Abstract
Background
In patients with Parkinson’s disease (PD), oral dosing of extended-release carbidopa-levodopa (Rytary, IPX066 [ER CD-LD]) achieves peak levodopa plasma concentrations within 1 hour and maintains them for 4–6 hours.
Aims
To compare the onset and duration of ER CD-LD benefit with those of immediate-release carbidopa-levodopa (IR CD-LD) in PD patients with motor fluctuations, using crossover data, and to evaluate which threshold values of improvement in finger-tapping and Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores yield results most similar to those for trained raters’ “on”/“off” assessments.
Methods
Patients underwent serial “on”/“off” rating and provided serial finger-tapping and UPDRS motor scores after receiving, in an “off” state, their usual morning IR dose or an ER dose designed to produce a similar levodopa peak concentration. Predefined improvement thresholds for analysis were 10%, 15%, and 20% increases in finger-tapping score and 2.5, 5, 7, and 11-point decreases in UPDRS motor score. Serial plasma samples were assayed for levodopa.
Results
Among 27 patients, mean time to onset of an “on” state was similar for ER compared with IR CD-LD (0.83 vs 0.81 hour), but mean duration was significantly longer for ER CD-LD than for IR CD-LD (5.56 vs 2.69 hours; P<0.0001). Duration was best matched by a $20% improvement in finger-tapping, a $11-point improvement in UPDRS motor score, and a levodopa plasma concentration $1,000 ng/mL.
Conclusion
For ER CD-LD, observer assessments of “on” state were corroborated by sustained treatment effects. Correlations among “on”-state duration, finger-tapping score, and UPDRS motor score may suggest clinically relevant thresholds for acute assessment of treatment benefit.
Keywords: Rytary, carbidopa-levodopa, Parkinson’s disease, treatment, duration of effect, motor fluctuations
Introduction
Carbidopa and levodopa extended-release capsules1 (ER CD-LD, Rytary, IPX066) are a multiparticulate oral formulation of carbidopa-levodopa (CD-LD) consisting of immediate-release (IR) and extended-release (ER) components, as well as the functional excipient tartaric acid.2 In patients with Parkinson’s disease (PD), the formulation achieves peak levodopa plasma concentrations within 1 hour after dosing and maintains them for 4–6 hours.3–6 Because of its distinct immediate and extended levodopa-releasing properties in the gastrointestinal tract, the plasma levodopa profile produced by an ER CD-LD dose differs markedly from those of other oral levodopa products.7 In a study of patients with advanced PD, single doses resulted in ~70% of the levodopa exposure seen after a similar milligram dose of IR CD-LD (as measured by area under the plasma concentration curve) and ~30% of the peak levodopa plasma levels (Cmax).8 For doses providing similar peak levodopa levels, the onset of improvement of motor function observed for ER CD-LD resembled that observed for IR CD-LD, as judged by change in score on Part III of the Unified Parkinson’s Disease Rating Scale (UPDRS) and by an investigator rating of motor state, and the duration of effect was longer by both measures.8
For the post hoc analyses presented here, we were interested in comparing the onset and duration of benefit of ER CD-LD and those of IR CD-LD in patients with motor fluctuations, using data from a Phase II crossover study.8 Trained rater assessments of “on” and “off” states provide a straightforward means to determine onset and duration of benefit, but finger-tapping and UPDRS motor scores improve and decline gradually, with no clearly defined thresholds that separate “benefit” from “no benefit”. Improvements of 10%–20% in finger-tapping rate9–13 and 3–11 points in UPDRS motor scores14,15 have been considered clinically relevant in PD and have been employed to evaluate the efficacy of dopamine agonists16–18 and rasagiline.19 In the present analyses, we determined time to onset and duration of benefit using trained rater assessments of “on” and “off”, finger-tapping scores, and UPDRS motor scores. We then evaluated which tapping-score and UPDRS motor-score thresholds provided results most similar to those derived from “on” and “off” assessments.
