Abstract
Introduction
Liver function is affected during ischemia/reperfusion (IR). The current state of knowledge about liver aging processes during IR is incomplete. We evaluated the effects of aging on liver structure and function under IR conditions.
Material and methods
Animals were divided into control (C-2) and ischemia/reperfusion (IR-2) groups of young rats (2–4 months old) and C-12 and IR-12 groups of old rats (12–14 months old). The livers from IR-2 and IR-12 groups were subjected to partial ischemia (60 min), followed by global reperfusion (4 h). Blood samples were obtained during reperfusion (0, 30 and 240 min) to estimate the activity of aminotransferases (ALT, AST). After IR, tumor necrosis factor-α (TNF-α), interleukin-1b (IL-1b), malondialdehyde (MDA), and superoxide dismutase (SOD) were determined in liver homogenates.
Results
At all points of reperfusion, an increase in aminotransferase activity levels in the ischemic groups was observed; mainly between IR-12 and C-12 rats. The concentration of TNF-α was significantly higher in young animals (in non-ischemic groups: p = 0.09, in ischemic groups: p = 0.05). Under IR conditions, the concentration of IL-1b dropped (p = 0.05). The concentration of MDA was significantly higher in mature animals (in non-ischemic groups: p = 0.09, in ischemic groups: p = 0.05). In ischemic groups an increase in necrosis rate was observed regardless of age. Rats in the IR-12 group showed the most pronounced changes in hepatic architecture, including increased micro- and macrosteatosis and parenchymal cell destruction.
Conclusions
The function and structure of mature livers slightly deteriorate with age and these differences are more noticeable under IR conditions.
Keywords: ageing, ischemia/reperfusion, liver, rat, oxidative stress, inflammation
Introduction
Ischemia/reperfusion (IR) is considered to be the main cause of organ injury during such procedures as liver transplantation or hepatectomy [1]. This damage is initially caused by ischemia, and further aggravated by reperfusion. The reperfusion stage can be divided into the acute phase (3–6 h), involving generation of reactive oxygen species (ROS) and nitric oxide (NO) and activation of T-cells and Kupffer cells (KCs), and the subacute phase (18–24 h), characterized by neutrophil infiltration leading to continuous oxidant, cytokine, and chemokine production [2, 3].
During IR-evoked oxidative stress, generation of ROS may exceed the capacity of antioxidative systems. Malondialdehyde (MDA) is the most commonly studied product of polyunsaturated fatty acid peroxidation, and it is widely used as an indicator of oxidative damage. Superoxide dismutases (SODs) are antioxidant enzymes that catalyze the dismutation of superoxide (O2 •–) into oxygen or hydrogen peroxide [2].
Tumor necrosis factor-α (TNF-α), released from KCs in the initial phase of reperfusion, intensifies the expression of adhesion molecules and release of other cytokines. These changes are responsible for neutrophil activation and their accumulation in the later phase [4, 5]. Interleukin-1β (IL-1β) is a proinflammatory cytokine released from KCs in response to TNF-α. Similarly to TNF-α, IL-1β can up-regulate adhesion proteins on neutrophils and induce IL-8 synthesis. It can also interact with neutrophils and stimulate the release of ROS. Another property of this molecule is the augmentation of TNF-α synthesis by KCs [5].
The liver function appears to be well maintained in old age [6]. However, the risk of failure of the mature liver after IR appears to be increased [7]. Increased morbidity and mortality after vascular clamping [8, 9], as well as poor long-term survival after transplantation [10], have been observed. Although in some papers age-related differences in the liver response to IR have been reported [11–13], our knowledge is still incomplete.
The aim of the study was to evaluate age-related differences in function and structure of rat livers subjected to IR and to investigate how aging influences selected parameters of inflammation and oxidative stress under both physiological and IR conditions.
Material and methods
Animals
The study was carried out on Wistar male rats obtained from the Animal Laboratory of the Department of Pathological Anatomy, Wroclaw Medical University. Before the experiment, the animals had been housed in standard conditions (12 : 12 day/night cycle, stable temperature 19–21°C, humidity 45–60%, and continuous ventilation) and had ad libitum access to food and water. The experiment was performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and was approved by the First Local Ethics Committee on Animal Research of the Institute of Immunology and Experimental Therapy, Polish Academy of Sciences in Wroclaw.
