Abstract
Objective
Whether moderate alcohol consumption has health benefits remains controversial, but the harmful effects of excessive alcohol consumption on behavior and brain function are well recognized. The aim of this study was to investigate alcohol-induced regional brain activities and their relationships with behavioral factors.
Subjects and methods
A total of 29 alcohol-dependent subjects (9 females and 20 males) and 29 status-matched healthy controls (11 females and 18 males) were recruited. Severity of alcohol dependence questionnaire (SADQ) and alcohol use disorders identification test (AUDIT) were used to evaluate the severity of alcohol craving. Regional homogeneity (ReHo) analysis was used to explore the alcohol-induced regional brain changes. Receiver operating characteristic (ROC) curve was used to investigate the ability of regional brain activities to distinguish alcohol-dependent subjects from healthy controls. Pearson correlations were used to investigate the relationships between alcohol-induced ReHo differences and behavioral factors.
Results
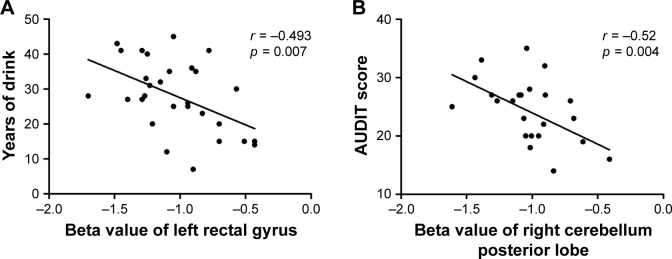
Alcohol-dependent subjects related to healthy controls showed higher ReHo areas in the right superior frontal gyrus (SFG), bilateral medial frontal gyrus (MFG), left precentral gyrus (PG), bilateral middle temporal gyrus (MTG), and right inferior temporal gyrus (ITG) and lower ReHo areas in the right cerebellum posterior lobe (CPL), left rectal gyrus (RG), and right cluster of pons and cerebellum anterior lobe (CAL). ROC curve revealed high area under the curve (AUC) values (mean ± SD: 0.864 ± 0.028; range: 0.828–0.911) of ReHo differences. Diagnostic analysis showed that these areas alone discriminated alcohol-dependent subjects from healthy controls with high degree of sensitivities (mean ± SD: 81.25% ± 11.49%; range: 62.5%–100%) and specificities (mean ± SD: 81.75% ± 12.36%; range: 67.5%–100%). Years of drink showed negative correlation with left RG (r = −0.493, p = 0.007), the same finding was shown between AUDIT and right CPL (r = −0.52, p = 0.004).
Conclusion
Alcohol dependence is associated with aberrant regional activities in multiple brain areas. ReHo analysis may be a useful biological indicator for the detection of regional brain activities in individuals with alcohol dependence.
Keywords: alcohol dependence, regional homogeneity, receiver operating characteristic
Introduction
Alcohol is the widest substance addiction worldwide. Whether moderate alcohol consumption has health benefits remains controversial, but the harmful effects of excessive alcohol consumption on behavior and brain function are well recognized. Alcohol dependence is a psychiatric disorder with adversely interpersonal, social, and health consequences. Individuals with alcohol dependence always showed the inability to inhibit the crave of alcohol consumption. The neuroimaging methods as a tool to highlight the importance of combining neuroimaging and alcohol use will advance the development of improving treatment strategies, as well as elucidating the biological mechanism. The neural mechanism of alcohol dependence is complex due to functional impairment of multiple brain areas. Although studies about the alcohol dependence underlying neurobiology have increased tremendously,1,2 the neural mechanism underlying altered functional network patterns remains ambiguous.
