Oxytocin, a hypothalamic hormone that is secreted directly into the brain and enters the peripheral circulation through the posterior pituitary gland, regulates a range of physiologic processes, including eating behaviour and metabolism. In rodents and nonhuman primates, chronic oxytocin administration leads to sustained weight reduction by reducing food intake, increasing energy expenditure and inducing lipolysis. Oxytocin might improve glucose homeostasis, independently of its effects on weight. Clinical studies are beginning to translate these important preclinical findings to humans. This Review describes key data linking oxytocin to eating behaviours and metabolism in humans. For example, a single intranasal dose of oxytocin can reduce caloric intake, increase fat oxidation and improve insulin sensitivity in men. Furthermore, a pilot study of 8 weeks of oxytocin treatment in adults with obesity or overweight led to substantial weight loss. Together, these data support further investigation of interventions that target pathways involving oxytocin as potential therapeutics in metabolic disorders, including obesity and diabetes mellitus. Therapeutic considerations and areas for further research are also discussed.
Oxytocin is a nine amino acid peptide hormone that binds to the oxytocin receptor, which is a member of the G-protein coupled receptor family, to regulate a range of physiologic processes, including eating behaviour and metabolism. Produced in the supraoptic nucleus and paraventricular nucleus (PVN) of the hypothalamus, oxytocin is released to brain regions involved in energy metabolism via dendritic diffusion (for example, the ventromedial nucleus (VMH)1,2), as well as axonal connections of parvocellular oxytocin neurons to other regions, including the arcuate nucleus (ARC) 3, ventral tegmental area (VTA)4, nucleus accumbens 5, nucleus tractus solitarius (NTS) 6,7 and spinal cord 7,8. There are also axonal connections of magnocellular oxytocin neurons to the posterior pituitary gland, which secretes oxytocin to the peripheral circulation. In addition, oxytocin is produced and released in local tissues, such as the gastrointestinal tract 9, where oxytocin has autocrine and paracrine effects via oxytocin receptors10,11.
Although endogenous oxytocin does not readily cross the blood–brain barrier, behavioural studies indicate that circulating oxytocin might directly enter the hindbrain or act on the vagus nerve 12-15, and a study in nonhuman primates indicates that oxytocin at supraphysiologic concentrations administered intravenously or intranasally enters the cerebrospinal fluid (CSF) 16. Furthermore, exogenous oxytocin administration might promote endogenous oxytocin secretion either directly through PVN oxytocin autoreceptors or indirectly through peripheral oxytocin receptors 17,18. The oxytocin receptor is widely distributed in the central nervous system (for example, the hypothalamus, basal ganglia, VTA, nucleus accumbens, frontal cortex, insula, NTS and spinal cord) and peripheral regions that are key for the control of food intake and metabolism (for example, vagus nerve, anterior pituitary gland, adipocytes, gastrointestinal tract and pancreas) 8,10,11,19-30,8,31.
Animal studies indicate that oxytocin is a potent regulator of caloric intake and metabolism 8,14. Oxytocin or oxytocin receptor knockout mice gain weight and develop late-onset obesity 32-34 and impaired glucose homeostasis 34. In addition, mice with haploinsufficiency of single minded 1 (encoded by Sim1; mutations in SIM1 are associated with hyperphagic obesity in humans) have low levels of oxytocin mRNA in the hypothalamus, and oxytocin administration reverses their excessive food intake and weight gain 35. Exogenous oxytocin administration in mice, rats and monkeys, whether given centrally or peripherally, leads to sustained weight suppression by reducing food intake, increasing or maintaining energy expenditure, and promoting lipolysis 8,14,18,36-38. Independent of effects on weight, oxytocin administration can improve glucose homeostasis in diet-induced obese or diabetic rodents 18,36. These findings in animals indicate that pathways involving oxytocin might provide novel therapeutic targets for obesity and metabolic disorders. Clinical investigations are beginning to translate these important findings to humans.
Measurement of oxytocin in samples
In interpreting human data, it is important to recognize that measurement of oxytocin levels in blood samples is controversial and rigorous validations of assays have not been performed 39,40. Older radioimmunoassays yielded results comparable to bio-assays and typically reported oxytocin levels after extraction of <10 pg/ml in healthy young men and non-pregnant or lactating women, with levels up to 24 pg/ml during lactation and 114 pg/ml during labour39.
Unfortunately, using newer commercially available radioimmunoassays, oxytocin levels can be falsely elevated due to interference of other substances 41. In the past decade, most publications reporting oxytocin in serum or plasma samples use commercially available enzyme immunoassays (ELISA), which yield levels that are up to two orders of magnitude higher than traditional radioimmunoassays when extraction is not performed39. Extraction before the ELISA filters out some, but not all, of the interfering substances and yields overall levels of similar magnitude to the traditional radioimmunoassays 41. The ELISA might detect oxytocin fragments or degradation products, and the biological activity of these molecules is unknown 41. In a paper published in 2016, it was posited that extraction might result in falsely low oxytocin levels due to elimination of protein-bound oxytocin42. In sum, studies reporting oxytocin levels using immunoassays are measuring oxytocin immunoreactive products, not absolute values of oxytocin, and levels cannot be compared between studies. However, these assays might be useful for the comparison of relative levels of peripheral oxytocin between groups and investigating the relationship between oxytocin levels and relevant clinical endpoints.
Newer techniques free oxytocin from protein binding by reduction–alkylation reactions, followed by liquid chromatography-mass spectrometry (LC-MS)42. After interfering substances are removed by solid phase extraction, the samples undergo high-performance liquid chromatography separation and measurement by mass spectrometry, which is highly selective and sensitive. The addition of a stable isotope of oxytocin (5x deuterated oxytocin) as the first step during LC-MS analysis enables accurate corrections for any loss of analytes or gain in signal strength due to concentration. Using this methodology, oxytocin levels in the ng/ml range (which is ≥1,000 times the levels reported using traditional techniques) have been reported42. These data suggest that much of oxytocin is protein-bound and not measured using traditional approaches. The biological relevance of protein-bound versus free oxytocin is an important question to be investigated.
Several other caveats need to be considered when interpreting data on oxytocin levels. Firstly, central and peripheral oxytocin secretion might be coordinated or independent of one another, and therefore peripheral levels might not reflect central functioning 43-46. Secondly, CSF levels of oxytocin might not mirror activity in specific brain circuits. Thirdly, blood levels of oxytocin might not reflect local autocrine and/or paracrine oxytocin activity. Finally, the measurement of oxytocin in other biological samples (for example, urine and saliva) is not well-validated and the biological importance of these data is not yet clear39.
Oxytocin dose
Oxytocin is typically given intravenously at doses of 1-30 mIU/min during labour and 10 IU post-partum to prevent haemorrhage (1 mg oxytocin is equivalent to 600 IU (WHO International Standard); therefore a dose of 1 mIU/min is approximately 1.7 ng/min). Metabolic studies in humans have often used doses that are higher than the 1 mIU/min required for inducing contractions in pregnancy and the estimated 14 IU oxytocin contained in the human posterior pituitary gland 47. Doses used in these studies are therefore supraphysiologic, even when accounting for differences in routes of administration 48. Evidence indicates that oxytocin at a supraphysiologic dose (80 IU) delivered either intranasally or intravenously crosses the blood brain barrier 16 and therefore might have direct central effects, in addition to peripheral effects. Some of the effects seen at supraphysiologic doses might be due to oxytocin acting at vasopressin receptors 49. Studies are needed to clarify the relative contributions of these receptors in mediating the effects of exogenous oxytocin.
Eating behaviour
Experiments in rodents, nonhuman primates and humans consistently show that oxytocin reduces caloric consumption 14,18,35,50-52. These effects are almost immediate and in animal models are sustained with repeated dosing 14,18,37,38,50,51. With the exception of one study demonstrating that oxytocin reduces food intake and weight gain in female Sim1 haploinsufficent mice 35, most studies have included only males, leaving the question of whether oxytocin also reduces food intake in females unclear. Interestingly, obese rodents seem to be more sensitive to oxytocin treatment than their lean counterparts 38. Preclinical studies have demonstrated a preferential effect of oxytocin in reducing carbohydrate consumption 14,53-56. Although some rodent studies have not found that oxytocin effects intake of lipid emulsions 54,55, others have demonstrated that oxytocin suppresses consumption of high-fat diets 17,18,37,38. Preclinical studies implicate both central (homeostatic, for example, VMH and ARC 1,3, and hedonic (for example, VTA and nucleus accumbens) 4,5, autonomic (NTS and dorsal motor nucleus of vagus) 6,7 and peripheral (gastrointestinal system and vagus nerve) 37,57-60 signalling pathways in the modulation of food intake. Furthermore, oxytocin might affect appetite indirectly by altering levels of other appetite-regulating hormones (for example, increased levels of the anorexigenic hormones CCK and GLP1) 57,61,62.
