Abstract
With the growing availability and use of copper based nanomaterials (Cu-NMs), there is increasing concern regarding their release and potential impact on the environment. In this study, the short term (≤ 5 days) ageing profile and the long term (4 months) speciation of dissolved Cu, copper oxide (CuO-) and copper sulfide nanoparticles (CuS-NPs) were investigated in five different soils using X-ray absorption spectroscopy (XAS). Soil pH was found to strongly influence the short term chemistry of the Cu-NMs added at 100 mg/kg above background. Low pH soils promoted rapid dissolution of CuO-NPs that effectively aligned their behaviour to that of dissolved Cu within 3 days. In higher pH soils, CuO-NPs persisted longer due to slower dissolution in the soil and resulted in contrasting short term speciation compared to dissolved Cu, which formed copper hydroxides and carbonates that were reflective of the soil chemistry. Organic matter appeared to slow the dissolution process but in the long term, the speciation of Cu added as dissolved Cu, CuO-NPs and CuS-NPs were found to be same for each soil. The results imply that in the short term Cu-NMs may exhibit unique behaviour in alkaline soils compared to their conventional forms (e.g. in the event of an adverse leaching event), but in the long term (≥ 4 months), their fates are dictated by the soil properties and are independent of the initial Cu form, and are likely to present minimal risk of nano-specific Cu-NM impact in the soil environment for the concentration studied here.
Keywords: Copper nanomaterials, speciation, ageing, soil, X-ray Absorption Spectroscopy
INTRODUCTION
The advent of nanotechnology has introduced novel products containing engineered nanomaterials (ENMs) to many industries around the world. ENMs include metallic nanoparticles such as silver or gold, metal oxide nanomaterials (e.g. zinc or titanium oxides), as well as composite or polymeric nanomaterials (Forbrugerrådet Tænk, 2013, Woodrow Wilson Institute, 2005, rev. 2013). The increasing abundance and availability of ENMs has led to concerns regarding their behaviour in the environment, largely because their unique, specific properties have created uncertainties about their likely environmental fate and effects. Many studies have now focussed on comparing the environmental chemistry and ecotoxicology of metal ions against their nanoparticulate counterparts, but the vast majority of studies to date have focussed on silver and zinc. Far less is known about copper-based nanomaterials (Cu-NMs), yet these are used increasingly widely, particularly as antifungal/antifouling agents (Anyaogu, Fedorov, et al., 2008), and also in electronics, ceramics, health and personal care products (PCP) (Forbrugerrådet Tænk, 2013).
Soil is one of the critical environmental endpoints in the environmental risk assessment of copper. Copper, in its variety of forms, has been used in agriculture for a long time. Bordeaux mixture (copper hydroxide and calcium sulfate) has been used as an agricultural fungicide since the 1800s (Flores-VÉLez, Ducaroir, et al., 1996), and chromated copper arsenate (CCA) has long been used to treat timber as it provides highly effective protection against termites and fungi (Hingston, Collins, et al., 2001). CCA itself is no longer widely used due to its potential to leach toxic arsenic and chromium (Hingston, Collins, et al., 2001), but other copper based materials, and increasingly Cu-NMs, (Civardi, Schubert, et al., 2015, Evans, Matsunaga, et al., 2008), are still used to treat timber. They also continue to be used as active ingredients in agricultural fungicides (e.g. Kocide®) as copper-based fungicides are accepted for use on organically certified products in many countries (Bruggen and Finckh, 2016). Copper from these sources could potentially be released into the environment in its native form (e.g. CuO or Cu(OH)2) or through leaching of more soluble or mobile forms (e.g. Cu(OH)+, organically bound Cu). In other scenarios, Cu-NMs from PCPs and supplements (e.g. MesoCopper®, Ionic Colloidal Copper) may enter the sewerage system (as other ENMs from consumer products have been shown to) where they are likely to partition into the solid phase throughout the wastewater treatment process and hence become a component of the sewage sludge/biosolids. In these scenarios it is likely that they would exist in the biosolids as a mixture of copper sulfides (CuxSy) and humic acid bound Cu (Donner, Brunetti, et al., 2013, Donner, Howard, et al., 2011). Since copper can exert toxic effects on microbial communities (Frenk, Ben-Moshe, et al., 2013, Li, Ma, et al., 2015, Moore, Stegemeier, et al., 2016), nematodes (e.g. Amorim and Scott-Fordsmand, 2012, Unrine, Tsyusko, et al., 2010), and plants (Anjum, Adam, et al., 2015), that may depend on both form and speciation, understanding the fate of Cu-NMs as influenced both by the initial form and subsequent transformation is of key importance in understanding their ecological impact.
Copper is an essential micronutrient for plants and understanding Cu-NM chemistry in soil environments could thus play a crucial role in optimising their beneficial use and appropriate application as fertilisers. However, Cu is also a known contaminant, and due to its reactive, redox-active nature, various soil properties may affect its behaviour over time. For example, pH has a strong influence on Cu soil chemistry and was found to have a significant impact on its speciation and lability (Ma, Lombi, et al., 2006a, Ma, Lombi, et al., 2006b). In these studies, precipitation as Cu(OH)2 or malachite (Cu2CO3(OH)2) was suggested to be important in high pH soils (e.g. calcarosols), and Mamindy-Pajany et al. (2014) recently identified malachite by X-ray absorption near-edge structure (XANES) spectroscopy in a biosolid-amended calcarosol. Liu and Wang (2004) found that CuCO3, CuO and Cu2O were also identified using XANES analysis. Another important factor in the regulation of soil Cu chemistry, particularly in the long term, is soil organic matter. Cu has been found to associate strongly with SOM across a wide range of soils (Strawn and Baker, 2008, Strawn and Baker, 2009), and the soluble fraction of Cu is almost exclusively complexed to organic ligands (Sauvé, McBride, et al., 1997). Adsorption to inorganic mineral surfaces (e.g. iron oxyhydroxide, calcium carbonate) is also known to occur but to a lesser extent, and this process depends on both the pH (McBride, Martínez, et al., 1998) and the ratio of the inorganic to organic adsorbent (Alcacio, Hesterberg, et al., 2001). Through these studies and others, conventional Cu speciation in soils has been studied extensively, however, very few studies have focused on Cu-NM behaviour and speciation in soils (Collins, Luxton, et al., 2012, Julich and Gäth, 2014, Shah, Luxton, et al., 2016).
In this study, we have examined the behaviour of Cu applied to soils as dissolved copper (Cu(NO3)2), copper oxide (CuO-) and copper sulfide nanoparticles (CuS-NPs), using XANES spectroscopy to determine their reaction profiles and eventual speciation in five soils of varying properties. Both the soil pH and the form of Cu was found to influence the short term reactions in soil: dissolved Cu partitioned very quickly into major compartments, while pH-dependent dissolution of CuO-NPs controlled the behaviour of Cu applied in this form. However, in the long term (4 months) Cu speciation was the same regardless of the initial form for each soil, with the major differences arising from soil pH and organic carbon content.