Resting-state functional magnetic resonance imaging (rfMRI) does not need exposure to radioactive tracers and can combine functional and structural images, which makes it suitable for mechanism exploration and insights into the pathophysiology of disease.3 Furthermore, the rfMRI can be used to find the altered neuronal spontaneous brain activity.3 Synchronous neuronal activity is conducive to integration and coordination of information processing.4 Once the neuronal activity is out of synchrony, the speed and efficiency of information processing break down, resulting in functional impairment of brain areas.5 Each voxel is temporally similar to those of its neighbors, based on which, the widely used regional homogeneity (ReHo) analysis evaluates the similarity and consistency of whole brain areas by calculating the value of Kendall’s coefficient concordance (KCC).6
The hemodynamic characteristics of each voxel are dynamic synchronization and similar within a functional cluster, and thus the ReHo analysis may be useful to explore the altered neuronal spontaneous brain activity.6–8 Higher ReHo may indicate a consistent increase in regional neuronal activity (in time series) with good synchronization; however, lower ReHo may indicate that the regional neuronal activity is out of order.7 The ReHo analysis has good test–retest reliability and does not require prior knowledge or external stimulus. Therefore, the reliable and simple characterization makes it useful for investigating altered intrinsic neuronal spontaneous brain activities associated with alcohol dependence.9 Recently, the ReHo analysis has been successfully applied to evaluate the regional brain activities in sleep disorders7,9,10 and various neuropsychiatric disorders.11–13 However, to our knowledge, the ReHo analysis has hitherto not been applied in alcohol dependence.
Changes in various behaviors, brain structure, and regional functional brain activity were found in alcohol dependence. However, the nature of these changes has not been well explored. We hypothesize that distinct regional brain activity in alcohol dependence is a function of duration and severity of alcohol dependence. Therefore, in this study, altered regional intrinsic functional network hubs in alcohol dependence and its relationships with the duration and severity of alcohol dependence were identified.
Subjects and methods
Subjects
Twenty-nine alcohol-dependent subjects (9 females, 20 males; mean age, 48.62 ± 6.81 years; mean education, 9.52 ± 2.87 years) and 29 age-, education-, and sex status-matched healthy subjects (11 females, 18 males; mean age, 48.48 ± 7.05 years; mean education, 8.48 ± 3.1 years) were recruited. The life history of psychiatric disorders, severity of alcohol dependence questionnaire (SADQ), and alcohol use disorders identification test (AUDIT) were evaluated by an experienced psychiatrist. The daily alcohol consumption and mean years of drink were recorded. The drink history duration of alcohol dependence was from 7 years to 45 years (mean ± SD: 27.93 ± 10.28 years). The daily alcohol consumption and SADQ score of alcohol dependence was 239.66 ± 107.22 mL and 20.34 ± 6.89, respectively.
All subjects with alcohol dependence met the following inclusion criteria: 1) met the diagnostic criteria of alcohol dependence as defined by Diagnostic and Statistical Manual of Mental Disorders, version 4 (DSM-4); 2) were the first-time visitors and had never taken medications treatment before for the alcohol dependence; 3) without other substance dependence or abuse; 4) had not any family history of alcohol dependence; 5) had not any sleep disorders nor any history of major psychiatric disorders; and 6) had not any foreign implants, pathological brain MRI findings, and inborn or acquired diseases. This study was approved by Medical Research Ethical Committee of Seventh People’s Hospital of Wenzhou City in accordance with the Declaration of Helsinki, and written informed consent was obtained from all subjects.
MRI parameters
The MRI scan was performed on a Siemens 3.0T MRI scanner using a 12-channel array coil. High-resolution three-dimensional T1-weighted anatomical images with 176 images in an sagittal orientation (repetition time [TR] = 1,950 ms, echo time [TE] = 2.3 ms, gap = 0 mm, thickness = 1 mm, acquisition matrix = 248 × 256, field of view [FOV] = 244 × 252 mm, and flip angle = 9°) and 240 functional images using a blood oxygenation level dependent-fMRI sequence covering the whole brain (TR = 3,000 ms, TE = 25 ms, gap = 0.5 mm, thickness = 5.0 mm, acquisition matrix = 32 × 32, flip angle = 90°, and FOV = 210 × 210 mm) were collected.
Data analysis
Based on MATLAB2010a (Mathworks, Natick, MA, USA), the MRI data pre-processing was analyzed by Data Processing & Analysis for Brain Imaging (DPABI 2.1, http://rfmri.org/DPABI) toolbox. First, the Digital Imaging and Communications in Medicine (DICOM) format of the functional data was performed standards for form transformation. Second, the first 10 time points were discarded. Third, for the remaining data, slice timing, head motion correction, and spatial normalization were performed.
Friston 24 head motion parameters were applied to regress out head motion effects. The functional data of the subjects with translation motion ≥1.5 mm in x, y, or z and/or rotation motion ≥1.5° would be rejected. Linear regression analysis was used to remove the effects of white matter, cerebrospinal fluid signal, and global mean signal.
