10.1124/jpet.105.094482 [DOI] [PubMed] [Google Scholar]
- 83.Fujimoto Y, Hayashi S, Azuma YT, Mukai K, Nishiyama K, Kita S, et al. Overexpression of Na+/Ca2+ exchanger 1 display enhanced relaxation in the gastric fundus. J Pharmacol Sci (2016) 132:181–6. 10.1016/j.jphs.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 84.Boeckxstaens GE, Hollmann M, Heisterkamp SH, Robberecht P, de Jonge WJ, van Den Wijngaard RM, et al. Evidence for VIP(1)/PACAP receptors in the afferent pathway mediating surgery-induced fundic relaxation in the rat. Br J Pharmacol (2000) 131:705–10. 10.1038/sj.bjp.0703625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toyoshima M, Takeuchi T, Goto H, Mukai K, Shintani N, Hashimoto H, et al. Roles of PACAP and PHI as inhibitory neurotransmitters in the circular muscle of mouse antrum. Pflugers Arch (2006) 451:559–68. 10.1007/s00424-005-1491-6 [DOI] [PubMed] [Google Scholar]
- 86.Hagi K, Azuma YT, Nakajima H, Shintani N, Hashimoto H, Baba A, et al. Involvements of PHI-nitric oxide and PACAP-BK channel in the sustained relaxation of mouse gastric fundus. Eur J Pharmacol (2008) 590:80–6. 10.1016/j.ejphar.2008.05.045 [DOI] [PubMed] [Google Scholar]
- 87.Ishiguchi T, Takahashi T, Itoh H, Owyang C. Nitrergic and purinergic regulation of the rat pylorus. Am J Physiol Gastrointest Liver Physiol (2000) 279:G740–7. 10.1152/ajpgi.2000.279.4.G740 [DOI] [PubMed] [Google Scholar]
- 88.Mizumoto A, Fujimura M, Ohtawa M, Ueki S, Hayashi N, Itoh Z, et al. Pituitary adenylate cyclase activating polypeptide stimulates gallbladder motility in conscious dogs. Regul Pept (1992) 42:39–50. 10.1016/0167-0115(92)90022-M [DOI] [PubMed] [Google Scholar]
- 89.Ishiguchi T, Nishioka S, Takahashi T. Inhibitory neural pathway regulating gastric emptying in rats. J Auton Nerv Syst (2000) 79:45–51. 10.1016/S0165-1838(99)00103-4 [DOI] [PubMed] [Google Scholar]
- 90.Vamos Z, Ivic I, Cseplo P, Toth G, Tamas A, Reglodi D, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) induces relaxations of peripheral and cerebral arteries, which are differentially impaired by aging. J Mol Neurosci (2014) 54:535–42. 10.1007/s12031-014-0349-9 [DOI] [PubMed] [Google Scholar]
- 91.Banki E, Hajna Z, Kemeny A, Botz B, Nagy P, Bolcskei K, et al. The selective PAC1 receptor agonist maxadilan inhibits neurogenic vasodilation and edema formation in the mouse skin. Neuropharmacology (2014) 85:538–47. 10.1016/j.neuropharm.2014.06.019 [DOI] [PubMed] [Google Scholar]
- 92.Bhatt DK, Gupta S, Olesen J, Jansen-Olesen I. PACAP-38 infusion causes sustained vasodilation of the middle meningeal artery in the rat: possible involvement of mast cells. Cephalalgia (2014) 34:877–86. 10.1177/0333102414523846 [DOI] [PubMed] [Google Scholar]
- 93.Lamine A, Letourneau M, Doan ND, Maucotel J, Couvineau A, Vaudry H, et al. Characterizations of a synthetic pituitary adenylate cyclase-activating polypeptide analog displaying potent neuroprotective activity and reduced in vivo cardiovascular side effects in a Parkinson’s disease model. Neuropharmacology (2016) 108:440–50. 10.1016/j.neuropharm.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 94.Wei MX, Hu P, Wang P, Naruse S, Nokihara K, Wray V, et al. Possible key residues that determine left gastric artery blood flow response to PACAP in dogs. World J Gastroenterol (2010) 16:4865–70. 10.3748/wjg.v16.i38.4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Naruse S, Nakamura T, Wei M, Ando E, Nokihara K, Wray V, et al. Effects of PACAP-VIP hybrid peptides on gastric blood flow in conscious dogs. Ann N Y Acad Sci (1996) 805:511–5. 10.1111/j.1749-6632.1996.tb17512.x [DOI] [PubMed] [Google Scholar]
