Abstract
Introduction
Bisphosphonates are widely used in metastatic cancer such as prostate and breast cancer, and their nephrotoxic effects have been established previously. In this study we aimed to evaluate both the nephrotoxic effects of zoledronic acid (ZA) and the protective effects of vitamin E (Vit-E) on this process under light and electron microscopy.
Material and methods
A total of 30 male Sprague-Dawley rats were divided into 3 groups. The first group constituted the control group. The second group was given i.v. ZA of 3 mg/kg once every 3 weeks for 12 weeks from the tail vein. The third group received the same dosage of ZA with an additional i.m. injection of 15 mg Vit-E every week for 12 weeks. Tissues were taken 4 days after the last dose of ZA for histopathological and ultrastructural evaluation. Paller score, tubular epithelial thickness and basal membrane thickness were calculated for each group.
Results
For group 2, the p-values are all < 0.001 for Paller score, epitelial thickness, and basal membrane thickness. For group 3 (ZA + Vit. E), the p-values are < 0.001 for Paller score, 0.996 for epitelial thickness, and < 0.001 basal membrane thickness. Significant differences were also observed in ultrastructural changes for group 2. However, adding Vit-E to ZA administration reversed all the histopathological changes to some degree, with statistical significance.
Conclusions
Administration of ZA had nephrotoxic effects on rat kidney observed under both light and electron microscopy. Concomitant administration of Vit-E significantly reduces toxic histopathological effects of ZA.
Keywords: zoledronic acid, vitamin E, nephrotoxicity, renal function, rat
Introduction
Patients with advanced cancers, especially prostate and breast cancers, are at high risk for bone metastases leading to accelerated bone resorption and clinically significant skeletal morbidity [1]. Bisphosphonates are a standard treatment to counteract the symptoms associated with bone metastases, including pathological fractures or spinal cord compression. However, bisphosphonates differ with respect to their renal safety [2].
Zoledronic acid (ZA) is a third-generation, double nitrogen-containing, cyclic bisphosphonate administered by intravenous infusion; and it has demonstrated the broadest clinical activity in patients with bone metastases originating from various malignancies including prostate, breast and lung cancers, and other solid tumors as well as multiple myeloma [3–5]. Although the higher antiresorptive capacity and the relatively low renal toxicity of ZA have been shown in thyroparathyroidectomized rats by Green et al., many cases of ZA-associated renal toxicity or renal failure have been reported related to the dosage used [6–8]. For this reason, patients treated with ZA should be monitored closely for any potential deterioration in renal functions.
Vitamin E (Vit E – α-tocopherol), as a fat-soluble antioxidant, stops the production of reactive oxygen species formed when fat undergoes oxidation, and is incorporated into cell membranes to protect against oxidative damage [9]. Like verapamil and selenium, Vit E is being studied for its antioxidative effects in the kidney [10, 11].
In this study, we evaluated the renal protective effects of Vit E shown by both light and electron microscopy in an experimental rat model of ZA-induced renal injury.
Material and methods
This experimental study was approved by the Local Ethics Committee of Animal Experiments of Selcuk University (approval number: 20005-32), and the studies were carried out in accordance with the principles of laboratory animal care of the Veterinary School of Selcuk University. A total of 30 male Sprague-Dawley rats (weighing 300–350 g) were included in the study and were divided equally and randomly into 3 groups. All animals were housed in large cages with three rats per cage, and were individually numbered using ear punches. The cages were kept under standard laboratory conditions (22 ±2°C temperature, 50 ±10% relative humidity, a 12 h light/dark cycle with free access to water and normal rodent food). All animals were acclimated for 1 week prior to the beginning of the study.
The first group constituted the control group in which 10 male rats were given nothing. The second group of 10 male rats was given i.v. ZA (Zometa, Novartis Pharma Stein AG, Switzerland) of 3 mg/kg once every 3 weeks for 12 weeks from the tail vein under i.p. ketamine anesthesia (8 mg/rat) as performed by Pfister et al. [12]. In the third group, rats received the same dosage of ZA with an additional i.m. injection of 15 mg Vit E (α-Tocopherol, Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) every week for 12 weeks. Four days after the last dose of ZA, all rats were anesthetized by i.p. administration of xylazine hydrochloride (10 mg/kg) and ketamine hydrochloride (50 mg/kg) for performing nephrectomy. The rats were sacrificed by an overdose of anesthesia after the operation had been completed.