After the head motion correction, the rest functional images were spatially normalized and resampled to Montreal Neurological Institute (MNI) space at a resolution of 3 × 3 × 3 mm3. Next, each voxel’s time series were performed bandpass filter (0.01–0.1 Hz) and linear detrend. The KCC of one given voxel’ time series and its nearest neighbor voxels (here, n = 26) was calculated, and then individual ReHo map was generated. The KCC among each voxel was divided by averaged KCC, and the normalized ReHo maps were generated. Finally, for the remaining images, spatial smoothing with Gaussian kernel of 8 × 8 × 8 mm3 full-width at half-maximum (FWHM) was performed.
Statistical analysis
Chi-square (χ2) test was used for categorical data. Demographic factors (age, sex, years of education, and AUDIT score) were analyzed using independent sample t-tests. Data are presented as mean ± standard deviation. The statistical threshold was set at p < 0.05 with the two-tailed t-test.
For ReHo index, two-sample t-test was used to evaluate the ReHo differences in regional brain activities between alcohol-dependent subjects and healthy controls with age, sex, and years of education as nuisance covariates of no interest. A corrected significance level of individual voxel p < 0.001 and the contiguous voxel size ≥13, using an AlphaSim corrected threshold of cluster p < 0.05, were used to determine the statistical significance.
Receiver operating characteristic (ROC) curve was used to evaluate the abilities of these ReHo differences in brain areas to distinguish the alcohol-dependent subjects from the healthy controls.
Pearson correlation analysis was used to explore the correlations between clinical factors and beta value of ReHo differences in brain areas. p < 0.05 was considered to be significant differences.
Results
Sample characteristics
We did not find any significant differences in age (t = 0.076, p = 0.94), sex (χ2 = 0.305, p = 0.581), and education (t = 1.318, p = 0.193) between alcohol-dependent subjects and healthy controls; however, significant difference was found in the AUDIT score between groups (t = 20.353, p < 0.001). The demographic characteristics are presented in Table 1.
Table 1.
Characteristics of alcohol-dependent subjects and healthy controls
| Demographic factors | Alcohol-dependent subjects | Healthy controls | t/χ2 value | p-value |
|---|---|---|---|---|
| Mean age, years | 48.62 ± 6.81 | 48.48 ± 7.05 | 0.076 | 0.94 |
| Sex (male, female) | 29 (20, 9) | 29 (18, 11) | 0.305a | 0.581 |
| Education, years | 9.52 ± 2.87 | 8.48 ± 3.1 | 1.318 | 0.193 |
| Years of drink | 27.93 ± 10.28 | N/A | N/A | N/A |
| SADQ score | 20.34 ± 6.89 | N/A | N/A | N/A |
| AUDIT score | 23.83 ± 5.55 | 2.55 ± 0.95 | 20.353 | <0.001 |
| Daily alcohol consumption, mL | 239.66 ± 107.22 | N/A | N/A | N/A |
Notes:
Chi-square (χ2) test; data are mean ± standard deviation values.
Abbreviations: AUDIT, alcohol use disorders identification test; N/A, not applicable; SADQ, severity of alcohol dependence questionnaire.
ReHo differences between groups
Compared with healthy controls, alcohol-dependent subjects had significantly higher ReHo areas in the right superior frontal gyrus (SFG) (Brodmann’s area [BA] 6), left precentral gyrus (PG) (BA 6), bilateral medial frontal gyrus (MFG) (BA 6), bilateral middle temporal gyrus (MTG) (BA 21), and right inferior temporal gyrus (ITG) (BA 37) and lower ReHo areas in the right cerebellum posterior lobe (CPL), left rectal gyrus (RG) (BA 11), and right cluster of pons and cerebellum anterior lobe (CAL) (Table 2 and Figure 1). The mean ReHo value of these altered areas was extracted (Figure 2).
Table 2.
