Abstract
Introduction
The standard therapy for exocrine pancreatic insufficiency (EPI) is porcine-derived pancreatic enzyme replacement therapy (PERT). In the present study we tested a new approach with a mixture of pancreatic-like enzymes of microbial origin (PLEM) in a 1-week efficacy study in EPI pigs. In addition to the conventionally used coefficient of fat and nitrogen absorption (CFA and CNA), parameters that more accurately reflect the nutritional and health status, such as changes in the lipemic index (LI), plasma triglyceride (TG) and non-esterified fatty acid (NEFA) levels, and somatic growth, were determined.
Material and methods
A PLEM dose containing 120 000 active lipase units, 80 000 active protease units and 12 000 active amylase units (all from Sigma, St. Louis, MO) was given as a powder, twice daily with a meal (40 g fat/meal) to 8 EPI pigs for 7 days. Ten healthy pigs were used as a comparator.
Results
The PLEM enhanced fat and protein digestion, and reversed growth impairment in EPI pigs. With treatment, CFA and CNA increased by 59% and 43% (p < 0.05), respectively. Although fat and protein absorption were lower than in the comparator, the postprandial blood lipid profile was normal as in healthy pigs. The mucosal thickness significantly increased by 27%, 50% and 26%, in the proximal, middle, and distal jejunum (p < 0.05) with treatment and resembled that of healthy animals.
Conclusions
Pancreatic-like enzymes of microbial origin supported somatic growth and normalized the postprandial lipid profile. As a measure of efficacy, postprandial LI, TG and NEFA are viable endpoints to be explored in human trials.
Keywords: pancreatic-like enzymes of microbial origin, exocrine pancreatic insufficiency, pigs
Introduction
Exocrine pancreatic insufficiency (EPI) is a chronic condition resulting from pancreatic disease and/or surgery in which compromised exocrine pancreatic function results in reduced production and secretion of both digestive enzymes and bicarbonate. Exocrine pancreatic insufficiency is a major consequence of diseases that lead to the loss of pancreatic parenchyma (pancreatitis, cystic fibrosis (CF) or obstruction of the main pancreatic duct; decreased pancreatic stimulation, celiac disease) and/or the acid-mediated inactivation of pancreatic enzymes (Zollinger-Ellison syndrome). In addition, gastrointestinal and pancreatic surgical resections (e.g. gastrectomy, duodenopancreatectomy, gastric bypass surgery) are frequent causes of EPI [1]. The major symptoms of EPI include steatorrhea, weight loss, fatigue, flatulence, abdominal distention, edema, and, in some cases, anemia. The most common symptomatic complaint is diarrhea, which is frequently watery, reflecting the osmotic load received by the intestine. Weight loss and fatigue are common and may be pronounced, especially in patients with CF [2, 3]; however, patients may compensate by increasing their caloric consumption, and as a result, weight loss from malabsorption may be masked. Edema may result from hypoalbuminemia caused by chronic protein malabsorption; loss of protein into the intestinal lumen can cause peripheral edema. With severe protein depletion, ascites may develop. Anemia resulting from malabsorption can be either microcytic (related to iron deficiency) or macrocytic (related to vitamin B12 deficiency). Anemia may also be associated with the underlying disease causing EPI. For instance, iron deficiency anemia is often a manifestation of celiac disease. Bleeding disorders are usually a consequence of vitamin K malabsorption and subsequent hypoprothrombinemia. Ecchymosis usually is the manifesting symptom, though melena and hematuria may occur on occasion [4].
The diagnosis of EPI is largely clinical. This condition may go undetected, both because the signs and symptoms are similar to those of other mucosal and luminal gastrointestinal diseases that may interfere with fat digestion and absorption and because the signs and symptoms of EPI in some cases may be obscured by dietary restrictions. A complete laboratory evaluation (including pancreatic function testing) is required not only to diagnose EPI but also to determine the extent of the malabsorption and assess the manifestations of the underlying disease, if present [4, 5]. Management of EPI is based primarily on pancreatic enzyme replacement therapy (PERT) but may also include lifestyle modifications and vitamin supplementation as appropriate. Conventional treatment of EPI involves replacement of pancreatic enzymes with a pancreatic enzyme preparation from pigs. But despite high doses of pancreatic enzymes used during therapy, normalization of digestion does not often occur and only partial corrections of the malnutrition have been reported [6]. Recent studies on an EPI pig model have shown a strong deteriorative effect of EPI on brain function and morphology [7]. However, it was also demonstrated that enrichment of the diet with pancreatic-like enzymes of microbial origin (PLEM) restored morpho-functional brain parameters [7–9]. Treatment of the malabsorption and gastrointestinal (GI) symptoms in EPI patients using the standard of porcine enzyme replacement therapy (PERT) is highly patient-dependent and only partially corrects the symptoms [10]. Problems associated with these current PERT include poor enzyme delivery to the duodenum due to enteric coating formulations, fibrosing colonopathy, and batch-to-batch impurity with the potential for viral contamination, in addition to a high pill burden [11–13].