Experimental protocol
After adaptation, the animals were divided into two groups (C-2 and IR-2) of young rats (2–4 months old) and two groups (C-12 and IR-12) of old rats (12–14 months old). Rats belonging to the C-2 (n = 10) and the C-12 (n = 9) groups were not subjected to IR, and rats from the IR-2 (n = 9) and the IR-12 (n = 9) groups were subjected to 60 min of partial ischemia followed by 4 h of global reperfusion.
Preparation of the liver IR injury model
The rats were weighed and anesthetized with intramuscular injection of ketamine hydrochloride (7 mg/kg) (Bioketan, Vetoquinol Biowet, Poland), medetomidine hydrochloride (0.1 mg/kg) (Domitor, amp. 1 mg/ml, Orion Pharma, Finland), and butorphanol tartrate (2 mg/kg) (Morphasol, amp. 4 mg/ml, aniMedica GmbH Germany). After a suitable level of anesthesia was achieved, rats from IR-2 and IR-12 groups underwent midline laparotomy. Seventy percent liver ischemia (left lateral and median lobes) was achieved by occluding the branches of the portal vein and hepatic artery using a microvascular clip. The rats were given heparin (200 U/kg) (Heparinum WZF – amp. 25 000 U/5 ml, Polfa Warszawa, Poland), to prevent blood coagulation. After 60 min of ischemia, the clip was removed to allow reperfusion for 4 h. The abdomen was closed and the rats were observed during reperfusion. During whole reperfusion rats remained asleep. In the rats from C-2 and C-12 groups, the branches of the portal vein and the hepatic artery were isolated, but not occluded. Blood samples (0.8 ml) were obtained after catheterization of the tail vein, just before ischemia and after 30 and 240 min of reperfusion to determine the levels of alanine and asparagine aminotransferases (ALT, AST). All blood samples were replaced by the same volume of saline solution (sol. 0.9% sodium chloride (Polpharma S.A., Poland)). When the experiment was terminated, the livers were weighted and ischemic lobes were isolated.
After the reperfusion, one batch of ischemic hepatic tissues was fixed in 0.1 M phosphate buffer with 2% paraformaldehyde and 2% glutaraldehyde (pH 7.6), and the other batch of the ischemic tissues was homogenized on ice with the lysis buffer (140 mM NaCl, 10 mM EDTA, 10% glycerol, 1% NP40, 20 mM Tris base, pH 7.5). The homogenized tissues were subsequently centrifuged at 14 000 rpm for 25 min, at 4°C and the resulting supernatants were collected [14].
Parameters of oxidative stress and inflammation
Malondialdehyde and SOD were estimated in the liver homogenates using colorimetric methods (spectrophotometer MARCEL S350 PRO). SOD activity was assayed using a RANSOD kit (Randox Laboratories, Crumlin, UK). Malondialdehyde concentration was determined with BIOXYTECH-MDA-586 (OxisResearch, USA), according to the manufacturer’s instructions. TNF-α and IL-1β plasma levels were determined using commercially available Rat TNF-α ELISA and Rat IL-1β ELISA kits (Diaclone SAS, France).
Biochemical analyses
Serum activity of ALT and AST and protein concentration in the homogenates were assayed using commercial enzymatic methods in a certified laboratory.
Histological examination
Different regions of the ischemic and non-ischemic livers were fixed in 10% formalin and embedded in paraffin. Sections of 4.5 μm were made and stained with hematoxylin-eosin. Then, they were histologically evaluated under a light microscope for the severity of ischemic necrosis, degree of steatosis as percentage of the microscopic field (small cytoplasmic vacuoles containing lipids or single fat droplets displacing the nuclei of hepatocytes), neutrophil infiltration and destruction of hepatic architecture [15]. The necrosis rate was semi-quantitatively determined as follows: absent – 0; 0–25% necrosis per microscopic field – 1; 25–50% necrosis per microscopic field – 2; 50–75% necrosis per microscopic field – 3; 75–100% necrosis per microscopic field – 4.