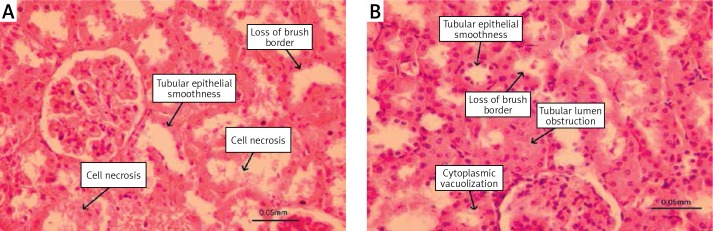
All histopathological examinations under the light microscope were performed by the same pathologist without prior knowledge of the groups after all kidney tissues were fixed in buffered 10% formaldehyde solution for a minimum of 24 h. Fixed tissue samples were processed with an autotechnicon and then embedded in paraffin. A section of 5-μm thickness was prepared with a microtome from all samples, and then was investigated with a light microscope (Nikon Eclipse E400, Nikon Corp., Tokyo, Japan). Histopathologically, tubular epithelial smoothness (tes), loss of brush border (lbb), cytoplasmic vacuolization (cv), tubular lumen obstruction (ob) and cell necrosis (cn) were investigated (Figures 1 A, B).
Figure 1.
A, B. Histopathological changes in rat kidney caused by zoledronic acid (hematoxylin-eosin)
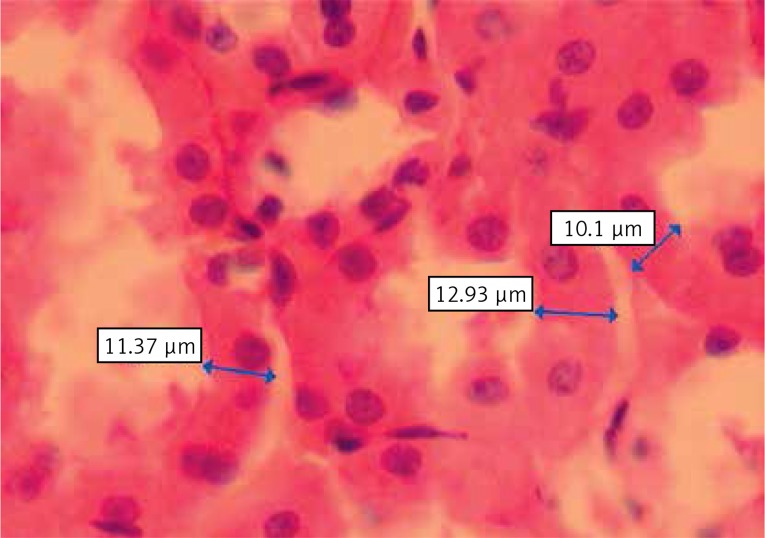
For each specimen, the same area was photographed using a Nikon Coolpix 5000 photograph attachment (Nikon Corp., Tokyo, Japan). A photograph of a Nikon micrometer microscope slide (Stage Micrometer Type A MBM11100, Japan) was also taken during the procedure. All photographs were then transferred into the personal computer environment and analyzed using the Clemex Vision Lite 3.5 Image Analysis program (Clemex Technologies Inc., Quebec, Canada). The length was calibrated by comparing the photograph of the specimen with the photograph of the Nikon micrometer microscope slide, which was taken under the same magnification. Tubular epithelial thickness and tubular lumen enlargement were measured with the Image Analysis system (Figure 2).
Figure 2.
Image of tubular epithelium under light microscopy showing the measurements of tubular epithelium thickness using Clemex Vision Lite 3.5 Image Analysis program (hematoxylin-eosin)
The modified Paller score was calculated according to given points for tubular epithelial smoothness (0,1), loss of brush border (0,1,2), cytoplasmic vacuolization (0,1), tubular lumen obstruction (0,1,2) and cell necrosis (0,1,2), with a minimum value of 0 and a maximum value of 8 [13].