- 96.Nemeth J, Reglodi D, Pozsgai G, Szabo A, Elekes K, Pinter E, et al. Effect of pituitary adenylate cyclase activating polypeptide-38 on sensory neuropeptide release and neurogenic inflammation in rats and mice. Neuroscience (2006) 143:223–30. 10.1016/j.neuroscience.2006.07.028 [DOI] [PubMed] [Google Scholar]
- 97.Zhao CM, Kodama Y, Flatberg A, Beisvag V, Kulseng B, Sandvik AK, et al. Gene expression profiling of gastric mucosa in mice lacking CCK and gastrin receptors. Regul Pept (2014) 19(2–193):35–44. 10.1016/j.regpep.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 98.Ganko M, Calka J. Localization and chemical coding of the dorsal motor vagal nucleus (DMX) neurons projecting to the porcine stomach prepyloric area in the physiological state and after stomach partial resection. J Mol Neurosci (2014) 52:90–100. 10.1007/s12031-013-0102-9 [DOI] [PubMed] [Google Scholar]
- 99.Ganko M, Calka J. Prolonged acetylsalicylic-acid-supplementation-induced gastritis affects the chemical coding of the stomach innervating vagal efferent neurons in the porcine dorsal motor vagal nucleus (DMX). J Mol Neurosci (2014) 54:188–98. 10.1007/s12031-014-0274-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gonkowski S, Calka J. Changes in pituitary adenylate cyclase-activating peptide 27-like immunoreactive nervous structures in the porcine descending colon during selected pathological processes. J Mol Neurosci (2012) 48:777–87. 10.1007/s12031-012-9838-x [DOI] [PubMed] [Google Scholar]
- 101.Majewski M, Bossowska A, Gonkowski S, Wojtkiewicz J, Brouns I, Scheuermann DW, et al. Neither axotomy nor target-tissue inflammation changes the NOS- or VIP-synthesis rate in distal bowel-projecting neurons of the porcine inferior mesenteric ganglion (IMG). Folia Histochem Cytobiol (2002) 40:151–2. [PubMed] [Google Scholar]
- 102.Mei Q, Sundler F. Changes in pituitary adenylate cyclase activating peptide and vasoactive intestinal peptide innervation of rat oxyntic mucosa during ulcer healing. Neuropeptides (1998) 32:527–35. 10.1016/S0143-4179(98)90081-6 [DOI] [PubMed] [Google Scholar]
- 103.Yagi K, Takehara K, Kitamura M, Takeuchi K. Effects of pituitary adenylate cyclase activating polypeptide-27 on alkaline secretory and mucosal ulcerogenic responses in rat duodenum. Life Sci (1998) 63:317–25. 10.1016/S0024-3205(98)00280-X [DOI] [PubMed] [Google Scholar]
- 104.Konturek PC, Konturek SJ, Hahn EG. Duodenal alkaline secretion: its mechanisms and role in mucosal protection against gastric acid. Dig Liver Dis (2004) 36:505–12. 10.1016/j.dld.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 105.Takeuchi K, Yagi K, Sugamoto S, Furukawa O, Kawauchi S. Involvement of PACAP in acid-induced HCO3- response in rat duodenums. Pharmacol Res (1998) 38:475–80. 10.1006/phrs.1998.0393 [DOI] [PubMed] [Google Scholar]
- 106.Konturek SJ, Konturek PC, Pawlik T, Sliwowski Z, Ochmanski W, Hahn EG. Duodenal mucosal protection by bicarbonate secretion and its mechanisms. J Physiol Pharmacol (2004) 55(Suppl 2):5–17. [PubMed] [Google Scholar]
- 107.Glad H, Ainsworth MA, Svendsen P, Fahrenkrug J, Schaffalitzky de Muckadell OB. Effect of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide on pancreatic, hepatic and duodenal mucosal bicarbonate secretion in the pig. Digestion (2003) 67:56–66. 10.1159/000069707 [DOI] [PubMed] [Google Scholar]
- 108.Moody TW, Jensen RT. PACAP and cancer. In: Reglodi D, Tamas A, editors. Current Topics of Neurotoxicity 11 Pituitary Adenylate Cyclase Activating Polypeptide-PACAP. Switzerland: Springer International Publishing AG; (2016). p. 795–814. [Google Scholar]
