Abstract
Introduction
The aim was to devise an animal model showing some of the neuropathological changes seen in senile dementia, and to investigate the effect of celastrol on cognition neuropathology in this model.
Material and methods
Forty male Sprague Dawley rats weighing 300–350 g were randomly divided into 5 groups (n = 8 each): control (Con); inhaled sevoflurane (Sev); diabetes mellitus (DM); diabetes mellitus + inhaled sevoflurane (DM/Sev); diabetes + inhaled sevoflurane + celastrol (Cel). Diabetes was induced by an intraperitoneal injection of streptozotocin (STZ). After 20 days, the Sev, DM/Sev and Cel group rats inhaled 3% sevoflurane for 2 h, while the control and DM groups inhaled air. Cel group rats were given intraperitoneal injections of celastrol (0.7 mg/kg) daily for 4 days, while the control group received intraperitoneal injections of an equal volume of dimethylsulfoxide. The Morris water maze test was performed to test cognition. Animals were killed after the last water maze test and Congo red staining was used to observe deposition of amyloid substance in the hippocampus. The expression of GFAP and IGF-1 in the hippocampus was observed by immunohistochemistry.
Results
Diabetes decreased cognition, increased amyloid substance and GFAP expression, and decreased IGF-1 expression in the hippocampus (all p-values < 0.05). Sevoflurane administration intensified and celastrol decreased these changes (all p-values < 0.05).
Conclusions
Sev/DM rats showed cognitive and neurochemical changes similar to those seen in senile dementia. Celastrol decreased these changes and should be evaluated further as a possible clinical agent in dementia.
Keywords: diabetic, sevoflurane, celastrol, Morris water maze, hippocampus, GFAP, IGF-1
Introduction
The neurodegeneration, memory loss, and decrease in cognition seen during aging are thought to be due, at least in part, to neuroinflammation, reactive oxygen species, and in Alzheimer’s disease (AD) to the accumulation of β amyloid protein [1, 2].
Diabetes mellitus is a common clinical condition, and has been associated in a epidemiological study with an increased risk of AD [3]. The inhalation anesthetic sevoflurane has been shown to decrease cognition when given to neonatal rats [4] and to increase β amyloid protein levels in human neuroglioma cells and in mice [5]. Administering sevoflurane to adult rats with diabetes decreases cognition in a manner exhibiting some of the characteristics (neuroinflammation and increased β amyloid protein) seen in the decreased cognition of aging, but provides a potential contrast to the characteristics seen in animal models of aging or AD.
Celastrol is a naturally occurring compound separated from the root bark of Tripterygium wilfordii. It has antioxidant and anti-inflammatory activities, and has been suggested to have potential as a treatment for AD [6, 7]. However, although a number of cell and animal studies of the biochemical effects of celastrol that are related to prevention of neurodegeneration have been performed [6–8], a study of both biochemistry and prevention of cognitive decline in an animal model with neurodegenerative conditions similar to those seen in aging has not been done. Administering celastrol to sevoflurane-treated diabetic rats might enable us to evaluate the effect of celastrol in vivo on both a decrease in cognition and the accompanying cellular events.
In the present study, intraperitoneal injection of streptozotocin (STZ) was performed establish a diabetes mellitus (DM) model in rats that were then subjected to a period of sevoflurane inhalation. Their post-anesthesia cognition, brain histology, and brain biochemistry were observed, and the influence of celastrol on these parameters was investigated. It was intended as a preliminary study to find a short-term acute model with Alzheimer’s-like physiological changes that might be expanded in the future to a chronic model of these changes using aged animals.
Material and methods
Animals
Healthy male Sprague Dawley rats (n = 40) aged 10 weeks and weighing 300–400 g were purchased from the Medical Experimental Animal Center of Guangdong Province. Young, rather than elderly rats were used because the influence of aging on the brain is hard to differentiate from that of DM, and both conditions may cause damage to the brain. Animals were housed in a specific pathogen-free environment (humidity 40–70%; good ventilation: constant temperature: 21–24°C; 12 h light-dark cycle) of the Medical Experimental Center of Guangzhou University of Chinese Medicine, and fed with standardized sterile chow and water. This study was approved by the Institutional Animal Care and Use Committees of our institute.
Grouping and establishment of DM model
Rats were randomly assigned to the following groups (n = 8 per group): control (Con), sevoflurane (Sev), DM (DM), DM + sevoflurane (DM/Sev), DM + sevoflurane + celastrol (Cel). In the 3 diabetes groups (DM, DM/Sev, and Cel), rats were given intraperitoneal injections of 10% STZ (1 mg/ml; S0130; Sigma, USA) in citrate buffer at 60 mg/kg to induce DM [9, 10]. The preparation and injection of STZ were done under aseptic conditions. The proficient injection procedure avoided accidental injection into the intestine. Rectal temperature was measured daily and features of sepsis were not observed in these rats. Three days later and daily afterward, blood was collected from the tail vein and blood glucose was measured. Intake of water and food, urine volume, body weight, and general condition were also monitored. When the blood glucose was ≥ 16.7 mmol/l and rats had developed symptoms of DM (such as polydipsia, polyphagia, less activity, dry hair, and weight loss), DM was confirmed. In the 2 remaining groups, rats were given intraperitoneal injections of an equal volume of citrate buffer.
