Abstract
PURPOSE
We investigated the prognostic utility of onset age at first signs and symptoms (SS) to predict onset age at loss of ambulation (LOA) for childhood-onset Duchenne and Becker Muscular Dystrophies (DBMD).
METHODS
Our cohort comprised male cases with DBMD ascertained by the population-based Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards models for associations between onset ages of first SS and LOA. Covariates controlled for were corticosteroid use, family history of DBMD, birth year, race/ethnicity, and MD STARnet site. Onset age at first SS was considered as a continuous and as a categorical variable.
RESULTS
A one-year increase in onset age at first SS was significantly associated with a 10% reduction in annual risk of LOA (HR = 0.90, CI = 0.87–0.94). Treating onset age at first SS as a categorical variable yielded a similar association (≥ 5 years: referent; ≥ 3 to < 5 years: HR = 1.36, CI = 1.02–1.81; 18 months to < 3 years: HR = 1.72, CI = 1.31–2.26; < 18 months: HR = 1.52, CI = 1.14–2.02).
CONCLUSIONS
Earlier onset age at first SS is associated with earlier onset age at LOA and may have clinical utility in differentiating childhood-onset Duchenne and Becker muscular dystrophies.
Keywords: Duchenne Muscular Dystrophy, Becker Muscular Dystrophy, ambulation
1. Introduction
Childhood-onset dystrophinopathies, such as Duchenne and Becker Muscular Dystrophies (DBMD), are allelic, X-linked recessive neuromuscular disorders caused by mutations in the dystrophin gene. Mutations that disrupt the mRNA reading frame lead to complete lack of dystrophin and cause the severe Duchenne (DMD) phenotype, whereas in-frame mutations that allow for translation of some shorter but partially functional dystrophin cause the milder Becker (BMD) phenotype. This “reading frame” rule is accurate in predicting phenotype severity in about 92% of cases [1]. Exceptions to this rule exist and are likely due to alternative initiation sites [2], exon-skipping, and differences in intronic deletion breakpoints as well as genetic and environmental modifiers [3–5].
Historically, loss of ambulation (LOA) has been used as the key clinical milestone to describe disease progression with early LOA associated with a more severe disease manifestation [6]. Clinically, DMD patients are defined by onset of symptoms by age 5 years and, historically, LOA before age 12, whereas BMD patients have onset of symptoms after age 5 years (mean age 11 years) and remain ambulatory beyond age 15 [6]. Patients who lose ambulation between ages 12 and 15 years have been clinically classified as intermediate cases (IMD). Treatment with prednisone and deflazacort has been found to prolong ambulation by one to three years for DMD patients thereby slowing disease progression and complicating clinical diagnosis [7–9]. As a result, age at LOA alone may no longer be a useful diagnostic indicator of disease severity. An alternative approach would be to identify risk factors for LOA identified in the early stages of disease onset and use these factors along with LOA to describe progression of muscle weakness. One such indicator would be the age at which the first signs of muscle weakness are observed. Although onset of such symptoms has been used clinically, the prognostic utility of onset age at first signs or symptoms (SS) has not been studied in a large cohort of patients. As such, we investigated the association between age at first SS and age at LOA using a large population-based cohort of males with childhood-onset dystrophinopathy identi-fied by the Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet).
2. Methods
2.1. Study population and case classification
The MD STARnet cohort is the first and only population-based cohort of childhood-onset dystrophinopathy cases in the United States (U.S.) and is funded by the Centers for Disease Control and Prevention (CDC) [10]. Starting in 2004, the MD STARnet retrospectively identified and longitudinally followed cases with DBMD who were diagnosed before age 21 years, born since 1982, and resided in an MD STARnet site (Arizona, Colorado, Iowa, Western New York State); Georgia and Hawaii joined the MD STARnet in 2005 and 2008, respectively. Multiple sources were used to identify potential cases including neuromuscular clinics, hospitals and hospital discharge databases, private physicians, service units for children with special healthcare needs, and birth defects surveillance programs. Since the start of surveillance in 2004, all health and vital status information was systematically collected annually by trained abstractors from medical records for cases in the cohort until December 31, 2011, or sooner if the case was deceased or moved out of a surveillance catchment area. A full description of the MD STARnet surveillance methodology has been published [10,11]. Colorado, Georgia, Iowa, and New York State expanded public health authority to permit active case finding and record abstraction; institutional review board approval was obtained for these surveillance activities in Arizona (University of Arizona) and Hawaii (Hawaii Department of Health).