Methods
Study design
Details of the original study design have been published previously.8 Briefly, the data for the present analyses were derived from a Phase II crossover study of two open-label treatments administered in a randomized order: ER CD-LD followed by IR CD-LD, or the reverse. Each treatment was taken for 1 week. Between treatment periods, patients used their prestudy IR CD-LD regimen for ~1 week. At the beginning of each treatment period, all patients underwent 8 hours of serial pharmacokinetic/pharmacodynamic assessments after receiving, in a fasted state, their prestudy morning dose of IR CD-LD or a dose of ER CD-LD designed to provide a levodopa Cmax similar to that of the IR treatment. Each treatment was taken in an “off” state. Patients experiencing three consecutive hours of “off” time during the 8 hours following dosing could be redosed at the investigator’s discretion.
Dose selection
During the IR CD-LD treatment, patients received their pre-study IR CD-LD regimen. During the ER CD-LD treatment, patients received a dose regimen of ER CD-LD using a conversion table that was based on the patient’s morning IR dose. The conversion was based on pharmacokinetic data of healthy volunteers and was designed to achieve similar (within ~20%) peak levodopa concentrations following the morning IR CD-LD and ER CD-LD doses.7
Study participants
All patients had been taking commercially available IR CD-LD at least four times/day (excluding nighttime dosing), with a levodopa dosage totaling 500–1,600 mg/day, in a regimen that had been stable for at least 1 month. Patients were also required to be experiencing at least 3 hours/day of “off” time. Their “on” state was required to be predictable, as documented in UPDRS Questions 37 and 38. In the “on” state, patients were required to show a $10% increase in finger-tapping rate, as compared with the “off” state. Patients were also required to be experiencing dyskinesia, as documented in UPDRS Question 32 (score $1). The study was conducted in accordance with Good Clinical Practice ethical guidelines and was approved by the appropriate institutional review boards: Western Institutional Review Board, Inc., 3535 Seventh Avenue SE, Olympia, WA 98502-5010, USA; QUORUM Review Inc., 1601 Fifth Avenue, Suite 1000, Seattle, WA 98101, USA; or Rush University Medical Center, Research and Clinical Trials Administrative Office, 1653 West Congress Parkway, Chicago, IL 60612-3833, USA. Before any study procedures, each patient provided written informed consent.
Efficacy measures
On each pharmacokinetic/pharmacodynamic day, each patient was assessed by a trained rater at approximate half-hour intervals, three times pre-dose and for 8 hours post-dose, as being “on” with no dyskinesia, “on” with non-troublesome dyskinesia, “on” with troublesome dyskinesia, or “off”.20 For each patient, the same rater performed all assessments. In addition, finger-tapping was measured three times pre-dose and half-hourly for 8 hours post-dose. Each test was scored as the number of times the patient could alternately tap two keys 20 cm apart in 1 minute, using the index finger of the arm more affected by PD, as determined at screening. A UPDRS Part III score was obtained hourly by qualified study-site personnel, twice pre-dose and for 8 hours post-dose.
Efficacy analyses
For ER CD-LD and IR CD-LD, the half-hourly “on/off” classifications made by trained raters were utilized to calculate the mean time to onset and the mean duration of an “on” state. Differences between ER CD-LD and IR CD-LD were tested for statistical significance, set at P<0.05, using a mixed-model analysis of covariance (ANCOVA) with baseline value as a covariate.
Details on the finger-tapping scores have been reported previously.8 In the present post hoc analysis, to assess the proportions of patients attaining various levels of motor-function improvement, thresholds of improvement from a patient’s pre-dose value (obtained in an “off” state) were defined as increases of 10%, 15%, and 20% in finger-tapping score and decreases of 2.5, 5, 7, and 11 points in UPDRS Part III score. For each such threshold, the time at which each patient achieved the threshold was defined as the time to onset of effect, and the time at which the response subsequently fell below the threshold for the first time during the 8-hour post-dose period was defined as the loss of effect. Duration of effect was defined as time from onset of effect to time of loss of effect. For finger-tapping, each patient’s baseline was the average of the patient’s three pre-dose values. For UPDRS Part III score, it was the average of the two pre-dose values. Differences between ER CD-LD and IR CD-LD were tested for statistical significance by the ANCOVA model. Only the patients who reached each threshold were included in each analysis. No adjustments were made for multiple testing. For patients who were redosed during the 8 hours after their pre-study morning dose, any measurement obtained 10 minutes or more after taking a second dose was assigned a value of the pre-dose average of the treatment period and treated as if there was no benefit.