Twenty days after establishment of the DM model, the inhalation procedure was begun. By 20 days after induction of DM, the DM-induced damage becomes stable and obvious. Brain injury may not be evident and blood glucose levels may be unstable or even return to normal during the early period after STZ injection. If the time allowed to elapse before beginning the inhalation procedure is too long, some diabetic rats may die of unknown causes or die during the exposure to sevoflurane. For these reasons and on the basis of findings from pilot study data reported by others [9, 11], we began the inhalation procedure 20 days after intraperitoneal (IP) injection of STZ.
We used 3% sevoflurane for inhalation, according to previous studies [12–14]. This is a low concentration from a clinical point of view, but the diabetic rats are weak and unable to tolerate high concentrations of sevoflurane. The inhalation period of 2 h approximated the median time period seen in clinical surgery. Longer exposure in these weak diabetic rats can cause high mortality.
Anesthesia with sevoflurane
Twenty days after establishment of the DM model, rats were placed in a Plexiglas box (40 cm × 30 cm × 15 cm) for the inhalation procedure. In the Sev, DM/Sev, and cel groups, rats inhaled 3.0% sevoflurane (Maruishi Pharmaceutical Co., Ltd, Osaka, Japan), for 2 h at 1.5 l/min. In the Con and DM groups, rats inhaled air while in the box for the same time period. A multifunction monitor (Datex-Ohmeda, Madison, WI, USA) was used to monitor the concentration of sevoflurane in the box. In rats, the minimum alveolar concentration (MAC) for sevoflurane is about 2.3% [15]. In clinical anesthesia, the 99% effective concentration (ED99) is about 1.3 MAC, and 2.3–3.5% sevoflurane has no influence on the cardiovascular function and blood perfusion in major organs of rats [12]. Thus, in the present study, 3.0% sevoflurane was used.
Drug administration
In the cel group, rats were given intraperitoneal injections of celastrol beginning on the day before sevoflurane anesthesia. In the other groups, rats were given intraperitoneal injections of an equal volume of 0.5% dimethylsulfoxide (DMSO) in the same manner.
Cognition testing
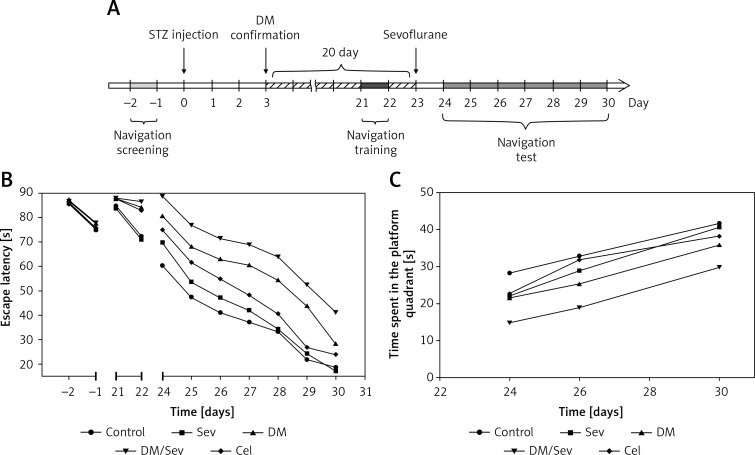
Figure 1 A shows the experimental schedule for cognition testing. During the 7 days after sevoflurane anesthesia, the place navigation and spatial exploration portions of the Morris water maze test (Feidi Biotech Co., Ltd, Guangzhou, China) were performed to evaluate treatment differences in cognition [16–18]. To provide baseline data, cognition was also evaluated 2 days before diabetes induction and 2 days before sevoflurane anesthesia.
Figure 1.
Morris water maze test. A – Experimental schedule from 2 days before streptozotocin (STZ) injection and diabetes mellitus (DM) induction to 30 days after DM induction. B – Escape latency in Morris water maze test between groups across days of test in rats. C – Time spent in the platform quadrant in Morris water maze test between groups across days of test in rats. N = 8 per group
Place navigation test
In this test, used daily as a test of learning ability, a transparent escape platform was placed underwater in the second quadrant of a circular swimming pool. Rats were placed in quadrants from I to IV and the time taken to find the platform (escape latency) was recorded. If the rats did not find the platform within 90 s, the escape latency was recorded as 90 s, and rats were placed on the platform and allowed to stay there for 20 s to familiarize themselves with the location before continuing with the testing. The mean escape latency was calculated after 4 tests each day during days 1 to 7 after sevoflurane administration.
Spatial exploration test
This test, performed to evaluate the rat’s memory of the previous location of the escape platform, was performed on days 1, 3, and 7. For this test, the hidden platform was removed, rats were placed in any quadrant, and the time in the next 90 s spent swimming in the quadrant where the platform had been located was recorded.
Sample collection, staining, and immunohistochemistry
Sample collection
On the day of the last water maze test, rats were intraperitoneally anesthetized with 10% chloral hydrate. After thoracotomy, a tube was inserted through the left ventricle to the ascending aorta. The right atrial appendage was opened, and 0.01 mol/l phosphate-buffered saline (PBS) was injected for flushing, followed by injection of 4% paraformaldehyde. Two hours later, the brain was collected and fixed in 4% paraformaldehyde for further immunohistochemistry.
