AUXIN RESPONSE FACTOR3 integrates the activities of AGAMOUS and auxin to control cytokinin biosynthesis and signaling, thereby coordinately regulating floral meristem maintenance and termination.
Abstract
Successful floral meristem (FM) determinacy is critical for subsequent reproductive development and the plant life cycle. Although the phytohormones cytokinin and auxin interact to coregulate many aspects of plant development, whether and how cytokinin and auxin function in FM determinacy remain unclear. Here, we show that in Arabidopsis thaliana, cytokinin homeostasis is critical for FM determinacy. In this developmental context, auxin promotes the expression of AUXIN RESPONSE FACTOR3 (ARF3) to repress cytokinin activity. ARF3 directly represses the expression of ISOPENTENYLTRANSFERASE (IPT) family genes and indirectly represses LONELY GUY (LOG) family genes, both of which encode enzymes required for cytokinin biosynthesis. ARF3 also directly inhibits the expression of ARABIDOPSIS HISTIDINE KINASE4, a cytokinin receptor gene, resulting in reduced cytokinin activity. Consequently, ARF3 controls cell division by regulating cell cycle gene expression through cytokinin. In flowers, we show that AGAMOUS (AG) dynamically regulates the expression of ARF3 and IPTs, resulting in coordinated regulation of FM maintenance and termination through cell division. Moreover, genome-wide transcriptional profiling revealed both repressive and active roles for ARF3 in early flower development. Our findings establish a molecular link between AG and auxin/cytokinin and shed light on the mechanisms of stem cell maintenance and termination in the FM.
INTRODUCTION
Plant meristems are responsible for producing all postembryonic parts of the plant body. While the shoot apical meristem (SAM) has a permanent stem cell pool that results in the formation of the indeterminate shoot, the floral meristem (FM) produces a defined number of floral organs and subsequently undergoes a programmed termination in a process termed FM determinacy (Sablowski, 2007a). Successful FM determinacy ensures normal reproductive development and life cycle progression. Thus, meristem maintenance and termination are critical for plant organogenesis and metagenesis. At the same time, stem cells located at the centers of meristems have the capacity for both self-renewal and the production of daughter cells that undergo differentiation (Laux, 2003), while the SAM has a permanent stem cell pool that results in the formation of the indeterminate shoot (Sablowski, 2007a; Chandler, 2012). The FM is therefore a good model for understanding the temporal regulation of stem cell activity (Ji et al., 2011; Liu et al., 2011).
In plants and animals, stem cells reside in stem cell niches, a specialized microenvironment that produces intercellular signals to sustain the self-renewing capacity of adjacent stem cells (Sablowski, 2004; Scheres, 2007; Aichinger et al., 2012). In the model plant Arabidopsis thaliana, the homeobox transcription factor gene WUSHCEL (WUS) is exclusively expressed in a group of cells called the organizing center (OC) located under the stem cells of the SAM (Mayer et al., 1998). While the null wus-1 mutation leads to premature termination of meristems, ectopic WUS expression is sufficient to induce surrounding cells to form stem cell-like cells, indicating that WUS is critical for the establishment, maintenance and termination of stem cells (Laux et al., 1996; Schoof et al., 2000; Gallois et al., 2004). WUS diffuses into upper stem cell layers through intercellular movement, where WUS activates CLAVATA3 (CLV3) expression in a dose-dependent manner (Yadav et al., 2011; Daum et al., 2014; Perales et al., 2016). CLV3, in turn, encodes a signal peptide that binds to plasma membrane-localized receptor-like kinases, receptor-like proteins, and receptor-like cytoplasmic kinases in OC cells to inhibit WUS expression (reviewed in Somssich et al., 2016). Thus, the CLV/WUS feedback regulatory pathway ensures the maintenance of the stem cell pool in the SAM and FM. However, this negative feedback loop does not trigger the programmed termination of the FM, which instead is primarily regulated by AGAMOUS (AG), a MADS domain protein with critical roles in floral organ identity and FM determinacy (reviewed in Irish, 2010). AG functions to ensure that WUS expression is shut off (Lenhard et al., 2001) by stage 6 of flower development (stages according to Smyth et al., 1990), when the carpel primordia have formed. In ag-1 plants, prolonged WUS expression in the FM results in a flower-in-flower phenotype, indicating that WUS is normally repressed by AG (Bowman et al., 1989; Lenhard et al., 2001). On the other hand, LEAFY and WUS directly activate AG expression at stage 3 of flower development (Lohmann et al., 2001; Hong et al., 2003), with the time gap between stage 3 and stage 6 indicating that the role of AG in inhibiting WUS expression is indirect (Lenhard et al., 2001). How AG represses WUS has been an intriguing puzzle during the past two decades. While several FM determinacy factors have been isolated as regulators of WUS expression whose functions are dependent or independent of the AG pathway, the intricate mechanisms underlying the role of AG in repressing WUS expression have yet to be fully resolved (Sablowski, 2007a; Cao et al., 2015). AG has been shown to repress WUS through both indirect and direct means: WUS is indirectly inhibited by AG through the AG target gene KNUCKLES (KNU) at stage 6 and directly repressed by AG through AG-dependent recruitment of the Polycomb group protein-terminal FLOWER2 to the WUS locus (Sun et al., 2009; Liu et al., 2011). Nevertheless, the weak FM determinacy defects of knu and ag-10 tfl2-2 suggest that other coeffectors exist in the AG genetic pathway that await further characterization (Payne et al., 2004; Liu et al., 2011).
Plant hormones regulate many aspects of plant growth and development. For example, the formation and maintenance of the SAM are mainly controlled by the paradoxically antagonistic and complementary interaction between cytokinin and auxin at the levels of biosynthesis, transport, metabolism, and signaling (reviewed in Pernisová et al., 2011; Su et al., 2011; Chandler and Werr, 2015; Schaller et al., 2015). Cytokinin is biosynthesized by members of the ISOPENTENYLTRANSFERASE (IPT) and LONELY GUY (LOG) enzyme families (Kieber and Schaller, 2014). In the Arabidopsis SAM and FM, LOG4 expression is restricted to layer 1 (L1) epidermal cells, suggesting that apically derived active cytokinin is biosynthesized in the cells of the upper layer (Chickarmane et al., 2012). By contrast, the expression of the cytokinin receptor gene ARABIDOPSIS HISTIDINE KINASE4 (AHK4) is concentrated in the OC cells that overlap the WUS expression domain (Gordon et al., 2009), which is consistent with the maxima of cytokinin activity in the OC based on ProTCSn:GFP (Two Component Signaling Sensor [new]:Green Fluorescent Protein) reporter analysis (Chickarmane et al., 2012; Zürcher et al., 2013). Accordingly, cytokinin is required for the activation of WUS expression in an AHK2/AHK4-dependent manner (Gordon et al., 2009). WUS, in turn, represses the expression of several A-type response regulator (ARR) genes, such as ARR5, ARR7, and ARR15, which encode negative regulators of cytokinin signaling, resulting in increased cytokinin activity in the OC (Leibfried et al., 2005; Zhao et al., 2010). Exogenous cytokinin treatment increases the SAM surface area in wild-type plants by altering cell division; it also increases WUS expression to levels sufficient enough to restore inner floral organ development in the hypomorphic wus-6 mutant, indicating that cytokinin promotes cell division and WUS expression (Chickarmane et al., 2012). A very recent study demonstrated that cytokinin activates WUS expression de novo during axillary meristem initiation (Wang et al., 2017). Cytokinin oxidase/dehydrogenase (CKX) enzymes degrade cytokinin, and mutations in CKX3 and CKX5 lead to enlarged inflorescences and FMs along with an increased WUS expression domain, further indicating that cytokinin plays an important role in FM maintenance and activity (Bartrina et al., 2011). Thus, cytokinin is a key element in SAM/FM maintenance through a WUS/cytokinin signaling regulatory loop. However, the role of cytokinin in FM determinacy is still unclear.
In the SAM, the maxima of auxin activity occur at the initiation sites of organ primordia based on analysis of ProDR5:VENUS reporter expression (Murray et al., 2012). Auxin induces cellular differentiation and organ outgrowth and is therefore critical for SAM maintenance (reviewed in Vernoux et al., 2010). Auxin signaling is mediated by two transcription factor families: The auxin response factors (ARFs) activate or repress auxin response gene expression by binding to the promoters of target genes and interacting with members of the second transcription factor family, the AUX/IAA repressors, which are auxin-responsive (Ulmasov et al., 1999b; Liscum and Reed, 2002). Arabidopsis contains 23 ARFs with distinct expression patterns and functions during plant development (Okushima et al., 2005; Li et al., 2016b). ARF5/MONOPTEROS, for example, is essential for leaf initiation and vein pattern formation as well as the formation and maintenance of the SAM (Berleth and Jurgens, 1993; Hardtke and Berleth, 1998; Ckurshumova et al., 2014; Krogan et al., 2016). ARF5 mediates the crosstalk between auxin and cytokinin signaling required for SAM maintenance; specifically, ARF5 represses ARR7 and ARR15 expression in the central and peripheral zones of the meristem, indicating that ARR7 and ARR15 integrate cytokinin and auxin signaling to maintain the SAM (Zhao et al., 2010). However, it remains enigmatic whether auxin functions in FM determinacy and, if so, how auxin and cytokinin interact in this process.
ARF3, also known as ETTIN, was functionally characterized through its roles in regulating gynoecium morphogenesis, self-incompatibility, de novo organ regeneration, and organ polarity (Sessions et al., 1997; Nemhauser et al., 2000; Chitwood et al., 2009; Tantikanjana and Nasrallah, 2012; Cheng et al., 2013). Using genetic and in situ hybridization analyses, we previously showed that ARF3 promotes FM determinacy by repressing WUS expression (Liu et al., 2014). Further studies revealed that ARF3 is an APETALA2 (AP2) target gene whose expression is dynamically regulated by AG (Ng et al., 2009; Liu et al., 2014). Thus, ARF3 integrates the functions of AP2 and AG to regulate FM determinacy, but the molecular mechanism by which ARF3 promotes FM determinacy is still unclear. Here, we report that in Arabidopsis, cytokinin functions in regulating FM determinacy and that ARF3 coordinates with AG to repress the expression of IPT3, IPT5, IPT7, LOGs, and AHK4; this repression inhibits cytokinin activity, resulting in the timely termination of WUS expression. Intriguingly, while ARF3 partially mediates the function of auxin in repressing the activity of cytokinin to regulate FM determinacy, this protein might also act in the AG pathway to fine-tune cytokinin activity and coordinate FM maintenance and termination through cell division during early flower development.
RESULTS
Exogenous Cytokinin Treatment Enhances the FM Determinacy Defect of ag-10
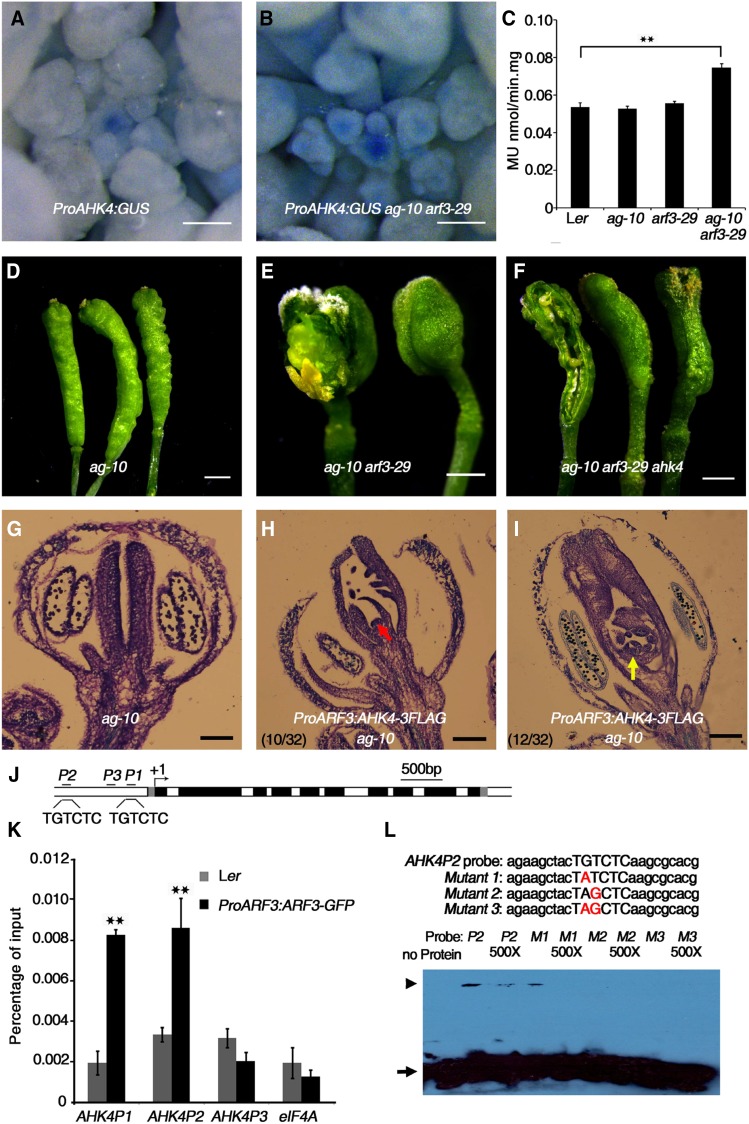
To investigate the roles of cytokinin in regulating meristem activity, we treated Arabidopsis Ler, ag-10, arf3-29 (Liu et al., 2014), and ag-10 arf3-29 inflorescences with 6-benzylaminopurine (6-BA), a synthetic cytokinin, once daily for 5 d. Compared with the respective untreated plants, the cytokinin-treated plants had a significantly larger SAM (Supplemental Figures 1A to 1H). This finding is consistent with previous reports that increased active cytokinin levels reinforce SAM activity (Werner et al., 2003; Kurakawa et al., 2007; Chickarmane et al., 2012) and with the known functions of cytokinin in promoting cell division and positively regulating SAM growth (Chickarmane et al., 2012). Statistical analysis confirmed the expansion of SAM size in cytokinin-treated plants (Supplemental Figure 1I). SAM size in the arf3 mutant background was also noticeably larger than in control plants (arf3-29 versus Ler and ag-10 arf3-29 versus ag-10) (Supplemental Figure 1I). This finding, together with the results of the cytokinin treatment assay described above, suggests that ARF3 might regulate SAM activity via cytokinin.
We then investigated the role of cytokinin in FM termination. Analysis of longitudinal silique sections showed that cytokinin-treated wild-type plants produced normal siliques with the same FM determinacy phenotype as untreated plants (Figures 1A and 1B), indicating that cytokinin has no effect on FM determinacy during flower development. However, we could not rule out the possibility that such a role might be masked by cytokinin signaling homeostasis accomplished via negative feedback regulation (Kieber and Schaller, 2014). We then focused on ag-10 and ag-10 arf3-29 plants. Most ag-10 siliques exhibit normal FM determinacy (Ji et al., 2011), and only a few genes have significantly altered expression (fold change >2) in the ag-10 mutant versus the wild type based on genome-wide gene expression profiling (Li et al., 2016a). Nevertheless, we previously isolated and functionally characterized several FM determinacy regulators via EMS mutagenesis of ag-10 (Cao et al., 2015; Li et al., 2016a). We therefore reasoned that the FM homeostasis that is perturbed in ag-10 is critical for FM maintenance and termination (Liu et al., 2016). Analysis of longitudinal sections revealed that the siliques of cytokinin-treated ag-10 had more bulges compared with untreated plants and produced additional tissues within the primary carpel, indicating a severe FM determinacy defect due to exogenous cytokinin treatment (Figures 1C and 1D). We also detected a slight enhancement in terms of extra tissue growing within siliques in cytokinin-treated ag-10 arf3-29 compared with untreated plants (Figures 1E and 1F). One possible reason that the enhancement was not as obvious as in treated ag-10 is that cytokinin homeostasis may have been sufficiently impaired in ag-10 arf3-29 so that exogenous cytokinin treatment had less of an effect on FM determinacy. In other words, ARF3 may genetically interact with cytokinin to regulate FM determinacy.