Binarized degree centrality differences between alcohol-dependent subjects and healthy controls
| Brain regions of peak coordinates | R/L | BA | Voxel size | t-score of peak voxel | Peak MNI coordinates
|
|---|---|---|---|---|---|
| x, y, z | |||||
| Superior frontal gyrus | R | 6 | 13 | 4.6918 | 9, 9, 60 |
| Precentral gyrus | L | 6 | 14 | 4.5918 | −48, −3, 45 |
| Medial frontal gyrus | L | 6 | 16 | 4.9293 | −6, −3, 57 |
| Middle frontal gyrus | R | 6 | 46 | 4.563 | 45, 0, 48 |
| Middle temporal gyrus | L | 21 | 17 | 5.6139 | −69, −18, −15 |
| Middle temporal gyrus | R | 21 | 13 | 4.0442 | 57, −51, 0 |
| Inferior temporal gyrus | R | 37 | 14 | 4.9242 | 57, −57, −15 |
| Pons, cerebellum anterior lobe | R | N/A | 30 | −6.3384 | 15, −27, −24 |
| Cerebellum posterior lobe | R | N/A | 17 | −4.1926 | 27, −21, −45 |
| Rectal gyrus | L | 11 | 19 | −5.1733 | −6, 18, −24 |
Note: The statistical threshold was set at corrected significance level of individual voxel p < 0.001 using an AlphaSim-corrected threshold of cluster p < 0.05.
Abbreviations: BA, Brodmann’s area; L, left; MNI, Montreal Neurological Institute; N/A, not applicable; R, right.
Figure 1.
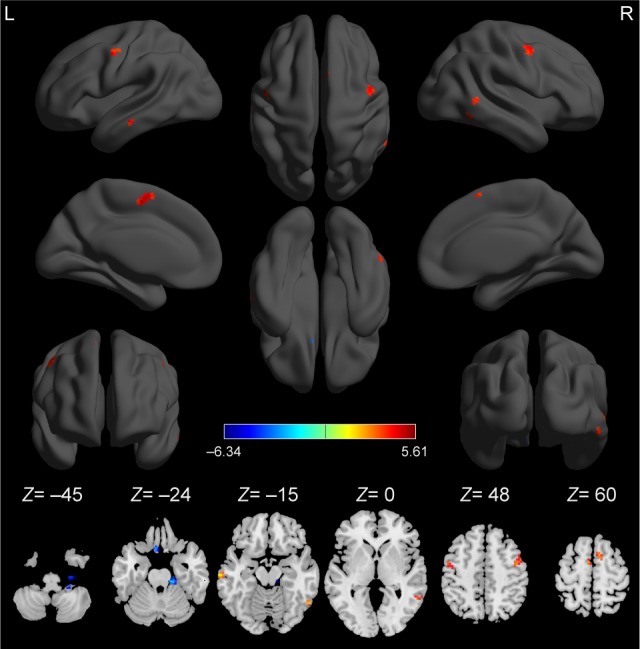
Compared to healthy controls, alcohol-dependent subjects showed altered ReHo areas.
Note: The red color represents an increased ReHo area, and the blue color represents a decreased ReHo area.
Abbreviations: L, left; R, right; ReHo, regional homogeneity.
Figure 2.
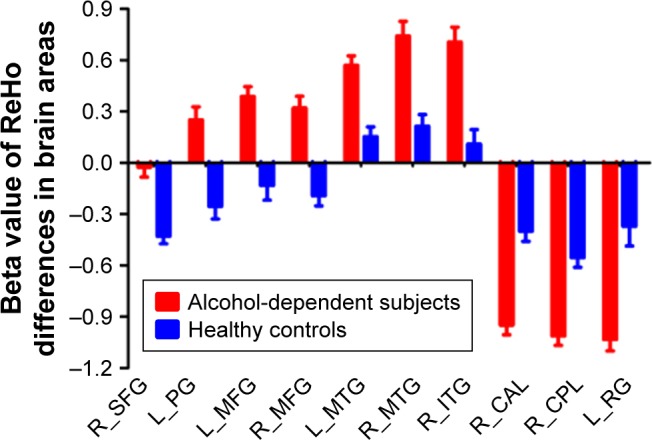
Mean beta value of ReHo differences in regional brain areas.
Note: Significant differences were found for beta value of all ReHo brain areas between alcohol-dependent subjects and healthy controls (p < 0.001).
Abbreviations: CAL, cerebellum anterior lobe; CPL, cerebellum posterior lobe; ITG, inferior temporal gyrus; L, left; MFG, medial frontal gyrus; MTG, middle temporal gyrus; PG, precentral gyrus; R, right; ReHo, regional homogeneity; RG, rectal gyrus; SFG, superior frontal gyrus.