Aβ staining
Congo red staining was used to observe the deposition of Aβ in the hippocampus. The brain was taken out of 4% paraformaldehyde, embedded in paraffin, sectioned, deparaffinized and hydrated, followed by hematoxylin staining for 2 min. Sections were treated with 0.5% hydrochloric acid in ethanol for 2 s, washed in water and stained with Congo red for 25 min. After dehydration in absolute ethanol twice, sections were made transparent with xylene, and then mounted and dried.
Immunohistochemistry for GFAP and IGF-1
Brain sections were prepared, deparaffinized and hydrated. Sections were then placed in PBS (pH = 6.0) and heated at 92°C for 15 min in a microwave oven and allowed to cool to room temperature. After washing in PBS (pH = 7.2) 3 times (2 min for each), one section was subjected to HE staining. Remaining sections were treated with primary antibody (GFAP: 1 : 200; IGF-1: 1 : 200) at 4°C overnight. Rabbit anti-rat GFAP antibody (bs-0199R; Bioss Biotech Co., Ltd, Beijing, China) and rabbit anti-rat IGF-1 antibody (bs-0014R; Bioss Biotech Co., Ltd) were used.
After incubation with primary antibody and washing in PBS three times (2 min for each), sections were treated with secondary antibody at room temperature for 20 min. After washing in PBS three times (2 min for each), sections were subjected to 3,3′-diaminobenzidine (DAB) staining (2–5 min). Counterstaining was done with Mayer’s hematoxylin, followed by dehydration, transparentization and mounting. Three sections were randomly selected from each rat and 3 fields were randomly selected from each section. Positive cells were counted at a magnification of 400 and averages were obtained.
Statistical analysis
Data are presented as mean and standard deviation (SD) according to groups and days of test. Since the Morris water maze tests for each rat were performed repeatedly, two-way repeated measures analysis of variance (rANOVA) was used to examine differences between the five groups in escape latency and time spent in the platform quadrant. Post hoc multiple comparisons with Bonferroni correction were made to identify whether pair-wise differences between any two groups existed. Comparisons of GFAP and IGF-1 expression between groups were performed by one-way analysis of variance. Post hoc pair-wise comparisons were also made with Bonferroni correction. Post hoc power analyses were performed for the Morris water test, Aβ deposit biochemistry, and GFAP and IGF1 expression. Given the mean, standard deviation, and sample size for each of these outcomes, the estimated power for each outcome was 0.8, which was adequate to detect the group differences that were seen.
All statistical analyses were carried out with IBM SPSS statistical software version 22 for Windows (IBM Corp., Armonk, NY, USA). A two-tailed p-value less than 0.05 was considered significant.
Results
Cognition
Time course of learning
Baseline data showed place location learning to be similar in all groups in the testing performed before diabetes induction, but poorer in the 3 diabetic groups than in the other 2 groups in the testing performed before sevoflurane anesthesia (Figure 1 B). In all groups, both place location learning and place location memory improved with time during the 7 days of post-sevoflurane testing (Figures 1 B and C). Both a decreasing time trend in escape latency (F(6,210) = 269.49, p < 0.001) and an increasing time trend in time spent in the platform quadrant (F(2,70) = 71.74, p < 0.001) were seen.
Place location learning
Control rats and control rats treated with sevoflurane learned the location of the hidden escape platform at the same rate. It took diabetic rats a longer time to learn this location and diabetic rats treated with sevoflurane a longer time still. Treatment with celastrol decreased the learning time of sevoflurane-treated diabetic rats toward control.
After consideration of repeated measurements, significant differences between treatment groups were seen in mean escape latency time (F(4, 35) = 77.06, p < 0.001). Post hoc multiple comparisons showed that escape latency time over the entire experiment period was longer in the 3 diabetic groups (DM, DM/Sev and Cel) than in the control group (all p < 0.001). Celastrol-treated diabetic rats anesthetized with sevoflurane had a decreased time of escape latency compared to diabetic rats anesthetized with sevoflurane who were not given celastrol (p < 0.001), the results suggesting efficacy of celastrol in lessening the increase in post-sevoflurane escape latencies seen in diabetic rats. Sevoflurane did not alter escape latencies when administered to non-diabetic rats (Sev vs. Con, Figure 1 B).
Place memory
In searching for the absent escape platform, diabetic rats spent less time than controls searching in the correct quadrant, and administration of sevoflurane to diabetic rats decreased still further the amount of time spent searching in the quadrant where the escape platform had been. Administration of celastrol to the sevoflurane-treated diabetic rats increased the amount of time searching in the correct quadrant compared to that of control rats (Figure 1 C).
As shown in Figure 1 C, significant group differences were seen in time spent in the platform quadrant in the Morris water maze test (F(4, 35) = 14.92, p < 0.001). Rats in both the DM and the DM/Sev groups spent a shorter time in the platform quadrant over the entire experimental period than rats in the control group (DM vs. Con: p = 0.009; DM/Sev vs. Con: p < 0.001). The time spent in the platform quadrant was longer in the Cel group compared to the DM/Sev group (p < 0.001). Furthermore, on the third day of the Morris water maze test, differences in time spent searching in the platform quadrant became significant between DM/Sev and Cel groups (p = 0.138, p = 0.002 and p = 0.042 for the first, third and seventh day of the test). No significant difference was found between Sev and control groups at any time (Figure 1 B).