Pharmacokinetic analyses
Venous blood was collected pre-dose and for 8 hours post-dose, half-hourly for 6 hours and at hours 7 and 8. The resulting plasma samples were stored at −70°C or below until analysis for levodopa concentration by liquid chromatography coupled to tandem mass spectrometry, using a validated bioanalytical method with a minimum quantifiable levodopa concentration of 10.0 ng/mL. If patients were redosed, their pharmacokinetic data were censored after the redosing time point.
Results
Study participants
In total, 27 patients were randomized at six study sites, all in the US. Their baseline characteristics are summarized in Table 1. All patients completed the study.
Table 1.
Patients’ baseline characteristics (safety population)
| Variables | Values |
|---|---|
| N | 27 |
| Age (years) | |
| Mean (SD) | 62.7 (8.6) |
| Median (range) | 62 (48–81) |
| Sex, n (%) | |
| Male | 21 (78) |
| Female | 6 (22) |
| Race, n (%) | |
| Caucasian | 24 (89) |
| Asian | 3 (11) |
| Weight (kg) | |
| Mean (SD) | 84.5 (14.8) |
| Median (range) | 85 (51–120) |
| Age at PD onset (years) | |
| Mean (SD) | 52.3 (8.7) |
| Median (range) | 50 (36–74) |
| “Off” time (h/day) | |
| Mean (SD) | 5.9 (2.2) |
| Median (range) | 5.7 (2.0–11.0) |
| Hoehn & Yahr stage,a n (%) | |
| 2 | 13 (48) |
| 3 | 10 (37) |
| 4 | 4 (15) |
| Levodopa IR dosage (mg/day) | |
| Mean (SD) | 815.7 (249.7) |
| Median (range) | 800 (500–1,250) |
| Levodopa IR dosing frequency (doses/day) | |
| Mean (SD) | 5.4 (1.6) |
| Median (range) | 5 (4–10) |
| Finger-tapping score (taps/min) | |
| “On” state | |
| Mean (SD) | 170.4 (64.6) |
| Median (range) | 163 (73–327) |
| “Off” state | |
| Mean (SD) | 117.9 (44.1) |
| Median (range) | 116 (44–229) |
| UPDRS Part III score | |
| “On” state | |
| Mean (SD) | 17.6 (8.5) |
| Median (range) | 17 (1–32) |
| “Off” state | |
| Mean (SD) | 35.9 (9.4) |
| Median (range) | 36 (20–51) |
Note:
During “on” time.
Abbreviations: IR, immediate release; PD, Parkinson’s disease; SD, standard deviation; UPDRS, Unified Parkinson’s Disease Rating Scale.
Dosing
Each patient received individualized regimens of ER CD-LD and IR CD-LD. On day 1 of each treatment, the mean (SD) levodopa morning doses were 663.9 (185.2) mg for ER CD-LD and 164.8 (61.7) mg for IR CD-LD. The medians were 585.0 mg for ER CD-LD and 200.0 mg for IR CD-LD. In total, six patients on ER CD-LD and ten patients on IR CD-LD were redosed before the end of the 8-hour pharmacokinetic/pharmacodynamic assessment period. One ER CD-LD patient and two IR CD-LD patients were redosed before hour 4, three IR CD-LD patients were redosed between hours 4 and 6, and five patients in each group were redosed between hours 6 and 8.