In light of the uncertainties and potential health risks for PERT, pancreatic-like enzyme replacement therapy (PLERT) is under development. Studies on humans using PLEM demonstrated significant improvements in coefficients of fat (CFA) and protein (CNA) absorption, together with good growth, and maintenance of nutritional status [14].
Since in human patients the fat digestion and absorption are the most crucial issues in patients with EPI, the aim of the study was to determine whether PLERT can produce any quickly measurable changes in blood parameters related to fat digestion and absorption. The main task was to explore whether PLERT can be as good as PERT in EPI treatment. In the current study we monitored postprandial changes in fat and protein absorption expressed as the lipemic index (LI), plasma levels of triglycerides (TG) and non-esterified fatty acids (NEFA) as quickly measured parameters [15–17].
Material and methods
Animals and surgical procedures
The present study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved (approval No. M124-09) by Malmö/Lunds district court upon the recommendation of the local Lund University ethical committee. All efforts were made to minimize animal suffering. EPI was induced in 12 male pigs (breed: Swedish Landrace x Yorkshire x Hampshire) that were 10 ±4 weeks of age and weighed 10.0 ±1.1 kg. Typically, 3–5 weeks after surgery pigs develop EPI with steatorrhea and arrested growth even when fed a high-fat diet (HFD) [15]. In addition, 10 non-operated healthy pigs of the same age and breed were included as a comparator group. All pigs were catheterized in the jugular vein a week before the start of the study for easy blood collection, and placed into individual collection cages for easy and accurate feeding and collection of feces before any enzyme treatment and during the treatment periods.
Feeding
Following surgery and during the treatment periods, all pigs were fed a HFD twice daily (2% of body mass/meal) in the morning (8–9 a.m.) and in the evening (4–5 p.m.). The HFD consisted of standard cereal-based pelleted feed (Växtill, Lantmännen, Sweden) enriched with 15% extra fat composed of 40% rape seed oil (“Rapsolja”, Karlshaman, Sweden), and 60% cream from cow’s milk (“Vispgrädde”, Lantmejerer, Sweden (40% fat content)) resulting in a final fat content of around 18% [15]. Pigs were allowed to drink water ad libitum.
Study design
Eight pigs out of 12 operated were randomized for treatment with PLEM into two groups (first n = 3, second n = 5) and the 4 remaining EPI pigs were kept aside without treatment for histopathology analysis. Each animal served as its own control. Prior to PLEM dosing, pigs were placed into collection cages for a 7-day adaptation period followed by a 7-day control period where animals received no treatment. Thereafter, the EPI pigs were treated with PLEM for 7 days, followed by a 7-day wash-out period. Similar to EPI piglets, 10 healthy pigs (not operated) were divided into two groups (first n = 4; second n = 6). These healthy pigs were kept in collection cages for a 7-day adaptation period followed by a 7-day control period with a HFD. Food intake was measured daily, and body weight was measured at the beginning of each study phase. Twenty-four hour fecal samples were collected on days 5, 6 and 7 of the control, treatment, and wash-out weeks. Blood was collected on the last day of the respective control and treatment weeks at the following time points: 1 h before the morning meal (basal), +30 min, 1, 2, 3, 4, 5, 6 and 8 h after the meal. On the last day of the experiment, the pigs were euthanized by intravenous injection of pentobarbital (Mebumal, Nordvace, Sweden).
Administration of PLEM
A PLEM dose containing 120 000 active lipase units, 80 000 active protease units and 12 000 active amylase units (all from Sigma, St. Louis, MO, USA) was mixed with 20 ml of drinkable yogurt (0.5% fat, Skane Mejerier AB, Sweden), and this mixture was administered twice daily, in the middle of the morning and evening meal. Once the PLEM yogurt mix was consumed, the pigs were given the remaining portion of their meal.
Sample analysis
Feed and 24 h fecal samples were collected for nutrient content and fecal balance measurements. After homogenization and weight determination, all samples were processed for fat and protein content using standard gravimetric [18] and Kjeldahl methods [19, 20] in a specialized laboratory (Eurofins, Lidkoping, Sweden). CFA and CNA measures were calculated as previously described [15]. Blood lipid profiles were analyzed for LI and NEFA using a turbidimetric procedure [15] and colorimetric kit (Wako Chemicals GmbH, Germany), respectively. Plasma TG and total cholesterol were measured at Medilab, Tarnaby, Sweden.
Histology
After sacrification, samples of the proximal, middle and distal parts of the small intestine obtained from 4 EPI and 4 healthy pigs were embedded into paraffin, and stained with hematoxylin and eosin (H + E) according to the standard histological techniques. Analysis was performed using light microscopy (Olympus PROVIS [10×]) and the Image J 1.36 program (NIH, Bethesda, Maryland, USA). Mucosal thickness, defined as the distance from the muscularis mucosae to the top of the villus, was measured in 25–30 replicates for each part of the small intestine (mean of 60–100 measurements for each segment). Goblet cells were counted per mucosal area in the proximal part of the small intestine from EPI pigs, EPI pigs treated with PLEM, and healthy pigs.