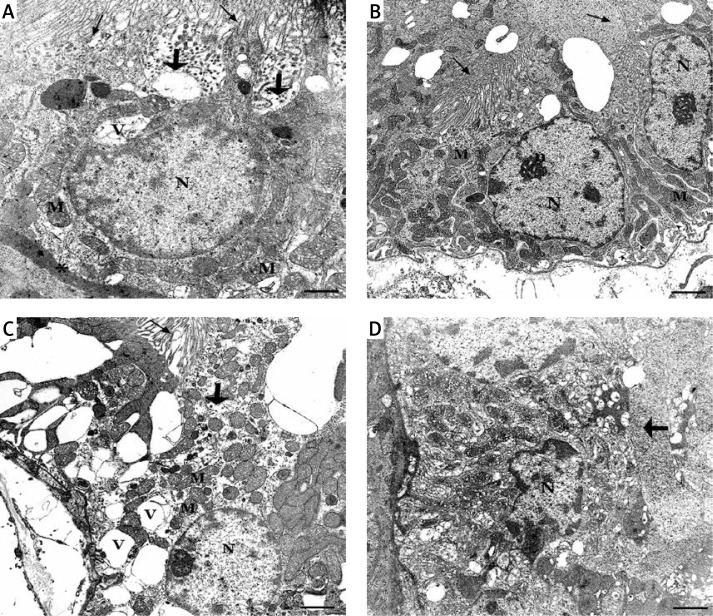
For electron microscopic study, tissues were perfused with 0.2 M phosphate buffer containing 2% (w/v) paraformaldehyde and 2.5% (v/v) glutaraldehyde, and kidneys were removed and immersed in the same fixative for 3 h at 4°C. Samples were rinsed 3 times in 0.1 M phosphate buffer then post-fixed with 1% osmium tetroxide in 0.1 M phosphate buffer for 1 h at room temperature. Following 3 washes in buffer, specimens were dehydrated in graded ethanol and finally embedded in Araldite. One-micrometer semi-thin sections were stained with 1% toluidine blue for light microscopic localization, and ultra-thin sections (60–80 nm) were cut with a Leica Ultracut R microtome and double-stained with uranyl acetate and lead citrate. They were examined and photographed using a LEO 906E electron microscope (80 kV, LEO Electronenmicroskopie GmbH, Oberkochen, Germany) by the same histologist without prior knowledge of the groups (Figure 3).
Figure 3.
A – In group 1 showing healthy nucleus (N), prominent nucleolus (n), preserved apical microvilli (↑), basal infoldings (▲) consisting of elongate mitochondria (M) (uranyl acetate-lead citrate, original mag. 4646×). B – In the ultrastructural image of group 3, shortened proximal convoluted tubule (PCT) epithelium cell, thickened basal lamina (*), dispersal of basal infoldings, partial loss of microvilli (thick arrow) and intracytoplasmic vacuole (V) (uranyl acetate-lead citrate, original mag. 7750×). C – In group 2, a swelling cell that is characterized by single cell necrosis with dispersed basal infoldings and mitochondria, large intracytoplasmic vacuoles, complete loss of microvilli (uranyl acetate-lead citrate, original mag. 4646×). D – An apoptotic cell in group 2, PCT epithelium with typical nucleus which has heterochromatin clumping under nucleolemma (uranyl acetate-lead citrate, original mag. 6000×)
Statistical analysis
Conformity of the data to a normal distribution was assessed with the Shapiro-Wilk test. While the tubular epithelial thickness parameter conformed to a normal distribution, the other parameters did not. The descriptive statistics were presented as mean ± standard deviation (SD) and median. For the comparison of groups, one-way ANOVA and Bonferroni post-hoc tests were used for epithelial thickness, while Kruskal-Wallis variant analysis and Mann-Whitney U test with Bonferroni correction were used for the other parameters. All statistical analysis tests were performed with the Statistical Package for the Social Sciences (SPSS) for Windows version 15.0 (SPSS Inc., Chicago, Illinois, USA). A p-value < 0.05 was considered statistically significant.