Time to “on” and duration of “on”
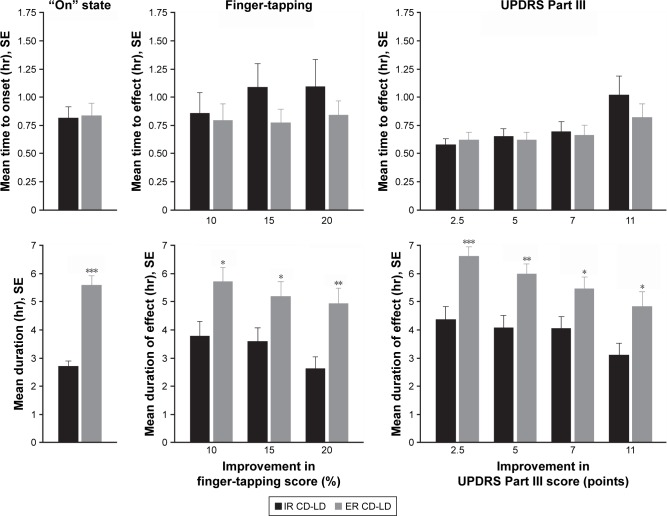
For patients rated as achieving an “on” state, the mean time to onset and the mean duration are displayed in Figure 1 (leftmost charts). The mean (SD) time to onset showed no significant difference between ER CD-LD and IR CD-LD (0.83 [0.52] vs 0.81 [0.45] hours; P=0.89), but the mean duration was significantly longer for ER CD-LD than for IR CD-LD (5.56 [1.78] vs 2.69 [0.92] hours; P<0.0001).
Figure 1.
Mean time to onset (top graphs) and mean duration (bottom graphs) for an “on” state, finger-tapping score improvement, and UPDRS Part III score improvement after single doses of ER CD-LD vs IR CD-LD.a
Notes: *P<0.05, **P<0.01, ***P<0.001 (ANCOVA). aEach analysis includes only the patients who reached each outcome.
Abbreviations: ANCOVA, analysis of covariance; CD-LD, carbidopa-levodopa; ER, extended release; IR, immediate release; SE, standard error; UPDRS, Unified Parkinson’s Disease Rating Scale.
Time to onset of effect and duration of effect
For patients achieving each predefined threshold level of improvement in finger-tapping score or in UPDRS Part III score, the mean time to onset of effect and the mean duration of effect are displayed in Figure 1 (center and rightmost charts). For all improvement levels, the mean time to onset of effect showed no significant difference between ER CD-LD and IR CD-LD. However, the mean duration of effect was significantly longer for ER CD-LD than for IR CD-LD.
Pharmacokinetics
Mean plasma levodopa concentrations following single doses of ER CD-LD and IR CD-LD are presented in Figure 2. The initial concentrations increased rapidly after both treatments, but were sustained longer by ER CD-LD than by IR CD-LD. The fluctuation index is a measure of the magnitude of rise and fall of levodopa plasma concentrations relative to the average concentration and may be of particular interest for an ER formulation. The lower the fluctuation index, the more likely the Cmax is blunted relative to the trough, thus improving the pharmacodynamic profile and minimizing Cmax-related adverse effects. The fluctuation index measured as (Cmax−Cmin)/Cave was 1.5 and 3.2 for ER CD-LD and IR CD-LD, respectively.2
Figure 2.
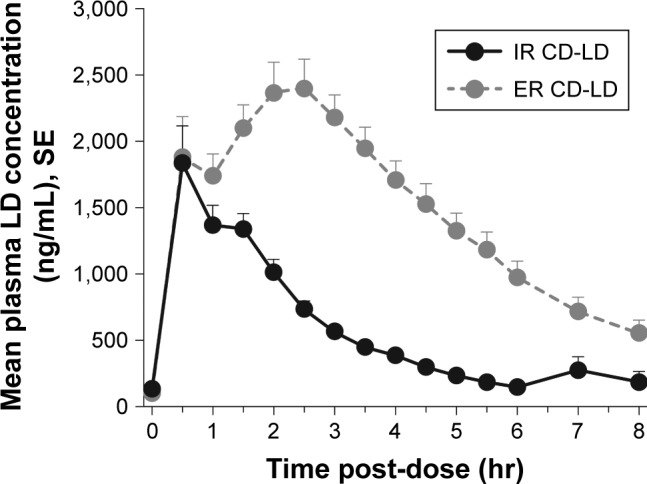
Mean plasma levodopa levels after single doses of ER CD-LD vs IR CD-LD.a
Notes: aFor each treatment, n=27. However, for six patients who required ER CD-LD redosing and for ten who required IR CD-LD redosing, data are included only until the redosing (see Pharmacokinetic Analyses in the Methods section and Dosing in the Results section).