Statistical analysis
Data were expressed as mean values ± SD. Statistical analysis of the age and IR effects on the oxidative stress and inflammatory parameters was performed using a two-way analysis of variance (ANOVA). Statistical analysis of the effect of rat age and time of reperfusion on ALT and AST levels was performed using MANOVA with repetitions. Contrast analysis was used for specific comparisons. Hypotheses were considered positively verified if p < 0.05.
Results
ALT and AST activity
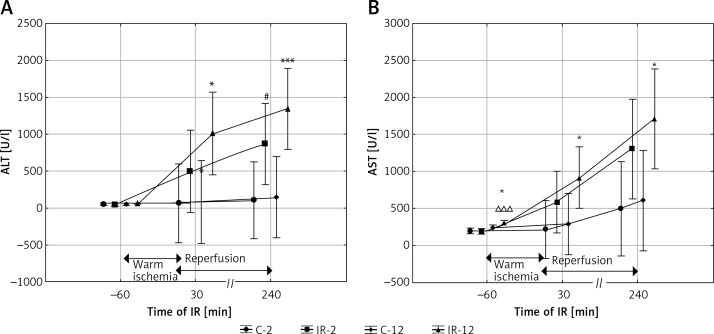
Alanine aminotransferase activity measured before IR was comparable in all groups of rats regardless of age, but AST activity was the highest in the IR-12 group (IR-12 vs. C-2 and IR-2, p < 0.001, and IR-12 vs. C-12, p < 0.05). During reperfusion, an increase in both ALT and AST activity in the ischemic groups was observed. After 30 min of reperfusion, the activity of both aminotransferases was significantly higher in the IR-12 than in the C-12 group (IR-12 vs. C-12, p < 0.05, for both enzymes). After 240 min of reperfusion, the activity of both aminotransferases was also significantly different in mature groups (IR-12 vs. C-12, p < 0.05 for AST and IR-12 vs. C-12, p < 0.005 for ALT). At this point in time the increase in ALT activity was also significant in the younger group that underwent IR (IR-2 vs. C-2, p < 0.05) and the increase in AST activity was bordering on significance (IR-2 vs. C-2, p = 0.08) (Figures 1 A, B).
Figure 1.
The effect of IR and aging on ALT (A) and AST (B) activity
The values are presented as mean ± SD. C-2 – young rats non-subjected to IR, C-12 – mature rats not subjected to IR, IR-2 – young rats subjected to IR, IR-12 – mature rats subjected to IR; *p < 0.05 and ***p < 0.005 (IR-12 vs. C-12), #p < 0.05 (IR-2 vs. C-2), ∆∆∆p < 0.005 (IR-12 vs. IR-2).
Cytokine levels
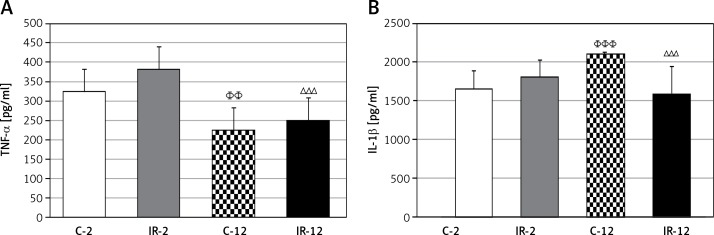
In the ischemic groups the concentration of TNF-α was slightly higher than in the corresponding non-ischemic groups. In both non-ischemic and ischemic groups, the concentration of TNF-α was significantly higher in young animals than in the older ones (C-12 vs. C-2, p < 0.01, and IR-12 vs. IR-2, p < 0.005) (Figure 2 A). Concentration of IL-1β in the non-ischemic groups depended on age and it was significantly higher in the mature group (C-12 vs. C-2, p < 0.005). Under IR conditions, the concentration of IL-1β dropped in the IR-12 group (IR-12 vs. C-12, p < 0.005) and increased in the IR-2 group, which is why the difference between young and mature ischemic rats was on the border of significance (IR-12 vs. IR-2, p = 0.053) (Figure 2 B).
Figure 2.
The effect of IR and aging on cytokine (TNF-α (A) and IL-1b (B)) levels
The values are presented as mean ± SD. C-2 – young rats non-subjected to IR, C-12 – mature rats not-subjected to IR, IR-2 – young rats subjected to IR, IR-12 – mature rats subjected to IR; ∆∆∆ p < 0.005 (IR-12 vs. IR-2), ΦΦp < 0.01 and ΦΦΦp < 0.005 (C-12 vs. C-2).