Evidence increasingly suggests that in humans, oxytocin is involved in normal and aberrant eating behaviours. In anorexia nervosa, an eating disorder characterized by severe chronic food restriction despite self-starvation and markedly low weight, low CSF levels of oxytocin were found in five women with low-weight restrictive 63 (but not low-weight 63 or weight restored binge-purge64 subtypes) anorexia nervosa compared with controls. In addition, peripheral levels of oxytocin in response to stimulation were suppressed in seven low-weight women with anorexia nervosa; levels normalized with weight restoration 65. Low basal serum levels of oxytocin have also been reported in acute (low-weight) and partially-recovered (normal weight) women with anorexia nervosa 66-68. Furthermore, post-prandial oxytocin levels have been linked to the severity of eating disorder psychopathology and functional magnetic resonance imaging (fMRI) hypoactivation of food motivation neural circuitry in low-weight and weight-restored women with anorexia nervosa, which suggests that oxytocin might have a role in the aetiology of anorexia nervosa69. By contrast, several studies have not demonstrated differences in CSF levels of oxytocin between women with active 63 or recovered 64 bulimia nervosa and healthy individuals. Furthermore, no differences were found in basal or stimulated peripheral levels of oxytocin in women with bulimia nervosa compared with controls65,68. In the past 5 years, studies in women have identified associations between genetic variation in the oxytocin receptor and eating disorder diagnosis (bulimia nervosa) 70, eating disorder thoughts and behaviours (in those with anorexia nervosa as well as a community sample of women) 71-73, food preoccupation (in anorexia nervosa) 71 and reported nutrient intake (in a community sample of women) 72.
A single-dose randomized placebo-controlled crossover study of 40 IU intranasal administration of oxytocin in women showed that oxytocin reduced self-reported food intake in the subsequent 24 h in women with bulimia nervosa, but not in women with anorexia nervosa or healthy controls 74; however, a test meal was not performed. In a study of intranasal administration of oxytocin (12-24 IU twice a day) in boys and girls with Prader–Willi syndrome (a genetic syndrome characterized by severe early onset obesity and hyperphagia), 4 weeks of oxytocin treatment reduced the incidence of abnormal food-related behaviours (for instance, preoccupation with food) in a subset of younger children (those aged 6-10 years), but not in those 11-14 years old75. Oxytocin did not affect food intake at a test meal in these children; however, the ability to detect a difference was hindered by the fairly small size of the meal, which was finished in its entirety by nearly all participants, who had hyperphagia75.
To date, three human studies – all exclusively in men – that investigated the effects of intranasal administration of oxytocin on food intake in individuals without eating disorders have been published 51,52,76. In a randomized, placebo-controlled cross-over study of a single intranasal dose of 24 IU oxytocin in 25 healthy men without diabetes mellitus (13 normal-weight and 12 with overweight or obesity), oxytocin reduced caloric intake at a breakfast test meal by 122 kcal with a preferential effect on fat consumption; intake of fat was significantly reduced, while intake of carbohydrates and protein was not significantly reduced 51. Although initial work using the same intranasal dose of oxytocin in normal-weight men did not show suppression of caloric intake at a breakfast buffet 52, a follow up study demonstrated a reduction of 147 kcal in men with obesity76, which is consistent with the animal data that suggests that obesity might be a particularly oxytocin-sensitive state 38. The authors found that oxytocin reduced consumption of a post-prandial snack, particularly chocolate cookies, to a similar extent in men who were normal-weight or had obesity76.
Each of the three human studies63,64,90 assessed for changes in appetite using visual analogue scales and found no evidence that oxytocin effected participants’ self-report of fasting or post-prandial appetite, which demonstrates that oxytocin might alter eating behaviour without the awareness of the individual. This finding is consistent with reports from smaller studies showing no effects of intravenous administration of oxytocin on subjective satiety in healthy individuals 77 or in patients with diabetic gastroparesis or functional dyspepsia 78, but is in contrast to one study that reported reduced satiety in healthy individuals 79. Furthermore, several small studies using intravenous infusions of oxytocin (20-80 mIU/min) in men and women who were healthy 79 or had functional dyspepsia 78 found that oxytocin had no effect on the volume of liquid meal intake required to induce satiety. These data highlight the importance of obtaining objective measures, such as food intake, at a test meal and/or brain imaging in this line of research.
The relationship between postprandial serum levels of oxytocin and hypoactivation detected with fMRI of the hypothalamus – a brain region critical to homeostatic regulation of food consumption – as well as hypoactivation of areas responsible for reward-driven eating, such as the orbitofrontal cortex, in response to visual food stimuli in women with anorexia nervosa 69 supports preclinical evidence of oxytocin’s role in both homeostatic and hedonic food motivation 8. In addition, the preferential effects of intranasal administration of oxytocin in reducing consumption of more palatable foods, such as fats and carbohydrates, by men 51,52,76 is consistent with the concept that oxytocin might inhibit food intake in part by modulating reward-related food motivation and/or impulse control neurocircuitry. This concept is in line with a study published in 2016 showing that a single intranasal dose of 24 IU oxytocin increased activation of brain circuitry involved in cognitive control (detected using fMRI) and, at the trend level, reduced food craving when women were asked to cognitively control the urge to eat while viewing images of palatable foods 80. This finding is also consistent with animal studies demonstrating that administration of oxytocin to the VTA56 or nucleus accumbens81, which are regions of the brain involved in reward that receive oxytocin projections from the PVN4 and express oxytocin receptors23-25, inhibits sucrose intake. Further research examining the specific effects of oxytocin on caloric intake and underlying mechanisms in humans will be important (Figure 1).
Figure 1. Hypothesized mechanisms underlying the effects of oxytocin on caloric intake.
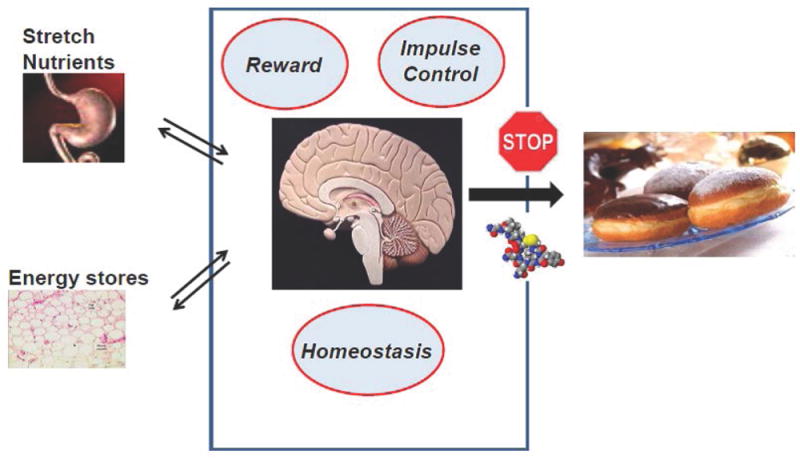
In response to peripheral signals that indicate energy availability, oxytocin acts on homeostatic, reward and impulse control brain circuitry to reduce caloric intake, particularly of more palatable foods.
Peripheral sites of oxytocin action have also been implicated as potential modulators of appetitive behaviours. Studies in rats suggest that oxytocin might induce satiety in part by slowing gastric emptying 57-60. The data in humans, however, are inconsistent with one study in patients with diabetic gastroparesis reporting prolonged gastric emptying time (40-80 mIU/min) 78, and studies in healthy individuals showing no effects (40 mIU/min)77, or accelerated gastric emptying (0.33 IU/min) 82 following intravenous administration of oxytocin. Although a study in healthy men and women found that the oxytocin antagonist atosiban increased gastric emptying time 77, the authors speculate that these effects could have been due to actions at the vasopressin receptor. These conflicting results might reflect differences in the populations studied and/or small sample sizes. Two studies have examined the effects of oxytocin on the colon in humans. In women, intravenous administration of oxytocin (20 mIU/min or 40 mIU/min) increased colonic motility in response to a lipid infusion by feeding tube 83. In a study of 26 patients with irritable bowel syndrome and constipation, intravenous administration of oxytocin at doses of 20-50 mIU/min reduced their perception of colonic distention 84. The clinical significance of these findings is unclear and further studies are needed to determine dose and population-specific effects of oxytocin on gastroenterological function and appetitive behaviours.