Abbreviations: CD-LD, carbidopa-levodopa; ER, extended release; IR, immediate release; LD, levodopa; SE, standard error.
Similarities among outcomes
By observers’ assessments, the mean duration of an “on” state was ~5.6 hours following ER CD-LD dosing and ~2.7 hours following IR CD-LD dosing (Figure 1). By inspection of mean durations of motor-function improvement, the thresholds that yielded the most similar set of durations were a 20% increase from baseline for finger-tapping and a 11-point decrease from baseline for UPDRS Part III score (Figure 1). In the study’s pharmacokinetic data, a levodopa plasma level $1,000 ng/mL provided a similar set of durations (Figure 2).
Discussion
In this post hoc analysis of ER CD-LD and IR CD-LD in patients with advanced PD, the onset of clinical effect following a single dose was similar for both treatments, but the duration of effect was significantly longer for ER CD-LD than for IR CD-LD, as documented by investigator assessments of motor state, finger-tapping, and UPDRS Part III scores. Consistent with these clinical endpoints, plasma levodopa concentrations showed a similar time to reach a threshold level $1,000 ng/mL for both treatments, but were maintained for a longer duration by ER CD-LD.
Pharmacokinetic data in healthy subjects had indicated that on a dose-adjusted basis (mg ER CD-LD:mg IR CD-LD), the ER CD-LD treatment resulted in a mean Cmax ratio of 0.34.2 Thus, an approximately threefold higher dose of ER CD-LD compared to IR CD-LD would result in similar Cmax values. Recognizing that there may be differences in the pharmacokinetics of ER CD-LD in healthy subjects and patients, and the importance of achieving an “on” state in both treatments to allow for pharmacodynamic comparisons, a conservative approach was adopted for the initial conversion, allowing physicians to subsequently titrate the ER CD-LD dose for the first 3 days as needed to optimize the therapeutic effect. Similarly, physicians could also adjust the IR CD-LD regimen for the first 3 days as needed. Subsequent pharmacokinetic data from patients in this study confirmed the dose conversion. Following a single IR CD-LD dose of 50–200 mg, the mean levodopa peak concentration was 2,331 ng/mL compared to 2,424 ng/mL following 122.5–490 mg ER CD-LD.2 In addition, multiple dose pharmacokinetic data indicated that the Cmax and average levodopa concentration following the IR 50–200 mg CD-LD were 3,057 ng/mL and 969 ng/mL, respectively.2 In comparison, following multiple doses of 490 mg ER CD-LD, the Cmax and average levodopa concentration were 3,227 ng/mL and 1,623 ng/mL, respectively. These data indicate that similar to observations in healthy subjects, a 2.45-fold higher levodopa dose with ER CD-LD compared to IR CD-LD resulted in very comparable (ie, within 4%–6%) mean peak levodopa concentrations following single and multiple doses. The higher average levodopa concentration with ER CD-LD is due to its pharmacokinetic profile with more sustained levodopa concentration and contributes to the longer duration of effect for this treatment.
Of the three efficacy measures utilized in the present analyses – observers’ “on”/“off” assessments, finger-tapping scores, and UPDRS Part III scores – the “on”/“off” assessments may come closest to being considered a “gold standard” for judging a patient’s clinical status. The observation of an “on” state within 1 hour after levodopa dosing is consistent with that reported by others. For IR CD-LD, Lewitt et al reported that the mean (SD) time to “on”, as assessed via patient diary, was 0.95 (0.55) hours.21 Similarly, Merims et al reported that the mean (SD) time to beginning of an “on” state after levodopa dosing, as judged by the patient, was 46 (21) minutes.22 The original study did not use a stopwatch method to measure time to “on” and its duration. Investigator assessments of motor state were done every 30 minutes, and measurements of the time to “on” and the duration of “on” may be expected to be influenced by the frequency of assessment.