Statistical analysis
All data were analyzed using the paired Student t-test, and one-way ANOVA multiple comparisons and the multiple comparisons with a Bonferroni correction (Student-Newman Keuls test; Sigma Stat 2.0, Jandel Scientific, SIGMA Scan Pro, USA). Data are expressed as mean ± standard deviation (SD). Any difference was considered statistically significant if the p-value was less than 0.05.
Results
Food consumption, lipid and protein digestion and somatic growth
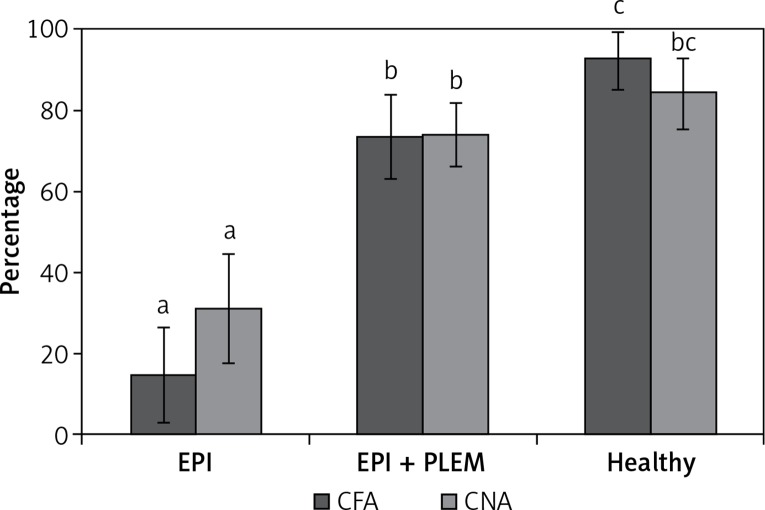
Figure 1 shows the mean percent of CFA (%CFA) and CNA (%CNA) on days 5–7 during PLEM treatment in EPI pigs compared to the EPI pigs and healthy pigs. During PLEM therapy, CFA and CNA increased by 59% and 43% (p < 0.05), respectively.
Figure 1.
CFA and CNA percentage in experimental animals at end of treatment week
CFA – coefficient of fat absorption, CNA – coefficient of nitrogen absorption, EPI group – pigs with pancreatic duct ligation (n = 4), EPI + PLEM group – pigs with pancreatic duct ligation and dietary supplementation with PLEM (pancreatic like enzymes of microbial origin) for 7 days (n = 5), Healthy group – pigs with intact pancreas (n = 6). Data are presented as mean ± SD. Different letters given with result bars describe significant differences when p < 0.05.
This correlated with a 38% (p < 0.05) reduction in the 24-hour stool weight in EPI pigs obtaining PLEM mixture. The stool appearance changed from soft, fatty looking, to a more normal appearance (data not shown). There were observed no changes in feed intake during the PLEM treatment period compared to the non-treatment period – all pigs consumed an amount of food equal to 4% of their body weight (280 g for EPI pigs, 320 for EPI + PLEM and 600 for healthy pigs). The feed conversion rate (FCR) was calculated as the amount of feed consumed to gain 1 kg of body weight. In EPI pigs FCR was undefined since animals do not gain weight. In EPI pigs treated with PLEM the FCR value was 2.9 ±1. Thus, the FCR value after PLEM treatment was essentially improved and was close to the one observed in healthy pigs (2.0 ±0.2), and EPI pigs treated with Creon [20]. Body mass in the group of EPI pigs treated with PLEM increased by 9% compared to baseline (10.6 ±2.0 kg to 11.6 ±2.5 kg, p < 0.05). This increase was slightly below the 14% increase recorded in comparator healthy pigs (from 14.8 ±3.7 to 16.9 ±4.1 kg). Furthermore, the removal of PLEM during the wash-out period in the sub-cohort of 4 EPI pigs resulted in a body mass reduction (–0.22 ±0.16 kg).
Postprandial plasma lipid profile
After 1 week of PLEM treatment, significant differences in LI were found between various sampling points before and after a meal in EPI and EPI + PLEM groups, and the postprandial LI curve of EPI pigs treated with PLEM resembled that of healthy pigs fed HFD (Table I). Lipemic index peaked 3 h after a meal in PLEM-treated pigs, at a level similar to the LI peak in healthy pigs and significantly higher than in untreated EPI pigs (Cmax: EPI + PLEM 2.82 ±0.57 vs. healthy 2.10 ±0.65, EPI untreated 0.92 ±0.12; Table I). Comparable to LI, fasting TG, NEFA and cholesterol levels were low and remained unchanged in EPI pigs after the morning HFD meal (Table I). However, after 1 week of PLEM treatment, postprandial changes in TG, NEFA, and cholesterol were observed. The mean TG level in EPI pigs fed a HFD and PLEM doubled 2 h after the morning meal (Cmax 0.91 ±0.25 mmol/l), similar to that in healthy pigs (Cmax 0.73 ±0.07 mmol/l), and higher than that in untreated EPI pigs (Cmax 0.54 ±0.11 mmol/l; Table I). The NEFA levels in PLEM-treated EPI pigs were comparable to the levels in healthy pigs, and peaked 2.5-fold above that of the untreated EPI pigs 3 h after a meal (EPI + PLEM Cmax 0.25 ±0.10 mmol/l, vs. healthy Cmax 0.19 ±0.06 mmol/l; EPI untreated Cmax 0.06 ±0.05 mmol/l; Table I).