Several studies in humans have examined the effects of oxytocin on other appetite-regulating hormones. While intravenous administration of oxytocin reduces the typical lipopolysaccharide-induced increase in levels of ghrelin (which is orexigenic) 85, 24 IU intranasal administration of oxytocin did not result in statistically significant changes in fasting or post-prandial levels of ghrelin 51,52. Oxytocin increased CCK levels, but this change was not related to differences in caloric consumption between oxytocin and placebo conditions 51. Oxytocin had no statistically significant effects on the levels of anorexigenic hormones leptin, GLP1 or PYY 51,52. Reduced levels of cortisol, which have been previously demonstrated in animal and human studies, were associated with reduced consumption of chocolate cookies in normal weight (but not obese) men receiving oxytocin 52,76. In future studies, it will be important to examine the effects of oxytocin on other appetite-regulating hormones and whether some of the effects of oxytocin on caloric intake could be mediated by other hormones.
Lipid metabolism and body fat
Preclinical studies indicate that oxytocin induces lipolysis 14,18 and fat oxidation 18,37,86, which in turn, independent of food intake, lead to reduced body fat and weight 18. Notably, oxytocin reduces visceral and liver fat 37, which are metabolically important fat depots that are associated with an increased risk of the metabolic syndrome and cardiovascular disease 87.
In men and women, endogenous peripheral levels of oxytocin are high in those with obesity 88,89 and oxytocin levels in different populations across the weight spectrum are correlated with BMI and levels of body fat 66,89-91. A notable exception is a study showing low levels of oxytocin in adults with obesity compared with adults of a normal weight; thus, oxytocin levels were negatively associated with BMI. However, these results might have been confounded by the fact that a large proportion of participants had type 2 diabetes mellitus, a condition associated with low oxytocin levels92. Published in 2016, results from the MINOS study of men aged 50-85 yrs demonstrated higher endogenous levels of oxytocin in men with the metabolic syndrome compared to those without metabolic syndrome. Furthermore, higher oxytocin levels were associated with greater odds of metabolic syndrome after adjusting for confounders including leptin levels. 91. High oxytocin levels were associated with increased central fat and waist circumference, which are surrogate markers for visceral fat 91.
In the 1960s, an experiment in women showed that intravenous administration of oxytocin (10 mIU/kg) increased plasma levels of nonesterified free fatty acids and reduced plasma levels of triglycerides93, which is consistent with the lipolytic effect of oxytocin that has been demonstrated in animal models 14,18. Several human studies published in the past 5 years have used intranasal oxytocin as a physiologic probe to study the effects of oxytocin on lipid metabolism and body fat 36,51. A single dose of intranasal oxytocin (24 IU) in men resulted in a drop in respiratory quotient as assessed by indirect calorimetry 30 min after oxytocin administration, which is consistent with an increase in fat oxidation 51. In a small study of 8 weeks of sustained intranasal administration of oxytocin (24 IU before meals and at bedtime) in men and women who were overweight or had obesity, oxytocin led to weight loss with an improved lipid profile (lower levels of total cholesterol and LDL cholesterol) and reduced waist circumference 36. Imaging was not performed to assess for changes in total body fat or specific fat depots 36. The study authors noted that oxytocin resulted in slightly improved results from liver function tests, which could reflect reduced hepatic steatosis 36. Further studies are warranted to examine the effects of oxytocin on lipid metabolism and body fat depots.
Energy expenditure
A major barrier to maintenance of weight loss in humans is the physiologic reduction in energy expenditure that occurs with reduced food intake and weight 94. Unlike existing pharmacological options, which primarily target food consumption or absorption, oxytocin not only reduces food intake but, in diet induced obese rodent and nonhuman primate models, also promotes energy expenditure, which enables maintenance of weight reduction 14,18,38,86. How oxytocin increases energy expenditure is not yet clear; however, some preclinical evidence suggests that oxytocin may lead to preferential preservation of lean body mass, a key determinant of energy expenditure, despite weight loss 86; activation of brown fat 32-34; and/or the conversion of white adipose tissue to beige fat that is capable of thermogenesis 90,95.
In young female athletes and non-athletes aged 14-21 yrs, fasting levels of oxytocin were positively associated with resting energy expenditure, providing the first evidence for such a link in humans 90. However, studies of a single intranasal dose of oxytocin (24 IU) in men of a normal weight, or who had overweight or obesity found no acute effect of oxytocin on resting or post-prandial energy expenditure measured by indirect calorimetry 51,52. It is possible that chronic oxytocin exposure is required to induce changes in energy expenditure, and therefore effects on energy expenditure were not captured in these single dose experiments. Whether sustained oxytocin will increase energy expenditure in humans as it does in animal models will be an important area of investigation.
Body weight
Oxytocin is emerging as a promising novel weight-loss therapy for obesity, as evidenced by review articles published on this topic over the past several years 8,96,97 and current clinical research studies registered on ClinicalTrials.gov (NCT 02849743, 03043053, 03119610). Experiments in rodents and nonhuman primates demonstrate that chronic peripheral or central administration of oxytocin results in sustained weight loss attributed to reduced food intake, maintenance of energy expenditure despite weight loss and increased lipolysis 14,18,37,38,86.
Human studies have shown that peripheral levels of oxytocin are low in patients with anorexia nervosa 66,68, high in those with obesity 88,89 and positively associated with BMI and fat mass 66,89. Furthermore, oxytocin levels drop with weight loss following gastric banding 88. Together, these preclinical and clinical data suggest that oxytocin could act as a signal of energy availability: high oxytocin levels (for example, in obesity) appropriately signal the need to reduce caloric intake and increase energy expenditure.
Dysfunctional oxytocin signalling might contribute to weight gain in human genetic syndromes of severe obesity. In a genome-wide association study of white children in the UK, copy number variation in genes that encode proteins in the oxytocin receptor pathway were associated with severe early onset obesity 98. Furthermore, hypothalamic oxytocin deficiency has been implicated in the pathogenesis of Prader–Willi syndrome99,100. In these patients, fairly low levels of oxytocin have been reported in the CSF 99, and post-mortem studies have identified a reduced number of oxytocin-expressing neurons in the PVN of the hypothalamus 100. In addition, polymorphisms of the oxytocin receptor (A-allele of rs53576) are associated with increased risk of obesity in children of low socioeconomic status101.
To date, only one study has examined whether oxytocin administration reduces body weight in humans who have overweight or obesity but are otherwise healthy. This randomized, placebo-controlled study of 24 IU oxytocin delivered intranasally four times per day in men and women who were overweight or had obesity demonstrated that oxytocin led to substantial weight loss over 8 weeks 36. This study was limited by a small sample size (9 participants randomly assigned to receive oxytocin) and the groups were not well-matched for age or baseline weight. However, the body weight loss of approximately 9 kg (or 9%) with oxytocin was similar to the weight loss seen with the most effective FDA-approved medications for obesity 102,103.
In contrast to the previous study 36, two randomized, placebo-controlled cross-over studies of intranasal administration of oxytocin (12-40 IU twice daily) in children and adults with Prader–Willi syndrome did not result in statistically significant weight loss 75,104. Several potential explanations for the failure of oxytocin to reduce weight in these studies exist. Firstly, the lower daily dose and frequency of oxytocin administration than in the previous study. Secondly, Prader-Willi syndrome is associated with structural abnormalities of the PVN, including reduced number of oxytocin neurons 100; it is therefore plausible that these patients may not be able to respond to exogenous oxytocin (for example, there may be a reduced number of hypothalamic oxytocin receptors and/or an inability to respond to oxytocin stimulation with endogenous oxytocin release).104 Thirdly, children might have a different response to oxytocin compared with adults. Fourthly, in one of the studies, weight was not a primary endpoint 75.