MATERIALS AND METHODS
Synthesis and characterisation of copper oxide and copper sulfide nanoparticles
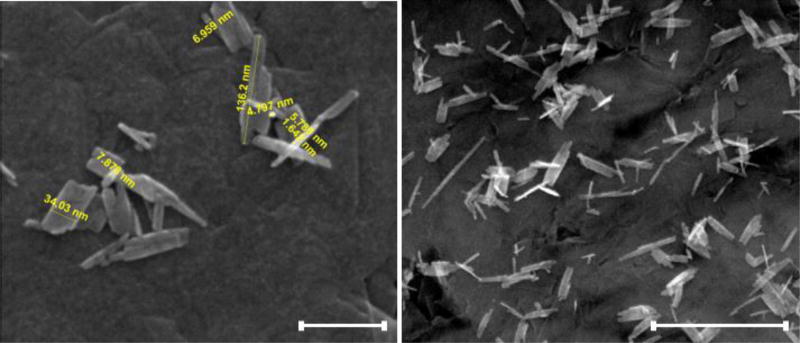
Copper oxide nanoparticles (CuO-NPs) were synthesised from copper chloride (CuCl2.2H2O) according to a wet chemistry method described by Misra et al. (2011). Briefly, 0.02 M CuCl2.2H2O was dissolved in 150 mL of ultrapure water. The solution was then heated to 100 °C followed by rapid addition of 0.6 g NaOH. This resulted in the formation of a black precipitate, which was centrifuged out and repeatedly washed several times with MilliQ water to obtain pure-phase CuO-NPs. The synthesised nanoparticles were characterised by dynamic light scattering (DLS; Nicomp 380 ZLS, Particle Sizing Systems) and scanning electron microscopy (SEM; Quanta 450 FEG-ESEM, FEI Company). SEM images show that the CuO-NPs took the form of elongated, non-spherical particles (Figure 1) and that the indicative hydrodynamic diameter (Dh) measured by DLS was 47.8 ± 6.2 nm.
Figure 1.
SEM images of the synthesized CuO-NPs showing the nanoscale needle like structures. Scale bars for the left and right images indicate 100 and 400 nm respectively.
Copper sulfide nanoparticles (CuS-NPs) were synthesized through the direct sulfidation of CuO NPs described by Moore et al. (2016). In short, 1 gram of ca. 50 nm CuO-NPs (Sigma Aldrich) were mixed with 2.5 g of gum arabic (Sigma Aldrich) in 900 mL of DI water and dispersed using a sonicating probe (Branson Model 250). To this suspension, 100 mL of of 0.1M Na2S (Sigma Aldrich) was added and allowed to react for one week under constant stirring. To remove excess gum arabic and Na2S, the suspensions were centrifuged and resuspended in DI three times. The CuS-NPs were characterized previously in filtered porewater by DLS (Malvern Zetasizer Nano) and transmission electron microscopy (TEM, FEI Tecnai G2 Twin). These particles were characterized previously: the CuS-NPs are aggregates of non-spherical particles (primary particle size of 12.4 ± 4.1 nm) and DLS confirmed that these particles exist as aggregates (185 ± 11 nm) in an aqueous suspension (Moore, Stegemeier, et al., 2016).
Five different soils were selected from Australian sites (South Australia: Lenswood, Barren Ground, Minnipa and Pimpinio; Victoria: Hamilton) to cover a range of soil parameters including pH (5 – 8), total organic carbon (TOC; 1.3 – 5.5 %), water holding capacity (WHC, 26.5 – 43.7 % w/w) and background copper concentration (1 – 9.6 mg/kg) (Table 1). Five grams of each soil were brought to 30 % WHC for each treatment prior to adding Cu. Dissolved copper, CuO- or CuS-NPs were added to give final Cu concentrations in each soil of 100 mg/kg above background (Table 1) and WHC was increased to 60%. The samples were aged at 18 °C and 60% WHC in the dark for 4 months, and subsampled for analysis at 3 days, 5 days and 4 months. The samples were aerated by once daily mixing during the first part of the experiment (i.e. up until the 5 day sampling point), and by weekly mixing thereafter up until 4 months. The 0 day samples were prepared on site at the synchrotron immediately before spectral acquisition (t< 1h).
Table 1.
Selected properties of the soils used for Cu(NO3)2, CuO-NPs and CuS-NPs ageing. The extended range of soil parameters are available in Supplemental Table S1.
| Soil name | Soil type | pH | WHC (%) |
EC (dS/m) |
N (wt %) |
TOC (wt %) |
Na (wt %) |
Cu (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| Barren Grounds | Organosol | 5.0 | 26.5 | 0.03 | 0.22 | 5.48 | 0.0002 | 7.61 |
| Lenswood | Kurosol | 5.3 | 33.0 | 0.02 | 0.18 | 2.75 | 0.0010 | 3.91 |
| Hamilton | Ferrosol | 6.8 | 40.7 | 0.03 | 0.16 | 3.05 | 0.0132 | 1.09 |
| Pimpinio | Vertisol (red) | 8.0 | 43.7 | 0.22 | 0.14 | 1.27 | 0.0425 | 9.59 |
| Minnipa | Calcarosol | 8.0 | 40.7 | 0.12 | 0.11 | 1.48 | 1.8260 | -- |
Synchrotron X-ray Absorption Spectroscopy
X-ray absorption spectra were collected using the wiggler XAS Beamline at the Australian Synchrotron. The storage ring was operating at 3 GeV and 200 mA in top-up mode. Spectra were recorded at the Cu-K absorption edge (8979 eV) using a liquid N2 cooled Si (111) double crystal monochromator. The sample spectra were collected in fluorescence using a 100-element HP-Ge detector (Canberra), in duplicate, and Cu foil reference spectra were recorded simultaneously in transmission during sample analysis. The reference spectra were used for energy calibration and alignment purposes (1st derivative inflection point at 8979 eV, absorption peaks at 8981.5, 8993.5 and 9025 eV).
Inorganic Cu standards analysed included copper (I) –oxide (Cu2O), -sulfide (Cu2S), copper (II) -nitrate (Cu(NO3)2), -chloride (CuCl2), -sulfide (CuS, covellite), -sulfate (CuSO4), -oxide (CuO), -carbonate (CuCO3), and -phosphate (Cu3(PO4)2), as well as Cu bound to organic ligands: cysteine, histidine, aspartate and arginine. Cu sorbed to iron oxyhydroxides (ferrihydrite, Fh, and goethite, Gt) were used to model Cu sorbed on mineral surfaces. Native copper in Suwannee river natural organic matter (SR-NOM) was used as a model for copper bound to NOM, as well as SR-NOM that was allowed to dialyse (MWCO = 13.5 kDa) for 24 h against 100 mM Cu(NO3)2. The CuO-NPs standard spectrum was acquired from a paste made by mixing the suspension with cellulose powder. The spectra after 4 months were collected at the Materials Research Collaborative Access Team Beamline BM10, Sector 10, at the Advanced Photon Source (APS) of the Argonne National Laboratory (Kropf, Katsoudas, et al., 2010).