Parameters of oxidative stress
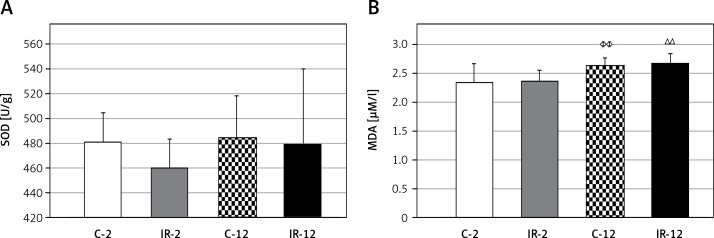
Under IR conditions, the changes in SOD activity were not significant, but were slightly less pronounced than in the corresponding non-ischemic age groups (Figure 3 A). The concentration of MDA depended on age and increased significantly in both ischemic and non-ischemic mature animals (C-12 vs. C-2, and IR-12 vs. IR-2, p < 0.01, in both variants). Only a negligible increase in MDA under IR conditions was observed (Figure 3 B).
Figure 3.
The effect of IR and aging on the activity of SOD (A) and the concentration of MDA (B)
The values are presented as mean ± SD. C-2 – young rats non-subjected to IR, C-12 – mature rats not-subjected to IR, IR-2 – young rats subjected to IR, IR-12 – mature rats subjected to IR; ∆∆p < 0.01 (IR-12 vs. IR-2), ΦΦp < 0.01 (C-12 vs. C-2).
Histological findings
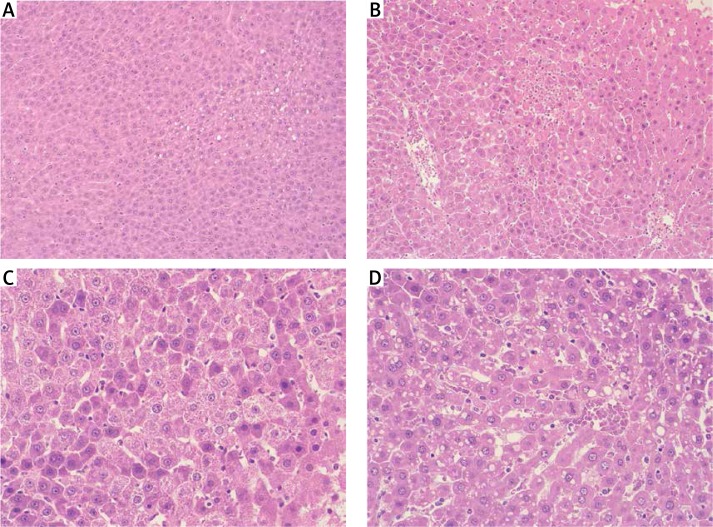
No significant differences in the hepatic structure were seen in either young or mature non-ischemic animals (C-2, C-12). Livers from those groups featured normal architecture and only a slight degree of steatosis. In the ischemic groups (IR-2, IR-12), an increase in the necrosis rate associated with intense neutrophil recruitment was observed regardless of age (Figure 4). Given the necrosis score value (NSV), the difference between young ischemic and non-ischemic rats was bordering on significance (IR-2 vs. C-2, p = 0.06). More pronounced differences were observed between the mature groups (IR-12 vs. C-12, p < 0.05). Adult ischemic rats (IR-12) showed the most noticeable changes in the hepatic architecture, including increased micro- and macrosteatosis and parenchymal cell destruction. Also the percentage of steatosis was the highest in this group (IR-12 vs. C-2 and C-12, p < 0.01, and IR-12 vs. IR-2, p < 0.05) (Table I).
Figure 4.
Histopathological examination of liver tissue (stained with hematoxylin-eosin) from group C-2 – young rats non-subjected to IR (A), group IR-2 – young rats subjected to IR (B), group C-12 – mature rats not subjected to IR (C), and from group IR-12 – mature rats subjected to IR (D). Livers from C-2 and C-12 groups featured normal architecture and only low degree of steatosis, but from IR-2 and IR-12 groups presented higher rate of the necrosis associated with intense neutrophil infiltrate
Table I.