Glucose homeostasis
Oxytocin receptors are present on α cells and β cells in pancreatic islets 29. Furthermore, oxytocin administered at physiologic concentrations increases insulin release in the isolated rat pancreas 105. Studies examining the effects of oxytocin on glucose homeostasis have yielded conflicting results and are very much dependent on the experimental model used. While some studies of central or peripheral administration of oxytocin in animal models (for example, in lean rats or obese diabetic ob/ob mice) have shown that oxytocin induces hyperglycaemia 26,106, others (for example, in lean or diet-induced obese mice, diet-induced obese rats, obese diabetic db/db mice, or streptozotocin-induced diabetic mice with impaired beta-cell function) have clearly demonstrated the therapeutic potential of using oxytocin or oxytocin analogues to increase insulin secretion, insulin sensitivity and glucose tolerance independent of effects on weight or food intake 8,17,18,36,37,95,107,108.
Human studies show inconsistent effects of oxytocin on glucose homeostasis, which might reflect differences in route of administration (for instance, intravenous versus intranasal and bolus versus continuous infusion), dose and/or populations studied. Acute effects of oxytocin on glucose homeostasis in humans were first demonstrated using intravenous administration of oxytocin more than 50 years ago. The study demonstrated that an intravenous bolus of oxytocin (10 mIU/kg) in postpartum women or women who were not pregnant or postpartum resulted in a dramatic lowering of blood levels of glucose over the 3 h experiment 93. By contrast, another study of postpartum women found no effect of an intravenous dose of oxytocin (10 IU) on glucose or insulin levels 109. This discrepancy may have been due to differences in dose, which was more than ten times higher in the second experiment. In a small study of men, 6 IU (an initial dose of 2 IU plus an infusion of 4 IU over 1 h), but not 3 IU (an initial dose of 1 IU plus an infusion of 2 IU over 1 h) oxytocin intravenously increased insulin levels in response to intravenous administration of glucose (0.33 g/kg) without affecting glucose levels or the glucagon, growth hormone or cortisol responses 110. Another study reported that continuous intravenous infusion of oxytocin (0.2 IU/min over 60 min) resulted in hyperglycaemia, accompanied by increased release of glucagon, insulin and adrenaline and reduced release of cortisol in healthy, young men aged 21-26 yrs 111. Furthermore, in response to insulin-induced hypoglycaemia, oxytocin infused intravenously at this dose attenuated the drop in levels of glucose, increased levels of glucagon and adrenaline and reduced cortisol levels 111,112. Using a euglyceamic euinsulinaemic clamp, it was also shown that the same dose of intravenous oxytocin increased hepatic glucose output in healthy young men aged 22-28 yrs 113.
Studies published in the past few years that used intranasal administration of oxytocin in men without diabetes mellitus have reported consistently beneficial effects on glucose homeostasis 51,52,76,114. In healthy men without diabetes mellitus who had a normal weight, were overweight or had obesity, 24 IU oxytocin intranasally – the same dose that is under investigation for weight loss in obese adults 36(ClinTrials.gov, NCT03043053) – reduced fasting levels of insulin while maintaining normal levels of glucose, which resulted in improved insulin sensitivity 51. In the fed state, 24 IU oxytocin intranasally in separate groups of healthy normal-weight men and men with overweight or obesity reduced glucose levels without a change in insulin or C-peptide levels, independent of caloric intake 52,76. In response to a 75 g oral glucose tolerance test, 24 IU oxytocin intranasally blunted the rise in levels of glucose and resulted in a more rapid, but attenuated insulin and C-peptide peak in 29 normal-weight healthy men 114. The authors found that oxytocin improved β-cell responsivity to the glucose challenge 114. However, a pilot study of intranasal administration of oxytocin (24 IU before meals and bedtime) for 8 weeks in a small group of men and women without diabetes mellitus who had overweight or obesity did not show statistically significant changes in fasting or postprandial levels of glucose or insulin despite weight loss 36.
Men and women with type 2 diabetes mellitus 92 and women with type 1 diabetes mellitus 115 have been shown to have lower levels of oxytocin than controls. Furthermore, in those with type 2 diabetes mellitus, low fasting levels of oxytocin were associated with higher levels of glucose and insulin, both after fasting and after an oral glucose tolerance test, as well as higher HOMA-IR results and levels of HbA1c 92; these findings suggest that diabetes mellitus could be a state of oxytocin deficiency. However, an experiment administering continuous intravenous infusion of oxytocin (0.2 IU/min) to patients with type 1 diabetes mellitus resulted in hyperglycaemia 112, which is similar to findings with this dose and route of administration in healthy young men 111.
In summary, studies examining acute effects of intravenous oxytocin on glucose homeostasis in humans have shown mixed results with low dose (10 mIU/kg, i.e. ≤1 IU) oxytocin reducing glucose levels and higher dose oxytocin showing no effect (3-10 IU) or increasing (12 IU) glucose levels. While a number of studies of single dose intranasal oxytocin (24 IU) in non-diabetic men have resulted in improved glucose homeostasis, an eight week study of short-term intranasal oxytocin (24 IU four times per day) did not yield improvement in glucose parameters despite achieving weight loss. Further research examining dose-dependent effects of different routes of oxytocin administration, taking sex and physiologic context into account, is warranted. Studies in patients with prediabetes mellitus and diabetes mellitus will be particularly important.
Downregulation of the HPA axis
In addition to having direct beneficial metabolic actions, oxytocin might contribute additional complementary metabolic effects by downregulating activation of the hypothalamic–pituitary–adrenal (HPA) axis. The negative effects of cortisol on metabolism, including effects on lipid metabolism, body fat deposition and glucose homeostasis, are well-established 116. Oxytocin receptors are located in the anterior pituitary and adrenal glands 30,117, and intravenous or intranasal administration of oxytocin in humans reduces levels of adrenocorticotropic hormone and cortisol under basal and stress conditions 118-122. Intravenous administration of oxytocin completely blocked adrenocorticotropic hormone stimulation in response to corticotropin-releasing hormone in normal-weight men 123. Although oxytocin reduces cortisol levels, hypoadrenalism is not a reported adverse effect of this drug at therapeutic doses. Further research is needed to determine whether the effects of oxytocin on the HPA axis lead to an improved metabolic profile.
Therapeutic potential
Oxytocin and interventions targeting related pathways to increase the actions of the oxytocin signal are promising agents in the treatment of obesity and metabolic disorders, including diabetes mellitus (Figure 2). However, some important points must be considered when developing oxytocin therapeutics, including its short half-life31 and the current limitations in our knowledge about mechanisms and actions of oxytocin, as well as the safety of chronic administration in humans.
Figure 2. Proposed model of the effects of oxytocin on metabolic parameters.
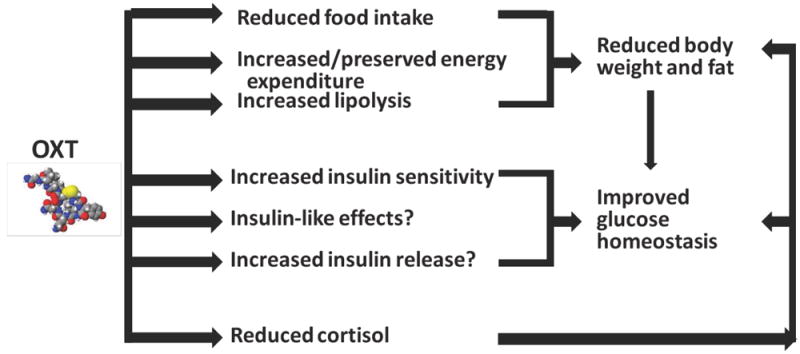
Oxytocin reduces body weight and fat and improves glucose homeostasis, highlighting its potential as a targeted therapy for metabolic disorders such as obesity and diabetes mellitus.