ROC curve
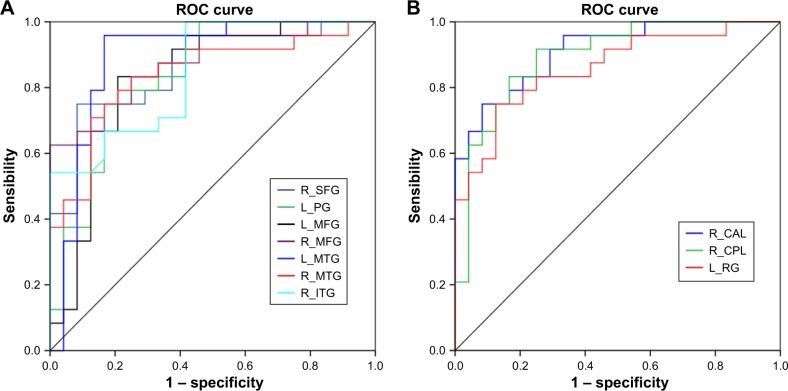
ROC curve revealed high area under the curve (AUC) values (mean ± SD: 0.864 ± 0.028; range: 0.828–0.911) of those specific ReHo differences in brain areas. Further diagnostic analysis exhibited that those specific ReHo differences in brain areas alone discriminated the alcohol-dependent subjects from the healthy controls with high degree of sensitivities (mean ± SD: 81.25% ± 11.49%; range: 62.5%–100%) and specificities (mean ± SD: 81.75% ± 12.36%; range: 67.5%– 100%). The details are presented in Table 3 and Figure 3.
Table 3.
ROC curve for the ReHo differences in brain areas between alcohol-dependent subjects and healthy controls
| Brain area | AUC | Sensitivity, % | Specificity, % | Cutoff pointa |
|---|---|---|---|---|
| R_superior frontal gyrus | 0.858 | 75 | 91.7 | −0.182 |
| L_precentral gyrus | 0.837 | 75 | 83.3 | 0.135 |
| L_medial frontal gyrus | 0.828 | 83.3 | 67.5 | 0.792 |
| R_middle frontal gyrus | 0.877 | 62.5 | 0.299 | |
| L_middle temporal gyrus | 0.891 | 95.8 | 0.251 | |
| R_middle temporal gyrus | 0.839 | 79.2 | 0.462 | |
| R_inferior temporal gyrus | 0.845 | 100 | 0.118 | |
| R_pons, cerebellum anterior lobe | 0.911 | 75 | −0.63 | |
| R_cerebellum posterior lobe | 0.894 | 91.7 | −0.8835 | |
| L_rectal gyrus | 0.859 | 75 | −0.6825 |
Note:
Cutoff point of mean
Abbreviations: A homogeneity; ROC
Figure 3.
ROC curve analysis of ReHo differences in regional brain areas.
Notes: ROC curve for differences in higher ReHo (A) and lower ReHo (B) in brain areas.
Abbreviations: CAL, cerebellum anterior lobe; CPL, cerebellum posterior lobe; ITG, inferior temporal gyrus; L, left; MFG, medial frontal gyrus; MTG, middle temporal gyrus; PG, precentral gyrus; R, right; ReHo, regional homogeneity; RG, rectal gyrus; ROC, receiver operating characteristic; SFG, superior frontal gyrus.
Pearson correlation analysis
Years of drink showed a negative correlation with the beta value of ReHo difference in the left RG (r = −0.493, p = 0.007; Figure 4A), and AUDIT showed a negative correlation with the beta value of ReHo difference in the right CPL (r = −0.52, p = 0.004; Figure 4B). No other significant correlations between the beta value of those ReHo differences in brain areas and the clinical features in alcohol dependence were found (p > 0.05).
Figure 4.
Pearson correlation between behavioral performances and beta value of ReHo differences in brain areas.
Notes: Years of drink (A) and AUDIT score (B) showed correlations with altered ReHo brain areas.
Abbreviations: AUDIT, alcohol use disorders identification test; ReHo, regional homogeneity.