Histology and staining of the CA1 area of the hippocampus
Histology
Diabetes mellitus rats showed a reduced and disordered neuron arrangement in the CA1 region of the hippocampus that was worsened after sevoflurane administration and improved when celastrol was also administered (Figure 2).
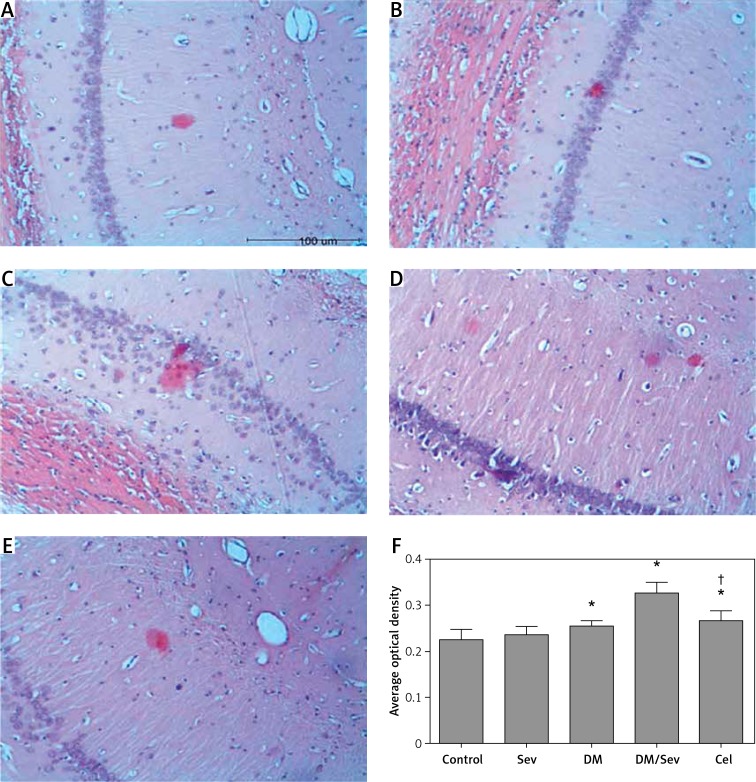
Figure 2.
Representative images of HE staining of CA1 region of hippocampus in different groups: A – control group (Con), B – DM group, C – sevoflurane group (Sev), D – DM + sevoflurane group (DM/ Sev), E – DM + sevoflurane + celastrol group (Cel)
In non-diabetic rats (Con and Sev groups) the morphology and structure of hippocampal neurons in the CA1 region were normal, neurons showed regular arrangement and even distribution, neurons were round or oval and had a clear borderline, the cytoplasm of neurons was dark-red, their nuclei were blue, large and round and had a clear nucleolus, and abnormal cells were not observed. In the DM group, neurons in this region showed reduced and disordered arrangement, the cell membrane showed shrinkage or the cell body showed swelling, cytoplasm was dark-stained, and nuclei were large and showed unclear structure. In the DM/Sev group, degenerative and disordered neurons increased as compared to the DM group. The degenerative, disordered cells were reduced in the Cel group when compared with the DM/Sev group (Figure 2).
Aβ deposition
Aβ deposition in the CA1 region of the hippocampus was increased in diabetic rats and further increased when sevoflurane was administered to these rats. Administration of celastrol decreased Aβ deposition (Figure 3).
Figure 3.
Histochemistry of Aβ deposits in CA1 region of hippocampus: A – control group (Con), B – DM group, C – sevoflurane group (Sev), D – DM + sevoflurane group (DM/Sev), E – DM + sevoflurane + celastrol group (Cel), F – expression of Aβ deposits by treatment group. N = 8 per group
Data are presented as mean ± standard deviation; *p < 0.05 as compared with control group, †p < 0.05 as compared with DM/Sev group.
Congo red staining showed a small amount of orange Aβ deposits in the hippocampus of the Con and Sev groups. In the DM and DM/Sev groups, the orange Aβ deposits in the hippocampus increased significantly, especially in the DM/Sev group. The orange Aβ deposits in the cel group were reduced when compared with the DM/Sev group. Figure 3 F shows these results in quantitative form, as average optical density.