Key clinical and diagnostic data were used to assign a case status (definite, probable, possible, female, asymptomatic, or not DBMD) and were then reviewed by a committee of neuromuscular clinicians representing each site to assign the final case status [12]. Cases that were definite or probable had either DNA or muscle biopsy confirmation.
2.2. Outcome definition
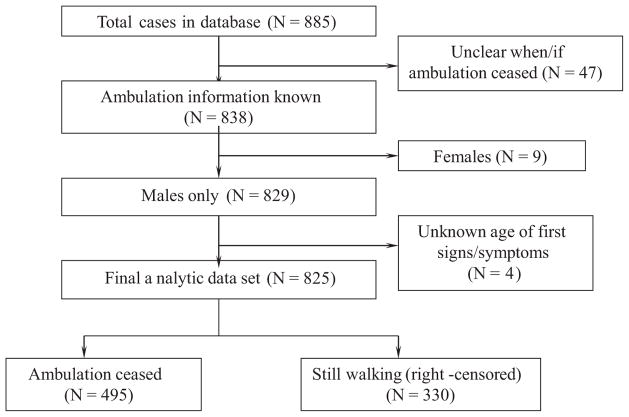
The primary outcome variable was onset age at LOA, calculated either from the earliest age at which full-time wheelchair use was recorded or from the earliest age when ambulation was recorded as ceased. Cases with conflicting information on earliest age when full-time wheelchair use began or when ambulation ceased were excluded (n = 47, 5.3%). Other exclusions were female cases diagnosed with DMD (n = 9, 1.0%) and those with unknown age at first SS (n = 4, 0.5%) (Fig. 1).
Fig. 1.
Selection of cases for final analytic data set.
2.3. Independent variable definition
The primary independent variable was the onset age at first SS, which was calculated from the earliest onset of the following signs and symptoms: trouble rising/Gowers’ sign, trouble walking/running/jumping, frequent falling/clumsiness, inability to keep up with peers, abnormal gait, loss of motor skills, gross motor delay, or muscle weakness. The onset age at first SS was analyzed as a continuous variable and as a categorical variable (< 18 months, ≥18 months to < 3 years, ≥ 3 years to < 5 years, and ≥ 5 years).
2.4. Covariates
The covariates examined and measured in cases, were corticosteroid use, family history of DBMD, birth year, race/ethnicity, and MD STARnet site, as well as maternal education at the time of birth of the case child. Corticosteroid use was categorized according to the age at start of corticosteroids and percentage of time on corticosteroids until ambulation ceased or last known age when ambulatory (never used corticosteroids, started after age 7 years and on ≥ 80% of the time, started after age 7 years and on < 80% of the time, started between ages 5–7 years and on ≥ 80% of the time, started between ages 5–7 years and on < 80% of the time, started before age 5 years and on ≥ 80% of the time, and started before age 5 years and on < 80% of the time). Family history was defined as ‘known’ if another family member was known to be diagnosed with DBMD before the birth of the case; otherwise, it was coded as known after the case child’s birth or as no known family history. Other covariates included: birth year (continuous), MD STARnet site (AZ, CO, GA, HI, IA, NY), race/ethnicity (White non-Hispanic, Black non-Hispanic, Hispanic/Latino, and Other), and maternal education at the time of the child’s birth (< high school, high school and/or GED, some college or two year degree, Bachelor’s degree or higher).
2.5. Statistical analyses
For cases who were still walking at the last follow-up contact (n = 330, 40.0%), their age was censored at date of this contact. Cox proportional hazards models were used to evaluate the association between the onset age at first SS and onset age at LOA, adjusting for covariates. Onset age at first SS was modeled as both a continuous variable and a categorical variable. To identify relevant covariates, multiple model selection procedures were used to select the most parsimonious model and included forward selection, backward elimination, and stepwise selection. Corticosteroid use was forced to be included in all models. A liberal significance level of 25% was used for variables to either enter or exit the model. After selection of the most parsimonious model for covariates, onset age at first SS was added to the model and a Wald test was performed to examine the statistical significance of this variable. Results for associations with onset age at LOA were reported as adjusted hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs). Data analyses were performed using SAS software (SAS Institute Inc., Cary, NC, USA) [13].