Inorganic compounds were used as purchased from commercial sources (Sigma Aldrich, , except for CuS which was freshly cleaved from its mineral (covellite) prior to spectral measurement. The organic Cu compounds were synthesised by reacting Cu(NO3)2 with each ligand and freeze-drying. Each material compound was diluted to ca. 200 mg/kg in cellulose powder for XANES acquisition. All standard spectra were acquired on the Australian Synchrotron except Cu-HA, Cu-goethite and malachite spectra, which were acquired at the APS.
The fluorescence XAS spectra were averaged using the pre-processing tool Sakura. Athena (Ravel and Newville, 2005) was used for background subtraction and normalisation over the range of −120 eV to +230 eV around the Cu K-edge. Principal Component Analysis and Target Transformation was performed was performed using the SixPack program (See Supplementary Information file for details). Linear Combination Fitting (LCF) over the XANES region (-15 eV to +100 eV) was performed with Athena, using a combination of up to 4 standards out of 15 standards selected for analysis, 11 of which were selected on the basis of low SPOIL values (<3.6) by Target Transformation (TT, see Supplemental Table S2), and 4 additional standards (Cu bound to SR-NOM and malachite) selected as likely to be relevant on the basis of related experiments and literature.
RESULTS AND DISCUSSION
Short term chemistry of dissolved Cu and CuO-NPs in soils
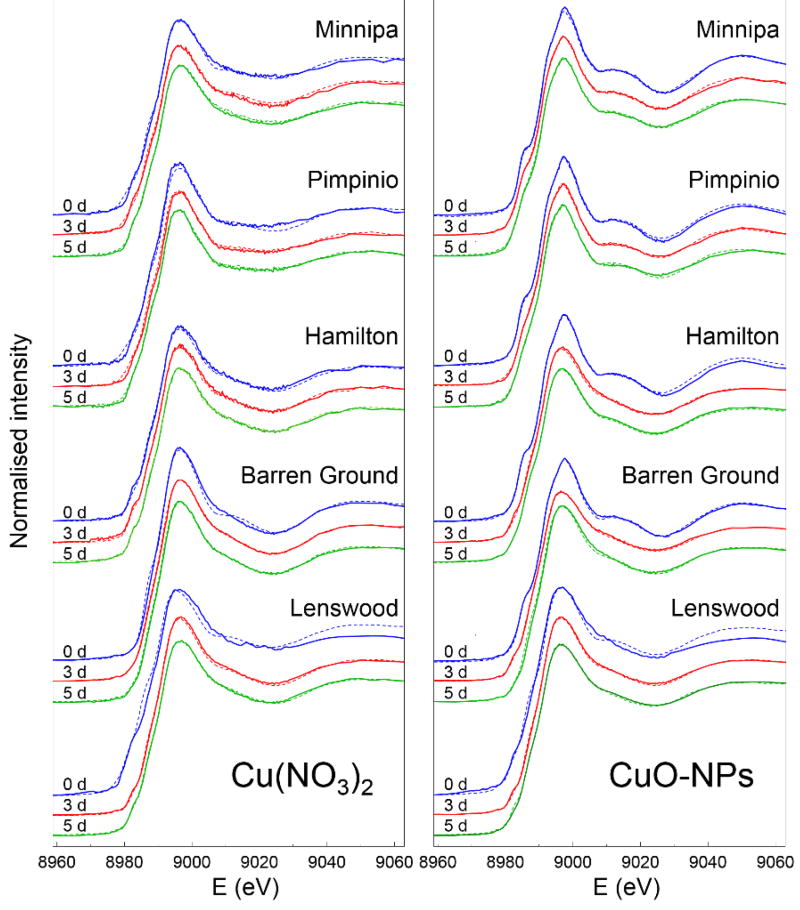
The Cu K-edge XANES spectra of the soils during the first 5 days after Cu addition indicated significant variation in Cu behaviour and speciation in these different soils (Figure 2). LCF analysis (Table 2) revealed that the short term reaction profiles of dissolved Cu and CuO-NPs were dependent on the soil properties (Table 1) and in particular, there was a strong dependence on the soil pH for both species. In low pH soils, CuO-NPs dissolved faster and behaved similarly to Cu(NO3)2 within the 5 days, while in more alkaline soils CuO-NPs reacted more slowly and persisted longer in the soil. This is particularly evident in the Lenswood soil, which was highly reactive towards CuO-NPs. Although characteristic CuO-NPs XANES spectra are evident in all other soils at Day 0, in the Lenswood soil, the CuO structure was immediately lost. By Day 3, the XANES profiles from the soils spiked with dissolved Cu and CuO-NPs were almost identical in appearance, and they did not change significantly by Day 5. This indicates that the fast reactions were largely completed by Day 3. LCF supports this observation, revealing near identical speciation (within the error of the LCF procedure) for both dissolved Cu and CuO-NPs at Days 3 and 5, consisting predominantly of Fh and NOM-bound Cu with very low residuals (R factor ≤ 1.2×10−4).
Figure 2.
Cu K-edge XANES spectra of Cu(NO3)2 (left) and CuO-NPs (right) after incubation in soil for 0 days (blue), 3 days (red) and 5 days (green). The LCF results are shown as dotted lines overlayed on each spectrum.
Table 2.
Linear combination fitting (LCF) results of the Cu K-edge XANES spectra acquired from soils spiked with dissolved Cu(NO3)2 (top) and CuO-NPs (bottom). The LCF results are shown as percentage composition of the total Cu in terms of the listed standards. FeO(OH) indicates any ferric oxyhydroxide (in this case, Cu-Fh and Cu-Gt have been summed). Note that LCF contributions of <10% are not considered significant due to the inherent errors in the LCF procedure. They have, however, been listed for completeness.