Discussion
To the best of our knowledge, this is the first study to utilize ReHo analysis to report on the underlying regional functional brain activity related to alcohol dependence and their correlations with clinical factors. Specifically, we found that alcohol dependence was associated with widespread regional brain activities with higher ReHo in the right ITG (BA 37), bilateral supplementary motor area (BA 6), and bilateral MTG (BA 21) and lower ReHo in the left orbitofrontal cortex (BA 11), right CPL, and right cluster of pons and CAL. Recently, more and more studies utilized ROC curve analysis to indirectly explore the reliability of one image technology in distinguishing the disease group from the healthy control group.3,14,15 The AUC value between 0.9 and 1 is considered as excellent, between 0.8 and 0.9 as good, between 0.7 and 0.8 as fair, between 0.6 and 0.7 as poor, and that between 0.5 and 0.6 as failed.16 In this study, the ROC curve revealed that the mean beta value of altered ReHo index in brain areas consistently showed high AUC value (>0.8). Further diagnostic analysis showed that these areas discriminated the alcohol-dependent subjects from the healthy controls with high degree of sensitivity and specificity. Furthermore, in the group of alcohol dependence, years of drink demonstrated a negative correlation with the beta value of ReHo difference in the left RG, and AUDIT demonstrated a negative correlation with the beta value of ReHo difference in the right CPL.
In this study, it was found that alcohol dependence was associated with significantly higher ReHo areas in the right ITG (BA 37), bilateral supplementary motor area (BA 6), and bilateral MTG (BA 21). The temporal cortex is associated with visual, verbal, and auditory function.17,18 The MTG (BA 21) is responsible for language-auditory processing. The ITG (BA37) is associated with the representation and detection of complex object features.17 Long duration alcohol dependence requiring sustained attention for compulsive drug-seeking behaviors may stimulate verbal, visual, and auditory brain regions. Furthermore, there are two accepted speculations for the higher brain activities.19 One speculation is a brain compensatory mechanism of these higher ReHo areas, which was consistent with previous findings of higher degree centrality in the supplementary motor area (BA 6).19 The individuals with alcohol dependence often present compulsive drug-seeking behaviors and reduced ability in language and auditory, which may be associated with the higher ReHo areas in the bilateral supplementary motor area and bilateral language-auditory areas, showing an excessive motor and auditory response in human addiction processing. Therefore, the cognitive motor and language-auditory-related brain areas may utilize additional resources to promote the subjects to achieve the same level of performance as before.19 Previous studies have shown long-term effects of alcohol on human brain structure both in gray and white matter.20,21 The other explanation is hyperactivation. These changes in regional brain activities may be interpreted as a compensation mechanism with enhanced neural effort to resist the brain structural damage. Therefore, the higher ReHo areas in these brain areas may offset the widespread brain structural damage associated with alcohol dependence.
The lower ReHo in brain areas may indicate a consistent decrease in regional neuronal activity with poor synchronization and out of order,7 showing impaired regional neural activity of these areas. Alcoholism-induced neuropathology results in disturbed functional networks associated with cognitive function deficits such as working memory.22,23 In this study, we found lower ReHo areas in the left orbitofrontal cortex in alcohol-dependent subjects compared to healthy controls. The orbitofrontal cortex is a major area of drive, motivation, and salience evaluation and is thought to play an important role in output of compulsive drug-seeking behaviors and executive functions such as self-control, adaptive responding in human addiction processing.24 Furthermore, years of drink showed a negative correlation with the beta value of ReHo difference in the left RG (r = −0.493, p = 0.007). Alcohol dependence is characterized by persistent alcohol-seeking despite negative consequences. In this framework, the lower ReHo area in the left orbitofrontal cortex observed in our study may be an important factor of drug-seeking behavior.
Poor regulation of behaviors and emotions are core features of alcohol dependence. The alcoholics were impaired on gaits and balances.25 The cerebellum is associated with coordinating movement and cognition7,14 and is particularly vulnerable to alcoholism-related damage.26 Cerebellar circuit is associated with normal motor behavior by integrating their overlapping motor control functions, which is disrupted by alcohol intoxication or alcohol dependence, in turn impaired driving behavior.19,26,27 Therefore, the lower ReHo area in the right cerebellum that showed a negative correlation with the AUDIT may be interpreted as functional impairment, which may further impair the driving behavior.
Conclusion
The ReHo analysis is useful for the detection of regional brain activities in individuals with alcohol dependence. The regional neural activity of the orbitofrontal cortex and cerebellum appears to be disturbed by alcohol dependence, which may be associated with the drug-seeking. Our findings may help expand our understanding of the pathophysiology underlying alcohol dependence.