Results
Of the 10 rats in each group, 9 rats in group 1 (control) and group 2 (ZA), and 10 rats in group 3 (ZA + Vit E) survived until the nephrectomy. The modified Paller score, epithelial thickness and basal membrane thickness of the rats in each group are shown in Table I. According to the Paller score, group 2 had the highest and group 1 had the lowest value. Differences between groups 1 and 2 (Z = 3.587, p < 0.001), groups 1 and 3 (Z = 3.440, p < 0.001), and groups 2 and 3 (Z = 3.243, p < 0.001) were statistically significant. Group 2 had the highest value for epithelial thickness, and its difference between groups 1 and 3 was statistically significant (p < 0.001). Although group 1 had lower values than group 3, there was no statistically significant difference (p = 0.996). Group 2 also had thicker basal membrane compared to group 1 (Z = 3.587, p < 0.001) and to group 3 (Z = 2.645, p = 0.001), with a significant difference. Basal membrane thickness was significantly higher in group 3 than group 1 (Z = 2.713, p < 0.001).
Table I.
Paller score, tubular epithelial thickness and basal membrane thickness of rats in each group
| Group | Parameter | Mean | SD | Median | Min. | Max. |
|---|---|---|---|---|---|---|
| Group 1 (n = 9) |
Paller score | 0.50 | 0.53 | 0.50 | 0.00 | 1.00 |
| Epithelial length | 11.30 | 0.72 | 11.45 | 10.30 | 12.10 | |
| Basal membrane thickness | 0.38 | 0.52 | 0.00 | 0.00 | 1.00 | |
| Group 2 (n = 9) |
Paller score | 5.78 | 0.83 | 6.00 | 4.00 | 7.00 |
| Epithelial length | 13.66 | 1.01 | 13.50 | 12.10 | 15.30 | |
| Basal membrane thickness | 2.11 | 0.60 | 2.00 | 1.00 | 3.00 | |
| Group 3 (n = 10) |
Paller score | 3.50 | 1.07 | 4.00 | 2.00 | 5.00 |
| Epithelial length | 11.34 | 1.01 | 11.55 | 10.10 | 13.10 | |
| Basal membrane thickness | 1.25 | 0.46 | 1.00 | 1.00 | 2.00 |
SD – standard deviation, Min. – minimum value, Max. – maximum value.
In the ultrastructural examinations of the groups, major histopathological changes were observed in proximal convoluted tubules (PCT). Degeneration of the PCT was characterized by total/partial loss of the long and straight microvilli, cell swelling and vacuolization, and occlusion of the tubular lumen. When compared to group 3, flattened epithelial cells with flattened nuclei and single cell necrosis of the PCT were markedly detected in group 2. Cell death by apoptosis was also seen in group 2. Tubular atrophy with minimal to moderate thickening of basal lamina was observed in both groups 2 and 3. Basal membrane infoldings, and long and narrow mitochondria, which were settled between them, were dispersed. Large intracytoplasmic vacuolizations, loss of microvilli and occlusion of PCT lumens were severe pathologic findings in group 2 (Figure 3).
These results show that administration of ZA caused an increase in kidney Paller score, epithelial thickness and basal lamina thickness. However, adding Vit E to ZA administration reversed all the histopathological changes to some degree, with statistical significance.
Discussion
Prostate and breast cancers, the most frequent cancers in males and females respectively, have a tendency to metastasize to bones in advanced stages [14]. Despite the general poor prognosis of metastatic prostate and breast cancer, new drugs – such as docetaxel, abiraterone, enzalutamide, and trastuzumab – that have potential to improve overall survival have been emerging recently [15, 16]. However, palliative and supportive treatments that increase the quality of life still play an important role in the prevention and/or the management of the systemic toxicities caused by these drugs [16, 17].
Bisphosphonates are non-hydrolyzed, organic pyrophosphate analogues that have an effect on bone metabolism in humans [18]. The majority of the bisphosphonates (40–60%) are rapidly bound to bone with high affinity after they reach the systemic circulation [19]. They are deposited in areas of bone remodeling (formation and destruction), and the skeletal uptake depends on bone turnover [18]. The remainder of the bisphosphonates are not metabolized and are eliminated unchanged by the kidneys through glomerular filtration and active tubular excretion [6].