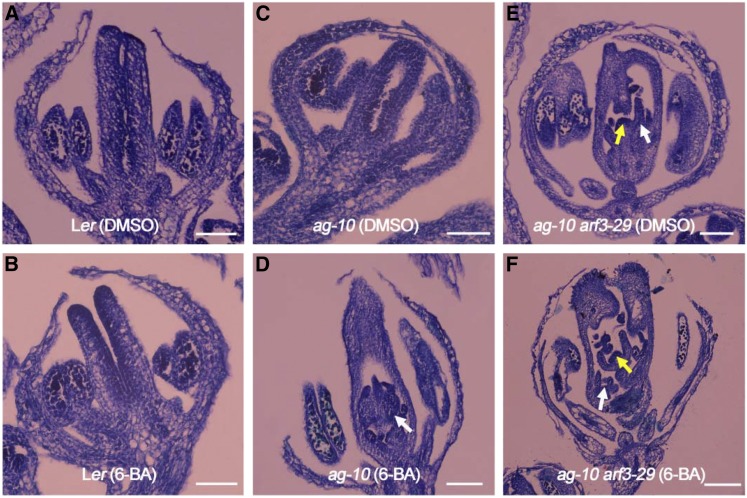
Figure 1.
Exogenous Cytokinin Treatment Enhances the FM Determinacy Defect of ag-10.
(A) and (B) Longitudinal sections of Ler siliques after DMSO (A) and 6-BA (B) treatment. No obvious FM determinacy defect was detected in 6-BA-treated siliques.
(C) and (D) Longitudinal sections of ag-10 siliques after DMSO (C) and 6-BA (D) treatment. Severe FM indeterminacy was observed in 6-BA-treated ag-10 siliques, as characterized by additional tissues in primary carpels (marked by a white arrow).
(E) and (F) Longitudinal sections of ag-10 arf3-29 siliques after DMSO (E) and 6-BA (F) treatment. Slightly enhanced FM indeterminacy was detected in the siliques of 6-BA-treated plants compared with DMSO-treated plants. Prolonged FMs and additional tissues are marked by yellow and white arrows, respectively.
Bars = 50 µm.
ARF3 Represses Cytokinin Levels and Activity during Early Flower Development
To investigate the relationship between ARF3 and cytokinin, we crossed the ProTCSn:GFP line, a synthetic reporter of endogenous cytokinin activity (Zürcher et al., 2013), with ag-10, arf3-29 and ag-10 arf3-29. In Ler and ag-10, we found high GFP fluorescence intensity in the FM, particularly in the OC regions of flower buds during early development, as previously described in the SAM (Zürcher et al., 2013), indicating that cytokinin activity is high in the OC regions of FMs (Figures 2A and 2B). Compared with Ler and ag-10 under the same imaging setup, we observed stronger GFP signals in the FMs of arf3-29 and ag-10 arf3-29 (Figures 2C and 2D). We also used RT-qPCR to measure GFP transcript levels in inflorescences containing stage 6 and younger flowers. In both arf3-29 versus Ler and ag-10 arf3-29 versus ag-10, GFP transcript levels were significantly elevated, with a more dramatic increase observed in the latter comparison (Figure 2E), indicating that cytokinin activity is derepressed in the FMs of arf3-29 and ag-10 arf3-29.
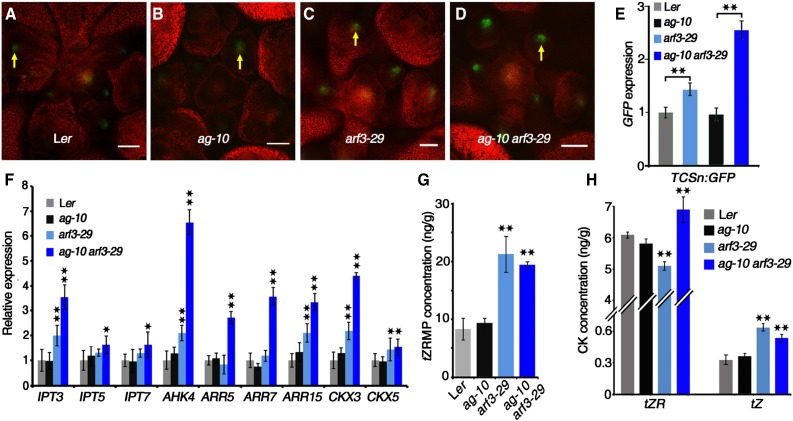
Figure 2.
ARF3 Represses Cytokinin Activity and Levels.
(A) to (D) Expression of ProTCSn:GFP (green) in FMs. Images show transverse sections of early flower buds of Ler (A), ag-10 (B), arf3-29 (C), and ag-10 arf3-29 (D). Yellow arrows indicate GFP in the OC regions of FMs. Bars = 50 µm.
(E) and (F) Transcript levels of GFP (E) and genes involved in cytokinin biosynthesis, signaling, and metabolism (F) in the indicated plants. RNA was extracted from inflorescences containing stage 6 and younger flowers for RT-qPCR. Error bars indicate the sd from three biological replicates with independently prepared inflorescence materials. *P < 0.05 and **P < 0.01 (Student’s t test).
(G) and (H) Mass spectrometric measurements of tZRMP (G) and tZR and tZ (H) in inflorescences of the indicated plants. Mean values of three replicates with independently prepared inflorescence materials containing unopened flowers are shown. Error bars indicate the sd of three biological experiments. **P < 0.01 (Student’s t test) between the inflorescences of wild type and the indicated mutants.
To dissect which aspects of cytokinin signaling were impaired by ARF3, we examined the expression of the following cytokinin-related genes with previously characterized functions in SAM maintenance: cytokinin biosynthesis genes IPT3, IPT5, and IPT7; signaling transduction genes AHK4, ARR5, ARR7, and ARR15; and CHX3 and CHX5, which are involved in cytokinin metabolism. All of these genes were upregulated in ag-10 arf3-29 inflorescences compared with Ler and ag-10 (Figure 2F). IPT3, AHK4, ARR15, CKX3, and CHX5 expression was also higher in arf3-29 inflorescences compared with Ler (Figure 2F). It is important to note that cytokinin signaling is regulated by a negative feedback loop in which increased cytokinin activity induces type-A ARRs and CKXs, which in turn negatively regulate cytokinin signaling (reviewed in Kieber and Schaller, 2014). To mimic the elevated cytokinin activity in arf3-29 and ag-10 arf3-29 plants, we treated Ler and ag-10 inflorescences with 6-BA. RT-qPCR showed increased levels of AHK4, ARR7, ARR15, CKX3, and CKX5 and decreased levels of IPT3, IPT5, and IPT7 expression after exogenous 6-BA treatment, which is consistent with previous reports (Supplemental Figure 2) (Miyawaki et al., 2004). We therefore hypothesized that in the wild type, ARF3 perturbs cytokinin activity by repressing the expression of IPT genes.
To test our hypothesis, we directly measured cytokinin levels in early-stage inflorescences by quantifying the endogenous levels of trans-zeatin riboside (tZR) and trans-zeatin (tZ) as well as their precursor trans-zeatin riboside 5′-monophosphate (tZRMP). tZ is the active and free base form of cytokinin, which is directly converted from tZRMP by the LOG family of enzymes (Kuroha et al., 2009). Alternatively, tZRMP may be converted to tZR and then to tZ through a two-step pathway (Kieber and Schaller, 2014). While Ler and ag-10 had comparable tZRMP, tZR, and tZ levels, ag-10 arf3-29 had dramatically increased levels of tZRMP, tZR, and tZ (Figures 2G and 2H). In arf3-29 inflorescences, tZRMP and tZ levels were similarly elevated, but tZR levels were significantly reduced (Figure 2H), indicating that cytokinin biosynthesis is impaired in the arf3-29 mutant. Collectively, these results suggest that ARF3 represses cytokinin levels and activity during early flower development.
ARF3 Directly Inhibits the Expression of IPT3, IPT5, and IPT7 to Regulate FM Determinacy
We previously demonstrated that the arf3-29 mutation severely enhances the FM determinacy defect of ag-10, as characterized by bulged and infertile siliques with additional tissues growing inside (Figures 3A and 3B) (Liu et al., 2014). To investigate the role of cytokinin in ARF3-promoted FM determinacy, we utilized the ipt3 ipt5 ipt7 triple mutant to introduce these mutations into ag-10 arf3-29. IPT3, IPT5, and IPT7 encode ATP/ADP IPTs that are responsible for the biosynthesis of tZ-type cytokinin (Miyawaki et al., 2006). While none of the single or double mutant combinations of ipt3, ipt5, and ipt7 exhibit visible phenotypes, the triple mutant has short and thin aboveground parts along with a reduced SAM size and thin inflorescence stems (Supplemental Figure 3A) (Miyawaki et al., 2006). The quintuple mutant ag-10 arf3-29 ipt3 ipt5 ipt7 was indistinguishable from the wild type at the seedling stage, but it was lethal after bolting, with only a few floral buds found in seedlings (Supplemental Figure 3B). All double mutant combinations (ipt3 ipt5, ipt3 ipt7, and ipt5 ipt7) partially rescued the severe FM indeterminacy of ag-10 arf3-29, as indicated by the presence of spindly siliques that produced a few seeds and contained less excess tissue in ag-10 arf3-29 ipt3 ipt5, ag-10 arf3-29 ipt3 ipt7, and ag-10 arf3-29 ipt5 ipt7 (Figures 3C to 3E).
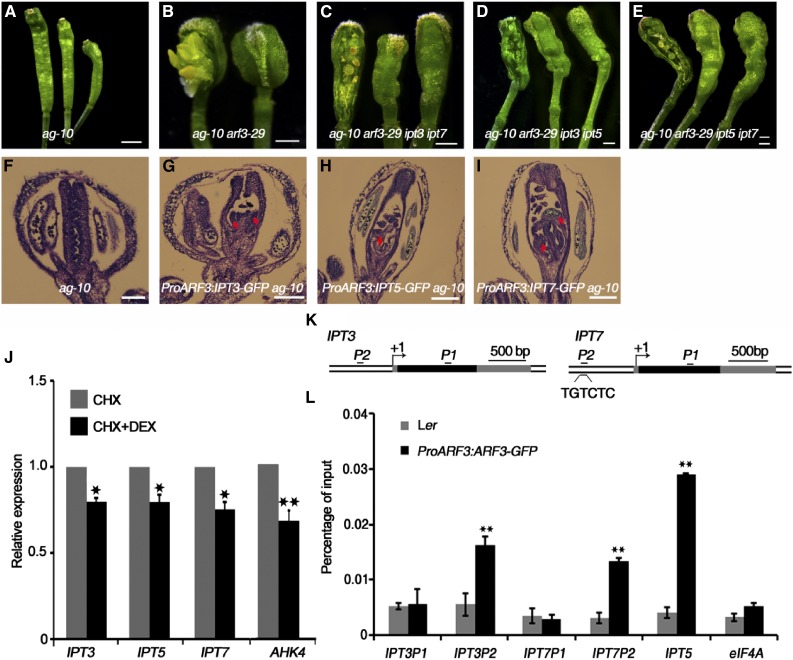
Figure 3.
ARF3 Directly Inhibits the Expression of IPT3, IPT5, and IPT7 to Regulate FM Determinacy.
(A) to (E) Representative silique phenotypes of ag-10 (A), ag-10 arf3-29 (B), ag-10 arf3-29 ipt3 ipt7 (C), ag-10 arf3-29 ipt3 ipt5 (D), and ag-10 arf3-29 ipt5 ipt7 (E). In (B) to (E), the leftmost silique has one carpel removed from the primary gynoecium to show the structures inside. ipt3 ipt7, ipt3 ipt5, and ipt5 ipt7 partially rescued the FM determinacy defect of ag-10 arf3-29, as characterized by a fertile and elongated ovary with fewer excess tissues inside.
(F) to (I) Longitudinal sections of representative siliques of ag-10 (F), ProARF3:IPT3-GFP ag-10 (G), ProARF3:IPT5-GFP ag-10 (H), and ProARF3:IPT7-GFP ag-10 (I). Increased IPT gene expression in ARF3 expression regions resulted in FM determinacy defects, such as extra tissues (marked by red arrows) growing within the primary ovaries of transgenic plants.
(J) Transcript levels of the indicated genes in ProARF3:ARF3-GR arf3-29 inflorescences after 8 h treatment with CHX or CHX+DEX. Inflorescences containing stage 6 and younger flowers were collected for RT-qPCR. Error bars indicate the sd from three biological replicates with independently prepared inflorescence materials. *P < 0.05 and **P < 0.01 (Student’s t test).
(K) Diagrams of the IPT3 and IPT7 genomic regions with an arrow indicating the transcription start site (+1). Black, coding sequences; dark gray, untranslated regions. Black bold lines with P1 and P2 indicate fragments examined by ChIP-qPCR. “TGTCTC” is the typical ARF binding motif. Bar = 500 bp.
(L) ChIP assay with anti-GFP antibody to examine ARF3 binding at IPT3 and IPT7 in Ler and ProARF3:ARF3-GFP inflorescences. The regions from IPT3 and IPT7 marked in Figure 3K were examined. IPT5, an ARF3 target gene, served as a positive control, and eIF4A (EUKARYOTIC TRANSLATION INITIATION FACTOR4A) served as a negative control. Error bars represent the sd from three biological repeats. **P < 0.01 (Student’s t test) between Ler and ProARF3:ARF3-GFP inflorescences.
Bars = 1 mm in (A) to (E) and 50 µm in (F) to (I).
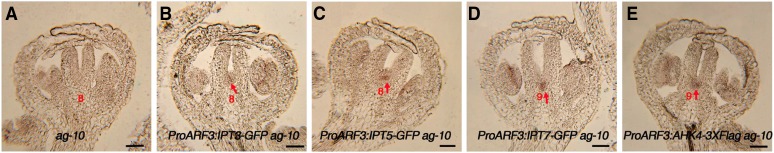
To increase endogenous cytokinin levels in ARF3 expression regions in ag-10 FMs, IPT3, IPT5, and IPT7 were each expressed under the control of the ARF3 promoter. Confocal microscopy imaging confirmed that the distribution patterns of IPT3-GFP, IPT5-GFP, and IPT7-GFP were similar to that of ARF3-GFP (Supplemental Figures 4A to 4C; Liu et al., 2014). The homozygous single-copy insertion lines in the ag-10 background exhibited enhanced FM determinacy defects (compare the longitudinal sections in Figures 3G to 3I with Figure 3F) that phenocopied the silique phenotype of ag-10 treated with exogenous cytokinin. During early development, the transgenic plants produced siliques with additional tissues growing inside the primary carpels, and at later stages, the siliques were composed of more than two fused carpels (Supplemental Figures 4D to 4F and Supplemental Table 1).