The necrosis score value (NSV) and the percentage of steatosis in microscopic fields in all examined groups
| Variable | Steatosis (% of microscopic field) | Necrosis (NSV) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| C-2 | 0.30 | 0.42 | 0.10 | 0.32 |
| IR-2 | 2.44# | 3.64 | 0.67# | 0.87 |
| C-12 | 0.22## | 0.36 | 0.11 | 0.33 |
| IR-12 | 11.56**,∆ | 16.48 | 0.78* | 0.83 |
The values are presented as mean ± SD. C-2 – young rats non-subjected to IR, C-12 – mature rats not-subjected to IR, IR-2 – young rats subjected to IR, IR-12 – mature rats subjected to IR; the difference in steatosis:
p < 0.01 (IR-12 vs. C-12)
p < 0.05 (IR-12 vs. IR-2); the difference in necrosis:
p = 0.06 (IR-2 vs. C-2)
p < 0.05 (IR-12 vs. C-12).
Discussion
In this study we focused on the age-dependent changes in the liver function and structure, as well as oxidative stress and proinflammatory parameters during IR. We observed that (1) the increase in ALT and AST activity was significantly higher in mature than in young animals, especially in the groups that underwent IR; (2) enzymatic changes were corroborated by histological findings, as a significant increase in necrosis rate in both ischemic groups, and severe micro- and macrosteatosis and parenchymal cell destruction in mature ischemic rats were observed; (3) changes in the proinflammatory parameters indicated the differentiated age-dependent reaction in IR conditions: the level of TNF-α was significantly higher in young rats, independent from IR, whereas the level of IL-1β was higher in old animals, but decreased under IR; (4) in mature rats oxidative stress was more pronounced as confirmed by significantly higher MDA concentration.
In non-ischemic groups, AST activity was significantly higher in mature than in young rats. Therefore, despite a lack of histological abnormalities, we may suspect a low-degree, age-related liver dysfunction in old animals. It is well known that liver function deteriorates during IR. As we found in a previous study [16], the most pronounced liver injury was detected in the groups exposed to IR – the activity of ALT and AST increased during hepatocyte damage. Similarly to other research [17], we also observed age-related susceptibility to IR. Differences in the enzymatic activities between the IR-exposed and non-exposed livers appeared at the time points of 30 and 240 min of reperfusion in the mature animals. In young animals, significant differences appeared only at 240 min of reperfusion. Hence, the conclusion is that the livers obtained from young animals are less susceptible to injury, than the livers harvested from mature rats. These findings were supported by histological analyses. The histological evaluation of liver specimens revealed necrotic foci with inflammatory infiltrates of similar magnitude, regardless of age. Age differences were associated with the degree of steatosis. Similarly to the study of Kireev et al. [18], the degree of steatosis after IR was greater in the group of mature animals. Our results are also consistent with those obtained by Okaya et al. [19]; however, these authors also revealed significant differences in leukocyte infiltration. In our study, leukocyte infiltration in the specimens was comparable in all ischemic groups. Steatosis is one of the determinants of reversible cell injury; therefore its significantly higher degree in ischemic organs derived from old rats, together with increased aminotransferase activity, indicates age-related injury.
It was observed that neutrophil accumulation in the lobes with sustained blood flow was the highest after 6 h of reperfusion [20], and their number correlated well with intensified ROS production in reperfused tissues [21]. Neutrophils adhere to the endothelium and pass into liver parenchyma, causing hepatocyte injury due to cell granule degradation and release of ROS and proteolytic enzymes, e.g. proinflammatory cytokines such as TNF-α [21, 22]. In our study, the time of reperfusion was shorter (4 h) and neutrophils accumulated in the ischemic tissue in similar amounts, regardless of age. Therefore, age-related differences in MDA and TNF-α concentrations suggest KCs as a probable source of ROS and proinflammatory cytokines [23]. KCs were shown to be activated during the first 6 h of reperfusion [24], and their activation in livers derived from older rats was impaired [25].