Table I.
Postprandial blood lipid profile in experimental animals at end of treatment week
| Variable | Basal | 30 min | 1 h | 2 h | 3 h | 4 h | 6 h | 8 h | AUC |
|---|---|---|---|---|---|---|---|---|---|
| Lipemic index: | |||||||||
| EPI | 0.90 ±0.06 | 0.73 ±0.08* | 0.75 ±0.07* | 0.83 ±0.10* | 0.92 ±0.12* | 0.71 ±0.05* | 0.73 ±0.34* | 0.70 ±0.21* | 6.37 ±0.71*,# |
| EPI + PLEM | 1.10 ±0.18 | 1.20 ±0.27 | 1.54 ±0.46 | 2.41 ±0.33 | 2.82 ±0.57 | 2.34 ±0.54 | 1.62 ±0.22 | 1.66 ±0.35 | 14.89 ±0.95* |
| Healthy | 0.98 ±0.13 | 1.01 ±0.17 | 1.20 ±0.29 | 1.85 ±0.41 | 2.10 ±0.65 | 1.75 ±0.48 | 1.61 ±0.45 | 1.31 ±0.23 | 12.86 ±0.43 |
| TG concentration [mmol/l]: | |||||||||
| EPI | 0.41 ±0.12 | 0.44 ±0.13 | 0.45 ±0.13 | 0.54 ±0.11* | 0.53 ±0.04* | 0.43 ±0.13 | 0.42 ±0.09 | 0.55 ±0.17 | 3.6 ±0.1* |
| EPI + PLEM | 0.43 ±0.09 | 0.05 ±0.07 | 0.76 ±0.16* | 0.91 ±0.25 | 0.75 ±0.11 | 0.65 ±0.11 | 0.50 ±0.10 | 0.51 ±0.11 | 5.1 ±0.5 |
| Healthy | 0.35 ±0.15 | 0.32 ±0.15 | 0.39 ±0.04 | 0.73 ±0.07 | 0.65 ±0.05 | 0.57 ±0.09 | 0.48 ±0.08 | 0.35 ±0.13 | 3.8 ±0.7 |
| NEFA concentration [mmol/l]: | |||||||||
| EPI | 0.12 ±0.07 | 0.07 ±0.04 | 0.07 ±0.01* | 0.06 ±0.05* | 0.10 ±0.07* | 0.10 ±0.05* | 0.12 ±0.07 | 0.11 ±0.02 | 0.8 ±0.3* |
| EPI + PLEM | 0.12 ±0.09 | 0.13 ±0.06 | 0.14 ±0.03 | 0.25 ±0.10 | 0.30 ±0.11 | 0.21 ±0.04 | 0.17 ±0.05 | 0.15 ±0.05 | 1.4 ±0.1 |
| Healthy | 0.21 ±0.11 | 0.07 ±0.05 | 0.1 ±0.01 | 0.19 ±0.06 | 0.30 ±0.09 | 0.20 ±0.03 | 0.12 ±0.05 | 0.18 ±0.04 | 1.4 ±0.1 |
| Cholesterol concentration [mmol/l]: | |||||||||
| EPI | 2.41 ±0.27 | 2.18 ±0.11* | 2.23 ±0.12* | 2.27 ±0.23* | 2.25 ±0.11* | 2.25 ±0.21 | 2.25 ±0.21 | 2.25 ±0.17* | 16.5 ±0.2*,# |
| EPI + PLEM | 2.93 ±0.31 | 2.55 ±0.22 | 2.73 ±0.22 | 2.79 ±0.16 | 2.65 ±0.23 | 2.65 ±0.35 | 2.74 ±0.39 | 2.83 ±0.28 | 20.1 ±0.1* |
| Healthy | 3.12 ±0.38 | 2.64 ±0.25 | 2.71 ±0.23 | 2.86 ±0.13 | 2.75 ±0.37 | 2.75 ±0.15 | 2.71 ±0.43 | 2.85 ±0.32 | 22.9 ±0.7 |
TG – triglycerides, NEFA – non-esterified fatty acids, AUC – area under the curve, EPI group – pigs with pancreatic duct ligation (n = 4), EPI + PLEM group – pigs with pancreatic duct ligation and dietary supplementation with PLEM (pancreatic like-enzymes of microbial origin) for 7 days (n = 5), healthy group – pigs with intact pancreas (n = 6). Data are presented as mean ± SD.