To investigate whether ARF3 directly regulates IPT3, IPT5, or IPT7, we generated a transgenic construct to express an ARF3-glucocorticoid receptor (GR) fusion protein under the control of the native ARF3 promoter. We introduced this construct into the arf3-29 mutant background to generate ProARF3:ARF3-GR arf3-29. Under dexamethasone (DEX) treatment, the ARF3-GR fusion protein translocated into the nucleus and rescued the arf3-29 phenotype (Supplemental Figure 5). We examined the effect of ARF3-GR on the expression of IPT3, IPT5, and IPT7 by RT-qPCR. DEX treatment for 8 h in the presence of the protein synthesis inhibitor cycloheximide (CHX) resulted in a slight but experimentally reproducible reduction in the transcript levels of IPT3, IPT5, and IPT7, indicating that ARF3 directly represses the expression of these three genes (Figure 3J). Since ARF3 binds to the IPT5 promoter (Cheng et al., 2013; Liu et al., 2014), we examined whether IPT3 and IPT7 are also ARF3 targets. ARFs specifically bind to TGTCTC auxin response elements (AuxRE) (Ulmasov et al., 1995,Ulmasov et al., 1999a). While the IPT3 promoter lacks an AuxRE, we found one TGTCTC sequence in the IPT7 promoter region ∼500 bp upstream of the transcription start site (Figure 3K). Using Ler and transgenic ProARF3:ARF3-GFP inflorescences (Liu et al., 2014), we examined ARF3 occupancy at the IPT3 and IPT7 loci using a chromatin immunoprecipitation (ChIP) assay. High ARF3 enrichment was detected at the IPT3 and IPT7 promoter regions but not at the coding regions, demonstrating that ARF3 targets IPT3 and IPT7 (Figure 3L).
ARF3 Represses the Expression of LOG Genes
To confirm the function of ARF3 in cytokinin biosynthesis, we treated ProARF3:ARF3-GR arf3-29 inflorescences with DEX or DMSO as a control, followed by the measurement of cytokinin levels. Compared with Ler, DMSO-treated ProARF3:ARF3-GR arf3-29 plants produced higher level of tZRMP, while the accumulation of tZRMP was reduced after DEX treatment (Figure 4A), suggesting that ARF3 induction may repress cytokinin biosynthesis. However, we cannot rule out the possibility that the changes in tZRMP level were due to defects in cytokinin metabolism, since the expression of CKX genes was impaired in arf3-29 and DEX-treated ProARF3:ARF3-GR arf3-29 plants (Figure 1F; Supplemental Data Sets 1 and 2). We then quantified tZR and tZ contents. Similar to the above results (Figure 2H), we detected lower tZR levels and higher tZ levels in DMSO-treated ProARF3:ARF3-GR arf3-29 plants compared with Ler (Figure 4B). Surprisingly, after DEX treatment, ProARF3:ARF3-GR arf3-29 plants produced significantly higher level of tZR, which consequently decreased tZ production compared with DMSO-treated plants (Figure 4B). These results suggest that the direct conversion of tZRMP to tZ was blocked in the DEX-treated ProARF3:ARF3-GR arf3-29 plants, resulting in the accumulation of tZR, suggesting that ARF3 might function in the biosynthesis of tZ. Since the LOG enzyme family is responsible for the conversion of tZRMP to tZ (Kuroha et al., 2009; Kieber and Schaller, 2014), we investigated LOG gene expression in wild-type and mutant plants. LOG1, LOG3, LOG4, LOG5, and LOG7 but not LOG2 and LOG8 expression was dramatically increased in ag-10 arf3-29 versus the wild type. Meanwhile, LOG3, LOG4, and LOG5 had derepressed expression in arf3-29, and LOG3 and LOG4 transcript levels increased in ag-10 compared with the wild type (Figure 4C). These results suggest that ARF3 and AG fine-tune cytokinin biosynthesis by repressing the expression of LOG genes.
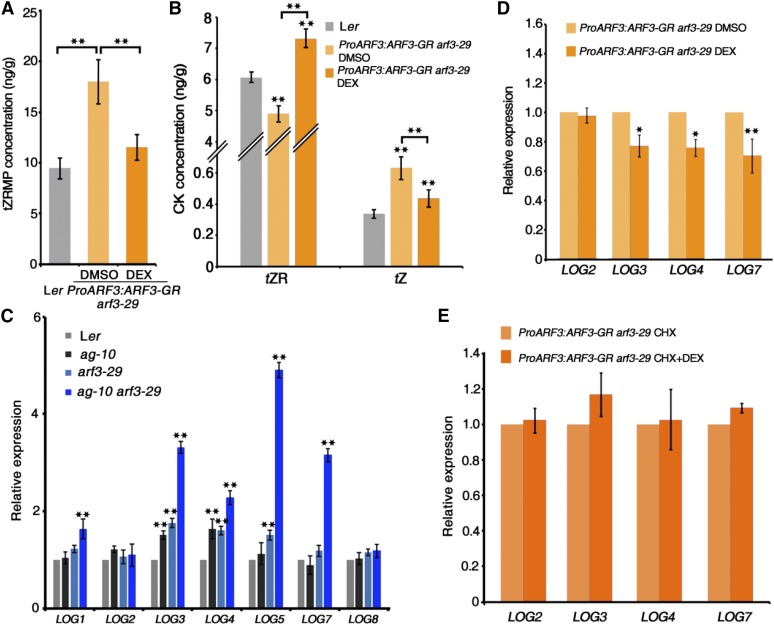
Figure 4.
ARF3 Represses LOG Gene Expression.
(A) and (B) Mass spectrometric measurements of tZRMP (A) and tZR and tZ (B) in inflorescences of Ler and ProARF3:ARF3-GR arf3-29 under DMSO and DEX treatment. Mean values of three replicates with independently prepared inflorescence materials containing unopened flowers are shown. Error bars indicate the sd of three biological experiments. **P < 0.01 (Student’s t test).
(C) Expression of LOG family genes in the inflorescences of the indicated plants. Inflorescences containing stage 6 and younger flowers were collected for RT-qPCR. Error bars indicate the sd from three biological replicates with independently prepared inflorescence materials. **P < 0.01 (Student’s t test) between Ler and mutant inflorescences.
(D) and (E) Expression of LOG2, LOG3, LOG4, and LOG7 in ProARF3:ARF3-GR arf3-29 inflorescences after 8 h treatment with the indicated chemical. Inflorescences containing stage 6 and younger flowers were collected for RT-qPCR. The DEX treatment data indicate that ARF3 represses the expression of some LOG genes, and the CHX+DEX treatment data indicate that the repression by ARF3 is indirect. Error bars indicate the sd from three biological replicates with independently prepared inflorescence materials. *P < 0.05 and **P < 0.01 (Student’s t test).
Previous functional characterization of LOG genes showed that transgenic plants overexpressing LOG2, LOG4, and LOG7 but not LOG5 and LOG8 displayed a semidwarf phenotype with more axillary stems than the wild type (Kuroha et al., 2009), suggesting that LOG2, LOG4, and LOG7 regulate SAM activity. Using DEX or DMSO control treatment of ProARF3:ARF3-GR arf3-29 inflorescences and RT-qPCR as described above, we investigated whether ARF3 directly regulates LOG gene expression. After DEX treatment for 8 h, LOG3, LOG4, and LOG7 transcript levels decreased, while LOG2 transcript levels did not obviously change (Figure 4D), indicating that ARF3-induced activation represses the expression of some LOG genes. Subsequently, we found no statistically significant changes in LOG gene expression in inflorescences treated with DEX+CHX versus CHX alone among the genes examined (Figure 4E), indicating their repression by ARF3 is indirect.
ARF3 Represses the Expression of Cytokinin Receptor Genes during FM Determinacy and Gynoecium Development
Both ARF3 and AHK4 are concentrated in the OC regions of meristems (Gordon et al., 2009; Liu et al., 2014). We noticed that AHK4 transcript levels were much higher in ag-10 arf3-29 plants than in 6-BA-treated ag-10 plants (compare Figure 2F with Supplemental Figure 2), suggesting that ARF3 might regulate AHK4. To test this hypothesis, we introduced a ProAHK4:GUS construct (i.e., the β-glucuronidase reporter gene under the control of the AHK4 promoter) into ag-10 arf3-29. In wild-type plants, strong GUS signals were mainly detected in meristematic tissues such as the SAM, root tips, and organ primordia (Figure 5A) (Nishimura et al., 2004). In ag-10 arf3-29 under the same histochemical staining conditions, we observed stronger staining in inflorescences, particularly in the SAM and FM regions (Figure 5B). A quantitative GUS activity assay using inflorescences containing stage 6 and younger flowers indicated that GUS activity was significantly higher in ag-10 arf3-29 than in Ler, ag-10, and arf3-29 (Figure 5C). For genetic analysis, we introduced the ahk4/cre1-10 mutation into ag-10 arf3-29. The ahk4/cre1-10 mutant exhibits indistinct phenotypic defects under normal growth conditions (Nishimura et al., 2004). In our analysis, the ahk4/cre1-10 mutation largely rescued the FM indeterminacy phenotype of ag-10 arf3-29, resulting in fertile siliques harboring less excess tissue growth, indicating that ahk4/cre1-10 is epistatic to arf3-29 in terms of FM determinacy (Figures 5D to 5F). We generated a ProARF3:AHK4-FLAG construct and transformed it into ag-10 to express AHK4 in ARF3 expression regions. In 69% of the transgenic plants (22 out of 32), we observed siliques with enhanced FM determinacy defects compared with ag-10. Among these, 45% (10 out of 22) exhibited a strong FM indeterminacy phenotype, with continuous prolonged FM activity, and 55% (12 out of 22) exhibited an intermediate FM indeterminacy phenotype characterized by multiple carpels (Figures 5G to 5I; Supplemental Table 1). These results suggest that ARF3 represses AHK4 expression to promote FM determinacy.
Figure 5.
ARF3 Directly Represses AHK4 to Regulate FM Determinacy.
(A) and (B) Expression of ProAHK4:GUS in Ler (A) and arf3-29 (B) inflorescences under the same staining conditions. The stronger GUS staining in arf3-29 compared with Ler indicates that AHK4 expression was derepressed in arf3-29.
(C) Quantitative GUS activity in inflorescences of the indicated plants. Mean values of three biological replicates with independently prepared inflorescence materials containing unopened flowers are shown. **P < 0.01 (Student’s t test).
(D) to (F) Representative silique phenotypes of ag-10 (D), ag-10 arf3-29 (E), and ag-10 arf3-29 ahk4 (F). In (E) and (F), the leftmost silique has one carpel of the primary gynoecium removed to show the structures contained therein. The ahk4 mutation partially rescued the severe FM determinacy defect of ag-10 arf3-29.
(G) to (I) Longitudinal sections of representative siliques of the following: ag-10 (G) and ProARF3:AHK4-3FLAG ag-10 with severe (H) and intermediate (I) FM indeterminacy. Increased AHK4 gene expression in ARF3 expression regions resulted in severe FM determinacy defects in 10 out of 32 plants, as characterized by a sustained FM (red arrow in [H]), and intermediate FM indeterminacy in 12 out of 32 plants, as indicated by excess tissues growing within the primary ovary (yellow arrow in [I]).
(J) Diagram of the AHK4 genomic region, with the arrow indicating the transcription start site (+1). Dark-gray rectangles, untranslated regions; black rectangles, exons; white rectangles, introns. Black bold lines with P1, P2, and P3 indicate fragments examined by ChIP-qPCR. “TGTCTC” is the typical ARF binding motif. Bar = 500 bp.
(K) ChIP assay with anti-GFP antibody to examine ARF3 binding at AHK4 in Ler and ProARF3:ARF3-GFP arf3-29 inflorescences. The regions marked in Figure 5J were examined. ARF3 could bind the P1 and P2 regions containing the “TGTCTC” motif but not the P3 region. eIF4A served as a negative control. Error bars represent the sd from three biological repeats with independently prepared inflorescence materials containing unopened flowers. **P < 0.01 (Student’s t test) between Ler and ProARF3:ARF3-GFP arf3-29 inflorescences.
(L) EMSA confirmation of ARF3-GST binding to the AHK4 P2 region. Upper panel: native and mutated AHK4P2 sequence used for the EMSA. The mutated nucleotides are shown in red. The arrowhead indicates band shifts (complexes of ARF3-GST protein and probe DNA), and the arrow indicates free probe. Nonlabeled oligonucleotides were used as a competitor.
Bars = 100 µm in (A) and (B), 1 mm in (D) to (F), and 50 µm in (G) to (I).
To investigate the mechanism by which ARF3 regulates AHK4 expression, we treated ProARF3:ARF3-GR arf3-29 transgenic plants with DEX+CHX or CHX alone as a control. After 8 h treatment, we collected inflorescences containing stage 6 and younger flowers and quantified AHK4 transcript levels in these tissues by RT-qPCR. AHK4 transcript levels were significantly lower in inflorescences treated with DEX+CHX compared with CHX treatment alone, indicating that ARF3 directly represses AHK4 expression (Figure 3J). We preformed sequence analysis to identify AuxRE TGTCTC motifs (Ulmasov et al., 1995) and found two typical ARF binding motifs in the AHK4 promoter (Figure 5J). ChIP analysis revealed high ARF3 occupancy at these two regions compared with other regions (Figure 5K). To verify the binding of ARF3 to AHK4, we performed an electrophoretic mobility shift assay (EMSA) using specific biotin-labeled probes containing native or mutated ARF3 binding sites, as shown in Figure 5L. For the competition assay, we added a 500-fold excess of unlabeled probe. We found that the A and G nucleotides were important for the binding of ARF3 to AHK4 in vitro, which is in agreement with previous findings (Ulmasov et al., 1999a).
Since AHK4 is a known cytokinin receptor (Inoue et al., 2001; Riefler et al., 2006), we performed further experiments to assess the roles of cytokinin receptors in ARF3-promoted FM determinacy. We treated ag-10 arf3-29 inflorescences with the phenylquinazoline compound S-4893, a noncompetitive cytokinin antagonist that targets cytokinin receptors (Arata et al., 2010; Wang et al., 2017). After 5 d of treatment, the FM determinacy defect of ag-10 arf3-29 was mostly rescued, suggesting that cytokinin receptors might function redundantly to regulate FM determinacy (Supplemental Figure 6A). Moreover, we noticed that the gynoecium developmental defect of the arf3 mutant was partially complemented in the siliques of ag-10 arf3-29 ahk4 and ag-10 arf3-29 treated with S-4893 (compare Figure 5F and Supplemental Figure 6A to Figure 5E), prompting us to investigate how cytokinin receptors might function in gynoecium development. While wild-type plants produce a bilocular ovary composed of two valves (when viewed abaxially) and capped with a stigma and style, the valve tissue of arf3/ett is lost (when viewed basally) and replaced by the structures between the abaxial style and the internode (Supplemental Figure 6B) (Sessions et al., 1997). We treated ProARF3:AFR3-GR arf3-29 with S-4893, DMSO as a negative control and DEX as a systemic control. As described above, DEX treatment fully complemented the developmental defect of arf3-29. Interestingly, ProARF3:AFR3-GR arf3-29 treated with S-4893 produced fertile siliques with almost normal gynoecium patterning, except for an elongated internode (Supplemental Figure 6B). These results provide evidence that cytokinin receptors are involved in regulating FM determinacy and gynoecium development.
Cytokinin Regulates Temporal Termination of WUS Expression to Modulate FM Determinacy
In the SAM, cytokinin regulates WUS expression by mediating the crosstalk between WUS and ARRs such as ARR7 and ARR15 (Zhao et al., 2010). Meanwhile, type-B ARRs such as ARR1, ARR10, and ARR12 activate WUS expression during shoot regeneration (Meng et al., 2017). We compared cytokinin levels in the inflorescences of ag-10 versus transgenic ProARF3:IPT3-GFP ag-10, ProARF3:IPT5-GFP ag-10, and ProARF3:IPT7-GFP ag-10 plants, finding that tZR and tZ levels were much higher in transgenic plants than in ag-10 (Supplemental Figure 7A). To investigate how cytokinin regulates WUS expression in the context of FM determinacy, we examined WUS expression in inflorescences containing stage 6 and younger flowers from each of the transgenic lines via RT-qPCR analysis. Surprisingly, WUS expression was not obviously perturbed in the inflorescences of ag-10 arf3-29, ProARF3:IPT3-GFP ag-10, ProARF3:IPT5-GFP ag-10, ProARF3:IPT7-GFP ag-10, or ProARF3:AHK4-FLAG ag-10 compared with ag-10 (Supplemental Figure 7B). Moreover, the sepal and petal numbers of the transgenic plants were the same as those of ag-10; however, the stamen number decreased and the carpel number increased in these lines compared with ag-10 (Supplemental Table 1). These results indicate that locally increased cytokinin levels in ARF3 expression regions did not increase WUS expression.