GFAP expression
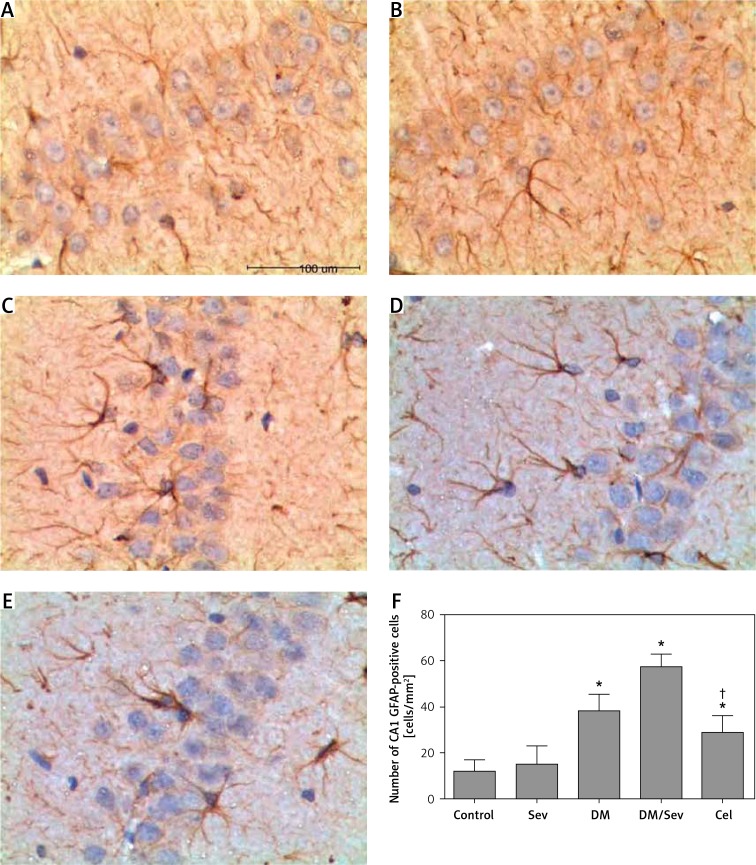
GFAP expression was increased in diabetic rats and further increased when sevoflurane was administered (Figure 4). Sevoflurane did not increase GFAP expression in normal rats, and celastrol administration to DM/Sev rats brought GFAP expression down toward levels seen in non-diabetic rats.
Figure 4.
Immunohistochemistry for GFAP in CA1 region of hippocampus of different groups: A – control group (Con), B – DM group, C – sevoflurane group (Sev), D – DM + sevoflurane group (DM/Sev), E – DM + sevoflurane + celastrol group (Cel), F – expression of GFAP in hippocampus of rats by treatment group. N = 8 per group
Data are presented as mean ± standard deviation; *p < 0.05 as compared with control group, †p < 0.05 as compared with DM/Sev group.
In DM and DM/Sev groups, astrocytes in the hippocampus increased, their cell body was enlarged, and the projections curled and became thickened (Figures 4 A–E). There were differences in GFAP expression among treatment groups (F(4, 35) = 62.94, p < 0.001). Expression of GFAP was higher in the DM, DM/Sev and Cel groups than in the control group, respectively (38.5 ±7.1 cells/mm2 in DM group, 57.6 ±5.5 cells/mm2 in DM/Sev group and 28.9 ±7.4 cells/mm2 in Cel group vs. 12.2 ±4.8 cells/mm2 in control group). No significant difference was found between control and Sev groups. Rats in the Cel group had fewer GFAP-positive cells than rats in the DM/Sev group (p < 0.05) (Figure 4 F).
IGF-1 expression
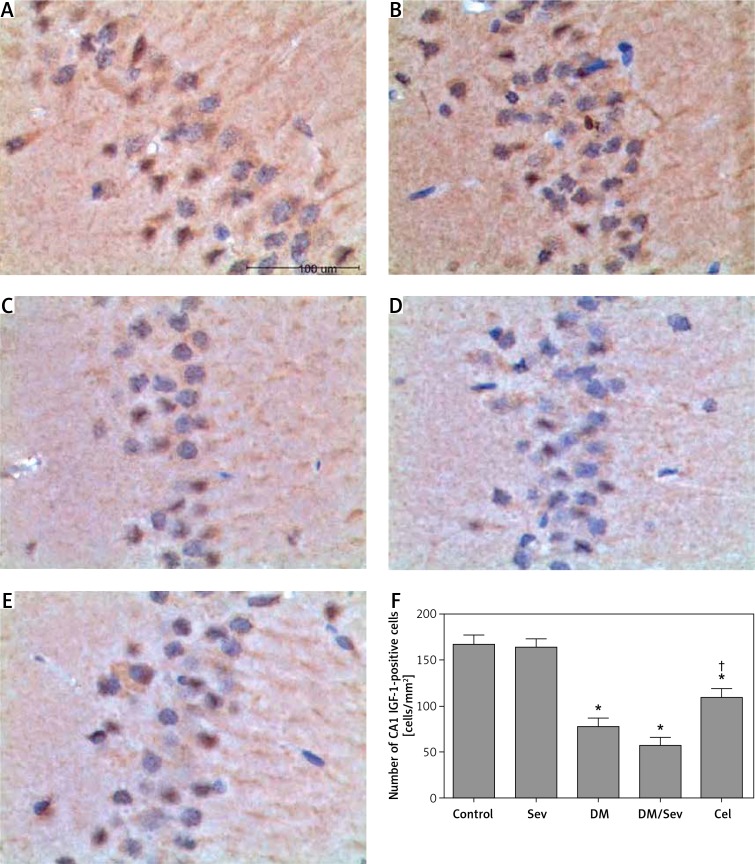
IGF-1 levels were decreased in diabetic rats, and decreased still further when sevoflurane was given (Figure 5). Sevoflurane administration did not affect IGF-1 levels in normal rats, and celastrol given to DM/Sev rats increased IGF-1 levels toward normal.
Figure 5.
Immunohistochemistry for IGF-1 in CA1 region of hippocampus of different groups: A – control group (Con), B – DM group, C – sevoflurane group (Sev), D – DM + sevoflurane group (DM/Sev), E – DM + sevoflurane + celastrol group (Cel), F – expression of IGF-1 in hippocampus of rats by treatment group. N = 8 per group
*p < 0.05 as compared with control group, †p < 0.05 as compared with DM/Sev group.