| Cu(NO3)2 | BARREN GROUND | LENSWOOD | HAMILTON | PIMPINIO | MINNIPA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Components | Day 0 | Day 3 | Day 5 | Day 0 | Day 3 | Day 5 | Day 0 | Day 3 | Day 5 | Day 0 | Day 3 | Day 5 | Day 0 | Day 3 | Day 5 |
| CuO (NPs) | |||||||||||||||
| Cu-FeO(OH) | 88.7 | 55.8 | 48.2 | 28.7 | 35.9 | 32.3 | 34 | 43.5 | 31.4 | 40.3 | 19.3 | ||||
| NOM† | 38.2 | 46.2 | 39.3 | 62.6 | 61.8 | 45.4 | 37.1 | 54.2 | 47.8 | 21.5 | 50.9 | ||||
| CuCO3 | 51.4 | 51.2 | 50.6 | 20.7 | |||||||||||
| Cu2S | 31.9 | 1.4 | 5.9 | 11.8 | 12.9 | 8.7 | 15 | 15.5 | 16.1 | 16.7 | 17.5 | 13.5 | |||
| Cu(OH)2 | 8.8 | 7.2 | 6.5 | 36.2 | 43.2 | 35.0 | 37.4 | 17.2 | |||||||
| Cu-HA | 11.3 | 6.2 | 6.1 | ||||||||||||
|
| |||||||||||||||
| Day 0 fraction‡ | 54.7 | 54.3 | 4.3 | 18.4 | 81.7 | 73.5 | 52.5 | 64.9 | 45.9 | ||||||
|
| |||||||||||||||
| SUM | 100.0 | 100.2 | 100.5 | 100.1 | 99.9 | 100.0 | 100.0 | 100.7 | 100.7 | 102.6 | 109.9 | 101.7 | 101.9 | 100.0 | 100.9 |
| R-factor | 5.0E-04 | 3.8E-05 | 4.7E-05 | 7.0E-04 | 7.6E-05 | 1E-04 | 1.9E-04 | 1.6E-04 | 9.8E-05 | 8.8E-04 | 2.6E-04 | 1.9E-04 | 2.8E-04 | 3.7E-04 | 7.7E-05 |
|
| |||||||||||||||
| CuO-NPs | BARREN GROUND | LENSWOOD | HAMILTON | PIMPINIO | MINNIPA | ||||||||||
| Components | Day 0 | Day 3 | Day 5 | Day 0 | Day 3 | Day 5 | Day 0 | Day 3 | Day 5 | Day 0 | Day 3 | Day 5 | Day 0 | Day 3 | Day 5 |
|
| |||||||||||||||
| CuO (NPs) | 73.2 | 83.9 | 81.2 | 41.1 | 26.5 | 97.1 | 60.7 | 46.2 | |||||||
| Cu-FeO(OH) | 22.9 | 40.8 | 24.3 | 33.5 | 28.4 | 13.7 | 39.1 | 40.7 | 17.6 | 39.4 | 60.7 | 4.3 | 20.9 | 28.9 | |
| NOM† | 75.7 | 59.2 | 67.3 | 67.2 | 67.4 | 56.7 | 51.2 | 13.0 | 10.8 | 16.8 | |||||
| CuCO3 | |||||||||||||||
| Cu2S | 5 | 8.4 | 4.2 | 5.4 | 4.2 | 8.1 | 3.2 | 6.4 | 14.0 | 7.7 | 10.1 | ||||
| Cu3(PO4)2 | 4.0 | ||||||||||||||
| Cu(OH)2 | 20.3 | ||||||||||||||
| Cu-HA | |||||||||||||||
|
| |||||||||||||||
| SUM | 101.1 | 100.0 | 100.0 | 100.0 | 100.7 | 100.0 | 103.0 | 100.0 | 100.0 | 102.0 | 99.9 | 101.2 | 101.4 | 100.1 | 102.0 |
| R-factor | 7.4E-05 | 7.8E-05 | 1.0E-04 | 4.7E-04 | 9.3E-05 | 1.2E-04 | 1.6E-04 | 1.0E-04 | 1.5E-04 | 2.2E-04 | 1.7E-04 | 2.8E-04 | 1.5E-04 | 6.7E-05 | 8.6E-05 |
NOM is the sum of the percentage contributions from native SR-NOM and SR-NOM dialysed with Cu(NO3)2.
Day 0 fraction is the percentage attributed by the LCF to the corresponding Day 0 spectrum before distributing to other components.
In comparison, alkaline soils (Pimpinio and Minnipa, both pH 8.0) reacted more slowly with CuO-NPs, with Minnipa retaining nearly half of the initial structure even after 5 days of incubation according to LCF decomposition. The slowly released Cu is then redistributed to the iron oxyhydroxide and NOM phases that were also observed in the low pH soils. Thus, while the rate of the CuO dissolution is different, the partitioning of Cu from CuO-NPs tend towards a similar fate in both low and high pH soils.
Evidently, pH alone cannot explain all the differences. For example, Barren Ground soil has a lower pH than Lenswood but the reactivity towards CuO-NPs are apparently suppressed in comparison; a clear CuO component is evident in the Barren Grounds Day 0 spectrum. This may be due to the higher soil organic matter (SOM, measured by total organic carbon, TOC) that may be providing some protection for CuO against dissolution by partially passivating the NP surface (Conway, Adeleye, et al., 2015) or by inducing aggregation (Sousa and Teixeira, 2013). Nevertheless, by Day 3 CuO is no longer identified in the LCF and it is clear that CuO-NPs behaviour in the short term are largely determined by the soil pH.
A slightly different observation is made for Cu introduced into soils as dissolved Cu(NO3)2. In low pH soils, the partitioning process is very fast and the Cu is rapidly distributed into similar Cu phases as those found in the CuO-NPs spiked soils. On the other hand, Cu(NO3)2 added to high pH soils tends to immediately form Cu(OH)2 or CuCO3, both of which are likely in alkaline environments, but are not observed in the CuO-NPs spiked soils. This can be rationalised based on solubility of the species Cu involved. When Cu is introduced as dissolved Cu, all of the added Cu is theoretically available to react with dominant moieties or surfaces. If all Cu was able to instantly dilute throughout the entire moisture content of the soil, Cu concentration would be ca. 6.5 mM (100 mg/kg = 1.57 mmol/kg, 1 kg soil has added 60% of soil WHC, which is ca. 40% for alkaline soils, so 1.57 (mmol/kg) ÷ (0.6×0.4) (L/kg) = 6.5 mmol/L). At pH > ca. 6, this concentration would result in Cu(OH)2 precipitation (Albrecht, Addai-Mensah, et al., 2011). In contrast, when Cu is added as CuO-NPs, the Cu release is slow and therefore does not precipitate to Cu(OH)2, thus it likely to sorb to FeO(OH) or NOM. It is also worth noting that although malachite (Cu2(CO3)(OH)2) can often be observed in calcarosols (Mamindy-Pajany, Sayen, et al., 2014, McBride and Bouldin, 1984), it nor the related CuCO3 was not observed in the calcarosol included in this study (Minnipa). However, the addition of free Cu to the Pimpinio soil, which has a higher EC (that may contain high dissolved carbonate), readily brought about CuCO3 formation. Thus, the short term speciation of Cu added as Cu(NO3)2 will more likely reflect the chemistry of the soil solution than will the short-term transformation products of Cu added as CuO-NPs.