Since the spatio-temporal patterning rather than the intensity of WUS expression is more important for FM determinacy (Prunet et al., 2009), we monitored WUS expression patterns by in situ hybridization. In the wild type, WUS expression is shut off by stage 6 of floral development when the carpel primordia have formed. In ag-10, a few flowers harbor slight WUS expression at stage 6-7 (Ji et al., 2011; Liu et al., 2011). However, in this study, in all of the transgenic flowers tested, WUS expression was detected even as late as stage 8-9 of floral development (Figure 6), indicating that cytokinin temporally regulates WUS expression to modulate FM determinacy.
Figure 6.
Expression Pattern of WUS in the Indicated Flowers.
In situ hybridization with a WUS antisense probe. The arrows indicate WUS signal. WUS expression was not detected in a stage 8 ag-10 flower (A), but it was detected in stage 8 or 9 flowers of ProARF3:IPT3-GFP ag-10 (B), ProARF3:IPT5-GFP ag-10 (C), ProARF3:IPT7-GFP ag-10 (D), and ProARF3:AHK4-3FLAG ag-10 (E). Red numbers indicate the floral developmental stage. Bars = 50 µm.
ARF3 Partially Mediates the Function of AG in Regulating Cytokinin Activity
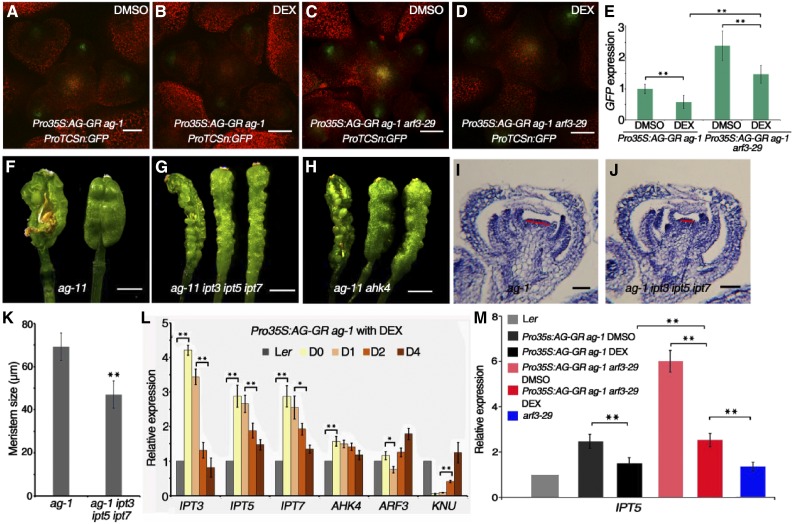
As described above, we found that cytokinin homeostasis was perturbed in ag-10 plants (Figures 1C and 1D). To explore the relationship between AG and cytokinin signaling during flower development, we introduced the ProTCSn:GFP reporter into Pro35S:AG-GR ag-1. Upon DEX treatment, AG activation fully rescued the floral development and FM determinacy defects of ag-1 (Liu et al., 2011). After 4 d of DEX treatment once per day, GFP fluorescence intensity was much lower in Pro35S:AG-GR ag-1 inflorescences compared with untreated plants under the same imaging conditions (Figures 7A and 7B), indicating that AG represses cytokinin activity in the FM. Since AG has been shown to dynamically regulate ARF3 expression (Ng et al., 2009; Liu et al., 2014), we also introduced ProTCSn:GFP into Pro35S:AG-GR ag-1 arf3-29 plants. Confocal imaging revealed that the GFP signals, which were stronger in DMSO-treated Pro35S:AG-GR ag-1 arf3-29 compared with Pro35S:AG-GR ag-1, were slightly reduced after DEX treatment but still stronger than that in the Pro35S:AG-GR ag-1 background (Figures 7C and 7D). These observations were confirmed by RT-qPCR quantification of GFP transcript levels (Figure 7E), suggesting that ARF3 partially mediates the repressive effect of AG on cytokinin activity.
Figure 7.
AG Regulates Cytokinin Activity.
(A) to (D) Expression of ProTCSn:GFP (green) in the inflorescences of Pro35S:AG-GR ag-1 under DMSO (A) and DEX (B) treatment and Pro35S:AG-GR ag-1 arf3-29 under DMSO (C) and DEX (D) treatment for 4 d.
(E) GFP transcript levels in inflorescences of the indicated plants. Inflorescences containing stage 6 and younger flowers were collected for RT-qPCR.
(F) to (H) Representative silique phenotypes of ag-11 (F), ag-11 ipt3 ipt5 ipt7 (G), and ag-11 ahk4 (H). The leftmost silique has one carpel of the primary gynoecium removed to show the structures contained therein. ag-11 produces short and bulged siliques with additional tissues growing inside. ipt3 ipt5 ipt7 and ahk4 largely rescued the severe FM determinacy defect of ag-11.
(I) and (J) Longitudinal sections of representative ag-1 (I) and ag-1 ipt3 ipt5 ipt7 (J) flowers at similar developmental stages. The FM size (marked by red lines) of ag-1 ipt3 ipt5 ipt7 is smaller than that of ag-1.
(K) Quantification of FM size (µm) in ag-1 (n = 15) and ag-1 ipt3 ipt5 ipt7 (n = 12). **P < 0.01 (Student’s t test).
(L) Time-course expression analysis of the indicated genes in Ler and Pro35S:AG-GR ag-1 inflorescences under DEX treatment. Ler inflorescences containing stage 6 and younger flowers and Pro35S:AG-GR ag-1 inflorescences containing unopened flowers were collected for RT-qPCR.
(M) IPT5 expression in inflorescences of the indicated plants. RNA was extracted from Ler and arf3-29 inflorescences containing stage 6 and younger flowers for RT-qPCR. Pro35S:AG-GR ag-1 and Pro35S:AG-GR ag-1 arf3-29 were treated with DEX or DMSO for 4 d, and inflorescences containing unopened flowers were collected for RT-qPCR.
Error bars in (E), (L), and (M) indicate the sd from three biological replicates with independently prepared inflorescence materials. *P < 0.05 and **P < 0.01 (Student’s t test). Bars = 50 µm in (A) to (D), (I), and (J) and 1 mm in (F) to (H).
Considering the direct repression of IPT3, IPT5, IPT7, and AHK4 expression by ARF3, we introduced the ipt3, ipt5, ipt7, and ahk4/cre1-10 mutations into the ag-11 mutant background to further examine the genetic connection between AG and cytokinin. ag-11 is as an intermediate-strength ag allele with more bulged and shorter siliques containing additional tissues inside than the wild type, which nevertheless produces only a few seeds (Figure 7F) (Huang et al., 2017), which is consistent with the finding that the expression of IPT5 and IPT7 was derepressed in ag-11 (Supplemental Figure 8). Both the ipt3 ipt5 ipt7 triple mutant combination and the ahk4/cre1-10 mutation fully repressed the FM determinacy defect of ag-11, resulting in longer and thinner gynoecia with two fused carpels (Figures 7G and 7H). We also introduced the ipt3 ipt5 ipt7 triple mutant combination into the ag-1 background. Although ipt3 ipt5 ipt7 failed to rescue the severe floral organ identity and FM determinacy defects of ag-1, the FMs ag-1 ipt3 ipt5 ipt7 were generally smaller than those of ag-1 (Figures 7I to 7K), indicating that decreased cytokinin activity disrupted the prolonged FM activity in ag-1. Moreover, these results suggest that cytokinin acts downstream of AG to regulate FM determinacy.
We further examined the expression of IPT3, IPT5, IPT7, AHK4, and ARF3 in DEX-treated Pro35S:AG-GR ag-1; the AG target gene KNU served as a positive control. Compared with the wild type, Pro35S:AG-GR ag-1 had significantly higher transcript levels of IPT3, IPT5, IPT7, and AHK4 before DEX treatment, indicating that these genes are derepressed in the ag-1 mutant (Figure 7L). After DEX treatment, IPT transcript levels dramatically decreased, indicating that AG represses cytokinin biosynthesis (Figure 7L). Interestingly, the significant reductions in IPT transcript levels occurred after one day of DEX treatment (Figure 7L, from day 1 [D1] to D2), indicating a delay between AG activation and reduced IPT expression. ARF3 levels decreased on D1 after AG activation and increased on subsequent days, which is consistent with previous reports (Ng et al., 2009; Liu et al., 2014), indicating that ARF3 expression is dynamically regulated by AG (Figure 7L). One possible explanation for the observed changes in ARF3 expression is that AG initially repressed ARF3 expression on D1 to sustain normal IPT expression and then activated ARF3 to repress IPT expression thereafter. To test this hypothesis, we treated Pro35S:AG-GR ag-1 and Pro35S:AG-GR ag-1 arf3-29 inflorescences with DEX and DMSO for 2 d. Inflorescences containing stage 6 and younger flowers were collected for RT-qPCR analysis of IPT5. IPT5 transcript levels were much higher in Pro35S:AG-GR ag-1 arf3-29 than in Pro35S:AG-GR ag-1, indicating that AG and ARF3 synergistically regulate IPT5 (Figure 7M). After DEX treatment, IPT5 transcript levels were reduced in both Pro35S:AG-GR ag-1 and Pro35S:AG-GR ag-1 arf3-29. However, IPT5 expression in DEX-treated Pro35S:AG-GR ag-1 arf3-29 was still much higher than in either arf3-29 or DEX-treated Pro35S:AG-GR ag-1, indicating that ARF3 is partially required for the AG-induced repression of IPT5 (Figure 7M).
Auxin Represses Cytokinin Biosynthesis through ARF3
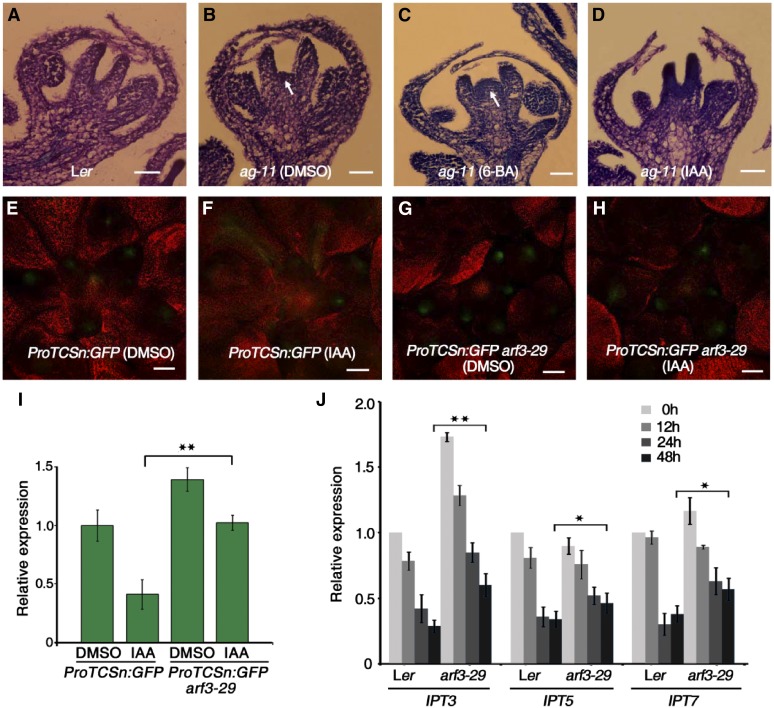
ARF3 expression is induced by auxin during shoot regeneration, and cytokinin biosynthesis relies on polar auxin transport (Cheng et al., 2013). We therefore treated the inflorescences of wild-type plants with indole-3-acetic acid (IAA), the most common, naturally occurring auxin hormone, followed by ARF3 expression analysis. ARF3 expression was induced by auxin and peaked at 4 h of treatment (Supplemental Figure 9). We treated ag-11 inflorescences with IAA and 6-BA to investigate the effects of auxin and cytokinin, respectively, on FM determinacy. In the wild type, the FM was terminated at stage 6, when the carpel primordia were generated (Figure 8A). In ag-11, prolonged WUS expression resulted in FM indeterminacy, marked by an active FM during later developmental stages (Figure 8B). While 6-BA treatment led to a more inflated, dome-like FM in ag-11, IAA treatment suppressed the FM indeterminacy of ag-11 by repressing FM activity (Figures 8C and 8D), indicating that cytokinin promotes FM activity, while IAA represses it.
Figure 8.
ARF3 Mediates Auxin-Mediated Repression of Cytokinin.
(A) to (D) Longitudinal sections of representative flowers of Ler (A) and ag-11 after 5 d of DMSO (B), 6-BA (C), and IAA (D) treatment. White arrows indicate prolonged FMs. Bars = 50 µm.
(E) to (H) Expression of ProTCSn:GFP (green) in Ler and arf3-29 inflorescences treated with DMSO ([E] and [G]) and IAA ([F] and [H]).
(I) GFP transcript levels in inflorescences of the indicated plants. RNA was extracted from inflorescences containing stage 6 and younger flowers for RT-qPCR. Error bars indicate the sd from three biological replicates with independently prepared inflorescence materials. **P < 0.01 (Student’s t test).
(J) Time-course expression analysis of IPT3, IPT5, and IPT7 in IAA-treated Ler and arf3-29 inflorescences. Inflorescences containing stage 6 and younger flowers were collected for RNA extraction and RT-qPCR. Error bars indicate the sd from three biological replicates with independently prepared inflorescence materials. *P < 0.05 and **P < 0.01 (Student’s t test).
To further investigate the role of auxin in regulating cytokinin activity, we treated ProTCSn:GFP and ProTCSn:GFP arf3-29 inflorescences with IAA or DMSO as a control. IAA treatment of ProTCSn:GFP led to reduced GFP fluorescence intensity, suggesting that auxin represses cytokinin activity (Figures 8E and 8F). GFP fluorescence intensity was stronger in ProTCSn:GFP arf3-29 than in ProTCSn:GFP, and IAA treatment did not appear to alter GFP fluorescence in ProTCSn:GFP arf3-29 (Figures 8G and 8H). These results were confirmed by RT-qPCR analysis of GFP transcript levels (Figure 8I), indicating that ARF3 is required in order for auxin to repress cytokinin activity. To follow up on this hypothesis, we quantified IPT3, IPT5, and IPT7 transcript levels in Ler and arf3-29 plants under IAA treatment. IAA treatment gradually reduced the transcript levels of all three genes in both Ler and arf3-29. However, IPT transcript levels still differed between Ler and arf3-29, even after prolonged IAA treatment (Figure 8J), further indicating that ARF3 mediates the function of auxin in repressing cytokinin biosynthesis.
ARF3 Plays Dual Roles in Early Flower Development
To investigate the molecular mechanisms underlying the roles of ARF3 in flower development, we performed genome-wide gene expression profiling analysis using RNA-seq. We treated ProARF3:ARF3-GR arf3-29 inflorescences with DMSO with or without DEX for 12 h and collected inflorescences containing flowers at stage 6 and younger for RNA-seq analysis. Three biological replicates with independently prepared inflorescence materials from ∼30 plants were performed for each treatment, which closely clustered with each other in the principal component analysis plot (Supplemental Figure 10A), indicating that our RNA-seq data were highly reproducible. We identified 1259 downregulated and 1881 upregulated differentially expressed genes (DEGs) using false discovery rate <0.01 and fold change >2 as significance cutoffs (Supplemental Data Sets 1 and 2), in DEX-treated plants compared with DMSO-treated plants.