Differences significant when p ≤ 0.05 in comparison with healthy group
differences significant when p ≤ 0.05 in comparison with EPI + PLEM group.
Mean cholesterol levels increased in PLEM-treated EPI pigs during postprandial period to that of healthy pigs fed a HFD (EPI + PLEM 2.79 ±0.16 mmol/l, vs. healthy 2.86 ±0.13 mmol/l, vs. EPI untreated; 2.27 ±0.23 mmol/l; Table I).
Significant differences in the area under the curve (AUC) values were demonstrated for LI, TG, and NEFA in EPI pigs treated with PLEM mixture (Table I), these parameters didn’t differ significantly from values recorded in healthy pigs.
Histomorphometric analysis
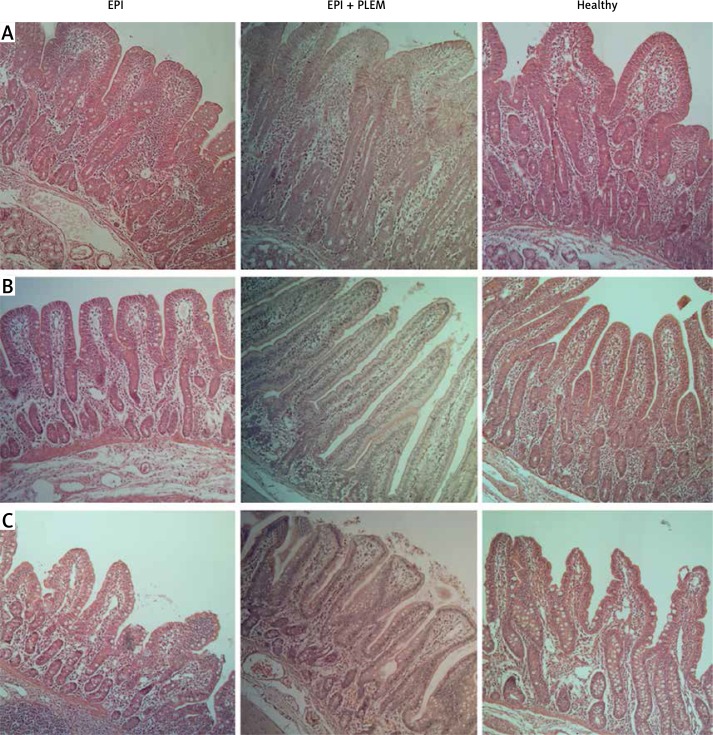
The length of the small intestine villi from the treated EPI pigs were not appreciably longer than of the villi of the EPI pigs (data not shown). However, the mucosal thickness significantly increased by 27%, 50% and 26%, in the proximal, middle, and distal small intestine in the EPI + PLEM pigs relative to the untreated EPI pigs, respectively (p < 0.05) (Figure 2, Table II). The analyzed sections of the small intestine of PLEM treated EPI animals were structurally similar to those seen in healthy pigs (Figure 2). In addition, morphometric analysis of the samples from proximal small intestine demonstrated a 54% decrease in the number of goblet cells after 7 days of treatment (p < 0.05) (Figure 2, Table II).
Figure 2.
Microphotographs of hematoxylin-eosin stained mucosal samples from proximal (A), middle (B) and distal (C) parts of small intestine
EPI group – pigs with pancreatic duct ligation (n = 4), EPI + PLEM group – pigs with pancreatic duct ligation and dietary supplementation with PLEM (pancreatic like-enzymes of microbial origin) for 7 days (n = 5), Healthy group – pigs with intact pancreas (n = 6). Magnification 400×. Bar 100 μm.
Table II.
Morphological parameters of small intestine in experimental animals at end of treatment week
| Variable | Mucosal thickness | Goblet cells/100 cells | ||
|---|---|---|---|---|
| Proximal | Middle | Distal | ||
| EPI | 0.63 ±0.05* | 0.51 ±0.07* | 0.58 ±0.06* | 65.22 ±3.71* |
| EPI + PLEM | 0.80 ±0.06 | 0.77 ±0.10 | 0.73 ±0.04 | 30.03 ±1.98 |
| Healthy | 0.97 ±0.12 | 0.83 ±0.13 | 0.72 ±0.07 | 22.6 ±2.24 |
EPI group – pigs with pancreatic duct ligation (n = 4), EPI + PLEM group – pigs with pancreatic duct ligation and dietary supplementation with PLEM (pancreatic like-enzymes of microbial origin) for 7 days (n = 5), Healthy group – pigs with intact pancreas (n = 6). Data are presented as mean ± SD.
Differences significant when p ≤ 0.05 in comparison with healthy group.
Discussion
Enteric-coated beads deliver enzymes poorly to the proximal duodenum and jejunum, where the majority of digestion of fats and proteins normally occurs [21]. Typically for PERTs, the standard efficacy measure is the percent change in fat and protein absorption. Interestingly, as a measure of efficacy, CFA and CNA have been inconsistent in patients with CF and EPI, but not in healthy individuals. This is probably due to the wide range of steatorrhea, compromised gastrointestinal function, or improper stool collection in patients with EPI [21, 22]. Therefore, the quick measurement of postprandial lipids, as a novel approach to efficacy for PLEM, was the main objective of this study, rather than conventional CFA. This was coupled to evaluated changes in the small bowel mucosal architecture, and determination of somatic growth.