As shown in Figures 5 A–E, IGF-1-positive cells had brown-yellow granules in the cytoplasm. Differences in IGF-1 expression were found between treatment groups (F(4, 35) = 223.713, p < 0.001). Lower levels of IGF-1 expression were found in the DM, DM/Sev and Cel groups than in the control group (77.9 ±8.9 cells/mm2 in DM group, 57.1 ±8.6 cells/mm2 in DM/Sev group and 109.0 ±10.4 cells/mm2 in Cel group vs. 166.9 ±9.7 cells/mm2 in control group). No significant difference in GFAP-positive cells was found between control and Sev groups. Rats in the Cel group had more IGF-1 positive cells than rats in the DM/Sev group (p < 0.05) (Figure 5 F).
Discussion
Summary of results
In the present study, DM rats showed impaired cognition that worsened after sevoflurane inhalation, and was accompanied by increased astrocyte numbers, increased Aβ deposits, and reduced IGF-1 expression in the hippocampus. After celastrol treatment, astrocyte numbers and Aβ deposits decreased and IGF-1 expression increased, changes that were accompanied by improvement in cognition. These findings suggest that celastrol inhibits Aβ production and inflammation in sevoflurane-treated DM rats, and that this action helps to improve cognition.
Sev/DM rat modeling and senile dementia
The combination in rats of sevoflurane inhalation and STZ-induced diabetes produced features similar to those seen in senile dementia – a decrease in cognition, neuronal degeneration, and an increase in Aβ, decrease in IGF1, and increase in astrocytes in the CA1 region of the hippocampus, a region of the brain vital for memory formation.
Our results and those of others suggest similarities between the neuropathology of DM and AD. Diabetes mellitus increases the risk of AD in humans [3], and type I diabetes increases AD pathology in a mouse model of AD [19]. In addition, expression of the gene for the astrocyte marker GFAP is increased [20] and expression of the gene for IGF1 is reduced [21] in AD brains, results parallel to our findings of increased GFAP and decreased IGF levels in DM rats.
Sevoflurane causes long-term cognitive dysfunction when given to young rats or mice [4, 22], induces apoptosis and increases Aβ levels in vitro and in vivo [5], and, in human neutrophils, increases oxidative stress [23]. It has also been shown to decrease cognitive function and the expression of IGF1 in old rats [14]. These actions may be partly responsible for the exacerbation by sevoflurane of the cognitive and neuropathological changes seen in the DM rats in our study. Thus, the combination in rats of sevoflurane and DM shows changes similar to the changes seen in senile dementia and may be useful in the future as an animal model for senile dementia. Sevoflurane has both short- and long-term effects on cognition, and our study was on the short-term effects, which are mainly found 1–3 days after surgery and have returned almost to normal by day 7 [14, 24]. Future studies of chronic cognition loss in aged animals are needed to validate the usefulness of this model.
Mechanisms of long-term cognitive impairment
Currently, the pathogenesis of cognition impairment is unclear. Some investigators propose that the Aβ accumulation in the brain is an initiator in the pathogenesis of cognition impairment, and is a major cause of neuronal loss [25]. However, the mechanisms underlying the Aβ-induced cognition impairment are diverse, and a widely accepted mechanism is Aβ-induced inflammation and oxidative injury [26, 27]. Aβ may act through causing an increase of the release of pro-inflammatory cytokines and NO; but it may also act through inhibiting the expression of HSP70 (a neuroprotective protein) and, by weakening this protective mechanism, cause damage to neurons that ultimately causes cognition impairment. Therefore inhibiting Aβ production and suppressing Aβ-induced inflammation may be an effective therapy against cognition impairment.
Celastrol action and potential mechanism
Celastrol, in our study, lessened the poor cognition and dementia-like pathology seen in sevoflurane-treated diabetic rats. As a non-steroidal anti-inflammatory drug that is a triterpenoid monomer, it may exert its anti-inflammatory and immunoregulatory effects in several different ways [6].
Aβ protein
Celastrol decreased Aβ protein in DM/Sev rats in our study, results similar to the decreased Aβ protein in a mouse model of AD reported previously [7]. Aβ protein is increased in AD, and one way in which this increase is thought to cause a cognitive deficit is to decrease cholinergic function in the brain [28].
Astrocytes
Celastrol decreased GFAP, a marker protein for astrocytes, cells thought to be involved in the formation of Aβ-rich plaques [29] and that are involved in the occurrence and progression of some nervous system diseases, coordinate neurovascular coupling, and are closely related to inflammation and injury [30]. Decreasing astrocyte activity may be another way in which celastrol improves cognition.
IGF-1
Celastrol diminished the depletion of IGF-1 seen in DM/Sev rat hippocampi. Studies on the pathogenesis of AD show that IGF-1 may regulate Aβ metabolism via PI3K/PKB, AMP-activated protein kinase (AMPK) and mitogen-activated protein kinase (MAPK) signaling pathways and is closely related to the neuronal apoptosis which may affect cognition [31, 32].
Other actions of celastrol
Celastrol has also been shown to activate the heat shock response against damaged and misfolded proteins [8], to decrease the production of inflammatory mediators [6], and to suppress microglial activation [6], all actions that would decrease the neuroinflammation thought to play a role in senile dementia. There have been no previous studies of the effect of celastrol on cognition under dementia-like conditions.