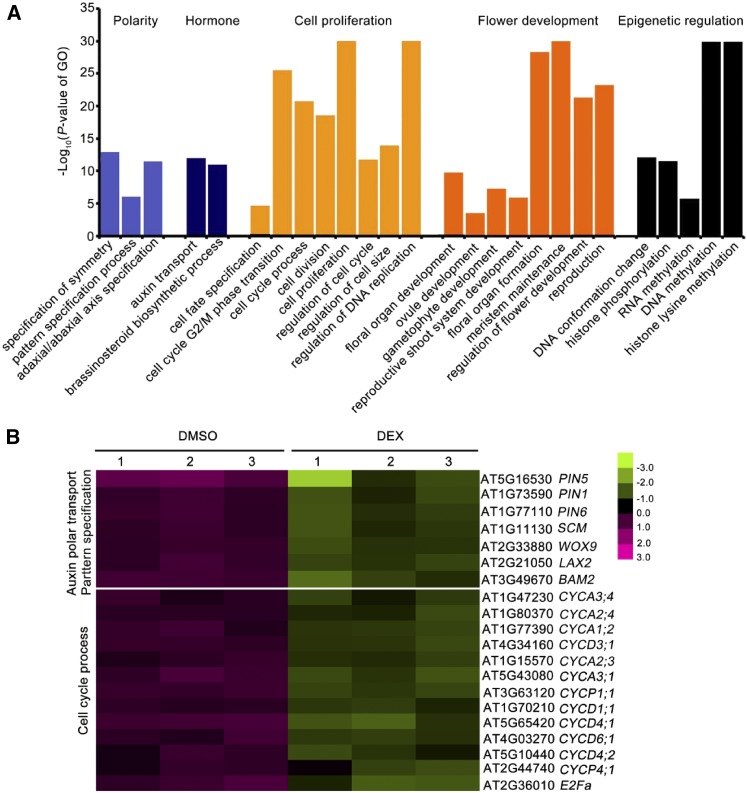
To investigate the roles of ARF3 in flower development, we performed Gene Ontology (GO) analysis of the DEGs. GO categories related to development, such as polarity specification, hormones, cell proliferation, and flower development, as well as epigenetic regulation, were highly enriched among ARF3-downregulated DEGs (Figure 9A; Supplemental Figure 10B). Notably, while several key genes involved in polar auxin transport and pattern specification, such as PIN-FORMED1 (PIN1), PIN5, LIKE AUXIN RESISTANT2, and WUSCHEL RELATED HOMEOBOX9 (WOX9) were repressed by ARF3, in line with its role in auxin signaling and pattern specification (Fahlgren et al., 2006; Garcia et al., 2006; Cheng et al., 2013), A-type and D-type cyclin genes were dramatically downregulated after ARF3 induction, indicating that ARF3 controls the cell cycle by regulating the expression of cyclin genes (Figure 9B). Interestingly, on the other hand, GO terms related to metabolic process and response to stimuli, particularly to environmental stimuli such as heat, salt, light, and insects, were highly enriched among ARF3 upregulated genes (Supplemental Figure 10C). These findings suggest that ARF3 plays a negative role in flower development but a positive role in plant stress responses, which is consistent with its transcriptional repressor activity and activator activity during flower development (Simonini et al., 2017).
Figure 9.
Repressive Role of ARF3 in Multiple Developmental Processes.
(A) Overrepresented GO categories in the downregulated genes in ProARF3:ARF3-GR arf3-29 inflorescence under the 12 h DEX treatment. The GO terms related to polarity specification, cell proliferation, flower development, and epigenetic regulation were enriched in ARF3 repressed genes (Supplemental Data Set 1).
(B) Expression of representative genes related to polar auxin transport and pattern specification as well as cell cycle progression after induction by ARF3. Red indicates increased expression, and green indicates reduced expression compared with the mean of all samples. The values from three biological replicates (1, 2, and 3) are listed for DMSO and DEX treatment, respectively.
ARF3 Controls Cell Cycle Genes and Cell Division during FM Development
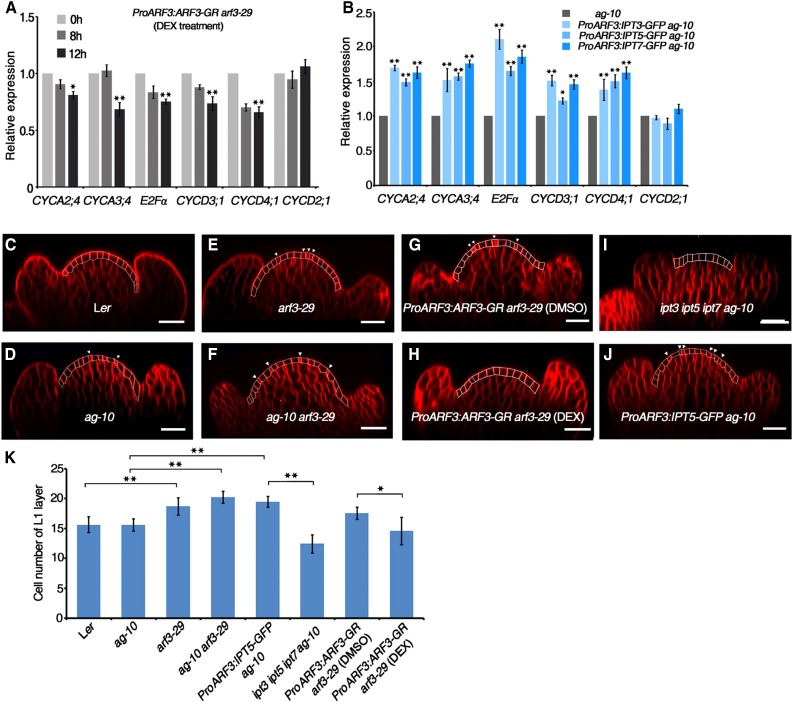
Whole genome-wide gene expression analysis revealed that ARF3 regulates cell cycle gene expression and that cytokinins are required to regulate cell cycle gene expression during the phase transition of the cell cycle for cell division (Figure 10A) (Riou-Khamlichi et al., 1999). We therefore investigated the impact of ARF3 on cell cycle gene expression using ProARF3:ARF3-GR arf3-29 transgenic plants under DEX treatment. The expression of CYCD3;1, CDKA;1, E2Fα, HISTONE H4 (HIS4), and CYCB1;1, but not CYCD2;1, decreased in ProARF3:ARF3-GR arf3-29 inflorescences after 12 h DEX treatment (Figure 10A), indicating that ARF3 inhibits the expression of cell cycle genes during flower development. Gene expression analysis after DEX+CHX treatment further indicated that the regulation of these genes by ARF3 was indirect, as their expression did not significantly change compared with those in plants treated with CHX alone (Supplemental Figure 11). The expression of CYCD3;1, CDKA;1, E2Fα, HIS4, and CYCB1;1 but not CYCD2;1 was significantly higher in the inflorescences of transgenic ProARF3:IPT3-GFP ag-10, ProARF3:IPT5-GFP ag-10, and ProARF3:IPT7-GFP ag-10 compared with ag-10 (Figure 10B), indicating that increased IPT gene expression in ARF3 expression regions enhances cell cycle gene expression.
Figure 10.
ARF3 Controls Cell Cycle Gene Expression and Cell Division in the FM.
(A) Expression of cell cycle genes in DEX-treated ProARF3:ARF3-GR arf3-29 inflorescences. ProARF3:ARF3-GR arf3-29 inflorescences were treated with DEX for the indicated amounts of time and collected under a microscope for RNA extraction and RT-qPCR. The transcript level at 0 h was used as a reference and was designated as 1.0. Error bars indicate the sd from three biological replicates with independently prepared inflorescence materials containing stage 6 and younger flowers. *P < 0.05 and **P < 0.01 (Student’s t test).
(B) Expression of cell cycle genes in inflorescences of the indicated plants. Inflorescences containing stage 6 and younger flowers were collected for RNA extraction and RT-qPCR. The transcript level in ag-10 was used as a reference and was designated as 1.0. Error bars indicate the sd from three biological replicates with independently prepared inflorescence materials. *P < 0.05 and **P < 0.01 (Student’s t test).
(C) to (J) Longitudinal sections of the inflorescence meristems of the indicated plants. Inflorescences containing stage 6 and younger flowers were stained with FM4-64 (red), and flowers at later stage 3 were selected for observation under a confocal microscope. The L1 layer cells are outlined in each panel, with cells undergoing division marked by a white arrowhead in (D) to (G) and (J). ProARF3:ARF3-GR arf3-29 inflorescences were treated with DMSO (G) and DEX (H) for 10 d prior to observation. Bars = 20 µm.
(K) Number of cells in the FM L1 layer. The numbers of FM L1 layer cells in the indicated plants were counted, and the mean values from multiple flowers (n = 12) are shown. *P < 0.05 and **P < 0.01 (Student’s t test).
Since L1 cells (the outermost layer of the SAM and FM) can clearly be distinguished, as the cell size is uniform throughout the layer without variation between the central zone and the peripheral zone (Laufs et al., 1998), we counted the number of L1 layer cells in longitudinal FM sections by confocal imaging. In Ler and ag-10 flowers, the FM had 15.5 ± 1.1 (n = 10) and 15.6 ± 1.1 (n = 10) L1 layer cells at stage 3-4, respectively (Figures 10C, 10D, and 10K). Consistent with the enlarged SAM of arf3-29 and ag-10 arf3-29 (Supplemental Figure 1), arf3-29 and ag-10 arf3-29 FMs were inflated and had more L1 layer cells at stage 3-4 compared with their respective controls (arf3-29, 18.8 ± 1.4, n = 10; ag-10 arf3-29, 20.3 ± 0.7, n = 10) (Figures 10E, 10F, and 10K). DEX treatment of ProARF3:ARF3-GR arf3-29 yielded a normal FM with a normal number of L1 layer cells (14.6 ± 2.3, n = 10) in contrast to DMSO-treated plants (17.6 ± 1.2, n = 10) (Figures 10G, 10H, and 10K). These results indicate that ARF3 ensures proper cell number during FM development. We also found that the ProARF3:IPT5-GFP ag-10 FM had a significantly increased number of L1 layer cells (19.5 ± 0.9, n = 10) compared with ipt3 ip5 ipt7 ag-10 (11.3 ± 1.1, n = 10) and ag-10 (Figures 10I and 10K), showing that decreased cytokinin biosynthesis resulted in reduced FM size and, conversely, locally increased cytokinin biosynthesis increased FM activity and cell proliferation. Consistently, we found several cells (indicated with white arrowheads) that were undergoing cell division in the FMs of ag-10, arf3-29, ag-10 arf3-29, ProARF3:ARF3-GR arf3-29, and ProARF3:IPT5-GFP ag-10 (Figures 10D to 10J). Collectively, the results indicate that ARF3 controls cell division by regulating cell cycle gene expression through cytokinin biosynthesis.
DISCUSSION
Cytokinin Functions in FM Determinacy through Regulating WUS Expression
Multiple lines of evidence suggest that cytokinin is involved in the formation, maintenance, and growth of plant meristems (Sablowski, 2007b). In the root apical meristem (RAM), cytokinin is concentrated in the columella cells of the root meristem and the vascular tissue at the transition zone; moreover, it is required for repressing the expression of WOX5, a key WUS homeodomain transcription factor gene required for RAM maintenance (Zhang et al., 2013). In contrast, the OCs of the SAM and FM harbor high levels of cytokinin, which are required for WUS expression and are mediated by interactions between WUS and type-A ARRs (Leibfried et al., 2005; Gordon et al., 2009). However, the involvement of cytokinin in FM determinacy is unclear.
In this study, we found that exogenous cytokinin treatment did not disrupt proper FM determinacy in wild-type Arabidopsis, but it greatly enhanced the FM determinacy defect of ag-10 (Figures 1B and 1D), indicating that cytokinin homeostasis is critical for normal FM determinacy. The increased cytokinin activity in the FMs of arf3-29 and ag-10 arf3-29 (Figures 2C and 2D), together with the increased cytokinin levels in these mutants (Figure 2G), suggests that increased cytokinin levels are responsible for the FM indeterminacy of ag-10 arf3-29. Furthermore, genetic and molecular lines of evidence indicate that increased cytokinin biosynthesis and reception enhanced the FM determinacy defect of ag-10, whereas attenuated cytokinin biosynthesis and reception rescued the FM indeterminacy of ag-10 arf3-29 (Figures 3 to 5). These results demonstrate that cytokinin functions in FM determinacy.
WUS is essential for meristem establishment, maintenance and termination (Laux et al., 1996). Recently, cytokinin was found to activate WUS expression to initiate the formation of axillary meristems, with exogenous cytokinin treatment altering the level but not the timing of WUS expression (Wang et al., 2017). Here, we showed that increased ectopic cytokinin levels prolonged WUS expression to later floral developmental stages rather than elevating WUS expression levels (Figure 6; Supplemental Figure 7B). In other words, during FM determinacy, cytokinin might regulate the timing of WUS expression but not its abundance. Thus, cytokinin appears to have tissue-specific functions in axillary meristem initiation, SAM maintenance and FM determinacy.
The Antagonistic Interaction of Cytokinin and Auxin Regulates FM Determinacy
Cytokinin and auxin are two important phytohormones that coordinately regulate many plant growth and developmental processes. The crosstalk between cytokinin and auxin is tissue- and context-specific (reviewed in Chandler and Werr, 2015; Schaller et al., 2015). In the RAM, cytokinin application promotes cell differentiation in the transition zone and reduces RAM size (Dello Ioio et al., 2007), while auxin treatment promotes cell division in the proximal meristem and increases RAM size (Blilou et al., 2005). The repressive effect of cytokinin on WOX5 expression is mediated in part by functions affecting auxin biosynthesis and transport (Ruzicka et al., 2009; Zhang et al., 2013). In the SAM, maxima of cytokinin activity in the OC promote the proliferation of meristem cells, and high auxin levels in the peripheral zone induce cellular differentiation and organ outgrowth. The interaction between cytokinin and auxin is mediated in part by ARF5/type-A ARR (Zhao et al., 2010). However, it is presently unclear whether and how cytokinin interacts with auxin in the context of FM determinacy.
IPT5 and IPT7 expression was derepressed in ag-11 inflorescences, suggesting that the determinacy defect of ag-11 might be attributed to increased cytokinin activity (Supplemental Figure 8). Cytokinin application enhanced the FM indeterminacy of ag-11, while exogenous auxin treatment led to normal FM termination (Figures 8C and 8D), suggesting that auxin functions antagonistically to cytokinin in controlling FM determinacy. This hypothesis is further supported by the finding that exogenous auxin treatment decreased cytokinin activity in FMs, as revealed using the ProTCSn:GFP reporter line (Figures 8F and 8I). Our findings also suggest that this process is partially mediated by ARF3, as auxin activates ARF3 expression, and ARF3 consequently represses the expression of IPT3, IPT5, and IPT7 (Figures 8G to 8J) (Cheng et al., 2013). Thus, unlike the ARF5-mediated auxin-cytokinin interaction, which increases cytokinin sensitivity in the SAM and type-B ARR-mediated auxin-cytokinin crosstalk, which decreases auxin biosynthesis during the reestablishment of the SAM (Zhao et al., 2010; Meng et al., 2017), ARF3 appears to mediate the role of auxin in repressing cytokinin activity, thus regulating FM determinacy.