Synthetic oxytocin is FDA-approved and widely used for induction of labour and control of postpartum uterine bleeding, and is readily available in intravenous and intramuscular formulations 124. Given the short half-life of oxytocin (on the order of 2-8 minutes in peripheral blood 125,126 and 19-28 minutes in CSF 127)128, intramuscular or intravenous bolus administration would not be feasible for chronic administration. Although not currently approved by the FDA, intranasal formulations of oxytocin are commercially available outside the USA (for instance, in Europe) to facilitate milk letdown in nursing mothers, and has been used in human investigations 129. However, the intranasal formulation is inconvenient (it consists of 24 IU or six sprays of 4 IU given four times per day) and results in imprecise dosing. Oxytocin is a pulsatile hormone 130-133 and the implications of basal (or continuous) versus pulsatile (or bolus) oxytocin exposure is not yet clear. Furthermore, the effect of oxytocin feed-forward regulation (that is, stimulation of oxytocin release by oxytocin) in extending effects of exogenously administered oxytocin is not well-defined. In diet-induced obese mice, continuous subcutaneous administration of oxytocin using a minipump is more than three times more effective in achieving weight reduction than a single daily injection of the same dose (13.0% vs. 3.6%) 37, which suggests that longer acting oxytocin formulations would have greater efficacy than current options. Longer-acting oxytocin analogues, such as carbetocin, [Ser4,Ile8]-oxytocin and [Asu1,6]-oxytocin have been developed, and improve metabolic parameters (such as weight loss and glucose homeostasis) in obese diabetic mice 26,36. Improving our currently limited mechanistic understanding of how oxytocin works in humans will be a critical step in bringing oxytocin therapeutics into the clinic. Elucidating the full range of on and off target effects in order to optimize efficacy and minimize tolerability and safety concerns will also be important. Many unanswered questions exist, including whether and how central and/or peripheral oxytocin mediates its metabolic effects. Optimal dosing will also need to be established. Pharmacokinetic studies are limited by challenges in accurately measuring oxytocin levels 39. Newer LC-MS methods may be useful in overcoming these limitations.
Although oxytocin is often administered to women around the time of childbirth, chronic use of oxytocin is not well studied and its safety for metabolic indications has not yet been established. A direct comparison of doses is difficult because oxytocin is given intravenously as a continuous infusion to induce labor, whereas single doses of intranasal oxytocin (four times per day) are under investigation for weight loss. However, the dose of intranasal oxytocin that reduces food intake, increases the use of fat as a fuel for the body, and improves glucose homeostasis in men (24 IU) 51,52,76,114 falls within the range of total intranasal oxytocin used in a series of patients receiving this for the induction and maintenance of labor in 1968-1970, when intranasal oxytocin was used clinically as an alternative to intravenous oxytocin 134. In one series, the mean total dose of oxytocin used for induction or stimulation of labor was 99 IU 135, similar to the daily dose that is under study for obesity (96 IU) 36(ClinTrials.gov, NCT03043053). Based primarily on our current knowledge of possible adverse effects in labour and delivery with oxytocin, key safety issues that will need to be assessed with chronic use for metabolic disease include cardiovascular risk including arrhythmias and changes in blood pressure, as well as hyponatraemia 124. Less concerning adverse effects that could limit tolerability include headache or nausea 124. Oxytocin for metabolic indications would be contraindicated in pregnancy given the risk of uterine contractions. Investigations of chronic intranasal administration of oxytocin in humans have extended up to 6 months with no serious safety concerns reported 36,75,104,129,136-139. Although oxytocin is under investigation as a potential therapy for neuropsychiatric disorders, such as autism spectrum disorders, in children 138, the long-term consequences of oxytocin administration in development are unknown and warrant investigation. Further study of chronic oxytocin use will be important, particularly in patients with obesity and its associated comorbidities, to demonstrate safety in this population.
Alternative ways to increase oxytocin signalling, for instance, by promoting oxytocin secretion, increasing oxytocin receptor activity or inhibiting oxytocin degradation, should also be explored.
Conclusions
Translational human studies are beginning to define the effects of oxytocin on eating behaviour and metabolism and support the therapeutic potential of oxytocin-based drugs for metabolic disorders, such as obesity and diabetes mellitus. Optimizing measurement techniques for biological samples is a critical step in improving our understanding of oxytocin physiology and translational applications. Further research examining dose-dependent effects and underlying mechanisms of oxytocin using different routes of administration (for example, intranasal versus intravenous and bolus versus continuous oxytocin exposure) and taking sex and physiologic context into account, will be important. The safety and tolerability of chronic oxytocin use in relevant clinical populations must also be established. Alternative ways to increase oxytocin signalling (for example, pharmacologic agents that increase secretion, act at the oxytocin receptor or inhibit oxytocin breakdown) should also be considered. Improving our mechanistic understanding of how and where oxytocin acts in humans will be critical to advancing clinical therapies.
Box 1. Endogenous peripheral levels of oxytocin in humans.
Positive associations
-
○
Severity of eating disorder psychopathology and hypoactivation of food motivation brain regions on fMRI (post-prandial levels of oxytocin in anorexia nervosa)
-
○
Body weight, BMI and body fat
-
○
Resting energy expenditure
Negative associations
-
○
Levels of glucose and insulin, HOMA-IR, HbA1c levels (type 2 diabetes mellitus) during fasting and after an oral glucose tolerance test.
Box 2. Effects of intranasal administration of oxytocin in humans.
Acute
-
○
Reduces caloric intake at test meal and post-meal snack
-
○
Reduces respiratory quotient, which is indicative of increased fat utilization
-
○
Increases fasting and post-prandial insulin sensitivity; improves β-cell responsivity to oral glucose tolerance test
-
○
Reduces hypothalamic–pituitary–adrenal activity and reactivity
Chronic
-
○
Reduces body weight and waist circumference
-
○
Reduces levels of total and LDL cholesterol
Key points.
Animal studies indicate that oxytocin is a potent regulator of caloric intake and metabolism; clinical investigations are beginning to translate these findings to humans.
A single dose of intranasal oxytocin reduces caloric intake in men, particularly of more palatable foods, and these effects could be increased in men with obesity.
Intranasal oxytocin acutely increases the use of fat as a fuel for the body, but effects of oxytocin in promoting energy expenditure have not been demonstrated in humans.
An 8-week pilot study of intranasal oxytocin in a small group of men and women with overweight or obesity led to substantial weight loss.
Independent of effects on body weight, oxytocin might improve glucose homeostasis, but data are conflicting.
Pathways that involve oxytocin offer novel therapeutic targets for obesity and metabolic disease.
Biography
Elizabeth A. Lawson is Director, Interdisciplinary Oxytocin Research Program in the Neuroendocrine Unit at Massachusetts General Hospital (MGH) and Associate Professor of Medicine at Harvard Medical School. She is also a practicing neuroendocrinologist at the Neuroendocrine Clinical Center at MGH. Her research focuses on the effects of oxytocin on eating behaviour and metabolism in humans.
Footnotes
Competing interests statement
E.A.L. is on the Scientific Advisory Board of OXT, Therapeutics, Inc.