Acknowledgments
This was not an industry-supported study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Everitt BJ, Belin D, Economidou D, et al. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai XJ, Liu CL, Zhou RL, et al. Long-term sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2015;11:761–772. doi: 10.2147/NDT.S78335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Liu Y, Jiang T, et al. Altered spontaneous activity in Alzheimer’s disease and mild cognitive impairment revealed by regional homogeneity. Neuroimage. 2012;59(2):1429–1440. doi: 10.1016/j.neuroimage.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 6.Tononi G, McIntosh AR, Russell DP, Edelman GM. Functional clustering: identifying strongly interactive brain regions in neuroimaging data. Neuroimage. 1998;7(2):133–149. doi: 10.1006/nimg.1997.0313. [DOI] [PubMed] [Google Scholar]
- 7.Dai XJ, Gong HH, Wang YX, et al. Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI study. Sleep Med. 2012;13(6):720–727. doi: 10.1016/j.sleep.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Dai XJ, Peng DC, Gong HH, et al. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2014;10:2163–2175. doi: 10.2147/NDT.S69681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng DC, Dai XJ, Gong HH, et al. Altered intrinsic regional brain activity in male patients with severe obstructive sleep apnoea: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2014;10:1819–1826. doi: 10.2147/NDT.S67805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Y, Huang X, Yang Z, Li B, Liu J, Wei D. Regional homogeneity of intrinsic brain activity in happy and unhappy individuals. PLoS One. 2014;9(1):e85181. doi: 10.1371/journal.pone.0085181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu C, Liao W, Ding J, et al. Regional homogeneity changes in social anxiety disorder: a resting-state fMRI study. Psychiatry Res. 2011;194(1):47–53. doi: 10.1016/j.pscychresns.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Philip NS, Kuras YI, Valentine TR, et al. Regional homogeneity and resting state functional connectivity: associations with exposure to early life stress. Psychiatry Res. 2013;214(3):247–253. doi: 10.1016/j.pscychresns.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai XJ, Nie X, Liu X, et al. Gender differences in regional brain activity in patients with chronic primary insomnia: evidence from a resting-state fMRI study. J Clin Sleep Med. 2016;12(3):363–374. doi: 10.5664/jcsm.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li HJ, Dai XJ, Gong HH, Nie X, Zhang W, Peng DC. Aberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRI. Neuropsychiatr Dis Treat. 2015;11:207–214. doi: 10.2147/NDT.S73730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Khouli RH, Macura KJ, Barker PB, Habba MR, Jacobs MA, Bluemke DA. Relationship of temporal resolution to diagnostic performance for dynamic contrast enhanced MRI of the breast. J Magn Reson Imaging. 2009;30(5):999–1004. doi: 10.1002/jmri.21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewald J, Staedtgen M, Sparing R, Meister IG. Processing of auditory motion in inferior parietal lobule: evidence from transcranial magnetic stimulation. Neuropsychologia. 2011;49(2):209–215. doi: 10.1016/j.neuropsychologia.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Robins DL, Hunyadi E, Schultz RT. Superior temporal activation in response to dynamic audio-visual emotional cues. Brain Cogn. 2009;69(2):269–278. doi: 10.1016/j.bandc.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo X, Guo L, Dai XJ, et al. Abnormal intrinsic functional hubs in alcohol dependence: evidence from a voxelwise degree centrality analysis. Neuropsychiatr Dis Treat. 2017;13:2011–2020. doi: 10.2147/NDT.S142742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durkee CA, Sarlls JE, Hommer DW, Momenan R. White matter microstructure alterations: a study of alcoholics with and without post-traumatic stress disorder. PLoS One. 2013;8(11):e80952. doi: 10.1371/journal.pone.0080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grodin EN, Lin H, Durkee CA, Hommer DW, Momenan R. Deficits in cortical, diencephalic and midbrain gray matter in alcoholism measured by VBM: effects of co-morbid substance abuse. Neuroimage Clin. 2013;2:469–476. doi: 10.1016/j.nicl.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17(3):239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180(4):583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- 24.Yucel M, Lubman DI. Neurocognitive and neuroimaging evidence of behavioural dysregulation in human drug addiction: implications for diagnosis, treatment and prevention. Drug Alcohol Rev. 2007;26(1):33–39. doi: 10.1080/09595230601036978. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24(5):611–621. [PubMed] [Google Scholar]
- 26.Rzepecki-Smith CI, Meda SA, Calhoun VD, et al. Disruptions in functional network connectivity during alcohol intoxicated driving. Alcohol Clin Exp Res. 2010;34(3):479–487. doi: 10.1111/j.1530-0277.2009.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21(10):2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]