Of the other two efficacy measures, finger-tapping scores have not been extensively validated, and various derivations of minimal clinically relevant changes in UPDRS scores have not resolved the issue of what clearly constitutes a meaningful change in a given patient or population.14,15,17–19 By observers’ assessments, the mean duration of an “on” state (~5.6 hours after ER CD-LD dosing compared with ~2.7 hours after IR CD-LD dosing) was matched most closely by a $20% improvement in finger-tapping, a $11-point improvement in UPDRS Part III score, and a levodopa plasma concentration $1,000 ng/mL, suggesting that these values may be the most clinically relevant thresholds for acute assessment of benefit, at least in this population of PD patients. Nonetheless, we note that none of the threshold values evaluated provided a perfect match to the “on”/“off” assessments. To some extent, this could be due to methodologic issues including the timing and frequency of the various evaluations and due to the fact that we only evaluated a few thresholds. It is also possible that there would always be some differences in duration of benefit as assessed by the various measures, no matter how carefully such a study were conducted. This could be because the transition from “on” to “off” and “off” to “on” is often gradual, so the designation of “on” or “off” during these transition periods is subjective. It is also possible that benefit occurs in the various signs and body parts at different times, such that finger-tapping could improve before, or after, walking and speech. These issues deserve further study. The concentration threshold of 1,000 ng/mL identified in the present study is comparable to the value of 900 ng/mL identified by Nelson et al23 as the median effective concentration typically needed to maintain control of Parkinsonian features.24
Limitations of the present analyses include the fact that the study was an open-label study and relatively small. In addition, although the doses administered were designed to have a similar Cmax according to pharmacokinetic information known at the time, results indicated that Cmax was higher in the ER CD-LD group than in the IR CD-LD group. Duration of benefit may depend on treatment dose, which was not tested, and it is possible that higher doses of IR CD-LD would yield a longer duration of benefit. However, it should be noted that the doses of IR CD-LD administered in the original study were the doses patients were typically taking prior to entering the study, and troublesome dyskinesia was not significantly increased with ER CD-LD compared to IR CD-LD. The identified thresholds for benefit are most likely to be applicable to patients with motor fluctuations of a severity similar to that required for original study entry.
The post hoc analyses presented here provide additional evidence that in a population of patients with motor fluctuations on levodopa, time to onset of benefit from ER CD-LD is similar to that for IR CD-LD, and duration of benefit is significantly longer. In PD patients such as those in the present study, for whom IR dosing provides benefit for only about 2.5 hours, ER dosing may provide benefit for about 5.6 hours. Nonetheless, clinicians should be aware that timing of administration may require adjustment based on the individual patient’s duration of benefit.
Acknowledgments
This study was supported by Impax Laboratories, Inc. Under the direction of the authors, editorial assistance was provided by Michael Feirtag of The Curry Rockefeller Group, LLC, which was funded by Impax Laboratories, Inc.