3. Results
The final dataset, which included birth years 1982–2009, comprised 825 cases of which over one-half were non-Hispanic white (59%). The onset age at first SS was > 5 years for 29% of cases and 69% of cases had no known family history of DBMD prior to case birth. Approximately one-half of cases had ever taken corticosteroids (Table 1). Of the 495 cases (60%) who had lost ambulation, 69% did so before age 12 years, 27% between ages 12 and 16, and 3% after age 16 (data not shown). Among these non-ambulatory cases, the average onset age at LOA was 11.1 years (standard deviation [SD] = 2.5 years, range = 5.5–24.3 years). The average age at the last follow-up visit for the 330 cases who were still walking was 10.6 years (SD = 5.3 years, range = 1.8–27.9 years).
Table 1.
Characteristics of boys with DBMD in the MD STARnet cohort, 1982–2009
| Characteristics | N (%) |
|---|---|
| Age at first signs/symptoms | |
| < 18 months | 182 (22.1%) |
| 18 months to < 3 years | 220 (26.7%) |
| ≥ 3 years to < 5 years | 185 (22.4%) |
| ≥ 5 years | 238 (28.9%) |
| Corticosteroid use | |
| None | 417 (50.6%) |
| Age > 7, < 80% of the time | 52 (6.3%) |
| Age 5–7, < 80% of the time | 28 (3.4%) |
| Age < 5, < 80% of the time | 8 (1.0%) |
| Age > 7, ≥ 80% of the time | 146 (17.7%) |
| Age 5–7, ≥ 80% of the time | 119 (14.4%) |
| Age < 5, ≥ 80% of the time | 55 (6.7%) |
| Family history of DBMD | |
| Known before/during pregnancy | 207 (25.1%) |
| Known after child’s birth due to diagnosed relative | 32 (3.9%) |
| No known family history | 544 (65.9%) |
| Missing | 42 (5.1%) |
| Birth year | |
| 1982–1984 | 61 (7.4%) |
| 1985–1989 | 175 (21.2%) |
| 1990–1994 | 203 (24.6%) |
| 1995–1999 | 198 (24.0%) |
| 2000–2004 | 152 (18.4%) |
| 2005–2009 | 36 (4.4%) |
| Race/ethnicity | |
| White, non-Hispanic | 485 (58.8%) |
| Black, non-Hispanic | 61 (7.4%) |
| Hispanic/Latino | 165 (20.0%) |
| Other | 39 (4.7%) |
| Unknown | 75 (9.1%) |
| MD STARnet site | |
| Arizona | 177 (21.5%) |
| Colorado | 187 (22.7%) |
| Georgia | 225 (27.3%) |
| Hawaii | 16 (1.9%) |
| Iowa | 111 (13.5%) |
| New York | 109 (13.2%) |
| Mother’s education level | |
| < High school | 134 (16.2%) |
| High school | 184 (22.3%) |
| Some college | 110 (13.3%) |
| Bachelor’s degree or higher | 95 (11.5%) |
| Missing | 302 (36.6%) |
DBMD = Duchenne or Becker Muscular Dystrophy; MD STARnet = Muscular Dystrophy Surveillance, Tracking, and Research Network.
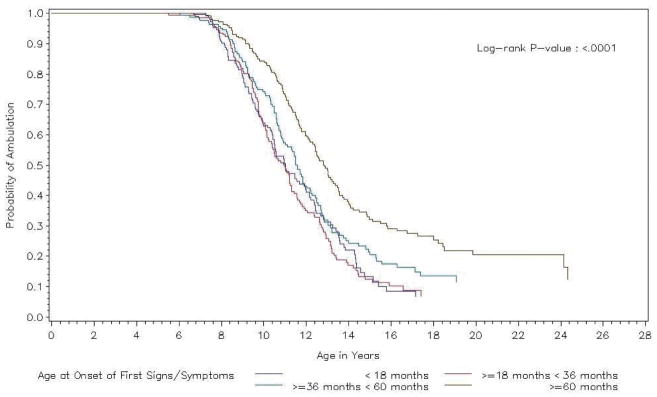
Unadjusted Kaplan-Meier curves showing the distribution of onset age at LOA by onset age at first SS are presented in Fig. 2. The median onset ages at LOA for the earliest two onset age at first SS groups (< 18 months and ≥ 18 months to < 3 years) were similar (median [interquartile range] ages = 11.02 years [10.41, 11.98] and 10.99 years [10.23, 11.30], respectively). For those cases whose onset age at first SS was between 3 and 5 years, a later median onset age at LOA was estimated (11.59 years [10.88, 12.18]). The median onset age at LOA was latest for those with onset age at first SS ≥ 5 years (12.83 years [12.17, 13.45]).