At the end of the experiment the level of TNF-α, was determined in liver homogenates. Its action in an ischemic liver is multidirectional. TNF-α influences, among other things, cell proliferation and apoptosis, proinflammatory cytokines and ROS release, and enhances neutrophil adhesion and blood coagulation [5]. In contrast to some other studies [12, 16, 26–28], in our study the level of TNF-α was only slightly increased in the livers subjected to IR. Detailed comparison of our results with the results obtained by other authors is difficult due to different duration of IR or different animal species. TNF-α is produced in extensive amounts during the sub-acute phase of reperfusion [29]. Our experiment was designed in such a way that the reperfusion phase lasted for only 4 h and probably was too short to show significant differences between the ischemic and non-ischemic groups. In our study, the level of TNF-α depended on age. Its values were significantly higher in the young than in the old rats. Okaya et al. also found that the production of this cytokine by KCs was greater in younger rats [19]. Kireev et al. described increased expression of mRNA for various proinflammatory cytokines under IR. In the case of TNF-α that increase was not as pronounced in the mature rats as in the younger ones [18]. IL-1β was another cytokine determined in our work in liver homogenates after the reperfusion period. The level of IL-1β was significantly higher in the mature animals than in the young ones. In response to IR, the level of this cytokine increased significantly in the younger livers, but decreased in the mature ones. Considering the extent of histological changes in the livers taken from old rats and the increase in aminotransferase activity in the same groups, it may be suspected that the lower proinflammatory cytokine level in these groups, as well as a lack of their increase under IR, may result from impaired KC and hepatocyte function in old rats.
While analyzing MDA concentration in the examined groups, it may be noted that the severity of oxidative stress was age-dependent and it was greater in the mature rats than the young ones. In the group of young rats characteristic changes in the oxidative stress parameters, such as increased level of MDA and reduced SOD activity, were found after IR. The observed changes were not statistically significant. In various reports, however, SOD activity also remained unaffected, suggesting that this enzyme is not a sensitive predictor of oxidative stress [30, 31]. The insignificant increase in MDA [32, 33] could be a result of too short duration of ischemia and reperfusion. In those conditions, liver injury could not be sufficiently manifested. Probably MDA or SOD levels should also be measured after 24 to 48 h, during the second phase of reperfusion. Another possibility of evoking more pronounced oxidative stress is development of another experimental model of global ischemia and reperfusion [34]. The lack of changes in MDA level in old livers in our experiment could be the result of an age-dependent impaired response of the organs to oxidative stress under IR conditions. Evaluation of post-ischemic ROS formation by chemiluminescent real-time imaging [35] indicated that age appears to be an important factor affecting liver sensitivity to oxidative stress. During reperfusion the livers of mature rats generated a lower amount of ROS, as compared to the livers obtained from young animals.
In conclusion, we conclude that the influence of IR on liver function and structure, as well as on oxidative stress and inflammation, depends on age. Based on both aminotransferases activity and histological findings, we found that hepatocyte damage was more pronounced in the mature livers than in the younger ones, especially under IR conditions. Age-related differences were also visible in the case of MDA and TNF-α, but only a slight increase in the levels of those parameters was observed following IR. Therefore, further experiments are probably needed, using a longer reperfusion period or a global ischemia model. An interesting question is why the changes of prooxidative and proinflammatory parameters were weaker in the groups of mature animals. A few possible explanations of the lower TNF-α and MDA levels in the mature animals and the drop in IL-1β concentration in the mature animals in response to IR could be offered. Similarly to the earlier suggestion of Gasbarrini et al. [35] the reasons for our results