Randomized and controlled studies have shown that i.v. administration of bisphosphonates with standard anticancer treatments has reduced skeletal complications significantly [3–5]. These drugs are not recommended for patients with severe renal failure (especially patients with a creatinine clearance < 30 ml/min) [20].
Nephrotoxicity is a well-known side effect of bisphosphonates, and renal failure after rapid i.v. administration has been observed in both animals and humans [21–23]. In animal studies, reduced excretion and high concentration of bisphosphonates have been associated with proteinuria and proximal tubular necrosis [20, 24]. Although it has been suggested that the mechanism of bisphosphonate-induced renal toxicity is related to bisphosphonate aggregation and calcium complexes in the kidney [19, 25], no corpuscular precipitations have been observed in histopathological studies in animals [12, 23, 24, 26, 27]. Therefore, it was concluded that the mechanism of bisphosphonate-induced renal toxicity is probably associated with the bisphosphonates’ induction of cell death in renal tubular cells, which is similar to their apoptotic effects in osteoclasts [26, 27].
Given that the pharmacodynamics of bisphosphonates have not been determined in rodents and there are different dosages and/or routes used in rat experiments, we determined the dosing according to the factors mentioned by Kim et al. [28], and the experimental setting by Pfister et al. [12]. It was reported that the factors including the oncologically relevant ZA doses in humans (which is 67 μg/kg/4 weeks), the relatively rapid bone metabolism of rodents, the lower plasma concentrations when using the subcutaneous route compared to the i.v. route, and maximizing drug exposure during the relatively short experimental period had an influence in determining the dosage of ZA in rats [28].
Markowitz et al. observed loss of brush border, hypereosinophilia, tubular degeneration characterized by luminal ectasia, tubular atrophy, interstitial fibrosis and inflammation in 6 patients with renal failure after ZA administration [23]. These severe tubular degenerative changes were confirmed with electron microscopy where epithelial simplification with reduced organellar content, cellular detachment from tubular basement membrane, apical blebbing, widened intercellular spaces, individual cell necrosis and focal shedding of cytoplasmic debris into the tubular lumen were observed in proximal tubules [23].
Pfister et al. studied the renal effects of i.v. administration of 1 mg/kg ibandronate and 1 or 3 mg/kg zoledronate with an interval of 3 weeks for a total of 25 weeks, and they observed degeneration of subcortical and cortical structures, single cell necrosis, loss of brush border and cytoplasmic swelling in 60% of the PCT [12]. Furthermore, they recorded basal membrane thickening and tubular atrophy in the medulla, and hypertrophy and hyperplasia in the distal tubules and collecting ducts in the inner medulla [12]. In another paper published by Pfister et al. in 2005, in which dose-finding and comparative studies as well as an electron microscopic study were performed, similar findings consistent with their previous study were observed, and the authors concluded that 3 mg/kg was the least nephrotoxic dose of ZA in rats, and maximal tissue concentration was achieved on the 4th day after i.v. administration [26].
Lühe et al. provided the first experimental evidence that inhibition of farnesyl diphosphate synthase with nitrogen-containing bisphosphonates, the mechanism also responsible for inhibition of osteoclasts, might cause the reduction in renal cell viability in vitro and is likely to contribute to in vivo nephrotoxicity with these drugs [29].
By applying the same experimental conditions of Pfister et al. [12, 26], we achieved a nephrotoxicity parallel to their study with a significant increase in Paller score, epithelial length and basal membrane thickness in light microscopy, and defined ultrastructural changes in electron microscopy, which in total prove the nephrotoxicity of ZA. Moreover, we examined the preventability of these nephrotoxic effects by administering a powerful antioxidant agent, Vit E, to one of the groups in accordance with the observation of Dillioglugil et al. [30]. They studied the efficacy of Vit E to prevent the negative effects of cisplatin, a well-known nephrotoxic chemotherapeutic agent, and observed that administration of Vit E statistically significantly decreased nephrotoxicity [30]. Parallel to their results, we also found that Vit E was efficacious to prevent the nephrotoxic effects of ZA. Similar results have been reported for Vit E in preventing the nephrotoxicity in rats induced by gentamicin [31], aluminum [32], colistin [33] and mercuric chloride [34].