Fig. 2.
Kaplan-Meier curves showing loss of ambulation (LOA) over time by age at onset of first signs/symptoms (SS).
The three model selection strategies (backward, forward, and stepwise) all yielded the identical covariate model for onset age at LOA, with corticosteroid use, family history of DBMD, birth year, race/ethnicity, and MD STARnet site included in the model. This analysis included 716 (87%) of the original 825 cases, due to missing values for case family history of DBMD (n = 42) and race/ethnicity (n = 75).
Table 2 displays the HR estimates for the association between onset age at first SS and onset age at LOA. When onset age at first SS was treated as a continuous variable, a one-year increase in this onset age was associated with a 10% reduction in annual risk of LOA (HR = 0.90, 95% CI = 0.87–0.94). When onset age at first SS was entered as a categorical variable, a generally progressive increased annual risk of LOA with earlier onset age at first SS was observed, except in the group with youngest onset age at first SS (≥ 5 years: HR = 1.00 [referent group]; ≥ 3 to < 5 years: HR = 1.36, 95% CI = 1.02–1.81; ≥ 18 months to < 3 years: HR = 1.72, 95% CI = 1.31–2.26; < 18 months: HR = 1.52, 95% CI = 1.14–2.02]) (Table 2).
Table 2.
Associations between age at onset of first signs or symptoms (SS) and age at loss of ambulation (LOA) among males with DBMD in the MD STARnet cohort, 1982–2009
| Variable | Hazard Ratio* | 95% CI | P-value |
|---|---|---|---|
| Model 1: Continuous | |||
| Age at first SS | 0.90 | (0.87, 0.94) | < 0.0001 |
| Model 2: Categorical | |||
| Age first SS | |||
| < 18 months | 1.52 | (1.14, 2.02) | 0.001 |
| 18 months ≥ SS < 3 years | 1.72 | (1.31, 2.26) | |
| 3 years ≥ SS < 5 years | 1.36 | (1.02, 1.81) | |
| ≥ 5 years | 1.00 | ||
Adjusted for race/ethnicity, family history of DBMD, year of birth, corticosteroid use, and MD STARnet site; CI = confidence interval; DBMD = Duchenne and Becker muscular dystrophies; MDSTARnet = Muscular Dystrophy Surveillance, Tracking, and Research Network.
4. Discussion
Our findings from this population-based cohort show that earlier onset age at first SS is a risk factor for a more rapid progression of muscle weakness, as measured by onset age at LOA, after controlling for corticosteroid use, family history of DBMD, birth year, race/ethnicity, and MD STARnet site. The model with onset age at first SS as a continuous variable showed that a one-year increase in this onset age was associated with a 10% reduction in annual risk of LOA. However, results from the model with onset age at first SS entered as a categorical variable suggested that the relationship between the log-hazard and onset age at first SS may not be linear. The HR representing the relative risk for LOA progressively increased in magnitude from the oldest onset age at first SS group (> 5 years, HR = 1.00) to the next-to-youngest onset age at first SS group (≥18 months to < 3 years, HR = 1.72), but the HR for the youngest onset age group at first SS was slightly lower in magnitude (< 18 months, HR = 1.52). Retrospective recall of onset age at first SS by the family may be difficult, particularly with a delay in diagnosis, and the associated measurement error may be one possible explanation for the observed non-linear association.
Comparison of our findings to available studies is difficult due to the absence of studies, and differences in study design and analysis among those reporting natural history data. A few other studies have examined early risk factors for clinical progression. For example, Humbertclaude et al. created disease severity groups based on onset age at LOA and reported on the earliest symptom of motor function loss across these groups [14]. The earliest symptom among the most severe classification (loss of running ability) occurred, on average, around age 5 years and 5 months among steroid-naïve DMD patients. Desguerre et al. used age and type of symptom onset, muscle strength at 8 years and intelligence quotient to identify four different subsets of DMD patients [15]. The group with earliest onset age at LOA (mean age = 9 years) had an average onset age at initial symptoms of 1.3 years. This study, however, did not conduct an analysis of onset age at LOA as an outcome variable and, thus, did not report differences in the distribution of onset age at LOA as a function of onset age at first SS. Finally, Ziter et al. found that the rapidity of loss of strength varied by individuals and could be clearly established by age seven, thus predicting future deterioration [16].