could also be reduced activity of KCs (the source of ROS in the initial period of reperfusion), impaired function of mitochondria (the main intracellular sources of ROS), and reduced blood flow in mature livers. Therefore, in order to better understand these results, our data should be supplemented by an analysis of additional parameters, such as KC activation or liver blood flow. Hence, the results of this study may just indicate that changes in liver function and structure, as well as particular parameters determining the redox and inflammatory state, depend on age and may be the reason for variable liver responses to such pathological conditions as those observed under IR, but some of our findings should be confirmed in subsequent studies.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Cobreros A, Sainz L, Lasheras B, Cenarruzabeitia E. Hepatotoxicity of ethanol: protective effect of calcium channel blockers in isolated hepatocytes. Liver. 1997;17:76–82. doi: 10.1111/j.1600-0676.1997.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 2.Fan C, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver. J Mol Med. 1999;77:577–96. doi: 10.1007/s001099900029. [DOI] [PubMed] [Google Scholar]
- 3.Hines IN, Harada H, Flores S, Gao B, McCord JM, Grisham MB. Endothelial nitric oxide synthase protects the post-ischemic liver: potential interactions with superoxide. Biomed Pharmacother. 2005;59:183–9. doi: 10.1016/j.biopha.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Peralta C, Fernández L, Panés J, et al. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology. 2001;33:100–13. doi: 10.1053/jhep.2001.20529. [DOI] [PubMed] [Google Scholar]
- 5.Perry BC, Soltys D, Toledo AH, Toledo-Pereyra LF. Tumor necrosis factor-alpha in liver ischemia/reperfusion injury. J Invest Surg. 2011;24:178–88. doi: 10.3109/08941939.2011.568594. [DOI] [PubMed] [Google Scholar]
- 6.Fu A, Nair KS. Age effect on fibrinogen and albumin synthesis in humans. Am J Physiol. 1998;275:E1023–30. doi: 10.1152/ajpendo.1998.275.6.E1023. [DOI] [PubMed] [Google Scholar]
- 7.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 8.Le Couteur DG, Rivory LP, Pond SM. The effects of aging and nutritional state on hypoxia-reoxygenation injury in the perfused rat liver. Transplantation. 1994;58:531–6. doi: 10.1097/00007890-199409150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Clavien PA, Selzner M, Rudiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–50. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins BH, Pirsch JD, Becker YT, et al. Long-term results of liver transplantation in patients 60 years of age and older. Transplantation. 2000;70:780–3. doi: 10.1097/00007890-200009150-00012. [DOI] [PubMed] [Google Scholar]
- 11.Trocha M, Szeląg A, Pieśniewska M, Fereniec-Gołębiewska L, Grotthus B, Merwid-Ląd A. Effect of aging process on liver function in extracorporeal rat liver perfusion. Hepatogastroenterology. 2007;54:1207–11. [PubMed] [Google Scholar]
- 12.Abe Y, Hines IN, Zibari G, et al. Mouse model of liver ischemia and reperfusion injury: method to study reactive oxygen and nitrogen metabolites in vivo. Free Radic Biol Med. 2009;46:1–7. doi: 10.1016/j.freeradbiomed.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kireev RA, Cuesta S, Ibarrola C, et al. Age-related differences in hepatic ischemia/reperfusion: gene activation, liver injury, and protective effect of melatonin. Surg Res. 2012;178:922–34. doi: 10.1016/j.jss.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 14.Morales AI, Vicente-Sanchez C, Jerkic M, Santiago JM, Sanchez-Gonzalez PD, Perez-Barriocanal F. Effect of quercetin on metallothionein, nitric oxide synthase and cyclooxygenase-2 expression on experimental chronic cadmium nephrotoxicity in rats. Toxicol Appl Pharmacol. 2006;210:128–35. doi: 10.1016/j.taap.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Takeda Y, Arii S, Kaido T, et al. Morphologic alteration of hepatocytes and sinusoidal endothelial cells in rat fatty liver during cold preservation and the protective effect of hepatocyte growth factor. Transplantation. 1999;67:820–8. doi: 10.1097/00007890-199903270-00007. [DOI] [PubMed] [Google Scholar]
- 16.Trocha M, Merwid-Ląd A, Chlebda E, et al. Influence of ezetimibe on selected parameters of oxidative stress in rat liver subjected to ischemia/reperfusion. Arch Med Sci. 2014;10:817–24. doi: 10.5114/aoms.2013.38087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selzner M, Selzner N, Chen L, et al. Exaggerated up-regulation of tumor necrosis factor alpha-dependent apoptosis in the older mouse liver following reperfusion injury: targeting liver protective strategies to patient age. Liver Transpl. 2009;15:1594–604. doi: 10.1002/lt.21864. [DOI] [PubMed] [Google Scholar]