To the best of our knowledge, our study is the first in the literature showing the preventive effects of Vit E in the nephrotoxicity induced by ZA. Nevertheless, it has some limitations that should be listed. Firstly, although 10 rats were included in each group, 1 or 2 rats from all groups could not have survived until the end of the experiment. This led to a decrease in total rat number at the end of the treatment, which may have affected the statistical analysis. Secondly, although the setting and the results of this study were consistent with the previous papers published, we still need to test these results in humans, as these nephrotoxic effects may be different in human kidneys.
In conclusion, in this experimental study, we found that concomitant administration of Vit E significantly reduces the toxic histopathological effects of ZA in rat kidney. With an appropriate dose and treatment schema, ZA is efficacious in patients with bone metastases. However, as this agent has nephrotoxic potential, all patients receiving ZA should be closely monitored throughout the treatment, and concurrent administration of Vit E should be kept in mind to minimize the potential toxicity to kidneys. Further studies are required to evaluate this protective effect in human kidneys.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Patrick DL, Cleeland CS, von Moos R, et al. Pain outcomes in patients with bone metastases from advanced cancer: assessment and management with bone-targeting agents. Support Care Cancer. 2015;23:1157–68. doi: 10.1007/s00520-014-2525-4. [DOI] [PubMed] [Google Scholar]
- 2.Bergner R, Diel IJ, Henrich D, Hoffman M, Uppenkamp M. Differences in nephrotoxicity of intravenous bisphosphonates for the treatment of malignancy-related bone disease. Onkologie. 2006;29:534–40. doi: 10.1159/000096056. [DOI] [PubMed] [Google Scholar]
- 3.Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (Alliance) J Clin Oncol. 2014;10:1143–50. doi: 10.1200/JCO.2013.51.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–32. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 5.Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25:820–8. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- 6.Green JR, Seltenmeyer Y, Jaeggi KA, Widler L. Renal tolerability profile of novel, potent bisphosphonates in two short-term rat models. Pharmacol Toxicol. 1997;80:225–30. doi: 10.1111/j.1600-0773.1997.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 7.Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91:1191–200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Reid IR, Brown JP, Burckhardt P, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–61. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 9.Herrera E, Barbas C. Vitamin E: action, metabolism and perspectives. J Physiol Biochem. 2001;57:43–56. [PubMed] [Google Scholar]
- 10.Haleem NYA, El-Aasar HM, Zaki SM, Sabry SM, El-Zainy AW. Concomitant protective and therapeutic role of verapamil in chronic mercury induced nephrotoxicity in the adult rat: histological, morphometric and ultrastructural study. Arch Med Sci. 2015;11:199–209. doi: 10.5114/aoms.2013.37342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun M, Venkataraman V, Razavian M, et al. Antioxidants for chronic kidney disease. Cochrane Database Syst Rev. 2012;10:CD008176. doi: 10.1002/14651858.CD008176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfister T, Atzpodien E, Bauss F. The renal effects of minimally nephrotoxic doses of ibandronate and zoledronate following single and intermittent intravenous administration in rats. Toxicology. 2003;191:159–67. doi: 10.1016/s0300-483x(03)00257-9. [DOI] [PubMed] [Google Scholar]