Footnotes
Author contributions
RAH: design and execution of the clinical study, conduct of data analyses, writing of the first draft, and review and critique of the manuscript; AE: design and execution of the clinical study, conduct of data analyses, and review and critique of the manuscript; SK: design of the clinical study, conduct of statistical and data analyses, and review and critique of the manuscript; SG: design and execution of the clinical study, conduct of data analyses, and review and critique of the manuscript; NBM: design and execution of the clinical study, conduct of data analyses, and review and critique of the manuscript. All authors contributed toward data analysis and drafting and revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
Robert A Hauser has received honoraria or payments for consulting, advisory services, or speaking services over the past 12 months from AbbVie, Allergan, AstraZeneca, Biotie Therapeutics, Ceregene, Chelsea Therapeutics, Cleveland Clinic, Eli Lilly, GE Healthcare, Impax Laboratories, Inc., Neurocrine, Indus, Ipsen Biopharmaceuticals, Lundbeck, Merck/MSD, Noven Pharmaceuticals, Pfizer, Straken Pharmaceuticals, Targacept, Teva Pharmaceutical Industries, Ltd., Teva Neuroscience, Upsher-Smith Laboratories, UCB, UCB Pharma SA, University of Houston, US WorldMeds, XenoPort, and Zambon Company SpA. Dr Hauser’s institution has received research support over the past 12 months from Abbott Laboratories, Addex Therapeutics, Allergan, AstraZeneca, Biotie Therapeutics, Chelsea Therapeutics, Civitas, GE Healthcare, Impax Laboratories, Inc., Ipsen Biopharmaceuticals, Merck/MSD, Merz, the Michael J. Fox Foundation for Parkinson’s Research, NINDS, the Parkinson Study Group, Schering-Plough, Teva Neuroscience, UCB, and Vita-Pharm. Dr Hauser has received royalties in the past 12 months from the University of South Florida. Aaron Ellenbogen has participated in a speaker bureau for Teva Pharmaceuticals and has been a study investigator and consultant for Impax Laboratories, Inc., clinical studies. Sarita Khanna, Suneel Gupta, and Nishit B Modi are employees and stockholders of Impax Laboratories, Inc. The authors report no other conflicts of interest in this work.
References
- 1.Impax Laboratories, Inc Rytary (carbidopa and levodopa) extended-release capsules: full prescribing information. 2016. [Accessed August 21, 2017]. Available from: http://documents.impaxlabs.com/rytary/pi.pdf.
- 2.Mittur A, Gupta S, Modi NB. Pharmacokinetics of Rytary®, an extended-release capsule formulation of carbidopa-levodopa. Clin Pharmacokinet. 2017;56(9):999–1014. doi: 10.1007/s40262-017-0511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser RA, Hsu A, Kell S, et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson’s disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol. 2013;12(4):346–356. doi: 10.1016/S1474-4422(13)70025-5. [DOI] [PubMed] [Google Scholar]
- 4.Waters CH, Nausieda P, Dzyak L, et al. Long-term treatment with extended-release carbidopa-levodopa (IPX066) in early and advanced Parkinson’s disease: a 9-month open-label extension trial. CNS Drugs. 2015;29(4):341–350. doi: 10.1007/s40263-015-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pahwa R, Lyons KE, Hauser RA, et al. Randomized trial of IPX066, carbidopa/levodopa extended release, in early Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(2):142–148. doi: 10.1016/j.parkreldis.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Stocchi F, Hsu A, Khanna S, et al. Comparison of IPX066 with carbidopa-levodopa plus entacapone in advanced PD patients. Parkinsonism Relat Disord. 2014;20(12):1335–1340. doi: 10.1016/j.parkreldis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Hsu A, Yao HM, Gupta S, Modi NB. Comparison of the pharmacokinetics of an oral extended-release capsule formulation of carbidopa-levodopa (IPX066) with immediate-release carbidopa-levodopa (Sinemet®), sustained-release carbidopa-levodopa (Sinemet® CR), and carbidopa-levodopa-entacapone (Stalevo®) J Clin Pharmacol. 2015;55(9):995–1003. doi: 10.1002/jcph.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauser RA, Ellenbogen AL, Metman LV, et al. Crossover comparison of IPX066 and a standard levodopa formulation in advanced Parkinson’s disease. Mov Disord. 2011;26(12):2246–2252. doi: 10.1002/mds.23861. [DOI] [PubMed] [Google Scholar]