Dual Roles of ARF3 in Flower Development
Previously, functional characterization of ARF3 revealed that this protein plays important roles in many aspects of plant development, such as gynoecium patterning, self-incompatibility, de novo organ regeneration, and organ polarity as well as FM determinacy (Sessions et al., 1997; Nemhauser et al., 2000; Chitwood et al., 2009; Tantikanjana and Nasrallah, 2012; Cheng et al., 2013; Liu et al., 2014). Based on the amino acid composition in its middle region, ARF3 was proposed to function as a repressing ARF (Guilfoyle and Hagen, 2007). However, the global functions of ARF3 in early flower development remain enigmatic. Using RNA-seq, we found that ARF3 mainly acts as a repressive regulator in the developmental context during early flower development. After induction, ARF3 represses the expression of genes related to pattern and polarity specification, polar auxin transport, cell proliferation, flower development, and epigenetic regulation (Figure 9A; Supplemental Figure 10B), in agreement with its known repressive function (Ulmasov et al., 1999b; Cheng et al., 2013; Liu et al., 2014). Surprisingly, ARF3 also functions as an activator to upregulate the expression of genes that respond to biotic and abiotic stimuli and various metabolic processes (Supplemental Figure 10C). Thus, our finding suggest that ARF3 plays a negative role in flower development but a positive role in plant stress responses during early floral development, which is consistent with the recent finding that ARF3 acts as transcriptional repressor and activator during flower development (Simonini et al., 2017). The tasiRNA-ARF pathway moderates floral architecture by up- or downregulating developmental gene expression under drought stress (Matsui et al., 2014); thus, it would be interesting to investigate whether ARF3 balances plant development and plant/environment interactions during early flower development.
The Repression of Cytokinin Biosynthesis and Signaling by ARF3 Is Critical for Its Function in FM Determinacy
We previously showed that ARF3 integrates the functions of AG and AP2 in promoting FM determinacy but did not characterize the underlying molecular mechanism (Liu et al., 2014). Using the ProTCSn:GFP reporter line, we found that cytokinin activity was elevated in the arf3-29 and ag-10 arf3-29 mutants (Figures 2C to 2E), showing that ARF3 represses cytokinin activity in the FM. Further molecular evidence and quantitative analysis of cytokinin levels revealed altered expression of cytokinin biosynthesis and signaling genes in arf3-29 and ag-10 arf3-29 and dramatic increases in the levels of the active form of cytokinin (Figures 2F and 2G). Given that cytokinin promotes WUS expression in the SAM (Gordon et al., 2009), we hypothesized that cytokinin mediates the role of ARF3 in FM determinacy. Indeed, ARF3 was able to directly bind to the promoters of IPT3, IPT5, and IPT7 and repress their expression in the FM, which is consistent with the increased cytokinin contents in arf3-29 and ag-10 arf3-29 (Figures 2G and 3J to 3L). Genetic analysis revealed that higher order combinations of the ipt3, ipt5, and ipt7 mutations largely rescued the indeterminacy of FMs in ag-10 arf3-29 and that ectopic expression of IPT3, IPT5, and IPT7 in ARF3 expression regions in ag-10 resulted in severe FM determinacy defects (Figures 3A to 3I; Supplemental Figure 4). Interestingly, using the ProARF3:ARF3-GR arf3-29 system, we found that ARF3 controlled the tZRMP-to-tZ conversion by regulating the expression of LOG family genes (Figures 4A to 4D). These results demonstrate that ARF3 fine-tunes cytokinin biosynthesis in concert with its functions in FM determinacy.
The distribution patterns of AHK4 and ARF3 overlap at the OC of the FM, and the cytokinin-induced activation of WUS expression is AHK2/AHK4 dependent (Gordon et al., 2009; Liu et al., 2014). Our analysis revealed that both AHK4 transcript and AHK4 protein levels were elevated in the ag-10 arf3-29 mutant, which is indicative of higher cytokinin sensitivity (Figures 2F and 5A to 5C). Genetic analysis showed that reducing cytokinin sensitivity by introducing the ahk4/cre1-10 mutation into ag-10 arf3-29 partially rescued its FM indeterminacy phenotype (Figures 5D to 5F), whereas expressing AHK4 specifically in ARF3 expression regions in ag-10 severely enhanced its FM determinacy defect (Figures 5G to 5I). ChIP and EMSA analysis revealed the direct binding of ARF3 to the AHK4 promoter and the requirement of the AuxRE motif (TGTCTC) in the AHK4 promoter for this binding (Figures 5J to 5L). Based on these results, together with the finding that activated ARF3-GR directly represses AHK4 expression (Figure 3J), we propose that ARF3 directly represses AHK4 expression to reduce cytokinin activity, thereby regulating FM determinacy. Moreover, treatment with the cytokinin receptor inhibitor S-4893 (Wang et al., 2017) largely rescued the FM determinacy defect of ag-10 arf3-29 and fully rescued the gynoecium developmental defect of arf3-29, indicating that cytokinin receptors play important roles in ARF3-regulated FM determinacy and gynoecium development. How other cytokinin receptors function in these processes awaits further investigation.
ARF3 Acts Synergistically with AG in FM Maintenance and Determinacy
AG plays a central role in promoting FM determinacy through regulating WUS expression (Bowman et al., 1989; Yanofsky et al., 1990; Lenhard et al., 2001). The molecular network surrounding AG in this developmental context was summarized recently (Cao et al., 2015), but the contribution of exogenous signals, such as phytohormones, light, and temperature, to FM determinacy is less established. In line with the repressive effect of AG-GR on cytokinin activity (Figures 7A, 7B, and 7E), the expression of IPT3, IPT5, and IPT7 was derepressed in ag-1, and activated AG-GR inhibited their expression (Figure 7L). Genetic analysis showed that reduced cytokinin biosynthesis or sensitivity rescued the FM determinacy defect of ag-11 or reduced the prolonged FM activity of ag-1 (Figures 7F to 7K). Thus, AG promotes FM determinacy in part through the control of cytokinin activity.
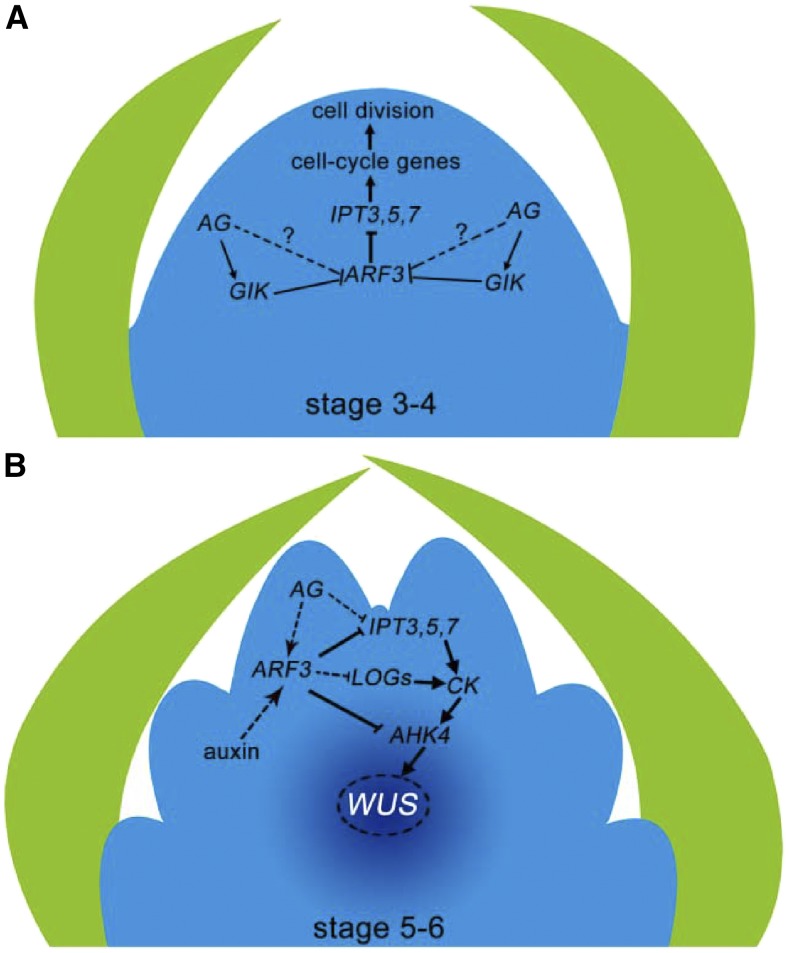
Besides regulating WUS expression directly and indirectly (Sun et al., 2009; Liu et al., 2011), AG helps maintain FM homeostasis. In terms of genome-wide gene expression, the impact of the ag-10 point mutation is minimal, but FM homeostasis is effectively destroyed: in the ag-10 genetic background, even small perturbations in the FM determinacy regulatory network are amplified and result in severe FM determinacy defects (Liu et al., 2016). For example, the expression of major cytokinin biosynthesis and signaling genes, such as IPTs, AHK4, ARRs, and LOGs, was significantly derepressed in ag-10 arf3-29 relative to the single ag-10 and arf3-29 mutants (Figures 2F and 4B), indicating that ARF3 and AG function synergistically to regulate cytokinin activity. On the other hand, the arf3-29 mutation blocked the repressive effect of activated AG-GR on cytokinin biosynthesis and activity (Figures 7E and 7M), indicating that ARF3 partially mediates the function of AG in regulating cytokinin activity. Meanwhile, AG dynamically regulates ARF3 expression during flower development. In Pro35S:AG-GR ag-1 plants, ARF3 expression was repressed 1 d after AG induction by GIANT KILLER (GIK), an AG target gene, and increased thereafter (Figure 7L) (Ng et al., 2009; Liu et al., 2014). Here, we showed that the expression of IPT genes dramatically decreased from D1 to D2 after AG induction (Figure 7L). Therefore, in light of the evidence that ARF3 controls cell cycle genes and cell proliferation during FM maintenance through the regulation of cytokinin biosynthesis (Figure 10), we propose the following model in which ARF3 acts with AG to coordinate FM maintenance and termination. At stage 3-4 of floral development, after AG induction AG represses ARF3 expression indirectly through GIK (Ng et al., 2009) or other factors to maintain normal cytokinin biosynthesis and cell cycle gene expression, thereby maintaining FM size and activity (Figure 11A). At stage 5-6 of floral development, AG and auxin activate ARF3 to inhibit the expression of IPTs, AHK4, and LOGs, either directly or indirectly; this regulation represses cytokinin biosynthesis and activity (Figure 11B), which is required for the temporal termination of WUS expression to regulate FM determinacy. Thus, our findings demonstrate that ARF3 functions as a molecular link between the phytohormones auxin/cytokinin and AG to regulate FM determinacy, shedding light on the mechanisms underlying FM maintenance and termination.
Figure 11.
A Model of How ARF3 Integrates the Repression of Cytokinin Biosynthesis by Auxin and AG to Regulate FM Determinacy.
(A) At stage 3-4 of floral development, AG indirectly represses ARF3 expression through GIK (Ng et al., 2009) and other factors and consequently enhances IPT and cell cycle gene expression to promote cell division and to maintain the meristem cell population, thereby favoring FM maintenance.
(B) At stage 5-6 of floral development, auxin and AG promote ARF3 expression, which in turn directly represses IPT3, IPT5, IPT7, and AHK4 and indirectly represses LOG gene expression. The resulting inhibition of cytokinin activity is required for the proper temporal termination of WUS expression during FM determinacy.
METHODS
Plant Materials and Growth Conditions
All mutants and transgenic Arabidopsis thaliana lines are in the Ler background except for ProTCSn:GFP (Zürcher et al., 2013), ipt3 ipt5 ipt7 (Miyawaki et al., 2006), ahk4/cre1-10, and ProAHK4:GUS (Higuchi et al., 2004), which are in the Col-0 background but were crossed into Ler three times. ag-1 (Bowman et al., 1989), ag-10 (Ji et al., 2011), arf3-29 (Liu et al., 2014), ag-11 (Huang et al., 2017), arf3-29 ag-10 (Liu et al., 2014), and Pro35S:AG-GR ag-1 (Liu et al., 2011) were described previously. All plants were grown at 23°C under long-day conditions (16 h light [100 µmol m−2 s−1]/8 h dark).
Generation of Mutant Combinations
To produce the ipt arf3-29 ag-10 and ahk4/cre1-10 arf3-29 ag-10 mutant combinations, arf3-29 ag-10 plants were crossed with ipt3 ipt5 ipt7 and cre1-10. In the F2 population, ipt3, ipt5, ipt7, ahk4/cre1-10, arf3-29, and ag-10 homozygous plants were identified by genotyping.
To generate the ipt ag-11, ipt ag-1, ahk4/cre1-10 ag-11, and ahk4/cre1-10 ag-1 mutant combinations, ipt3 ipt5 ipt7 and cre1-10 plants were crossed with ag-11 and ag-1. In the F2 population, ag-11 homozygotes were genotyped by PCR, and ag-1 was identified phenotypically.
To produce the ProTCSn:GFP ag-10, ProTCSn:GFP arf3-29, ProTCSn:GFP arf3-29 ag-10, and ProTCSn:GFP Pro35S:AG-GR ag-1 combinations, ProTCSn:GFP plants were crossed with arf3-29 ag-10 and Pro35S:AG-GR ag-1. In the F2 population, ProTCSn:GFP plants were identified based on Basta resistance, and Pro35S:AG-GR plants were selected on kanamycin. To generate ProTCSn:GFP Pro35S:AG-GR ag-1 arf3-29, arf3-29 plants were crossed with ProTCSn:GFP Pro35S:AG-GR ag-1.
All primers used for genotyping are listed in Supplemental Data Set 3.
Plasmid Construction
To construct ProARF3:IPT3/5/7-GFP, the ARF3 promoter was amplified by PCR with the primers ARF3pro F-BamHI and ARF3pro R-BamHI using ProARF3:ARF3-GFP plasmid DNA (Liu et al., 2014) as the template. The PCR product was cloned into pENTR1A-T after digestion with BamHI. As the genomic sequences of IPT3/5/7 lack introns, the IPT3/5/7 coding sequences (CDSs) were amplified by PCR with the primer pairs IPT3 F-AscI/IPT3 R-AscI, IPT5 F-XbaI/IPT5 R-XbaI, and IPT7 F-XbaI/IPT7 R-XbaI, respectively, using Ler genomic DNA as template. The IPT5/7 sequences were cloned into pENTR1A-T-ARF3pro after XbaI digestion, and the resulting plasmids were sequenced to ensure the integrity of the insertions. The plasmids were linearized and recombined into pMDC107 using a Gateway LR Clonase kit (Invitrogen). To construct ProARF3:IPT3-GFP, the pENTR1A-T-ARF3pro plasmid was linearized and recombined into pMDC107 using a Gateway LR Clonase kit (Invitrogen). The IPT3 CDS was cloned into pMDC107-ARF3pro after AscI digestion. The ProARF3:IPT3/5/7-GFP plasmids were transformed into ag-10 plants using the floral dip method (Clough and Bent, 1998).
To construct ProARF3:AHK4-3FLAG, the AHK4 CDS was amplified by PCR with the primers AHK4 F-XbaI and AHK4 R-XbaI using Ler cDNA as template. The PCR product was cloned into pENTR1A-T-ARF3pro after XbaI digestion, and the resulting plasmid was sequenced to ensure the integrity of the insertion. The plasmid was linearized and recombined into modified pEarleyGate100, in which the nucleotides corresponding to the 3FLAG epitopes (DYKDDDDKDYKDDDDKDYKDDDDK) were inserted in-frame into the AvrII and XbaI sites downstream of the attR2 recombination site, using a Gateway LR Clonase kit (Invitrogen) (Li et al., 2016a). ProARF3:AHK4-3FLAG was transformed into ag-10 plants via the floral dip method.