Copper speciation after 4 months
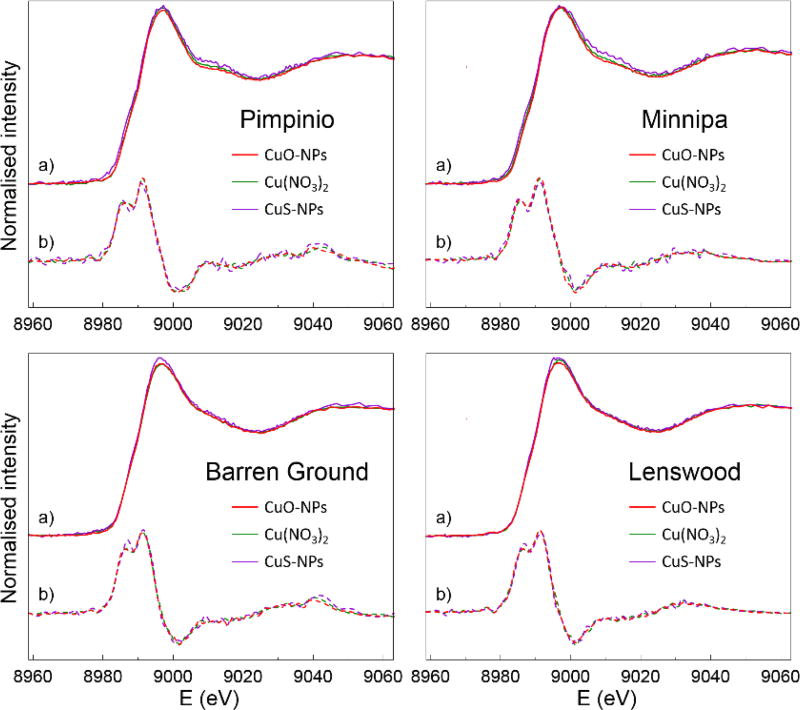
While the short term kinetics and speciation were highly dependent on the initial form of the Cu applied and soil properties, the long term fate of Cu in soil appears to be similar and therefore largely independent of the species of copper introduced. As shown in Figure 3, the XANES spectra from Cu(NO3)2, CuO-NPs and CuS-NPs aged in the soils for 4 months are near identical to each other. It is worth noting that despite their low Ksp (6×10−37), CuS-NPs are susceptible to oxidative dissolution under aerobic conditions and are reportedly more soluble than CuO-NPs (Ma, Stegemeier, et al., 2014). It has also been observed that while Cu was found as various sulfides in freshly prepared biosolids, a significant proportion had transformed to HA or iron oxyhydroxide-bound Cu after stockpiling (Donner, Howard, et al., 2011). Therefore, it is reasonable that CuS-NPs were not identified in the LCF (Supplemental Table S3). This contrasts with Ag2S-NPs, whose low Ksp and kinetic stability has a direct consequence on in its persistence in soil as Ag2S (Pradas Del Real, Castillo-Michel, et al., 2016, Sekine, Brunetti, et al., 2015, Wang, Menzies, et al., 2016).
Figure 3.
Cu K-edge XANES spectra of 4 different soils 4 months after spiking with CuO-NPs, CuS-NPs, or dissolved Cu(NO3)2. For each set of spectra (a) their derivative spectra (b) are also shown.
In general, the spectral profile for Cu in soils after 4 months is very similar to Cu bound to iron oxyhydroxides – both Cu-Gt and Cu-Fh could contribute to the final speciation according to LCF, with a smaller but consistent contribution from Cu bound to organic matter (NOM, HA). The predominance of Cu bound to iron oxyhydroxide is, however, in some disagreement with previous reports (for example (Nielsen, Scott-Fordsmand, et al., 2015, Strawn and Baker, 2009)), where SOM was found to be the dominant Cu partition. On the other hand, many of these studies focused on soils with pH < 7 that contained comparatively high SOM (as measured by TOC), so it could be that Cu bound to SOM had a stronger representation.
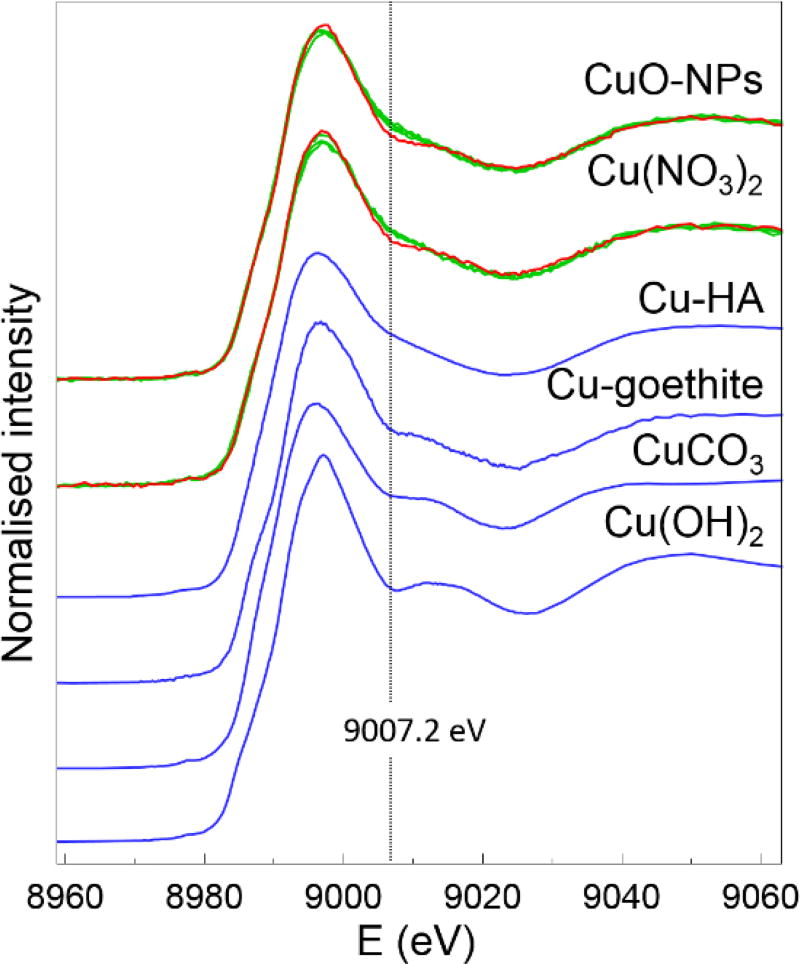
At the same time, the proportions predicted by LCF in this study may not provide the full picture. For example, a qualitative comparison of the Pimpinio spectra (pH 8.0) suggests a second “peak” is present and more distinctly visible in alkaline soils with an inflection point at 9008.5 eV. The latter inflection appears to be a common feature amongst our standards that have Cu(II) associated with an inorganic oxygen species as can be seen in Cu-Gt, -Fh, Cu(OH)2, CuCO3 for example (Figure 4). This may therefore indicate that there is more Cu bound to FeO(OH) in the alkaline soils contrary to the LCF results. This is plausible given that: i) Fh or Gt have a point of zero charge (PZC) around 6 to 9 (Schwertmann and Fechter, 1982), the binding of Cu (II) to FeO(OH) is expected to be more significant at higher pH; and ii) the ratio of Cu bound to FeO(OH) (Gt) versus NOM (HA) would increase with pH as reported by McBride et al. (1998) This feature is also present, although to a lesser extent, in the aged Minnipa soil but is not visibly apparent in the other soils where pH < 7. Possible explanations for the discrepancy between the visual vs LCF interpretation may be due to additional complexes formed at the FeO(OH)-NOM interface (e.g. “Type-A complex” similar to observations reported by Alcacio et al. (2001)), or the fact that the 4 month sample spectra were acquired on a different synchrotron beamline.