The PLEM dose used in the study was based on previously published work with porcine pancreatic enzymes shown to improve digestibility in the same model of the pancreatic duct-ligated pigs [15, 16]. Each pig was administered a daily dose of 120,000 units of lipase with a mean daily fat intake of 54.2 ±31.9 g, which correlates to the suggested dose of 4000 lipase units/g of dietary fat in humans [23]. The PLEM-food mix was well tolerated. Treated pigs became more alert, playful, and interactive, suggesting improvement in overall health (data not shown). This behavioral observation was similar to the observed behavior of healthy pigs, and very different from lethargic and cachexic untreated EPI pigs [7, 24].
Therapy diminished steatorrhea, reduced stool weight, and significantly increased, but did not normalize, CFA compared to healthy pigs. Similar to patients with CF and EPI, where patient-to-patient variations in CFA are pronounced, CFA measurements taken at 72 h before treatment (14.7 ±27.8%) and during treatment (73.6 ±10.2%) were very different between EPI piglets and healthy pigs (92.6 ±1.2%). This suggests that malabsorption is not only the result of impaired secretion of pancreatic enzymes, but also the confounding effect of gut function, such as intestinal acidification, decreased bile salt concentration with improper micelle formation, reduced mucosal thickness, and epithelial dysfunction [15, 25–27].
It is well documented in healthy humans that after a HFD meal, 95% of dietary TGs are absorbed by the intestine [28]. Thus, the efficacy of PLEM was measured by changes in postprandial lipids after a HFD meal composed primarily of cows’ cream and rape seed oil. The plasma lipid profile remained relatively constant on the day of the control collection (last day of the non-treatment week), but significantly improved with PLEM treatment (last day of the treatment week). In PLEM-treated pigs, the LI peaked 3 h after feeding, similar to that of healthy pigs. These positive changes in total lipid concentration were mirrored in plasma TG and NEFA (Table I), and were similar to healthy pigs and as seen in healthy humans who consume a HFD meal [29]. Postprandial LI, TG and NEFA curves superimposed with the curves of healthy pigs resulted in the same calculated AUC values (Table I). The total blood fat load was cleared by 7 h because TG and NEFA returned to their initial pre-prandial values, and again were similar to those of healthy animals. We found that mean postprandial plasma cholesterol increased in PLEM-treated EPI pigs (Table I). These changes suggest the proper digestion of fat in the small intestine, but also an effect of PLEM on the formation of cholesterol particles that is associated with TG-rich lipoprotein catabolism in the visceral tissue, e.g. liver [30].
PLEM treatment resulted in enhanced protein absorption, and a near normalized feed conversion rate. These changes led to a reversal of growth impairment despite CNA being 10% below the values seen in healthy pigs. This supports our notion that CNA measurements, similar to CFA, are an inadequate measure of the efficacy of EPI treatments. After only 1 week of PLEM treatment, body mass increased by 9%, which is comparable to published work using porcine-based supplements in growing EPI pigs and less than that seen in healthy pigs [16, 19]. This difference in growth could possibly be minimized with a longer treatment period whereby the severity of the EPI disease is reduced. In support of this, in a 1-year study with PLEM in humans, daily treatment supported good growth and micronutrient absorption [31].
It is well known that patients suffering from EPI, including patients with CF, have lesions in the small intestine despite relatively preserved mucosal architecture. The mucosal thickness is reduced with abundant mucus released from numerous goblet cells on the surface of the epithelium [27, 32]. The same phenotypic characteristics were demonstrated in EPI pigs (Table II). However, after only 1 week of PLEM therapy, re-healing was demonstrated by increased mucosa thickness together with a reduction in the number of mucin-producing cells. These results indicate that the structure and function of the small intestine have a direct dependence on pancreatic-like enzyme supplementation which facilitates efficient digestion, absorption, and utilization of nutrients. Moreover, PLEM may indirectly cause a release of hormones or other peptides which help to rebuild the mucosal structure of the small intestine. This is critical since the small bowel is the primary site for nutrient and vitamin absorption, and thus is worth further investigation. More studies are needed to further evaluate which enzyme(s) from the PLEM mixture are essential for re-healing.
In summary, comparative analysis (healthy vs. EPI-PLEM) demonstrated that although PLEM-treated EPI pigs did not achieve treatment CFA values comparable to those observed in healthy pigs, they had normal absorption of TG, NEFA and cholesterol. This suggests a limitation of the old gravimetric CFA method, which only measures how much fat is digested, and not where digestion and absorption occur. In addition, analyses indicate that the digestion of dietary lipids with exogenously administered lipase occurs in the duodenum and upper small intestine, while allowing absorption in the distal ileum. Therefore, PLEM digests fat similarly to endogenously secreted pancreatic lipase, and differs from enteric coated porcine supplements in that it has been shown to facilitate the digestion of fat in the ileum [33]. In this study, EPI pigs were fed PLEM mixed with drinkable yogurt, demonstrating that PLEM is stable, and can be given mixed with liquids. This is important for young children and patients who have difficulty swallowing capsules.