There is great interest in using genotyping, imaging techniques and other methods to identify markers that predict disease severity and possibly variability in disease progression [17]. For example, genetic studies have examined specific genotypes for LTBP4 and SSP1 and have shown these genotypes to modify disease progression in some series (3;4). Poorer motor outcomes, including onset age at LOA, have been shown to be associated with increased endomysial fibrosis identified through morphometry [18]. Cacchiarelli et al. found that high serum levels of muscle-specific microRNAs corresponded to low ambulant activity, and that microRNA quantification was a better diagnostic marker than CK activity [19]. Magnetic resonance imaging and spectroscopy are also showing great promise as techniques to measure muscle pathology (e.g., lipid infiltration, muscle damage and inflammation/edema) that might be used to predict progression [20]. The findings of more severe disease progression among those with early onset age at first SS may help refine disease phenotypes for studies using these techniques, thereby improving the prediction of progression.
4.1. Study strengths and limitations
To our knowledge, the MD STARnet cohort is the largest population-based cohort of DBMD in the U.S. Most other U.S. studies have used samples derived from single specialty clinics [7] or self-reports from patients enrolling in registries [21,22] and carry possible selection bias limiting generalization of the observed associations reported. The comprehensive, population-based surveillance approach used by the MD STARnet entails more systematic collection of clinical data and increases the generalizability of our findings to the larger DBMD population. Limitations of the MD STARnet are common to all observational studies based on medical record abstraction. Specifically, clinical records may not include all important information on factors that influence the natural history of DBMD. For example, details of home physical therapy programs and diet are difficult to extract. Also, the onset age at first SS is generally collected retrospectively and may be subject to recall bias, or inconsistent documentation in the medical record. Additionally, we used complete LOA as an objective endpoint that is consistently recorded in all medical records, but there could also be a subjective element in its determination if, for example, report of full-time wheelchair use relied occasionally on parent recall instead of clinical evaluation. Decisions a family makes regarding using walking aids longer instead of using a wheelchair earlier would also impact determination of age at LOA.
5. Conclusion
Individuals with childhood-onset dystrophinopathies display a continuum of clinical severity. Even among those with typical DMD, there is significant variability in the rate of progression [14,15]. Clinical decisions and counseling are required early in the course of disease; therefore, it is important to identify factors that can aid in predicting course, particularly ones that are non-invasive, inexpensive, and readily measured. We observed that earlier onset age at first SS was associated with earlier onset age at LOA in the MD STARnet cohort suggesting that early motor dysfunction is one factor with prognostic utility that could be considered when planning clinical management decisions. It might also be a factor to consider when stratifying boys in clinical trials given its prognostic significance.
Acknowledgments
This research was supported by Cooperative Agreement U01DD000191 from the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily reflect the official views of CDC.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Aartsma-Rus A, Van Deutekom JC, Fokkema IF, Van Ommen GJ, Den Dunnen JT. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006;34(2):135–144. doi: 10.1002/mus.20586. [DOI] [PubMed] [Google Scholar]
- 2.Winnard AV, Mendell JR, Prior TW, Florence J, Burghes AH. Frameshift deletions of exons 3–7 and revertant fibers in Duchenne muscular dystrophy: mechanisms of dystrophin production. Am J Hum Genet. 1995;56(1):158–166. [PMC free article] [PubMed] [Google Scholar]