Figure 4.
(Red) Cu K-edge XANES spectra of CuO-NPs and Cu(NO3)2 added to Pimpinio and aged for 4 months, overlayed against (Green) spectra from other soils. (Blue) XANES spectra of Cu standards identified in the LCF. The dotted line at ca. 9008.5 eV indicates the inflection observed in the Pimpinio spectra, aligning with a feature commonly observed in Cu (II) bound to inorganic oxygen.
Finally, it is important to note that Cu bound to FeO(OH) used here are still models for Cu bound to an iron-containing mineral species present in the soil. For a more detailed investigation, a microfocus beam is necessary so that elements and species can be co-localised to provide spatially resolved information, which may lead to further refinement and discrimination of individual Cu components (Proffit, Marin, et al., 2015).
Further discussions
The ageing process of Cu (as CuCl2) in soils have been investigated and successfully modelled previously via a combination of three main mechanisms: 1) precipitation of Cu on soil mineral surfaces, 2) occlusion within soil organic matter and 3) diffusion into the micropores of clays and other minerals (Ma, Lombi, et al., 2006a, Ma, Lombi, et al., 2006b). Soil pH was reported to be the key factor determining the lability of Cu in the soils covering a range of soil parameters, with lower lability observed at higher pH due to precipitation of Cu(OH)2 or malachite. Given the time scale in our study (up to 4 months), mechanisms 1 and 2 would be the main contributors to the ageing process, with a smaller contribution from diffusion based mechanism, 3. In addition, since the short term Cu speciation (up to 5 days) by LCF suggested that the ageing pathway were dependent on the initial form (i.e. dissolved or Cu-NMs), an extra term would be required to account for the form in which the Cu is introduced. Furthermore, this term would need to decay over time since the Cu speciation has converged to the same fate regardless of the initial form in the long term (4 months). While the development of a mathematical model to account for these additional, potentially complex “convergence terms” is beyond the scope of this study, it is likely that this additional term would have a dissolution-based component.
Clearly, this convergence term would depend on a range of soil parameters such as pH and organic matter content as evidenced by the short term reaction profile. In low pH soils, this would be negligible since CuO-NPs entering these soils quickly dissolve and behave as dissolved Cu. Thus, if Cu lability is taken as a direct indicator of adverse soil ecological impact, then CuO-NPs in these soils would largely present the same hazard as Cu introduced as dissolved Cu. On the other hand, in alkaline soils, where CuO-NPs persisted longer, the dissolution process would have a greater influence on the overall attenuation rate. Furthermore, it must be recognised that the dissolution process is also a function of the NPs properties such as the size, shape, surface coating and aggregation state, as well as external factors such as the temperature. It may also be affected by the NP concentration, especially for poorly soluble NPs if the porewater becomes saturated with respect to the solid NP phase. If occlusion of Cu within organic matter in its NP form (i.e. prior to dissolution) also occurs, that will slow the convergence time. All these factors would inevitably add significant complexity to a comprehensive attenuation model, but a survey of CuO/CuS-NPs dissolution rates across a range of soils may provide a semi-empirical term to extend the existing model to account for this process.
If Cu-NMs are to present any nano-specific risks, the results of this study imply that it would be greater in alkaline soils where CuO-NPs persisted for a longer time. For example, it has been reported that CuO-NPs can behave differently within soils not only in their speciation but also in their adsorption behaviour (Julich and Gäth, 2014) and its impact on soil (micro-)organisms (e.g. Buffet, Richard, et al., 2013, Unrine, Tsyusko, et al., 2010). These differences may be an important consideration in assessing their ecological risk under acute exposure scenarios in alkaline soils. Another possible scenario where such differences may manifest is where Cu-NMs are applied to soils in a matrix rich in organic matter such as biosolids (if Cu exist in nano form in biosolids) since it may be a contributor to their persistence in soil. In low pH soils however, they are unlikely to be significant unless under a considerably high exposure scenario in an organic matter rich soil. If the exposure occurs after the Cu-NMs have had time to age, the differences are likely to be minimal.
The result of this study also have implications for the efficacy of nano-enabled fertilizers of fungicides. If the nano-specific properties of Cu-NMs are to be exploited in such applications, their potential benefits are likely to be realised in alkaline soils or SOM-rich soils soon after application. In soils that do not meet this criteria, other amendments to raise the pH (e.g. liming) or its organic matter content (e.g. biosolid application) may increase the effectiveness of the applied Cu-NMs. However, such strategies will need to be examined carefully so that the potential environmental and ecological drawbacks do not outweigh the benefits, if any, gained from any amendments or Cu-NMs applications.
CONCLUSIONS
We have investigated the short term ageing profile and long term fate of dissolved Cu and Cu-NMs in five soils with different properties using Cu K-edge XANES spectroscopy. The short term reactions over the first 5 days of incubation were affected by both the initial form of Cu added and the soil characteristics. In particular, the soil pH had a significant impact on the behaviour of Cu in these early stages, with the low pH soils facilitating rapid dissolution of Cu-NMs that effectively aligned their behaviour to that of dissolved Cu. Soil organic matter is also likely to slow this process. At higher pH, Cu added as dissolved Cu immediately precipitated to form Cu(OH)2 and/or CuCO3 while CuO-NPs dissolved slowly and were partitioned directly into Cu bound to FeO(OH) or NOM. In the long term (4 months), the XANES spectra indicated that all Cu were a combination of inorganically bound Cu to FeO(OH) and organically bound copper to NOM with slightly different, pH-dependent proportions, with negligible contributions from other inorganic precipitates. Only minor differences were present in the spectra across the different Cu species in a given soil. These results imply that in the short term Cu-NMs may exhibit unique risks in alkaline soils compared to their conventional forms (e.g. in the event of an adverse leaching event), and there is a need to consider the effect of various factors relating to the Cu-NMs (e.g. concentration), the soil (e.g. SOM content) and external conditions (e.g. temperature). However, in the long term, it is likely that their fates are dictated by the soil properties, independent of the initial Cu form, and are thus likely to present minimal risk of nano-specific Cu-NP impact in the soil environment.
Supplementary Material
CORE IDEAS.