- 13.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–64. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 15.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James ND, Sydes MR, Mason MD, et al. Celecoxib plus hormone therapy versus hormone therapy alone for hormone-sensitive prostate cancer: first results from the STAMPEDE multiarm, multistage, randomized controlled trial. Lancet Oncol. 2012;13:549–58. doi: 10.1016/S1470-2045(12)70088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huszno J, Badora A, Nowara E. The influence of steroid receptor status on the cardiotoxicity risk in HER2-positive breast cancer patients receiving trastuzumab. Arch Med Sci. 2015;11:371–7. doi: 10.5114/aoms.2015.50969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu XL, Gou WL, Wang AY, et al. Basic research and clinical applications of bisphosphonates in bone disease: what have we learned over the last 40 years? J Transl Med. 2013;11:303. doi: 10.1186/1479-5876-11-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleisch H. Bisphosphonates in bone disease: from the laboratory to the patient. 4th ed. New York: Parthenon Publishing Group Ltd; 2000. pp. 1–212. [Google Scholar]
- 20.McKay RR, Taplin ME, Choueiri TK. Optimizing bone health and minimizing skeletal morbidity in men with prostate cancer. Hematol Oncol Clin North Am. 2013;27:1261–83. doi: 10.1016/j.hoc.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cal JC, Daley-Yates PT. Disposition and nephrotoxicity of 3-amino-1-hydroxypropylidene-1, bisphosphonate (APD), in rats and mice. Toxicology. 1990;65:179–97. doi: 10.1016/0300-483x(90)90088-x. [DOI] [PubMed] [Google Scholar]
- 22.Okazaki A, Sakai H, Matsuzawa T, Perkin CJ, East PW. Intravenous single and repeated dose toxicity studies of cimadronate (YM175), a novel bisphosphonate, in rats. J Toxicol Sci. 1995;20(Suppl 1):15–26. doi: 10.2131/jts.20.supplementi_15. [DOI] [PubMed] [Google Scholar]
- 23.Markowitz GS, Fine PL, Stack JI, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa) Kidney Int. 2003;64:281–9. doi: 10.1046/j.1523-1755.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 24.Alden CL, Parker RD, Eastman DF. Development of an acute model for the study of chloromethanediphosphonate nephrotoxicity. Toxicol Pathol. 1989;17:27–32. doi: 10.1177/01926233890171P104. [DOI] [PubMed] [Google Scholar]
- 25.Zojer N, Keck AV, Pecherstorfer M. Comparative tolerability of drug therapies for hypercalcemia of malignancy. Drug Saf. 1999;21:389–406. doi: 10.2165/00002018-199921050-00004. [DOI] [PubMed] [Google Scholar]
- 26.Pfister T, Atzpodien E, Bohrmann B, Bauss F. Acute renal effects of three intravenous bisphosphonates in the rat. Basic Clin Pharmacol Toxicol. 2005;97:374–81. doi: 10.1111/j.1742-7843.2005.pto_160.x. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee D, Asif A, Striker L, Preston RA, Bourgoignie JJ, Roth D. Short-term, high-dose pamidronate-induced acute tubular necrosis: the postulated mechanism of bisphosphonate nephrotoxicity. Am J Kidney Dis. 2003;41:E18. [PubMed] [Google Scholar]
- 28.Kim JW, Tatad JCI, Landayan MEA, Kim SJ, Kim MR. Animal model for medication-released osteonecrosis of the jaw with precedent metabolic bone disease. Bone. 2015;81:442–8. doi: 10.1016/j.bone.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Lühe A, Künkele KP, Haiker M, et al. Preclinical evidence for nitrogen-containing bisphosphonate inhibition of farnesyl diphosphate (FPP) synthase in the kidney: implications for renal safety. Toxicol Vitro. 2008;22:899–909. doi: 10.1016/j.tiv.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Dillioglugil MO, Maral Kir H, Gulkac MD, et al. Protective effects of increasing vitamin E and a doses on cisplatin-induced oxidative damage to kidney tissue in rats. Urol Int. 2005;75:340–4. doi: 10.1159/000089171. [DOI] [PubMed] [Google Scholar]
- 31.Patel Manali B, Deshpande S, Shah G. Evaluation of efficacy of vitamin E and N-acetyl cysteine in gentamicin-induced nephrotoxicity in rats. Ren Fail. 2011;33:341–7. doi: 10.3109/0886022X.2011.560987. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Hamid GA. Effect of vitamin E and selenium against aluminum-induced nephrotoxicity in pregnant rats. Folia Histochem Cytobiol. 2013;51:312–9. doi: 10.5603/FHC.2013.0042. [DOI] [PubMed] [Google Scholar]
- 33.Ghlissi Z, Hakim A, Sila A, et al. Evaluation of efficacy of natural astaxanthin and vitamin E in prevention of colistin-induced nephrotoxicity in the rat model. Environ Toxicol Pharmacol. 2014;37:960–6. doi: 10.1016/j.etap.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Aslanturk A, Uzunhisarcikli M, Kalender S, Demir F. Sodium selenite and vitamin E in preventing mercuric chloride induced renal toxicity in rats. Food Chem Toxicol. 2014;70:185–90. doi: 10.1016/j.fct.2014.05.010. [DOI] [PubMed] [Google Scholar]