In conclusion, PLEM treatment facilitated proper digestion and absorption of nutrients resulting in normalized postprandial plasma lipids, TG, NEFA and significant growth of EPI pigs. PLERT treatment does not require the creation of enteric coating formulations, and thus enzyme appearance in the duodenum is increased in comparison with conventional PERT. Also, as PLEM are products of microbial synthesis, the problem of batch-to-batch impurity with the potential for viral contamination disappears, which is an undeniable advantage of PLEM therapy in everyday practice. Thus, this therapy with non-porcine derived, microbial enzymes may be an important alternative to traditional PERT for the treatment of EPI.
Postprandial LI, TG and NEFA are perfect measures of efficacy, and are viable endpoints that should be explored in human trials instead of such parameters as CFA and CNA, which are complicated to measure and have limitations in the clinical setting.
Acknowledgments
Funding for this research was provided by a grant and financial contributions from Anara AB, Sweden, and Påhlsson Stiftelse, Sweden.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Zingg U, Oertli D. Functional syndromes after surgery of the upper gastrointestinal tract. Ther Umsch. 2012;69:39–47. doi: 10.1024/0040-5930/a000249. [DOI] [PubMed] [Google Scholar]
- 2.Monajemzadeh M, Ashtiani MT, Sadrian E, et al. Variation in plasma leptin levels in young Iranian children with cystic fibrosis. Arch Med Sci. 2013;9:883–7. doi: 10.5114/aoms.2013.38683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monajemzadeh M, Mokhtari S, Motamed F, et al. Plasma ghrelin levels in children with cystic fibrosis and healthy children. Arch Med Sci. 2013;9:93–7. doi: 10.5114/aoms.2012.28599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Kaade S. Exocrine pancreatic insufficiency. Medscape Reference. Drugs, Diseases & Procedures. Available at: http://emedicine.medscape.com/article/2121028-overview#showall. Assessed July 2015.
- 5.Wan C, Shen Y, Yang T, Wang T, Chen L, Wen F. Diagnostic value of microRNA for pancreatic cancer: a meta-analysis. Arch Med Sci. 2012;8:749–55. doi: 10.5114/aoms.2012.31609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalnins D, Wilschanski M. Maintenance of nutritional status in patients with cystic fibrosis: new and emergingtherapies. Drug Des Dev Ther. 2012;6:151–61. doi: 10.2147/DDDT.S9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goncharova K, Ushakova G, Kovalenko T, Osadchenko I, Skibo G, Pierzynowski SG. Diet supplemented with pancreatic-like enzymes of microbial origin restores the hippocampal neuronal plasticity and behaviour in young pigs with experimental exocrine pancreatic insufficiency. J Funct Foods. 2015;14:270–7. [Google Scholar]
- 8.Goncharova K, Skibo G, Kovalenko T, et al. Diet induced changes in brain structure and behaviour in old gerbils. Nutr Diab. 2015;5:e163. doi: 10.1038/nutd.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierzynowski S, Ushakova G, Kovalenko T, et al. Impact of colostrum and plasma immunoglobulin intake on hippocampus structure during early postnatal development in pigs. Int J Dev Neurosci. 2014;35:64–71. doi: 10.1016/j.ijdevneu.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Kalnins D, Ellis L, Corey M. Enteric-coated pancreatic enzymes with bicarbonate is equal to standard enetric-coated enzyme in treating malabsorption in cystic fibrosis. J Ped Gastroenterol Nutr. 2006;42:256–61. doi: 10.1097/01.mpg.0000189356.93784.01. [DOI] [PubMed] [Google Scholar]
- 11.Layer P, Keller J. Enzyme pellet size and luminal nutrients digestion in pancreatic insufficiency. Digestion. 1992;28:3–10. [Google Scholar]
- 12.Guarner L, Rodríguez R, Guarner F, Malagelada JR. Fate of oral enzymes in pancreatic insufficiency. Gut. 1993;34:708–12. doi: 10.1136/gut.34.5.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd-Still JD. Editorial: cystic fibrosis and colonic strictures. J Clin Gastroenterol. 1995;21:2–5. [PubMed] [Google Scholar]
- 14.Borowitz D, Goss CH, Limauro S, et al. Study of a novel pancreatic enzyme replacement therapy in pancreatic insufficient subjects with cystic fibrosis. J Pediatr. 2006;49:658–62. doi: 10.1016/j.jpeds.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Donaldson J, Fed’kiv O, Pawłowska M, et al. The effectiveness of enzymatic replacement therapy measured by turbidimetry and the lipaemic index in exocrine pancreatic insufficient young, growing pigs, fed a high-fat diet. Adv Med Sci. 2009;54:7–13. doi: 10.2478/v10039-009-0011-x. [DOI] [PubMed] [Google Scholar]