Short term reactions of Cu-NMs are dependent on the Cu form (dissolved vs nanoparticle) and soil chemistry
In low pH soils, CuO-NPs dissolved rapidly and behave similarly to dissolved Cu
CuO-NPs persisted longer in alkaline soils and in soils with high organic matter content
In the long term, Cu, CuO-NPs and CuS-NPs transform into Cu bound to iron oxyhydroxide or NOM and is largely independent of the original form
Acknowledgments
The funding support from the Australian Research Council Discovery Project DP120101115 is gratefully acknowledged. ED and EL are recipients of ARC Future Fellowships FT130101003 and FT100100337, respectively. RS is a recipient of an H2020 MSCA Individual Fellowship (2014-660565 MolNANOtox). The Australian Synchrotron is acknowledged for beamtime and travel funding (AS142 XAS7929), and the XAS Beamline staff are thanked for their assistance during the beamtime. We would like to thank Dr Sotirios Vasileiadis and Dr Euan Smith for providing the soils and their characterisation data, and Mr Stuart McClure for his assistance on the SEM. MRCAT operations are supported by the Department of Energy (DOE) and the MRCAT member institutions. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Although EPA contributed to this article, the research presented was not performed by or funded by EPA and was not subject to EPA’s quality system requirements. Consequently, the views, interpretations, and conclusions expressed in this article are solely those of the authors and do not necessarily reflect or represent EPA’s views or policies.
Abbreviations
- Cu-NMs
copper-based nanomaterials
- CuO-NPs
copper (II) oxide nanoparticles
- CuS-NPs
copper (II) sulfide nanoparticles
- Fh
ferrihydride
- Gt
goethite
- HA
humic acid
- LCF
linear combination fitting
- XANES
X-ray absorption near edge structure
- NOM
natural organic matter
References
- Albrecht TWJ, Addai-Mensah J, Fornasiero D. Effect of pH, concentration and temperature on copper and zinc hydroxide formation/precipitation in solution CHEMECA 2011 - Engineering A Better World. Engineers. Australia, Sydney: NSW; 2011. [Google Scholar]
- Alcacio TE, Hesterberg D, Chou JW, Martin JD, Beauchemin S, Sayers DE. Molecular scale characteristics of Cu(II) bonding in goethite-humate complexes. Geochim. Cosmochim. Acta. 2001;65:1355–1366. doi: http://dx.doi.org/10.1016/S0016-7037(01)00546-4. [Google Scholar]
- Amorim MJB, Scott-Fordsmand JJ. Toxicity of copper nanoparticles and CuCl2 salt to Enchytraeus albidus worms: Survival, reproduction and avoidance responses. Environ. Pollut. 2012;164:164–168. doi: 10.1016/j.envpol.2012.01.015. doi: http://dx.doi.org/10.1016/j.envpol.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Anjum NA, Adam V, Kizek R, Duarte AC, Pereira E, Iqbal M, et al. Nanoscale copper in the soil-plant system - toxicity and underlying potential mechanisms. Environ. Res. 2015;138:306–325. doi: 10.1016/j.envres.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Anyaogu KC, Fedorov AV, Neckers DC. Synthesis, Characterization, and Antifouling Potential of Functionalized Copper Nanoparticles. Langmuir. 2008;24:4340–4346. doi: 10.1021/la800102f. [DOI] [PubMed] [Google Scholar]
- Bruggen AHCv, Finckh MR. Plant Diseases and Management Approaches in Organic Farming Systems. Annu. Rev. Phytopathol. 2016;54:25–54. doi: 10.1146/annurev-phyto-080615-100123. [DOI] [PubMed] [Google Scholar]
- Buffet PE, Richard M, Caupos F, Vergnoux A, Perrein-Ettajani H, Luna-Acosta A, et al. A mesocosm study of fate and effects of CuO nanoparticles on endobenthic species (Scrobicularia plana, Hediste diversicolor) Environ. Sci. Technol. 2013;47:1620–1628. doi: 10.1021/es303513r. [DOI] [PubMed] [Google Scholar]
- Civardi C, Schubert M, Fey A, Wick P, Schwarze FWMR. Micronized Copper Wood Preservatives: Efficacy of Ion, Nano, and Bulk Copper against the Brown Rot Fungus Rhodonia placenta. PLoS ONE. 2015;10:e0142578. doi: 10.1371/journal.pone.0142578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Luxton T, Kumar N, Shah S, Walker VK, Shah V. Assessing the Impact of Copper and Zinc Oxide Nanoparticles on Soil: A Field Study. PLoS ONE. 2012;7:e42663. doi: 10.1371/journal.pone.0042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JR, Adeleye AS, Gardea-Torresdey J, Keller AA. Aggregation, Dissolution, and Transformation of Copper Nanoparticles in Natural Waters. Environ. Sci. Technol. 2015;49:2749–2756. doi: 10.1021/es504918q. [DOI] [PubMed] [Google Scholar]
- Donner E, Brunetti G, Zarcinas B, Harris P, Tavakkoli E, Naidu R, et al. Effects of Chemical Amendments on the Lability and Speciation of Metals in Anaerobically Digested Biosolids. Environ. Sci. Technol. 2013;47:11157–11165. doi: 10.1021/es400805j. [DOI] [PubMed] [Google Scholar]
- Donner E, Howard DL, Jonge MDd, Paterson D, Cheah MH, Naidu R, et al. X-ray Absorption and Micro X-ray Fluorescence Spectroscopy Investigation of Copper and Zinc Speciation in Biosolids. Environ. Sci. Technol. 2011;45:7249–7257. doi: 10.1021/es201710z. [DOI] [PubMed] [Google Scholar]
- Evans P, Matsunaga H, Kiguchi M. Large-scale application of nanotechnology for wood protection. Nat Nano. 2008;3:577–577. doi: 10.1038/nnano.2008.286. [DOI] [PubMed] [Google Scholar]
- Flores-VÉLez LM, Ducaroir J, Jaunet AM, Robert M. Study of the distribution of copper in an acid sandy vineyard soil by three different methods. Eur. J. Soil Sci. 1996;47:523–532. doi: 10.1111/j.1365-2389.1996.tb01852.x. [DOI] [Google Scholar]
- Forbrugerrådet Tænk. The Nanodatabase 2013 [Google Scholar]
- Frenk S, Ben-Moshe T, Dror I, Berkowitz B, Minz D. Effect of Metal Oxide Nanoparticles on Microbial Community Structure and Function in Two Different Soil Types. PLoS ONE. 2013;8:e84441. doi: 10.1371/journal.pone.0084441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingston JA, Collins CD, Murphy RJ, Lester JN. Leaching of chromated copper arsenate wood preservatives: a review. Environ. Pollut. 2001;111:53–66. doi: 10.1016/s0269-7491(00)00030-0. doi: http://dx.doi.org/10.1016/S0269-7491(00)00030-0. [DOI] [PubMed] [Google Scholar]