To construct ProARF3:ARF3-GR, the GR CDS was amplified by PCR with the primers GR F-AscI and GR R-AscI using DNA from the transgenic plant Pro35S:AG-GR as template. The PCR product was purified and cloned into pENTR/D-TOPO-ProARF3:ARF3genomic (Liu et al., 2014) with AscI. Sequencing was performed to ensure the integrity of the insertion. The plasmid was linearized and recombined into pEarleyGate303 using a Gateway LR Clonase kit (Invitrogen). ProARF3:ARF3-GR was transformed into arf3-29/+ plants via the floral dip method.
To construct pGEX-4T-1-ARF3-DBD, the ARF3-DBD+Auxin-resp fragment was amplified by PCR with primers ARF3CDS F and ARF3CDS R-1.2k using Ler cDNA as template. The PCR product was purified and cloned into pENTR1A-T. Sequencing was performed to ensure the integrity of the insertion. The insert was recombined into pGEX-4T-1 with BamHI. pGEX-4T-1-ARF3-DBD was transformed into BL21. All primers used for plasmid construction are listed in Supplemental Data Set 3.
Plant Treatment and Tissue Collection
For chemical treatment, inflorescences from 3-week-old plants were treated with 5 µM 6-BA (Sigma-Aldrich), 50 µM IAA (Sigma-Aldrich), 10 µM DEX (Sigma-Aldrich), 10 µM CHX (Sigma-Aldrich), and 60 µM S-4893 (3-[(6-chloro-4-phenylquinazolin-2-yl) amino] propan-1-ol; Vitas-M Laboratory) (Arata et al., 2010; Wang et al., 2017) dissolved in DMSO along with 0.015% Silwet L-77.
For RT-qPCR and RNA-seq analysis, inflorescences of the indicated plants were dissected under a stereomicroscope to remove stage 7 and older flowers; 30 flowers were pooled together for RNA extraction for each biological replicate with independently prepared inflorescence materials. For cytokinin quantification assays, inflorescences containing unopened flowers of the indicated plants were dissected without magnification, and 200 mg tissue was used for each biological replicate. Three independent harvests/biological triplicates were performed for each genotype.
RNA Extraction and Gene Expression Analysis
RNA was isolated with TransZol reagent (TransGen Biotech) after tissue collection. Contaminating DNA was eliminated with DNaseI (Roche) treatment, and reverse transcription was performed using M-MLV reverse transcriptase (Thermo Scientific). Quantitative RT-PCR was conducted in triplicate on the Bio-Rad CFX Connect real-time PCR system using SYBR Green PCR master mix (DBI Bioscience). Three to four biological replicates with independently prepared inflorescence materials were conducted, and the results were analyzed with SPSS statistics 17.0 (IBM).
RNA-Seq Analysis
ProARF3:ARF3-GR arf3-29 inflorescences were treated with 10 µM DEX dissolved in DMSO, and DMSO only as a control, for 12 h. Each treatment had three biological replicates with independently prepared inflorescence materials. After treatment, the inflorescences were harvested by dissecting under a stereomicroscope to remove stage 7 and older flowers. Total RNA was extracted with TransZol reagent (TransGen Biotech). Approximately 1.5 μg RNA per sample was used as input material for the RNA sequencing library construction with a NEBNext Ultra RNA Library Prep Kit for Illumina (NEB). Library quality was assessed on an Agilent 2100 Bioanalyzer system, and 150-bp paired-end reads were generated on an Illumina HiSeq 4000 sequencer.
For data analysis, SolexaQA (Cox et al., 2010) and cutadapt (Martin, 2011) were used to remove low quality regions and adapter sequences in the raw reads, and clean reads with length longer than 25 bp and phred score greater than 17 were mapped to the Arabidopsis Ler-0 genome (Gan et al., 2011) using TopHat 2.0.10 (Trapnell et al., 2009) with default parameters. The reads that were mapped to each annotated gene were counted by HTseq-count (Anders et al., 2015). Using edgeR (Robinson et al., 2010), the raw counts of each gene were normalized, and differentially expressed genes were identified using fold change >2 and false discovery rate <0.01 as significance cutoffs.
The up- and downregulated DEGs were subjected to GO enrichment analysis using topGO (Alexa and Rahnenfuhrer, 2016). The GO terms with a P value <0.001 were identified as significantly enriched GO terms.
ChIP Assay
ChIP was performed as previously described (Liu et al., 2011). Inflorescences were completely ground in liquid nitrogen and cross-linked in 1% formaldehyde (Sigma-Aldrich) for 10 min on ice. The chromatin was pelleted by centrifugation and sonicated into 500- to 1000-bp DNA fragments. The lysate was precleared with 50 μL protein-A agarose beads (Roche) for 1 h and incubated with anti-GFP (ab290; Abcam) (Liu et al., 2011) antibodies overnight. Anti-GFP was used at 5 µg per 0.5 g inflorescence tissue. Bound chromatin was purified on columns from the Qiagen Plasmid Extraction kit, and PCR was conducted on the input, no antibody control, and antibody-bound DNA in triplicate. Three biological replicates with independently prepared inflorescence materials containing unopened flowers were conducted to ensure reproducibility.
In Situ Hybridization
In situ hybridization was performed as previously described (Liu et al., 2011). For the WUS probe, the WUS coding region was amplified by RT-PCR and cloned into pGEM-T-easy (Promega). The resulting plasmid was digested with SpeI and transcribed with T7 RNA polymerase to generate the antisense probe.
EMSA
Wild-type and mutated oligonucleotides were commercially synthesized as single-stranded DNA and labeled with biotin at the 5′ end. The wild-type oligonucleotide sequence corresponds to the −1479 to −1504 region of the AtIPT5 promoter. For the mutated oligonucleotide, TGTCTC (−1489 to −1495) was replaced with TATCTC, TGGCTC, or TAGCTC. To generate double-stranded oligonucleotides, equal amounts of complementary single-stranded oligonucleotides were mixed, boiled for 2 min, and slowly cooled to 25°C. For the binding reaction, a Light Shift Chemiluminescent EMSA kit (Thermo Scientific) was used. Briefly, biotin-labeled probes [in 1× binding buffer, 2.5% glycerol, 5 mM MgCl2, and 50 ng/μL poly(dI-dC)] were incubated with purified ARF3-DBD proteins at room temperature for 20 min. For the competition experiments, different amounts of unlabeled wild-type and mutated double-stranded oligonucleotides were used for the binding reaction.
LC-MS/MS Quantification of Cytokinins
The levels of three cytokinins (tZ, tZR, and tZRMP) were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS 8030 plus; Shimadzu). Two hundred milligrams of each fresh sample frozen in liquid nitrogen was thoroughly homogenized using a TissueLyser homogenizer (Qiagen) with a small glass pestle in a 2-mL vial. After the addition of 1.0 mL 80% methanol (HPLC grade; Merck), the homogenates were mixed well in a KQ3200E ultrasonic bath (Kunshan Ultrasonic) and stored overnight at 4°C. After centrifugation at 15,200g (Eppendorf 5415R) for 10 min, the supernatant was collected and vacuum-dried in a RCT-60 concentrator (Jouan). Dried extract was dissolved in 200 μL sodium phosphate (Sigma-Aldrich) solution (0.1 mol/L, pH 7.8) and passed through a Sep-Pak C18 cartridge (Waters). The cartridge was eluted with 1.5 mL 80% methanol, and the eluate was once again vacuum-dried. After dissolving in 10 mL 10% methanol, 5 μL solution was injected into the LC-MS/MS system.
Liquid chromatography was performed using a 2.0-mm i.d. × 75 mm Shim-pack XR ODS I column (2.2 μm; Shimadzu) at a column temperature of 40°C. The mobile phase with an eluant flow rate of 0.3 mL/min consisting of solvent A (0.02%, v/v, aqueous acetic acid; Sigma-Aldrich) and solvent B (100%, v/v, methanol; Sigma-Aldrich) was employed in a gradient mode simply described as “min/%/%,” i.e., retention time/concentration of solvent A/concentration of solvent B. The gradient for tZ and tZR was 0/90/10, 5/50/50, 5.1/10/90, 6/10/90, and 6.1/90/10, while the gradient for tZRMP was 0/90/10, 6/60/40, 6.1/0/100, and 7.1/90/10.
The system was set to multiple-reaction-monitoring mode using electrospray ionization as the ion source, and positive ion mode was used. Deuterium-labeled 2H5-tZ and 2H5-tZR (Olchemim) were used as internal standards for tZ and tZR, respectively, and HPLC-grade tZRMP (Sigma-Aldrich) was used as an external standard for tZRMP. Optimized mass operation conditions were employed as follows: nebulizing gas flow (2.5 L/min), drying gas flow (15 L/min), heat block temperature (450°C), collision energy (−18V for tZ and 2H5-tZ; −19V for tZR, 2H5-tZR, and −20V for tZRMP), desolvation temperature (250°C for tZ and tZR; 200°C for tZRMP), Q1 Pre Bias and Q3 Pre Bias (−24 V and −24 V for tZ; −24 V and −25 V for 2H5-tZ; −17 V and −23 V for tZR and 2H5-tZR; −22 V and −22 V for tZRMP), m/z (220/136.1 for tZ, 225/137.1 for 2H5-tZ, 352.2/220.1 for tZR, 357.2/225.15 for 2H5-tZR, and 432.15/220.2 for tZRMP, respectively).
Quantitative GUS Analysis
For quantitative GUS analysis, 0.05 g plant tissue was ground into a fine powder in liquid nitrogen and dissolved in 0.5 mL protein extraction buffer (0.05 M phosphate buffer, pH 7.2, 0.01 M EDTA pH 8.0, 0.1% SDS, 0.1% Triton X-100, and Roche protease inhibitor). The samples were centrifuged for 10 min at 12,000 rpm at 4°C. The supernatants were transferred to new tubes as total protein, and the total protein concentration was determined using a BCA protein assay kit (Thermo 23227) via the microplate procedure. The following were combined and incubated at 37°C in the dark: 300 μL protein extraction buffer, 75 μL total protein, and 375 μL 2 mM 4-methylumbelliferyl-β-d-glucuronide hydrate (Sigma-Aldrich M9130) dissolved in protein extraction buffer. A 100-μL volume of the reaction mixture was transferred to a new tube containing 900 μL 0.2 M Na2CO3 at 0 min and 40 min to stop the reaction. The absorbance was measured at an excitation wavelength of 360 nm and an emission wavelength of 410 nm on a plate reader along with the 4-methylumbelliferone (MU; Sigma-Aldrich M1381) dilution standard. For each test sample, a standard curve was used to determine the amount of MU produced per minute. GUS activity was calculated as the amount of MU generated per minute normalized to total protein.
Histochemical Staining
GUS staining was performed as previously described (Liu et al., 2011). Inflorescences were fixed in 90% cold acetone for 15 to 20 min and rinsed with rinse solution [50 mM NaPO4, pH 7.2, 0.5 mM K3Fe(CN)6, and 0.5 mM K4Fe(CN)6]. After adding infiltration solution [50 mM NaPO4, pH 7.2, 0.5 mM K3Fe(CN)6, 0.5 mM K4Fe(CN)6, and 2 mM X-Gluc], the inflorescences were vacuum-infiltrated for 10 min, followed by incubation at 37°C overnight.
For toluidine blue staining, inflorescences were fixed, embedded, and sectioned in wax as performed for in situ hybridization. The sections were mounted on slides, briefly dipped in 0.1% toluidine blue dissolved in 0.1% sodium borate, and rinsed with water.
Microscopy
Optical photographs were taken under an Olympus CX31 microscope equipped with a Canon DS126281 camera or under a Leica M205d/DFC450 stereoscopic microscope. Confocal images were taken with a Leica TCS SP8 (except for Figure 9) or under a Nikon A1 confocal microscope (Figure 9). Explants were manually sectioned into thin slices. Proper filter sets and lasers were selected for fluorescence signal scanning. For GFP, the excitation light wavelength was 488 nm, and the detection range was 510 to 550 nm. For FM4-64, the excitation light wavelength was 561 nm, and the signal was detected in the 570 to 620 nm range. Autofluorescence was excited at 488 nm and detected in the 660- to 700-nm range.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: AG (AT4G18960), AHK4 (AT2G01830), ARF3 (AT2G33860), ARR5 (AT3G48100), ARR7 (AT1G19050), CDKA;1 (AT3G48750), CKX3 (AT5G56970), CKX5 (AT1G75450), CYCB1;1 (AT4G37490), CYCD2:1 (AT2G22490), CYCD3;1 (AT5G43080), E2Fa (AT2G36010), eIF4A1 (AT3G13920), HIS4 (AT2G28740), IPT3 (AT3G63110), IPT5 (AT5G19040), IPT7 (AT3G23630), LOG1 (AT2G28305), LOG2 (AT2G35990), LOG3 (AT2G37210), LOG4 (AT3G53450), LOG5 (AT4G35190), LOG7 (AT5G06300), LOG8 (AT5G11950), KNU (AT5G14010), RBR1 (AT3G12280), UBQ (AT3G62250), and WUS (AT2G17950). Sequencing data were deposited into the Gene Expression Omnibus database at the National Center for Biotechnology Information under accession number GSE103054.
Supplemental Data
Supplemental Figure 1. Exogenous cytokinin treatment enhances SAM size.
Supplemental Figure 2. Relative expression levels of genes involved in cytokinin biosynthesis and signaling in Ler and ag-10 treated with DMSO or 6-BA.
Supplemental Figure 3. The phenotypes of ipt3 ipt5 ipt7 and ag-10 arf3 ipt3 ipt5 ipt7 inflorescences.
Supplemental Figure 4. The expression patterns of the indicated transgenes and the phenotypes of siliques in transgenic plants.
Supplemental Figure 5. The ProARF3:ARF3-GR arf3-29 complementation system.
Supplemental Figure 6. Application of a cytokinin inhibitor rescues the FM determinacy defect of ag-10 arf3-29 and the gynoecium development defect of arf3-29.
Supplemental Figure 7. Ectopic expression of IPT genes does not alter the strength of WUS expression.
Supplemental Figure 8. Gene expression in Ler and ag-11 inflorescences.
Supplemental Figure 9. ARF3 expression in Ler inflorescences under IAA treatment.
Supplemental Figure 10. Functional examination of the role of ARF3 in flower development by RNA-seq analysis.
Supplemental Figure 11. The expression of cell-cycle genes in the inflorescences of ProARF3:ARF3-GR arf3-29 plants treated with CHX or CHX and DEX.
Supplemental Table 1. Floral organ number in wild-type and mutant plants.
Supplemental Data Set 1. List of genes that were downregulated in the inflorescences of ProARF3:ARF3-GR arf3-29 plants treated with DEX + DMSO compared with DMSO only.
Supplemental Data Set 2. List of genes that were up-regulated in the inflorescences of ProARF3:ARF3-GR arf3-29 plants treated with DEX + DMSO compared with DMSO only.
Supplemental Data Set 3. List of primers used in this study.
Acknowledgments
We thank Rae Eden Yumul for valuable advice and language editing, Bruno Müller for ProTCSn:GFP seeds, Tatsuo Kakimoto for ipt3 ipt5 ipt7 seeds, and Jianru Zuo for ProAHK4:GUS and ahk4/cre1-10 seeds. This work was funded by National Science Foundation of China (NSFC) (Project 31671354); the National Key Research and Development Program of China (2016YFD0100401); National Basic Research Program of China (973 program; Grant 2014CB138100); the CAS Pioneer Hundred Talents Program; and NSFC Grants 31401039, 31671777, and 31570372.