- 9.Contin M, Riva R, Martinelli P, Albani F, Baruzzi A. Relationship between levodopa concentration, dyskinesias, and motor effect in parkinsonian patients: a 3-year follow-up study. Clin Neuropharmacol. 1997;20(5):409–418. doi: 10.1097/00002826-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Contin M, Riva R, Martinelli P, Albani F, Baruzzi A. Effect of meal timing on the kinetic-dynamic profile of levodopa/carbidopa controlled release [corrected] in parkinsonian patients. Eur J Clin Pharmacol. 1998;54(4):303–308. doi: 10.1007/s002280050464. [DOI] [PubMed] [Google Scholar]
- 11.Contin M, Riva R, Martinelli P, et al. Response to a standard oral levodopa test in parkinsonian patients with and without motor fluctuations. Clin Neuropharmacol. 1990;13(1):19–28. doi: 10.1097/00002826-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Gancher ST, Nutt JG, Woodward W. Response to brief levodopa infusions in parkinsonian patients with and without motor fluctuations. Neurology. 1988;38(5):712–716. doi: 10.1212/wnl.38.5.712. [DOI] [PubMed] [Google Scholar]
- 13.Nutt JG, Carter JH, Van Houten L, Woodward WR. Short- and long-duration responses to levodopa during the first year of levodopa therapy. Ann Neurol. 1997;42(3):349–355. doi: 10.1002/ana.410420311. [DOI] [PubMed] [Google Scholar]
- 14.Rascol O. Defining a minimal clinically relevant difference for the unified Parkinson’s rating scale: an important but still unmet need. Mov Disord. 2006;21(8):1059–1061. doi: 10.1002/mds.20913. [DOI] [PubMed] [Google Scholar]
- 15.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the Unified Parkinson’s Disease Rating Scale. Arch Neurol. 2010;67(1):64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 16.Brodsky MA, Park BS, Nutt JG. Effects of a dopamine agonist on the pharmacodynamics of levodopa in Parkinson disease. Arch Neurol. 2010;67(1):27–32. doi: 10.1001/archneurol.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser RA, Gordon MF, Mizuno Y, et al. Minimal clinically important difference in Parkinson’s disease as assessed in pivotal trials of pramipexole extended release. Parkinsons Dis. 2014;2014:467131. doi: 10.1155/2014/467131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrag A, Sampaio C, Counsell N, Poewe W. Minimal clinically important change on the Unified Parkinson’s Disease Rating Scale. Mov Disord. 2006;21(8):1200–1207. doi: 10.1002/mds.20914. [DOI] [PubMed] [Google Scholar]
- 19.Hauser RA, Auinger P, Parkinson Study Group Determination of minimal clinically important change in early and advanced Parkinson’s disease. Mov Disord. 2011;26(5):813–818. doi: 10.1002/mds.23638. [DOI] [PubMed] [Google Scholar]
- 20.Hauser RA, Friedlander J, Zesiewicz TA, et al. A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol. 2000;23(2):75–81. doi: 10.1097/00002826-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Lewitt PA, Ellenbogen A, Chen D, et al. Actively transported levodopa prodrug XP21279: a study in patients with Parkinson disease who experience motor fluctuations. Clin Neuropharmacol. 2012;35(3):103–110. doi: 10.1097/WNF.0b013e31824e4d7d. [DOI] [PubMed] [Google Scholar]
- 22.Merims D, Djaldetti R, Melamed E. Waiting for ON: a major problem in patients with Parkinson disease and ON/OFF motor fluctuations. Clin Neuropharmacol. 2003;26(4):196–198. doi: 10.1097/00002826-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Nelson MV, Berchou RC, LeWitt PA, Kareti D, Galloway MP. Pharmacodynamic modeling of concentration-effect relationships after controlled-release carbidopa/levodopa (Sinemet CR4) in Parkinson’s disease. Neurology. 1990;40(1):70–74. doi: 10.1212/wnl.40.1.70. [DOI] [PubMed] [Google Scholar]
- 24.LeWitt PA, Jennings D, Lyons KE, et al. Pharmacokinetic-pharmacodynamic crossover comparison of two levodopa extension strategies. Mov Disord. 2009;24(9):1319–1324. doi: 10.1002/mds.22587. [DOI] [PubMed] [Google Scholar]