The effects of celastrol are thought to be due to its blockade of nuclear factor (NF)-κB, a transcription factor involved in the regulation of a variety of cellular processes, depending on the cell type [33, 34]. Previous studies have reported that celastrol may protect neurons against Aβ-induced injury by inhibiting the NF-κB pathway and inducing HSP70 expression. Allison et al. [6] found that celastrol at low nanomolar concentrations could cause the NF-κB pathway to attenuate Aβ accumulation-induced inflammation characterized by reduction in pro-inflammatory cytokines and NO release. Jung et al. [35] also reported that celastrol at a low dose could induce HSP70 expression and increase Aβ metabolism, exerting neuroprotective effects. In addition, celastrol is able to directly clear peroxide and oxygen radicals to protect neurons against Aβ-induced injury.
Limitations
Although we have clearly shown that celastrol inhibits the increased deposition of Aβ protein, increased GFAP expression, and decreased IGF-1 levels seen in the hippocampus of sevoflurane-treated DM rats, we did not examine the hippocampus for phenomena such as signs of cell death, oxidative stress, or plaque formation, or examine other brain areas involved in learning. Also, we did not investigate potential mechanisms for celastrol’s effect, such as actions on HSP70, TNF-α [36], release of inflammatory cytokines, or regulation of microglial activity. These are subjects for further study.
Moreover, we did not determine whether celastrol could decrease the neurodegenerative signs seen in DM rats themselves, that is, DM rats that were not given sevoflurane, because we did not include a group of celastrol-treated DM rats in the study.
Questions for the future
Two questions about the DM/Sev combination need to be answered by future research. In DM, it is not clear whether insulin resistance or high plasma glucose alone is involved in the mechanism for increasing the risk of AD. A genetic study has shown changes in diabetes-related genes in AD brains, suggesting that insulin resistance is involved [20]. On the other hand, Jolivalt et al. found that type I diabetes exaggerates Alzheimer’s features [19], which suggests that plasma glucose, rather than insulin resistance, exacerbates AD. Celastrol lowers insulin resistance and fasting blood glucose in mice with type 2 diabetes [37]; what effect these actions might have in lessening dementia needs to be studied.
Another question that needs study is why sevoflurane had a marked effect in DM rats, but no effect in control rats.
In conclusion, in the present study, sevoflurane given to DM rats produced acute changes similar to the chronic changes seen in senile dementia. Diabetes mellitus rats showed cognition impairment after sevoflurane anesthesia, accompanied by increased Aβ deposits, activation of astrocytes and reduced IGF-1 expression. After celastrol treatment, Aβ deposits were reduced, activated astrocytes decreased and IGF-1 expression increased, and these changes were accompanied by improvement of performance in the place navigation test and spatial exploration test (Morris water maze test). Our findings provide a foundation for further studies of a possible effect of celastrol in conditions in which an increase of Aβ deposits plays a role.
Acknowledgments
Wei-Tao Liao and Xiao-Yu Xiao contributed equally to this study.
This study was financially supported by the Administration of Traditional Chinese Medicine of Guangdong Province Scientific Research Fund Project (20121055).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Marcheezi VT. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 2011;25:5–13. doi: 10.1096/fj.11-0102ufm. [DOI] [PubMed] [Google Scholar]
- 2.Zhang M, Luo G, Zhou Y, Wang S, Zhong Z. Phenotypic screens targeting neurodegenerative diseases. J Biomol Screen. 2014;19:1–16. doi: 10.1177/1087057113499777. [DOI] [PubMed] [Google Scholar]
- 3.Wang KC, Woung LC, Tsai MT, Liu CC, Su YH, Li CY. Risk of Alzheimer’s disease in relation to diabetes: a population-based cohort study. Neuroepidemiology. 2012;38:237–44. doi: 10.1159/000337428. [DOI] [PubMed] [Google Scholar]
- 4.Wang SQ, Fang F, Xue ZG, Cang J, Zhang XG. Neonatal sevoflurane anesthesia induces long-term memory impairment and decreases hippocampal PSD-95 expression without neuronal loss. Eur Rev Med Pharmacol Sci. 2013;17:941–50. [PubMed] [Google Scholar]
- 5.Dong Y, Zhang G, Zhang B, et al. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66:620–31. doi: 10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–57. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 7.Paris D, Ganey NJ, Laporte V, et al. Reduction of beta amyloid pathology by celastrol in a transgenic mouse model of Alzheimer’s disease. J Neuroinflammation. 2010;7:17. doi: 10.1186/1742-2094-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westerheide SD, Bosman JD, Mbadugha BNA, et al. Celastrols as inducers of heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–60. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 9.Nam SM, Yi SS, Yoo DY, et al. Changes in cyclooxygenase-2 immunoreactivity in the hippocampus in a model of streptozotocin-induced type-1 diabetic rats. J Vet Med Sci. 2012;74:977–82. doi: 10.1292/jvms.12-0036. [DOI] [PubMed] [Google Scholar]
