Sun, X., Peng, L., Guo, J., Chi, W., Ma, J., Lu, C., and Zhang, L. (2007). Formation of DEG5 and DEG8 complexes and their involvement in the degradation of photodamaged photosystem II reaction center D1 protein in Arabidopsis. Plant Cell 19: 1347–1361.
The authors of the above article request the publication of corrections for Figures 5A and 5E, as described below. The original results and conclusions are unaffected by these corrections. The authors apologize and accept responsibility for not detecting these errors prior to publication. All authors approve these corrections.
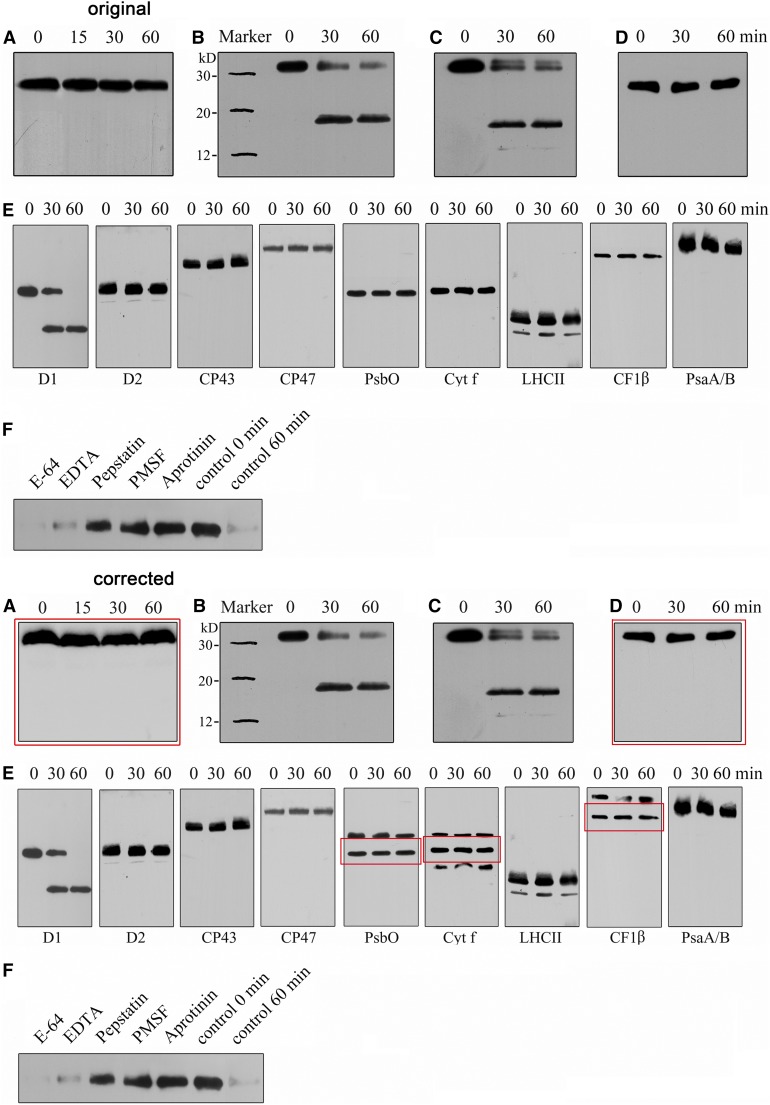
Figure 5.
Corrected: Degradation of the Photodamaged D1 Protein by Recombinant DEG8.
(A) to (C) Thylakoid membranes isolated from plants subjected to high-light treatment were incubated without (A) or with purified recombinant DEG8 ([B] and [C]), and the degradation of D1 was analyzed by immunoblots using antibodies directed against the DE loop ([A] and [B]) and the N-terminal amino acid residues (58 to 86) of D1 (C). The image of Figure 5A was derived from an independent repeated experiment.
(D) Thylakoid membranes from leaves that had not been subjected to high-light treatment were incubated with purified recombinant DEG8, and the thylakoid proteins were separated by SDS-PAGE and immunodetected with the antibody raised against the DE loop of the D1 protein. The PsbA band in (D) (which is intact; without high-light treatment) was aligned with the intact PsbA band in (B) and (C).
(E) High light–treated thylakoid membranes (10 µg chlorophyll) were incubated with purified recombinant DEG8 (0.5 µg), and the thylakoid proteins were separated and immunodetected with anti-D1, anti-D2, anti-CP43, anti-CP47, anti-cytochrome f, anti-CF1β, anti-PsaA/B, anti-PsbO, and anti-LHCII antibodies. For PsbO, Cyt f, and CF1β, the original image containing unspecific bands is presented and the legitimate bands are indicated by a red box.
(F) Purified recombinant DEG8 (1 µg protein) was added to heat-treated thylakoid membranes obtained following high-light illumination and incubated in the absence (control) or presence of the following protease inhibitors: 100 µg/mL PMSF, 1 µg/mL aprotinin, 1 µg/mL E64 [3-carboxy-trans-2,3-epoxypropyl-leucylamido(4-guanidino)butane], 1 µg/mL pepstatin, and 500 µg/mL EDTA. After these treatments, the thylakoid proteins were separated and immunodetected with specific antibodies raised against the DE loop of D1. These experiments were repeated three times independently, and similar results were obtained. Results from a representative experiment are shown.
Figure 5A: The background above the bands in the original image was improperly modified. The original image for Figure 5A was not found; therefore, it was reproduced from data from a repeated experiment. The legend was revised accordingly.
Figure 5D: The original image for Figure 5D was not aligned with the intact PsbA band in panels B and C; we have aligned the PsbA band in panel D (which is intact, without high-light treatment) with the intact PsbA band in panels B and C, corresponding to the molecular weight markers shown in panel B.
Figure 5E: The bands of PsbO, Cyt f, and CF1β were copied and then pasted into a “cloned” background in the original figure. In the corrected figure, the original images are presented and the legitimate bands are marked with red boxes. The figure legend was revised accordingly.
The legend for panels A, D, and E were amended and the text is in bold.
Editor’s note: The corrected figures and accompanying text were reviewed by members of The Plant Cell editorial board based on the information provided by the authors. The authors are responsible for providing a complete listing and accurate explanations for all known errors or instances of inappropriate data handling or image manipulation associated with the original publication.
Footnotes
Articles can be viewed without a subscription.