- Julich D, Gäth S. Sorption behavior of copper nanoparticles in soils compared to copper ions. Geoderma. 2014;235–236:127–132. doi: 10.1016/j.geoderma.2014.07.003. [DOI] [Google Scholar]
- Kropf AJ, Katsoudas J, Chattopadhyay S, Shibata T, Lang EA, Zyryanov VN, et al. The New MRCAT (Sector 10) Bending Magnet Beamline at the Advanced Photon Source. AIP Conference Proceedings. 2010;1234:299–302. doi:doi: http://dx.doi.org/10.1063/1.3463194. [Google Scholar]
- Li J, Ma Y-B, Hu H-W, Wang J-T, Liu Y-R, He J-Z. Field-based evidence for consistent responses of bacterial communities to copper contamination in two contrasting agricultural soils. Frontiers in Microbiology. 2015:6. doi: 10.3389/fmicb.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S-H, Wang HP. In Situ Speciation Studies of Copper-Humic Substances in a Contaminated Soil during Electrokinetic Remediation. J Environ Qual. 2004;33:1280–1287. doi: 10.2134/jeq2004.1280. [DOI] [PubMed] [Google Scholar]
- Ma R, Stegemeier J, Levard C, Dale JG, Noack CW, Yang T, et al. Sulfidation of copper oxide nanoparticles and properties of resulting copper sulfide. Environmental Science: Nano. 2014 doi: 10.1039/c4en00018h. [DOI] [Google Scholar]
- Ma Y, Lombi E, Nolan AL, McLaughlin MJ. Short-term natural attenuation of copper in soils: Effects of time, temperature, and soil characteristics. Environ. Toxicol. Chem. 2006a;25:652–658. doi: 10.1897/04-601R.1. [DOI] [PubMed] [Google Scholar]
- Ma Y, Lombi E, Oliver IW, Nolan AL, McLaughlin MJ. Long-Term Aging of Copper Added to Soils. Environ. Sci. Technol. 2006b;40:6310–6317. doi: 10.1021/es060306r. [DOI] [PubMed] [Google Scholar]
- Mamindy-Pajany Y, Sayen S, Mosselmans JFW, Guillon E. Copper, Nickel and Zinc Speciation in a Biosolid-Amended Soil: pH Adsorption Edge, µ-XRF and µ-XANES Investigations. Environ. Sci. Technol. 2014;48:7237–7244. doi: 10.1021/es5005522. [DOI] [PubMed] [Google Scholar]
- McBride M, Martínez CE, Sauvé S. Copper(II) Activity in Aged Suspensions of Goethite and Organic Matter. Soil Sci. Soc. Am. J. 1998;62:1542–1548. doi: 10.2136/sssaj1998.03615995006200060010x. [DOI] [Google Scholar]
- McBride MB, Bouldin DR. Long-Term Reactions of Copper(II) in a Contaminated Calcareous Soil1. Soil Sci. Soc. Am. J. 1984;48:56–59. doi: 10.2136/sssaj1984.03615995004800010010x. [DOI] [Google Scholar]
- Moore JD, Stegemeier JP, Bibby K, Marinakos SM, Lowry GV, Gregory KB. Impacts of Pristine and Transformed Ag and Cu Engineered Nanomaterials on Surficial Sediment Microbial Communities Appear Short-Lived. Environ. Sci. Technol. 2016;50:2641–2651. doi: 10.1021/acs.est.5b05054. [DOI] [PubMed] [Google Scholar]
- Nielsen MT, Scott-Fordsmand JJ, Murphy MW, Kristiansen SM. Speciation and solubility of copper along a soil contamination gradient. J. Soils Sed. 2015;15:1558–1570. doi: 10.1007/s11368-015-1109-3. [DOI] [Google Scholar]
- Pradas Del Real AE, Castillo-Michel H, Kaegi R, Sinnet B, Magnin V, Findling N, et al. Fate of Ag-NPs in Sewage Sludge after Application on Agricultural Soils. Environ Sci Technol. 2016;50:1759–1768. doi: 10.1021/acs.est.5b04550. [DOI] [PubMed] [Google Scholar]
- Proffit S, Marin B, Cances B, Ponthieu M, Sayen S, Guillon E. Using synthetic models to simulate aging of Cu contamination in soils. Environ Sci Pollut Res. 2015;22:7641–7652. doi: 10.1007/s11356-015-4291-3. [DOI] [PubMed] [Google Scholar]
- Ravel B, Newville M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. Journal of Synchrotron Radiation. 2005;12:537–541. doi: 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- Sauvé S, McBride MB, Norvell WA, Hendershot WH. Copper Solubility and Speciation of In Situ Contaminated Soils: Effects of Copper Level, pH and Organic Matter. Water, Air, Soil Pollut. 1997;100:133–149. doi: 10.1023/a:1018312109677. [DOI] [Google Scholar]
- Schwertmann U, Fechter H. The point of zero charge of natural and synthetic ferrihydrites and its relation to adsorbed silicate. Clay Minerals. 1982;17:471–476. [Google Scholar]
- Sekine R, Brunetti G, Donner E, Khaksar M, Vasilev K, Jamting A, et al. Speciation and Lability of Ag-, AgCl-, and Ag2S–Nanoparticles in Soil Determined by X-ray Absorption Spectroscopy and Diffusive Gradients in Thin Films. Environ. Sci. Technol. 2015;49:897–905. doi: 10.1021/es504229h. [DOI] [PubMed] [Google Scholar]
- Shah V, Luxton TP, Walker VK, Brumfield T, Yost J, Shah S, et al. Fate and impact of zero-valent copper nanoparticles on geographically-distinct soils. Sci. Total Environ. 2016;573:661–670. doi: 10.1016/j.scitotenv.2016.08.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa VS, Teixeira MR. Aggregation kinetics and surface charge of CuO nanoparticles: the influence of pH, ionic strength and humic acids. Environmental Chemistry. 2013 doi: http://dx.doi.org/10.1071/EN13001.
- Strawn DG, Baker LL. Speciation of Cu in a Contaminated Agricultural Soil Measured by XAFS, µ-XAFS, and µ-XRF. Environ. Sci. Technol. 2008;42:37–42. doi: 10.1021/es071605z. [DOI] [PubMed] [Google Scholar]
- Strawn DG, Baker LL. Molecular characterization of copper in soils using X-ray absorption spectroscopy. Environ. Pollut. 2009;157:2813–2821. doi: 10.1016/j.envpol.2009.04.018. doi: http://dx.doi.org/10.1016/j.envpol.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Unrine JM, Tsyusko OV, Hunyadi SE, Judy JD, Bertsch PM. Effects of particle size on chemical speciation and bioavailability of copper to earthworms (Eisenia fetida) exposed to copper nanoparticles. J Environ Qual. 2010;39:1942–1953. doi: 10.2134/jeq2009.0387. [DOI] [PubMed] [Google Scholar]
- Wang P, Menzies NW, Dennis PG, Guo J, Forstner C, Sekine R, et al. Silver Nanoparticles Entering Soils via the Wastewater-Sludge-Soil Pathway Pose Low Risk to Plants but Elevated Cl Concentrations Increase Ag Bioavailability. Environ. Sci. Technol. 2016;50:8274–8281. doi: 10.1021/acs.est.6b01180. [DOI] [PubMed] [Google Scholar]
- Woodrow Wilson Institute. The Project on Emerging Nanotechnologies. Woodrow Wilson International Center for Scholars, Pew Charitable Trusts; 2005. rev. 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.