- 3.Bello L, Piva L, Barp A, Taglia A, Picillo E, Vasco G, et al. Importance of SPP1 genotype as a covariate in clinical trials in Duchenne muscular dystrophy. Neurology. 2012;79(2):159–162. doi: 10.1212/WNL.0b013e31825f04ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flanigan KM, Ceco E, Lamar KM, Kaminoh Y, Dunn DM, Mendell JR, et al. LTBP4 genotype predicts age of ambulatory loss in Duchenne muscular dystrophy. Ann Neurol. 2013;73(4):481–488. doi: 10.1002/ana.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pegoraro E, Hoffman EP, Piva L, Gavassini BF, Cagnin S, Ermani M, et al. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy. Neurology. 2011;76(3):219–226. doi: 10.1212/WNL.0b013e318207afeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery AE, Skinner R, Holloway S. A study of possible heterogeneity in Duchenne muscular dystrophy. Clin Genet. 1979;15(5):444–449. doi: 10.1111/j.1399-0004.1979.tb01777.x. [DOI] [PubMed] [Google Scholar]
- 7.Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2004;(2):CD003725. doi: 10.1002/14651858.CD003725.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Moxley RT, III, Ashwal S, Pandya S, Connolly A, Florence J, Mathews K, et al. Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2005;64(1):13–20. doi: 10.1212/01.WNL.0000148485.00049.B7. [DOI] [PubMed] [Google Scholar]
- 9.Moxley RT, III, Pandya S, Ciafaloni E, Fox DJ, Campbell K. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J Child Neurol. 2010;25(9):1116–1129. doi: 10.1177/0883073810371004. [DOI] [PubMed] [Google Scholar]
- 10.Miller LA, Romitti PA, Cunniff C, Druschel C, Mathews KD, Meaney FJ, et al. The muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): surveillance methodology. Birth Defects Res A Clin Mol Teratol. 2006;76(11):793–797. doi: 10.1002/bdra.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romitti PA, Zhu Y, Puzhankara S, James KA, Nabukera SK, Zamba GK, et al. Prevalence of Duchenne and Becker Muscular Dystrophies in the United States. Pediatrics. 2015;135(3):513–521. doi: 10.1542/peds.2014-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews KD, Cunniff C, Kantamneni JR, Ciafaloni E, Miller T, Matthews D, et al. Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): case definition in surveillance for childhood-onset Duchenne/Becker muscular dystrophy. J Child Neurol. 2010;25(9):1098–1102. doi: 10.1177/0883073810371001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SAS Institute Inc. SAS/GRAPH ® 9.2 Reference. 2. Cary, NC: SAS Institute Inc; 2010. [Google Scholar]
- 14.Humbertclaude V, Hamroun D, Bezzou K, Berard C, Boespflug-Tanguy O, Bommelaer C, et al. Motor and respiratory heterogeneity in Duchenne patients: implication for clinical trials. Eur J Paediatr Neurol. 2012;16(2):149–160. doi: 10.1016/j.ejpn.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Desguerre I, Christov C, Mayer M, Zeller R, Becane HM, Bastuji-Garin S, et al. Clinical heterogeneity of duchenne muscular dystrophy (DMD): definition of sub-phenotypes and predictive criteria by long-term follow-up. PLoS One. 2009;4(2):e4347. doi: 10.1371/journal.pone.0004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziter FA, Allsop KG, Tyler FH. Assessment of muscle strength in Duchenne muscular dystrophy. Neurology. 1977;27(10):981–984. doi: 10.1212/wnl.27.10.981. [DOI] [PubMed] [Google Scholar]
- 17.Magri F, Govoni A, D’Angelo MG, Del BR, Ghezzi S, Sandra G, et al. Genotype and phenotype characterization in a large dystrophinopathic cohort with extended follow-up. J Neurol. 2011;258(9):1610–1623. doi: 10.1007/s00415-011-5979-z. [DOI] [PubMed] [Google Scholar]
- 18.Desguerre I, Mayer M, Leturq F, Barbet JP, Gherardi RK, Christov Endomysial fibrosis in Duchenne muscular dystrophy: a marker of ppor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol. 2009;68(7):762–73. doi: 10.1097/NEN.0b013e3181aa31c2. [DOI] [PubMed] [Google Scholar]
- 19.Cacchiarelli D, Legnini I, Martone J, Cazzella V, D’Amico A, Bertini E, Bozzoni I. miRNAs as serum biomarkers for Duchenne muscular dystrophy. EMBO Mol Med. 2011;3:258–265. doi: 10.1002/emmm.201100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forbes SC, Willcocks RJ, Triplett WT, Rooney WD, Lott DJ, Wang DJ, et al. Magnetic resonance imaging and spectroscopy assessment of lower extremity skeletal muscles in boys with Duchenne muscular dystrophy: a multicenter cross sectional study. PLoS One. 2014;9(9):e106435. doi: 10.1371/journal.pone.0106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang RT, Silverstein Fadlon CA, Ulm JW, Jankovic I, Eskin A, Lu A, et al. Online self-report data for Duchenne muscular dystrophy confirms natural history and can be used to assess for therapeutic benefits. PLoS Curr. 2014:6. doi: 10.1371/currents.md.e1e8f2be7c949f9ffe81ec6fca1cce6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangel V, Martin AS, Peay HL. DuchenneConnect Registry Report. PLoS Curr. 2012;4:RRN1309. doi: 10.1371/currents.RRN1309. [DOI] [PMC free article] [PubMed] [Google Scholar]