- 10.Baluchnejadmojarad T, Roghani M. Chronic epogallocatechin 3-gallate learning and memory deficits in diabetic rats via modulation of nitric oxide and oxidative stress. Behav Brain Res. 2011;224:305–10. doi: 10.1016/j.bbr.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Castilho AF, Liberal JT, Baptista FI, Gaspar JM, Carvalho AL, Ambrosio AF. Elevated glucose concentration changes the content and cellular localization of AMPA receptors in the retina but not in the hippocampus. Neuroscience. 2012;219:23–32. doi: 10.1016/j.neuroscience.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 12.Slemmer JE, Chacka IJ, Sweener MI, Weber JT. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury, and aging. Curr Med Chem. 2008;15:404–14. doi: 10.2174/092986708783497337. [DOI] [PubMed] [Google Scholar]
- 13.Wang WY, Wang H, Luo LJ, et al. The effects of metabatropic glutamate receptor 7 allosteric agonist N-di-benzhydryoethane-1,2-diaminendihydrochloride on developmental sevoflurane neurotoxicity: role of extracellular signal-regulated kinase 1 and 2 mitogen-activated protein kinase signaling pathway. Neuroscience. 2012;205:167–77. doi: 10.1016/j.neuroscience.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Peng S, Zhang Y, Sun DP, Zhang DX, Fang Q, Li GJ. The effect of sevoflurane anesthesia on cognitive function and the expression of Insulin-like Growth Factor-1 in CA1 region of hippocampus in old rats. Mol Biol Rep. 2011;38:1195–9. doi: 10.1007/s11033-010-0217-9. [DOI] [PubMed] [Google Scholar]
- 15.Obal D, Preckel B, Scharbatke H, et al. One MAC of sevoflurane provide against reperfusion injury in the heart in vivo. Br J Anaesth. 2001;87:905–11. doi: 10.1093/bja/87.6.905. [DOI] [PubMed] [Google Scholar]
- 16.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatgial and related procedures for assessing spatial and related forms of learning and memory. Nat Protocol. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhooge R, Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–9. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 18.Zheng X, Zhou J, Xia Y. The role of TNF-alpha in regulating ketamine-induced hippocampal neurotoxicity. Arch Med Sci. 2015;6:1296–302. doi: 10.5114/aoms.2015.56355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolivalt CG, Hurford R, Lee CA, Durnaop W, Rockenstein E, Masliah E. Type I diabetes exaggerates features of Alzheimer’s disease in APP transgenic mice. Exp Neurol. 2010;223:422–31. doi: 10.1016/j.expneurol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hokama M, Oka S, Leon J, et al. Altered expression of diabetes-related genes in Alzheimer’s disease brains: the Hisayama study. Cerebral Cortex. 2014;24:2476–88. doi: 10.1093/cercor/bht101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease is this type 3 diabetes? J Alzheimer’s Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 22.Satomoto M, Satoh Y, Teriu K, et al. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 23.Wong CH, Liu TZ, Chye SM, et al. Sevoflurane-induced oxidative stress and cellular injury in human peripheral polymorphonuclear neutrophils. Food Chem Toxicol. 2006;44:1399–407. doi: 10.1016/j.fct.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Le Freche H, Brouillette J, Fernandez-Gomez FJ, et al. Tau phosphorylation sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology. 2012;116:779–87. doi: 10.1097/ALN.0b013e31824be8c7. [DOI] [PubMed] [Google Scholar]
- 25.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 26.Farkas E, Luiten PG, Bari F. Permanent bilateral carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative disease. Brain Res Rev. 2007;54:162–80. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Cheng G, Whitehead SM, Hachinski V, Cechetto D. Effects of pyrrolidine dithiocarbamate in beta amyloid (25-35)-induced imflammatory responses and memory deficits in the rat. Neurobiol Dis. 2006;23:140–51. doi: 10.1016/j.nbd.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Schliebs R, Arendt T. The cholinergic system in aging and neurodegeneration. Behav Brain Res. 2011;221:555–63. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 29.Nagele RG, Weigel J, Venkataraman V, Imaki H, Wang KC, Weigel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging. 2004;25:663–74. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–9. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor-I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer’s disease. J Neurosci. 2007;27:824–31. doi: 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messier C, Teutenberg K. The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer’s disease. Neural Plast. 2005;12:311–28. doi: 10.1155/NP.2005.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakkar N, Guttridge DC. NFkappaB signaling: a tale of two pathways in skeletal myogenesis. Physiol Rev. 2010;90:495–511. doi: 10.1152/physrev.00040.2009. [DOI] [PubMed] [Google Scholar]
- 34.Sanz AB, Sanchez-Nino MD, Ramos AM, et al. NF-kappaB in renal inflammation. J Am Soc Nephrol. 2010;21:1254–62. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- 35.Jung HW, Chung YS, Kim YK. Celastrol inhibits production of nitric oxide and pro-inflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp Molec Med. 2007;6:715–21. doi: 10.1038/emm.2007.78. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Zhou J, Xia Y. The role of TNFalpha in regulating ketamine-induced hippocampal neurotoxicity. Arch Med Sci. 2015;11:1296–302. doi: 10.5114/aoms.2015.56355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JE, Lee MH, Nam DH, et al. Celastrol, an NF-kappaB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice. PLoS One. 2013;8:e62068. doi: 10.1371/journal.pone.0062068. [DOI] [PMC free article] [PubMed] [Google Scholar]