- 18.Kireev RA, Cuesta S, Ibarrola C, et al. Age-related differences in hepatic ischemia/reperfusion: gene activation, liver injury, and protective effect of melatonin. J Surg Res. 2012;178:922–34. doi: 10.1016/j.jss.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 19.Okaya T, Blanchard J, Schuster R, et al. Age-dependent responses to hepatic ischemia/reperfusion injury. Shock. 2005;24:421–7. doi: 10.1097/01.shk.0000181282.14050.11. [DOI] [PubMed] [Google Scholar]
- 20.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia-reperfusion injury in rat liver in vivo. FASEB. 1990;4:3355–9. [PubMed] [Google Scholar]
- 21.Lentsch AB, Yoshidone H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/injury in mice: roles of macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–7. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 22.Lentsch AB, Kato A, Yoshidone H, McMastres KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–73. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 23.Grewe M, Gausling R, Gyufko K, Hoffmann R, Decker K. Regulation of the mRNA expression for tumor necrosis factor alpha in rat liver macrophages. J Hepatol. 1994;20:811–8. doi: 10.1016/s0168-8278(05)80154-0. [DOI] [PubMed] [Google Scholar]
- 24.Nakamitsu A, Hiyama E, Imamura Y, Matsuura Y, Yokoyama T. Kupffer cell function in ischemic and nonischemic livers after hepatic partial ischemia/reperfusion. Surg Today. 2001;31:140–8. doi: 10.1007/s005950170198. [DOI] [PubMed] [Google Scholar]
- 25.Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman RG, Lemasters JJ. Kupffer cell activation and endothelial cell damage after storage of rat livers: effect of reperfusion. Hepatology. 1991;13:83–95. [PubMed] [Google Scholar]
- 26.Ma W, Wang ZR, Shi L, Yuan Y. Expression of macrophage inflammatory protein-1alpha in Kupffer cells following liver ischemia or reperfusion injury in rats. World J Gastroenterol. 2006;12:3854–8. doi: 10.3748/wjg.v12.i24.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aldemir D, Tufan H, Tecder-Unal M, et al. Age-related alterations of oxidative stress and arginase activity as a response to intestinal ischemia-reperfusion in rat kidney and liver. Transplantation Proceedings. 2003;35:2811–5. doi: 10.1016/j.transproceed.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 28.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA., Jr Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–43. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamitsu A, Hiyama E, Imamura Y, Matsuura Y, Yokoyama T. Kupffer cell function in ischemic and nonischemic livers after hepatic partial ischemia/reperfusion. Surg Today. 2001;31:140–8. doi: 10.1007/s005950170198. [DOI] [PubMed] [Google Scholar]
- 30.Ceylan A, Karasu C, Aktan F, Güven C, Can B, Ozansoy G. Effects of simvastatin treatment on oxidant/antioxidant state and ultrastructure of diabetic rat myocardium. Gen Physiol Biophys. 2003;22:535–47. [PubMed] [Google Scholar]
- 31.Ozansoy G, Akin B, Aktan F, Karasu Ç. Short-term gemfibrozil treatment reverses lipid profile and peroxidation but does not alter blood glucose and tissue antioxidant enzymes in chronically diabetic rats. Mol Cell Biochem. 2001;216:59–63. doi: 10.1023/a:1011000327529. [DOI] [PubMed] [Google Scholar]
- 32.Xue F, Zhang JJ, Xu LM, Zhang C, Xia Q. Protective effects of HGF-MSP chimer (metron factor-1) on liver ischemia-reperfusion injury in rat model. J Dig Dis. 2010;11:299–305. doi: 10.1111/j.1751-2980.2010.00453.x. [DOI] [PubMed] [Google Scholar]
- 33.Wu C, Wang P, Rao J, et al. Triptolide alleviates hepatic ischemia/reperfusion injury by attenuating oxidative stress and inhibiting NF-kappaB activity in mice. J Surg Res. 2011;166:e205–13. doi: 10.1016/j.jss.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Spiegel HU, Bahde R. Experimental models of temporary normothermic liver ischemia. J Invest Surg. 2006;19:113–23. doi: 10.1080/08941930600569704. [DOI] [PubMed] [Google Scholar]
- 35.Gasbarrini A, Pasini P, Nardo B, et al. Chemiluminescent real time imaging of post-ischemic oxygen free radicals formation in livers isolated from young and old rats. Free Radic Biol Med. 1998;24:211–6. doi: 10.1016/s0891-5849(97)00056-7. [DOI] [PubMed] [Google Scholar]