- 16.Rengman S, Fedkiv O, Botermans J, Svendsen J, Weström B, Pierzynowski S. An elemental diet fed, enteral or parenteral, does not support growth in young pigs with exocrine pancreatic insufficiency. Clin Nutr. 2009;28:325–30. doi: 10.1016/j.clnu.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Goncharova K, Pierzynowski SG, Grujic D, et al. A piglet with surgically induced exocrine pancreatic insufficiency as an animal model of newborns to study fat digestion. Br J Nutr. 2014;112:2060–7. doi: 10.1017/S0007114514003286. [DOI] [PubMed] [Google Scholar]
- 18.van de Kamer JH. Standard methods of clinical chemistry. Vol. 2. New York: Academic Press; 1958. Total fatty acids in stool; pp. 34–9. [Google Scholar]
- 19.Bradstreet RB. Kjeldahl method for organic nitrogen. Anal Chem. 1954;26:185–6. [Google Scholar]
- 20.Fedkiv O, Rengman S, Westrom BR. Growth is dependent on the exocrine pancreas function in young weaners but not in the growing-finishing pigs. J Physiol Pharmacol. 2009;60(Suppl 3):55–9. [PubMed] [Google Scholar]
- 21.Baker SS. Delayed release pancrelipase for the treatment of pancreatic exocrine insufficiency associated with cystic fibrosis. Ther Clin Risk Manag. 2008;4:1079–84. doi: 10.2147/tcrm.s3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borowitz D, Konstant MW, O’Rourke A. Coefficient of fat and nitrogen absorption in healthy subjects and individuals with cystic fibrosis. J Pediatr Pharmacol Ther. 2007;12:47–52. doi: 10.5863/1551-6776-12.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graff GR, Maguiness K, McNamara J. Efficacy and tolerability of a new formulation of pancrelipase delayed-release capsules in children aged 7 to 11 years with exocrine pancreatic insufficiency and cystic fibrosis: a multicenter, randomized, double-blind, placebo-controlled, two-period crossover, superiority study. Clin Ther. 2010;32:89–93. doi: 10.1016/j.clinthera.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Pierzynowski S, Swieboda P, Filip R, et al. Behavioral changes in response to feeding pancreatic-like enzymes to exocrine pancreatic insufficiency pigs. J Anim Sci. 2012;90:439–41. doi: 10.2527/jas.53868. [DOI] [PubMed] [Google Scholar]
- 25.Senegas-Balas F, Bastie MJ, Balas D, et al. Histological variations of the duodenal mucosa in chronic human pancreatitis. Dig Dis Sci. 1982;27:917–22. doi: 10.1007/BF01316576. [DOI] [PubMed] [Google Scholar]
- 26.Balas D, Senegas-Balas FS, Bertrand C, Frexinos J, Ribet A. Effects of pancreatic duct ligation on the hamster intestinal mucosa. Digestion. 1980;20:157–67. doi: 10.1159/000198435. [DOI] [PubMed] [Google Scholar]
- 27.Sbarbati A, Bertini M, Catassi C. Ultrastructural lesions in the small bowel of patients with cystic fibrosis. Ped Res. 1998;43:234–9. doi: 10.1203/00006450-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Van der Meer RW, Hammer S, Lamb HJ. Effects of the short term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J Clin Endocrinol Metab. 2008;93:2702–8. doi: 10.1210/jc.2007-2524. [DOI] [PubMed] [Google Scholar]
- 29.De Haene H, Taes Y, Christophe A, Delanghe J. Comparison of triglyceride concentration with lipemic index in disorders of triglyceride and glycerol metabolism. Clin Chem Lab Med. 2008;44:220–2. doi: 10.1515/CCLM.2006.040. [DOI] [PubMed] [Google Scholar]
- 30.Couillard C, Bergeron N, Bergeron J. Metabolic heterogeneity underlining postprandial lipaemia among men with low fasting high density lipoprotein cholesterol concentrations. J Clin Endoc Metab. 2000;85:4575–82. doi: 10.1210/jcem.85.12.7037. [DOI] [PubMed] [Google Scholar]
- 31.Borowitz D, Stevens C, Brettman LR, Campion M, Chatfield B, Cipolli M, Liprotamase 726 Study Group International phase III trial of liprotamase efficacy and safety in pancreatic-insufficient cystic fibrosis patients. J Cyst Fibros. 2011;10:443–52. doi: 10.1016/j.jcf.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Yang Q, Kock N. Intestinal adaptation following massive ileocecal resection in 20-day-old weanling rats. J Ped Gastroenerol. 2009;50:16–21. doi: 10.1097/MPG.0b013e3181c2c2af. [DOI] [PubMed] [Google Scholar]
- 33.Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clin Exp Gastroenterol. 2011;4:55–73. doi: 10.2147/CEG.S17634. [DOI] [PMC free article] [PubMed] [Google Scholar]