References
- 1.Tribollet E, Barberis C, Dubois-Dauphin M, Dreifuss JJ. Localization and characterization of binding sites for vasopressin and oxytocin in the brain of the guinea pig. Brain research. 1992;589:15–23. doi: 10.1016/0006-8993(92)91156-9. [DOI] [PubMed] [Google Scholar]
- 2.Sabatier N, Rowe I, Leng G. Central release of oxytocin and the ventromedial hypothalamus. Biochem Soc Trans. 2007;35:1247–1251. doi: 10.1042/BST0351247. [DOI] [PubMed] [Google Scholar]
- 3.Maejima Y, et al. Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS Lett. 2014;588:4404–4412. doi: 10.1016/j.febslet.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross HE, et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. The Journal of comparative neurology. 1998;399:101–109. doi: 10.1002/(sici)1096-9861(19980914)399:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. The Journal of comparative neurology. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 8.Blevins JE, Baskin DG. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: Insights from rodents, nonhuman primates and humans. Physiology & behavior. 2015 doi: 10.1016/j.physbeh.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohlsson B, Truedsson M, Djerf P, Sundler F. Oxytocin is expressed throughout the human gastrointestinal tract. Regulatory peptides. 2006;135:7–11. doi: 10.1016/j.regpep.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Qin J, et al. Oxytocin receptor expressed on the smooth muscle mediates the excitatory effect of oxytocin on gastric motility in rats. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21:430–438. doi: 10.1111/j.1365-2982.2009.01282.x. [DOI] [PubMed] [Google Scholar]
- 11.Feng M, et al. Estradiol upregulates the expression of oxytocin receptor in colon in rats. American journal of physiology Endocrinology and metabolism. 2009;296:E1059–1066. doi: 10.1152/ajpendo.90609.2008. [DOI] [PubMed] [Google Scholar]
- 12.Welch MG, et al. Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. The Journal of comparative neurology. 2009;512:256–270. doi: 10.1002/cne.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho JM, et al. Hindbrain oxytocin receptors contribute to the effects of circulating oxytocin on food intake in male rats. Endocrinology. 2014;155:2845–2857. doi: 10.1210/en.2014-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blevins JE, et al. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. American journal of physiology Regulatory, integrative and comparative physiology. 2014 doi: 10.1152/ajpregu.00441.2014. ajpregu 00441 02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasaki Y, et al. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. American journal of physiology Regulatory, integrative and comparative physiology. 2015;308:R360–369. doi: 10.1152/ajpregu.00344.2014. [DOI] [PubMed] [Google Scholar]
- 16.Lee MR, et al. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang G, Cai D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. American journal of physiology Endocrinology and metabolism. 2011;301:E1004–1012. doi: 10.1152/ajpendo.00196.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deblon N, et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PloS one. 2011;6:e25565. doi: 10.1371/journal.pone.0025565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould BR, Zingg HH. Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor-LacZ reporter mouse. Neuroscience. 2003;122:155–167. doi: 10.1016/s0306-4522(03)00283-5. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida M, et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrobel L, et al. Distribution and identity of neurons expressing the oxytocin receptor in the mouse spinal cord. Neurosci Lett. 2011;495:49–54. doi: 10.1016/j.neulet.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Hidema S, et al. Generation of Oxtr cDNA(HA)-Ires-Cre Mice for Gene Expression in an Oxytocin Receptor Specific Manner. J Cell Biochem. 2016;117:1099–1111. doi: 10.1002/jcb.25393. [DOI] [PubMed] [Google Scholar]
- 23.Peris J, et al. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. The Journal of comparative neurology. 2017;525:1094–1108. doi: 10.1002/cne.24116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaccari C, Lolait SJ, Ostrowski NL. Comparative Distribution of Vasopressin V1b and Oxytocin Receptor Messenger Ribonucleic Acids in Brain*. Endocrinology. 1998;139:5015–5033. doi: 10.1210/endo.139.12.6382. * Portions of the OTR mRNA distribution data were presented at the Wenner-Gren Conference, Stockholm, Sweden, 1996 This work was funded by the NIMH, NIH and by grant no MH-01050 to C S Carter that provided partial support to C Vaccari and E Gournelos, who were also recipients of Howard Hughes Undergraduate Fellowships from the University of Maryland, College Park. [DOI] [PubMed] [Google Scholar]
- 25.van Leeuwen FW, van Heerikhuize J, van der Meulen G, Wolters P. Light microscopic autoradiographic localization of [3H]oxytocin binding sites in the rat brain, pituitary and mammary gland. Brain research. 1985;359:320–325. doi: 10.1016/0006-8993(85)91443-x. [DOI] [PubMed] [Google Scholar]
- 26.Altirriba J, et al. Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology. 2014;155:4189–4201. doi: 10.1210/en.2014-1466. [DOI] [PubMed] [Google Scholar]
- 27.Gajdosechova L, et al. Hypooxytocinaemia in obese Zucker rats relates to oxytocin degradation in liver and adipose tissue. J Endocrinol. 2014;220:333–343. doi: 10.1530/JOE-13-0417. [DOI] [PubMed] [Google Scholar]
- 28.Muchmore DB, Little SA, de Haen C. A dual mechanism of action of ocytocin in rat epididymal fat cells. The Journal of biological chemistry. 1981;256:365–372. [PubMed] [Google Scholar]
- 29.Suzuki M, Honda Y, Li MZ, Masuko S, Murata Y. The localization of oxytocin receptors in the islets of Langerhans in the rat pancreas. Regulatory peptides. 2013;183:42–45. doi: 10.1016/j.regpep.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Antoni FA. Oxytocin receptors in rat adenohypophysis: evidence from radioligand binding studies. Endocrinology. 1986;119:2393–2395. doi: 10.1210/endo-119-5-2393. [DOI] [PubMed] [Google Scholar]
- 31.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 32.Kasahara Y, Takayanagi Y, Kawada T, Itoi K, Nishimori K. Impaired thermoregulatory ability of oxytocin-deficient mice during cold-exposure. Bioscience, biotechnology, and biochemistry. 2007;71:3122–3126. doi: 10.1271/bbb.70498. [DOI] [PubMed] [Google Scholar]
- 33.Takayanagi Y, et al. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- 34.Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity. 2009;17:980–984. doi: 10.1038/oby.2009.12. [DOI] [PubMed] [Google Scholar]
- 35.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Molecular endocrinology. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PloS one. 2013;8:e61477. doi: 10.1371/journal.pone.0061477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maejima Y, et al. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging. 2011;3:1169–1177. doi: 10.18632/aging.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morton GJ, et al. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. American journal of physiology Endocrinology and metabolism. 2012;302:E134–144. doi: 10.1152/ajpendo.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neuroscience and biobehavioral reviews. 2013;37:1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Leng G, Sabatier N. Measuring Oxytocin and Vasopressin: Bioassays, Immunoassays and Random Numbers. J Neuroendocrinol. 2016;28 doi: 10.1111/jne.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szeto A, et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandtzaeg OK, et al. Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci Rep. 2016;6:31693. doi: 10.1038/srep31693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabatier N, et al. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amico JA, Challinor SM, Cameron JL. Pattern of oxytocin concentrations in the plasma and cerebrospinal fluid of lactating rhesus monkeys (Macaca mulatta): evidence for functionally independent oxytocinergic pathways in primates. The Journal of clinical endocrinology and metabolism. 1990;71:1531–1535. doi: 10.1210/jcem-71-6-1531. [DOI] [PubMed] [Google Scholar]
- 45.Neumann I, Landgraf R, Takahashi Y, Pittman QJ, Russell JA. Stimulation of oxytocin release within the supraoptic nucleus and into blood by CCK-8. Am J Physiol. 1994;267:R1626–1631. doi: 10.1152/ajpregu.1994.267.6.R1626. [DOI] [PubMed] [Google Scholar]
- 46.Ivanyi T, Wiegant VM, de Wied D. Differential effects of emotional and physical stress on the central and peripheral secretion of neurohypophysial hormones in male rats. Life Sci. 1991;48:1309–1316. doi: 10.1016/0024-3205(91)90527-i. [DOI] [PubMed] [Google Scholar]
- 47.Heller H, Zaimis EJ. The antidiuretic and oxytocic hormones in the posterior pituitary glands of newborn infants and adults. The Journal of physiology. 1949;109:162–169. doi: 10.1113/jphysiol.1949.sp004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leng G, Ludwig M. Intranasal Oxytocin: Myths and Delusions. Biological psychiatry. 2016;79:243–250. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Hicks C, et al. Regional c-Fos expression induced by peripheral oxytocin administration is prevented by the vasopressin 1A receptor antagonist SR49059. Brain Res Bull. 2016;127:208–218. doi: 10.1016/j.brainresbull.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10:89–93. doi: 10.1016/0196-9781(89)90082-x. [DOI] [PubMed] [Google Scholar]