AUTHOR CONTRIBUTIONS
X.L. conceived and designed the experiments. K.Z., Y.L., D.L., and L.G. performed the experiments with the help of Y.P. R.W. and L.X. conducted the cytokinin quantification assay. H.Z., R.L., and X.L. analyzed data. X.C. and Y.J. performed the confocal imaging. K.Z., R.L., and X.L. wrote the manuscript.
References
- Aichinger E., Kornet N., Friedrich T., Laux T. (2012). Plant stem cell niches. Annu. Rev. Plant Biol. 63: 615–636. [DOI] [PubMed] [Google Scholar]
- Alexa A., Rahnenfuhrer J. (2016). topGO: Enrichment analysis for Gene Ontology. R package version 2.28.0, http://www.bioconductor.org/packages/2.11/bioc/html/topGO.html.
- Anders S., Pyl P.T., Huber W. (2015). HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arata Y., Nagasawa-Iida A., Uneme H., Nakajima H., Kakimoto T., Sato R. (2010). The phenylquinazoline compound S-4893 is a non-competitive cytokinin antagonist that targets Arabidopsis cytokinin receptor CRE1 and promotes root growth in Arabidopsis and rice. Plant Cell Physiol. 51: 2047–2059. [DOI] [PubMed] [Google Scholar]
- Bartrina I., Otto E., Strnad M., Werner T., Schmülling T. (2011). Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleth T., Jurgens G. (1993). The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 118: 575–587. [Google Scholar]
- Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heidstra R., Aida M., Palme K., Scheres B. (2005). The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44. [DOI] [PubMed] [Google Scholar]
- Bowman J.L., Smyth D.R., Meyerowitz E.M. (1989). Genes directing flower development in Arabidopsis. Plant Cell 1: 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., He Z., Guo L., Liu X. (2015). Epigenetic mechanisms are critical for the regulation of WUSCHEL expression in floral meristems. Plant Physiol. 168: 1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J.W. (2012). Floral meristem initiation and emergence in plants. Cell. Mol. Life Sci. 69: 3807–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J.W., Werr W. (2015). Cytokinin-auxin crosstalk in cell type specification. Trends Plant Sci. 20: 291–300. [DOI] [PubMed] [Google Scholar]
- Cheng Z.J., et al. (2013). Pattern of auxin and cytokinin responses for shoot meristem induction results from the regulation of cytokinin biosynthesis by AUXIN RESPONSE FACTOR3. Plant Physiol. 161: 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chickarmane V.S., Gordon S.P., Tarr P.T., Heisler M.G., Meyerowitz E.M. (2012). Cytokinin signaling as a positional cue for patterning the apical-basal axis of the growing Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA 109: 4002–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood D.H., Nogueira F.T., Howell M.D., Montgomery T.A., Carrington J.C., Timmermans M.C. (2009). Pattern formation via small RNA mobility. Genes Dev. 23: 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ckurshumova W., Smirnova T., Marcos D., Zayed Y., Berleth T. (2014). Irrepressible MONOPTEROS/ARF5 promotes de novo shoot formation. New Phytol. 204: 556–566. [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed]
- Cox M.P., Peterson D.A., Biggs P.J. (2010). SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinformatics 11: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Medzihradszky A., Suzaki T., Lohmann J.U. (2014). A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proc. Natl. Acad. Sci. USA 111: 14619–14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Ioio R., Linhares F.S., Scacchi E., Casamitjana-Martinez E., Heidstra R., Costantino P., Sabatini S. (2007). Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 17: 678–682. [DOI] [PubMed] [Google Scholar]
- Fahlgren N., Montgomery T.A., Howell M.D., Allen E., Dvorak S.K., Alexander A.L., Carrington J.C. (2006). Regulation of AUXIN RESPONSE FACTOR3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Curr. Biol. 16: 939–944. [DOI] [PubMed] [Google Scholar]
- Gallois J.L., Nora F.R., Mizukami Y., Sablowski R. (2004). WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev. 18: 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X., et al. (2011). Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature 477: 419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D., Collier S.A., Byrne M.E., Martienssen R.A. (2006). Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr. Biol. 16: 933–938. [DOI] [PubMed] [Google Scholar]
- Gordon S.P., Chickarmane V.S., Ohno C., Meyerowitz E.M. (2009). Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA 106: 16529–16534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle T.J., Hagen G. (2007). Auxin response factors. Curr. Opin. Plant Biol. 10: 453–460. [DOI] [PubMed] [Google Scholar]
- Hardtke C.S., Berleth T. (1998). The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 17: 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., et al. (2004). In planta functions of the Arabidopsis cytokinin receptor family. Proc. Natl. Acad. Sci. USA 101: 8821–8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong R.L., Hamaguchi L., Busch M.A., Weigel D. (2003). Regulatory elements of the floral homeotic gene AGAMOUS identified by phylogenetic footprinting and shadowing. Plant Cell 15: 1296–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Shi T., Zheng B., Yumul R.E., Liu X., You C., Gao Z., Xiao L., Chen X. (2017). APETALA2 antagonizes the transcriptional activity of AGAMOUS in regulating floral stem cells in Arabidopsis thaliana. New Phytol. 215: 1197–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Higuchi M., Hashimoto Y., Seki M., Kobayashi M., Kato T., Tabata S., Shinozaki K., Kakimoto T. (2001). Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409: 1060–1063. [DOI] [PubMed] [Google Scholar]
- Irish V.F. (2010). The flowering of Arabidopsis flower development. Plant J. 61: 1014–1028. [DOI] [PubMed]
- Ji L., et al. (2011). ARGONAUTE10 and ARGONAUTE1 regulate the termination of floral stem cells through two microRNAs in Arabidopsis. PLoS Genet. 7: e1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber J.J., Schaller G.E. (2014). Cytokinins. The Arabidopsis Book 12: e0168, doi/10.1199/tab.0168. [DOI] [PMC free article] [PubMed]
- Krogan N.T., Marcos D., Weiner A.I., Berleth T. (2016). The auxin response factor MONOPTEROS controls meristem function and organogenesis in both the shoot and root through the direct regulation of PIN genes. New Phytol. 212: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakawa T., Ueda N., Maekawa M., Kobayashi K., Kojima M., Nagato Y., Sakakibara H., Kyozuka J. (2007). Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445: 652–655. [DOI] [PubMed] [Google Scholar]
- Kuroha T., Tokunaga H., Kojima M., Ueda N., Ishida T., Nagawa S., Fukuda H., Sugimoto K., Sakakibara H. (2009). Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell 21: 3152–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs P., Grandjean O., Jonak C., Kiêu K., Traas J. (1998). Cellular parameters of the shoot apical meristem in Arabidopsis. Plant Cell 10: 1375–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux T. (2003). The stem cell concept in plants: a matter of debate. Cell 113: 281–283. [DOI] [PubMed] [Google Scholar]
- Laux T., Mayer K.F.X., Berger J., Jürgens G. (1996). The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96. [DOI] [PubMed] [Google Scholar]
- Leibfried A., To J.P., Busch W., Stehling S., Kehle A., Demar M., Kieber J.J., Lohmann J.U. (2005). WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438: 1172–1175. [DOI] [PubMed] [Google Scholar]
- Lenhard M., Bohnert A., Jürgens G., Laux T. (2001). Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 105: 805–814. [DOI] [PubMed] [Google Scholar]
- Li D., et al. (2016a). FAR-RED ELONGATED HYPOCOTYL3 activates SEPALLATA2 but inhibits CLAVATA3 to regulate meristem determinacy and maintenance in Arabidopsis. Proc. Natl. Acad. Sci. USA 113: 9375–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.B., Xie Z.Z., Hu C.G., Zhang J.Z. (2016b). A review of auxin response factors (ARFs) in plants. Front. Plant Sci. 7: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E., Reed J.W. (2002). Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 49: 387–400. [PubMed] [Google Scholar]
- Liu L., Li B., Liu X. (2016). FAR-RED ELONGATED HYPOCOTYL3 promotes floral meristem determinacy in Arabidopsis. Plant Signal. Behav. 11: e1238545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Dinh T.T., Li D., Shi B., Li Y., Cao X., Guo L., Pan Y., Jiao Y., Chen X. (2014). AUXIN RESPONSE FACTOR 3 integrates the functions of AGAMOUS and APETALA2 in floral meristem determinacy. Plant J. 80: 629–641. [DOI] [PMC free article] [PubMed]
- Liu X., Kim Y.J., Müller R., Yumul R.E., Liu C., Pan Y., Cao X., Goodrich J., Chen X. (2011). AGAMOUS terminates floral stem cell maintenance in Arabidopsis by directly repressing WUSCHEL through recruitment of Polycomb group proteins. Plant Cell 23: 3654–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann J.U., Hong R.L., Hobe M., Busch M.A., Parcy F., Simon R., Weigel D. (2001). A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105: 793–803. [DOI] [PubMed] [Google Scholar]
- Martin M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17: 10–12. [Google Scholar]
- Matsui A., Mizunashi K., Tanaka M., Kaminuma E., Nguyen A.H., Nakajima M., Kim J.M., Nguyen D.V., Toyoda T., Seki M. (2014). tasiRNA-ARF pathway moderates floral architecture in Arabidopsis plants subjected to drought stress. BioMed Res. Int. 2014: 303451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K.F.X., Schoof H., Haecker A., Lenhard M., Jürgens G., Laux T. (1998). Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815. [DOI] [PubMed] [Google Scholar]
- Meng W.J., Cheng Z.J., Sang Y.L., Zhang M.M., Rong X.F., Wang Z.W., Tang Y.Y., Zhang X.S. (2017). Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell 29: 1357–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K., Matsumoto-Kitano M., Kakimoto T. (2004). Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J. 37: 128–138. [DOI] [PubMed]
- Miyawaki K., Tarkowski P., Matsumoto-Kitano M., Kato T., Sato S., Tarkowska D., Tabata S., Sandberg G., Kakimoto T. (2006). Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 103: 16598–16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.A., Jones A., Godin C., Traas J. (2012). Systems analysis of shoot apical meristem growth and development: integrating hormonal and mechanical signaling. Plant Cell 24: 3907–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser J.L., Feldman L.J., Zambryski P.C. (2000). Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development 127: 3877–3888. [DOI] [PubMed] [Google Scholar]
- Ng K.H., Yu H., Ito T. (2009). AGAMOUS controls GIANT KILLER, a multifunctional chromatin modifier in reproductive organ patterning and differentiation. PLoS Biol. 7: e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura C., Ohashi Y., Sato S., Kato T., Tabata S., Ueguchi C. (2004). Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell 16: 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y., et al. (2005). Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17: 444–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne T., Johnson S.D., Koltunow A.M. (2004). KNUCKLES (KNU) encodes a C2H2 zinc-finger protein that regulates development of basal pattern elements of the Arabidopsis gynoecium. Development 131: 3737–3749. [DOI] [PubMed] [Google Scholar]
- Perales M., Rodriguez K., Snipes S., Yadav R.K., Diaz-Mendoza M., Reddy G.V. (2016). Threshold-dependent transcriptional discrimination underlies stem cell homeostasis. Proc. Natl. Acad. Sci. USA 113: E6298–E6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernisová M., Kuderová A., Hejátko J. (2011). Cytokinin and auxin interactions in plant development: metabolism, signalling, transport and gene expression. Curr. Protein Pept. Sci. 12: 137–147. [DOI] [PubMed] [Google Scholar]
- Prunet N., Morel P., Negrutiu I., Trehin C. (2009). Time to stop: flower meristem termination. Plant Physiol. 150: 1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler M., Novak O., Strnad M., Schmülling T. (2006). Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18: 40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C., Huntley R., Jacqmard A., Murray J.A. (1999). Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283: 1541–1544. [DOI] [PubMed] [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka K., Simásková M., Duclercq J., Petrásek J., Zazímalová E., Simon S., Friml J., Van Montagu M.C., Benková E. (2009). Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc. Natl. Acad. Sci. USA 106: 4284–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablowski R. (2004). Plant and animal stem cells: conceptually similar, molecularly distinct? Trends Cell Biol. 14: 605–611. [DOI] [PubMed] [Google Scholar]
- Sablowski R. (2007a). Flowering and determinacy in Arabidopsis. J. Exp. Bot. 58: 899–907. [DOI] [PubMed] [Google Scholar]
- Sablowski R. (2007b). The dynamic plant stem cell niches. Curr. Opin. Plant Biol. 10: 639–644. [DOI] [PubMed] [Google Scholar]
- Schaller G.E., Bishopp A., Kieber J.J. (2015). The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell; 27: 44–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B. (2007). Stem-cell niches: nursery rhymes across kingdoms. Nat. Rev. Mol. Cell Biol. 8: 345–354. [DOI] [PubMed] [Google Scholar]
- Schoof H., Lenhard M., Haecker A., Mayer K.F.X., Jürgens G., Laux T. (2000). The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644. [DOI] [PubMed] [Google Scholar]
- Sessions A., Nemhauser J.L., McColl A., Roe J.L., Feldmann K.A., Zambryski P.C. (1997). ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124: 4481–4491. [DOI] [PubMed] [Google Scholar]
- Simonini S., Bencivenga S., Trick M., Østergaard L. (2017). Auxin-induced modulation of ETTIN activity orchestrates gene expression in Arabidopsis. Plant Cell 29: 1864–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D.R., Bowman J.L., Meyerowitz E.M. (1990). Early flower development in Arabidopsis. Plant Cell 2: 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich M., Je B.I., Simon R., Jackson D. (2016). CLAVATA-WUSCHEL signaling in the shoot meristem. Development 143: 3238–3248. [DOI] [PubMed] [Google Scholar]
- Su Y.H., Liu Y.B., Zhang X.S. (2011). Auxin-cytokinin interaction regulates meristem development. Mol. Plant 4: 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Xu Y., Ng K.H., Ito T. (2009). A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes Dev. 23: 1791–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantikanjana T., Nasrallah J.B. (2012). Non-cell-autonomous regulation of crucifer self-incompatibility by Auxin Response Factor ARF3. Proc. Natl. Acad. Sci. USA 109: 19468–19473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S.L. (2009). TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T., Hagen G., Guilfoyle T.J. (1999a). Dimerization and DNA binding of auxin response factors. Plant J. 19: 309–319. [DOI] [PubMed]
- Ulmasov T., Hagen G., Guilfoyle T.J. (1999b). Activation and repression of transcription by auxin-response factors. Proc. Natl. Acad. Sci. USA 96: 5844–5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T., Liu Z.B., Hagen G., Guilfoyle T.J. (1995). Composite structure of auxin response elements. Plant Cell 7: 1611–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T., Besnard F., Traas J. (2010). Auxin at the shoot apical meristem. Cold Spring Harb. Perspect. Biol. 2: a001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Tian C., Zhang C., Shi B., Cao X., Zhang T.-Q., Zhao Z., Wang J.-W., Jiao Y. (2017). Cytokinin signaling activates WUSCHEL expression during axillary meristem initiation. Plant Cell 29: 1373–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T., Motyka V., Laucou V., Smets R., Van Onckelen H., Schmülling T. (2003). Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R.K., Perales M., Gruel J., Girke T., Jönsson H., Reddy G.V. (2011). WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev. 25: 2025–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky M.F., Ma H., Bowman J.L., Drews G.N., Feldmann K.A., Meyerowitz E.M. (1990). The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346: 35–39. [DOI] [PubMed] [Google Scholar]
- Zürcher E., Tavor-Deslex D., Lituiev D., Enkerli K., Tarr P.T., Müller B. (2013). A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta. Plant Physiol. 161: 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Swarup R., Bennett M., Schaller G.E., Kieber J.J. (2013). Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr. Biol. 23: 1979–1989. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Andersen S.U., Ljung K., Dolezal K., Miotk A., Schultheiss S.J., Lohmann J.U. (2010). Hormonal control of the shoot stem-cell niche. Nature 465: 1089–1092. [DOI] [PubMed] [Google Scholar]