- 109.Moody TW, Nuche-Berenguer B, Jensen RT. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr Opin Endocrinol Diabetes Obes (2016) 23:38–47. 10.1097/MED.0000000000000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu S, Zeng Y, Li Y, Guo W, Liu J, Ouyang N. VPAC1 overexpression is associated with poor differentiation in colon cancer. Tumour Biol (2014) 35:6397–404. 10.1007/s13277-014-1852-x [DOI] [PubMed] [Google Scholar]
- 111.Szanto Z, Sarszegi Z, Reglodi D, Nemeth J, Szabadfi K, Kiss P, et al. PACAP immunoreactivity in human malignant tumor samples and cardiac diseases. J Mol Neurosci (2012) 48:667–73. 10.1007/s12031-012-9815-4 [DOI] [PubMed] [Google Scholar]
- 112.Tamas A, Javorhazy A, Reglodi D, Sarlos DP, Banyai D, Semjen D, et al. Examination of PACAP-like immunoreactivity in urogenital tumor samples. J Mol Neurosci (2016) 59:177–83. 10.1007/s12031-015-0652-0 [DOI] [PubMed] [Google Scholar]
- 113.Reubi JC. In vitro identification of vasoactive intestinal peptide receptors in human tumors: implications for tumor imaging. J Nucl Med (1995) 36:1846–53. [PubMed] [Google Scholar]
- 114.Reubi JC, Laderach U, Waser B, Gebbers JO, Robberecht P, Laissue JA. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res (2000) 60:3105–12. [PubMed] [Google Scholar]
- 115.Reubi JC. In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues. Clinical implications. Ann N Y Acad Sci (2000) 921:1–25. 10.1111/j.1749-6632.2000.tb06946.x [DOI] [PubMed] [Google Scholar]
- 116.Cheng KT, Thakur ML. HSDAVFTDNYTKLRKQ-NIe-AVKK-(3-OCH3,4-OH)-FLNSSV-GABA-L-(Dap-(BMA)2)-64Cu. Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda, MD: National Center for Biotechnology Information (US) (2004–2013). [PubMed] [Google Scholar]
- 117.Kumar P, Tripathi SK, Chen CP, Mehta N, Paudyal B, Wickstrom E, et al. Evaluation of a PACAP peptide analogue labeled with (68)Ga using two different chelating agents. Cancer Biother Radiopharm (2016) 31:29–36. 10.1089/cbr.2015.1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schulz S, Mann A, Novakhov B, Piggins HD, Lupp A. VPAC2 receptor expression in human normal and neoplastic tissues: evaluation of the novel MAB SP235. Endocr Connect (2015) 4:18–26. 10.1530/EC-14-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schulz S, Rocken C, Mawrin C, Weise W, Hollt V, Schulz S. Immunocytochemical identification of VPAC1, VPAC2, and PAC1 receptors in normal and neoplastic human tissues with subtype-specific antibodies. Clin Cancer Res (2004) 10:8235–42. 10.1158/1078-0432.CCR-04-0939 [DOI] [PubMed] [Google Scholar]
- 120.Lieu SN, Oh DS, Pisegna JR, Germano PM. Neuroendocrine tumors express PAC1 receptors. Ann N Y Acad Sci (2006) 1070:399–404. 10.1196/annals.1317.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Germano PM, Lieu SN, Xue J, Cooke HJ, Christofi FL, Lu Y, et al. PACAP induces signaling and stimulation of 5-hydroxytryptamine release and growth in neuroendocrine tumor cells. J Mol Neurosci (2009) 39:391–401. 10.1007/s12031-009-9283-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lelievre V, Favrais G, Abad C, Adle-Biassette H, Lu Y, Germano PM, et al. Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung’s disease. Peptides (2007) 28:1688–99. 10.1016/j.peptides.2007.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garella R, Baccari MC. Contribution of endogenous nitrergic and peptidergic influences to the altered neurally-induced gastric contractile responses in strips from dystrophic (mdx) mice. Regul Pept (2010) 160:57–63. 10.1016/j.regpep.2009.12.012 [DOI] [PubMed] [Google Scholar]