- 51.Lawson EA, et al. Oxytocin reduces caloric intake in men. Obesity. 2015;23:950–956. doi: 10.1002/oby.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ott V, et al. Oxytocin reduces reward-driven food intake in humans. Diabetes. 2013;62:3418–3425. doi: 10.2337/db13-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. American journal of physiology Regulatory, integrative and comparative physiology. 2005;289:R1798–1806. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- 54.Sclafani A, Rinaman L, Vollmer RR, Amico JA. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. American journal of physiology Regulatory, integrative and comparative physiology. 2007;292:R1828–1833. doi: 10.1152/ajpregu.00826.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miedlar JA, Rinaman L, Vollmer RR, Amico JA. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. American journal of physiology Regulatory, integrative and comparative physiology. 2007;293:R1063–1068. doi: 10.1152/ajpregu.00228.2007. [DOI] [PubMed] [Google Scholar]
- 56.Mullis K, Kay K, Williams DL. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain research. 2013;1513:85–91. doi: 10.1016/j.brainres.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu CL, et al. Involvement of cholecystokinin receptor in the inhibition of gastric emptying by oxytocin in male rats. Pflugers Arch. 2002;445:187–193. doi: 10.1007/s00424-002-0925-7. [DOI] [PubMed] [Google Scholar]
- 58.Wu CL, Hung CR, Chang FY, Pau KY, Wang PS. Pharmacological effects of oxytocin on gastric emptying and intestinal transit of a non-nutritive liquid meal in female rats. Naunyn-Schmiedeberg’s archives of pharmacology. 2003;367:406–413. doi: 10.1007/s00210-003-0690-y. [DOI] [PubMed] [Google Scholar]
- 59.Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 1987;8:505–513. doi: 10.1016/0196-9781(87)90017-9. http://doi.org/10.1016/0196-9781(87)90017-9. [DOI] [PubMed] [Google Scholar]
- 60.Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain research. 1992;578:256–260. doi: 10.1016/0006-8993(92)90255-8. [DOI] [PubMed] [Google Scholar]
- 61.Rinaman L, Rothe EE. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. American journal of physiology Regulatory, integrative and comparative physiology. 2002;283:R99–106. doi: 10.1152/ajpregu.00008.2002. [DOI] [PubMed] [Google Scholar]
- 62.Wu CL, Doong ML, Wang PS. Involvement of cholecystokinin receptor in the inhibition of gastrointestinal motility by oxytocin in ovariectomized rats. Eur J Pharmacol. 2008;580:407–415. doi: 10.1016/j.ejphar.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 63.Demitrack MA, et al. CSF oxytocin in anorexia nervosa and bulimia nervosa: clinical and pathophysiologic considerations. Am J Psychiatry. 1990;147:882–886. doi: 10.1176/ajp.147.7.882. [DOI] [PubMed] [Google Scholar]
- 64.Frank GK, Kaye WH, Altemus M, Greeno CG. CSF oxytocin and vasopressin levels after recovery from bulimia nervosa and anorexia nervosa, bulimic subtype. Biological psychiatry. 2000;48:315–318. doi: 10.1016/s0006-3223(00)00243-2. [DOI] [PubMed] [Google Scholar]
- 65.Chiodera P, et al. Effect of estrogen or insulin-induced hypoglycemia on plasma oxytocin levels in bulimia and anorexia nervosa. Metabolism. 1991;40:1226–1230. doi: 10.1016/0026-0495(91)90220-q. [DOI] [PubMed] [Google Scholar]
- 66.Lawson EA, et al. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. The Journal of clinical psychiatry. 2011;72:1546–1551. doi: 10.4088/JCP.10m06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Afinogenova Y, et al. Low Fasting Oxytocin Levels Are Associated with Psychopathology in Anorexia Nervosa in Partial Recovery. Journal of Clinical Psychiatry. 2016 doi: 10.4088/JCP.15m10217. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monteleone AM, Scognamiglio P, Volpe U, Di Maso V, Monteleone P. Investigation of Oxytocin Secretion in Anorexia Nervosa and Bulimia Nervosa: Relationships to Temperament Personality Dimensions. European eating disorders review : the journal of the Eating Disorders Association. 2016;24:52–56. doi: 10.1002/erv.2391. [DOI] [PubMed] [Google Scholar]
- 69.Lawson EA, et al. Oxytocin secretion is associated with severity of disordered eating psychopathology and insular cortex hypoactivation in anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2012;97:E1898–1908. doi: 10.1210/jc.2012-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim YR, Kim JH, Kim CH, Shin JG, Treasure J. Association between the oxytocin receptor gene polymorphism (rs53576 and bulimia nervosa. European eating disorders review : the journal of the Eating Disorders Association. 2015;23:171–178. doi: 10.1002/erv.2354. [DOI] [PubMed] [Google Scholar]
- 71.Acevedo SF, Valencia C, Lutter M, McAdams CJ. Severity of eating disorder symptoms related to oxytocin receptor polymorphisms in anorexia nervosa. Psychiatry research. 2015;228:641–648. doi: 10.1016/j.psychres.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Connelly JJ, et al. Personality, behavior and environmental features associated with OXTR genetic variants in British mothers. PloS one. 2014;9:e90465. doi: 10.1371/journal.pone.0090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Micali N, Crous-Bou M, Treasure J, Lawson EA. Association Between Oxytocin Receptor Genotype, Maternal Care, and Eating Disorder Behaviours in a Community Sample of Women. European Eating Disorders Review. 2016:n/a–n/a. doi: 10.1002/erv.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim Y-R, Eom J-S, Yang J-W, Kang J, Treasure J. The Impact of Oxytocin on Food Intake and Emotion Recognition in Patients with Eating Disorders: A Double Blind Single Dose Within-Subject Cross-Over Design. PloS one. 2015;10:e0137514. doi: 10.1371/journal.pone.0137514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuppens RJ, Donze SH, Hokken-Koelega AC. Promising effects of oxytocin on social and food-related behaviour in young children with Prader-Willi syndrome: a randomized, double-blind, controlled crossover trial. Clinical endocrinology. 2016;85:979–987. doi: 10.1111/cen.13169. [DOI] [PubMed] [Google Scholar]
- 76.Thienel M, et al. Oxytocin/’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int J Obes. 2016 doi: 10.1038/ijo.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohlsson B, Bjorgell O, Ekberg O, Darwiche G. The oxytocin/vasopressin receptor antagonist atosiban delays the gastric emptying of a semisolid meal compared to saline in human. BMC Gastroenterol. 2006;6:11. doi: 10.1186/1471-230X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borg J, Ohlsson B. Oxytocin prolongs the gastric emptying time in patients with diabetes mellitus and gastroparesis, but does not affect satiety or volume intake in patients with functional dyspepsia. BMC Res Notes. 2012;5:148. doi: 10.1186/1756-0500-5-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borg J, Simren M, Ohlsson B. Oxytocin reduces satiety scores without affecting the volume of nutrient intake or gastric emptying rate in healthy subjects. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23:56–61, e55. doi: 10.1111/j.1365-2982.2010.01599.x. [DOI] [PubMed] [Google Scholar]
- 80.Striepens N, et al. Oxytocin enhances cognitive control of food craving in women. Human Brain Mapping. 2016;37:4276–4285. doi: 10.1002/hbm.23308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herisson FM, et al. Oxytocin Acting in the Nucleus Accumbens Core Decreases Food Intake. J Neuroendocrinol. 2016;28 doi: 10.1111/jne.12381. [DOI] [PubMed] [Google Scholar]
- 82.Petring OU. The effect of oxytocin on basal and pethidine-induced delayed gastric emptying. Br J Clin Pharmacol. 1989;28:329–332. doi: 10.1111/j.1365-2125.1989.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ohlsson B, Ringstrom G, Abrahamsson H, Simren M, Bjornsson ES. Oxytocin stimulates colonic motor activity in healthy women. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16:233–240. doi: 10.1111/j.1365-2982.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 84.Louvel D, et al. Oxytocin increases thresholds of colonic visceral perception in patients with irritable bowel syndrome. Gut. 1996;39:741–747. doi: 10.1136/gut.39.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vila G, et al. Systemic administration of oxytocin reduces basal and lipopolysaccharide-induced ghrelin levels in healthy men. J Endocrinol. 2009;203:175–179. doi: 10.1677/JOE-09-0227. [DOI] [PubMed] [Google Scholar]
- 86.Blevins JE, et al. Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. American journal of physiology Regulatory, integrative and comparative physiology. 2016;310:R640–658. doi: 10.1152/ajpregu.00220.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pischon T, et al. General and abdominal adiposity and risk of death in Europe. The New England journal of medicine. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 88.Stock S, Granstrom L, Backman L, Matthiesen AS, Uvnas-Moberg K. Elevated plasma levels of oxytocin in obese subjects before and after gastric banding. Int J Obes. 1989;13:213–222. [PubMed] [Google Scholar]
- 89.Schorr M, et al. Oxytocin and its relationship to bone mineral density and hip geometry across the weight spectrum. The Journal of clinical endocrinology and metabolism. 2017;102:2814–2824. doi: 10.1210/jc.2016-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawson EA, et al. Oxytocin secretion is related to measures of energy homeostasis in young amenorrheic athletes. The Journal of clinical endocrinology and metabolism. 2014 doi: 10.1210/jc.2013-4136. jc20134136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Szulc P, et al. High serum oxytocin is associated with metabolic syndrome in older men - The MINOS study. Diabetes research and clinical practice. 2016;122:17–27. doi: 10.1016/j.diabres.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 92.Qian W, et al. Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients. The Journal of clinical endocrinology and metabolism. 2014;99:4683–4689. doi: 10.1210/jc.2014-2206. [DOI] [PubMed] [Google Scholar]
- 93.Burt RL, Leake NH, Dannenburg WN. Effect of synthetic oxytocin on plasma nonesterified fatty acids, triglycerides, and blood glucose. Obstetrics and gynecology. 1963;21:708–712. [PubMed] [Google Scholar]
- 94.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. The New England journal of medicine. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 95.Plante E, et al. Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic male db/db mice. Endocrinology. 2015;156:1416–1428. doi: 10.1210/en.2014-1718. [DOI] [PubMed] [Google Scholar]
- 96.Altirriba J, Poher AL, Rohner-Jeanrenaud F. Chronic Oxytocin Administration as a Treatment Against Impaired Leptin Signaling or Leptin Resistance in Obesity. Frontiers in endocrinology. 2015;6:119. doi: 10.3389/fendo.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olszewski PK, Klockars A, Levine AS. Oxytocin and potential benefits for obesity treatment. Curr Opin Endocrinol Diabetes Obes. 2017 doi: 10.1097/MED.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 98.Wheeler E, et al. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nature genetics. 2013;45:513–517. doi: 10.1038/ng.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin A, et al. Cerebrospinal fluid levels of oxytocin in Prader-Willi syndrome: a preliminary report. Biological psychiatry. 1998;44:1349–1352. doi: 10.1016/s0006-3223(98)00190-5. [DOI] [PubMed] [Google Scholar]
- 100.Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. The Journal of clinical endocrinology and metabolism. 1995;80:573–579. doi: 10.1210/jcem.80.2.7852523. [DOI] [PubMed] [Google Scholar]
- 101.Bush NR, et al. Socioeconomic Disparities in Childhood Obesity Risk: Association With an Oxytocin Receptor Polymorphism. JAMA Pediatr. 2017;171:61–67. doi: 10.1001/jamapediatrics.2016.2332. [DOI] [PubMed] [Google Scholar]
- 102.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. Jama. 2014;311:74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bray GA. In: UpToDate. Pi-Sunyer FX, Mulder JE, editors. 1996. [Google Scholar]
- 104.Einfeld SL, et al. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. American journal of medical genetics Part A. 2014;164A:2232–2239. doi: 10.1002/ajmg.a.36653. [DOI] [PubMed] [Google Scholar]
- 105.Bobbioni-Harsch E, et al. Physiological concentrations of oxytocin powerfully stimulate insulin secretionin vitro. Endocrine. 1995;3:55–59. doi: 10.1007/BF02917449. [DOI] [PubMed] [Google Scholar]
- 106.Bjorkstrand E, Eriksson M, Uvnas-Moberg K. Evidence of a peripheral and a central effect of oxytocin on pancreatic hormone release in rats. Neuroendocrinology. 1996;63:377–383. doi: 10.1159/000126978. [DOI] [PubMed] [Google Scholar]
- 107.Maejima Y, et al. Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-Fos induction in limited brain areas. Neuroendocrinology. 2015;101:35–44. doi: 10.1159/000371636. [DOI] [PubMed] [Google Scholar]
- 108.Elabd S, Sabry I. Two Birds with One Stone: Possible Dual-Role of Oxytocin in the Treatment of Diabetes and Osteoporosis. Frontiers in endocrinology. 2015;6:121. doi: 10.3389/fendo.2015.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spellacy WN, Carlson KL, Birk SA. Effect of posterior pituitary hormones on blood glucose and plasma insulin levels in postpartum patients. Obstetrics and gynecology. 1966;28:355–359. [PubMed] [Google Scholar]
- 110.Chiodera P, et al. Effect of pharmacological doses of oxytocin on insulin response to glucose in normal man. Hormone research. 1984;20:150–154. doi: 10.1159/000179988. [DOI] [PubMed] [Google Scholar]
- 111.Paolisso G, et al. Pharmacological doses of oxytocin affect plasma hormone levels modulating glucose homeostasis in normal man. Hormone research. 1988;30:10–16. doi: 10.1159/000181018. [DOI] [PubMed] [Google Scholar]
- 112.Paolisso G, et al. Effects of oxytocin delivery on counter-regulatory hormone response in insulin-dependent (type 1) diabetic subjects. Hormone research. 1989;31:250–255. doi: 10.1159/000181126. [DOI] [PubMed] [Google Scholar]
- 113.Paolisso G, et al. Effects of oxytocin upon the endocrine pancreas secretion and glucose turnover in normal man. Acta endocrinologica. 1990;123:504–510. doi: 10.1530/acta.0.1230504. [DOI] [PubMed] [Google Scholar]
- 114.Klement J, et al. Oxytocin Improves Beta-Cell Responsivity and Glucose Tolerance in Healthy Men. Diabetes. 2016 doi: 10.2337/db16-0569. [DOI] [PubMed] [Google Scholar]
- 115.Kujath AS, et al. Oxytocin levels are lower in premenopausal women with type 1 diabetes mellitus compared with matched controls. Diabetes/metabolism research and reviews. 2015;31:102–112. doi: 10.1002/dmrr.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nieman LK. Cushing’s Syndrome: Update on signs, symptoms and biochemical screening. European journal of endocrinology / European Federation of Endocrine Societies. 2015;173:M33–M38. doi: 10.1530/EJE-15-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bathgate RA, Sernia C. Characterization of vasopressin and oxytocin receptors in an Australian marsupial. J Endocrinol. 1995;144:19–29. doi: 10.1677/joe.0.1440019. [DOI] [PubMed] [Google Scholar]
- 118.Legros JJ, Chiodera P, Geenen V, Smitz S, von Frenckell R. Dose-response relationship between plasma oxytocin and cortisol and adrenocorticotropin concentrations during oxytocin infusion in normal men. The Journal of clinical endocrinology and metabolism. 1984;58:105–109. doi: 10.1210/jcem-58-1-105. [DOI] [PubMed] [Google Scholar]
- 119.Legros JJ, Chiodera P, Demey-Ponsart E. Inhibitory influence of exogenous oxytocin on adrenocorticotropin secretion in normal human subjects. The Journal of clinical endocrinology and metabolism. 1982;55:1035–1039. doi: 10.1210/jcem-55-6-1035. [DOI] [PubMed] [Google Scholar]
- 120.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 121.Linnen AM, Ellenbogen MA, Cardoso C, Joober R. Intranasal oxytocin and salivary cortisol concentrations during social rejection in university students. Stress. 2012;15:393–402. doi: 10.3109/10253890.2011.631154. [DOI] [PubMed] [Google Scholar]
- 122.Cardoso C, Kingdon D, Ellenbogen MA. A meta-analytic review of the impact of intranasal oxytocin administration on cortisol concentrations during laboratory tasks: moderation by method and mental health. Psychoneuroendocrinology. 2014;49:161–170. doi: 10.1016/j.psyneuen.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 123.Page SR, et al. The effect of oxytocin infusion on adenohypophyseal function in man. Clinical endocrinology. 1990;32:307–313. doi: 10.1111/j.1365-2265.1990.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 124.Vallera C, Choi LO, Cha CM, Hong RW. Uterotonic Medications: Oxytocin, Methylergonovine, Carboprost, Misoprostol. Anesthesiol Clin. 2017;35:207–219. doi: 10.1016/j.anclin.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 125.Vankrieken L, Godart A, Thomas K. Oxytocin determination by radioimmunoassay. Gynecol Obstet Invest. 1983;16:180–185. doi: 10.1159/000299248. [DOI] [PubMed] [Google Scholar]
- 126.Fabian M, Forsling ML, Jones JJ, Pryor JS. The clearance and antidiuretic potency of neurohypophysial hormones in man, and their plasma binding and stability. The Journal of physiology. 1969;204:653–668. doi: 10.1113/jphysiol.1969.sp008937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain research. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- 128.Jones PM, Robinson IC. Differential clearance of neurophysin and neurohypophysial peptides from the cerebrospinal fluid in conscious guinea pigs. Neuroendocrinology. 1982;34:297–302. doi: 10.1159/000123316. [DOI] [PubMed] [Google Scholar]
- 129.MacDonald E, et al. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36:1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 130.Nissen E, et al. Different patterns of oxytocin, prolactin but not cortisol release during breastfeeding in women delivered by caesarean section or by the vaginal route. Early Hum Dev. 1996;45:103–118. doi: 10.1016/0378-3782(96)01725-2. [DOI] [PubMed] [Google Scholar]
- 131.Ueda T, Yokoyama Y, Irahara M, Aono T. Influence of psychological stress on suckling-induced pulsatile oxytocin release. Obstetrics and gynecology. 1994;84:259–262. [PubMed] [Google Scholar]
- 132.Fuchs AR, et al. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165:1515–1523. doi: 10.1016/0002-9378(91)90399-c. [DOI] [PubMed] [Google Scholar]
- 133.Baskaran C, et al. Oxytocin Secretion is Pulsatile in Men and is Related to Social-Emotional Functioning. Psychoneuroendocrinology. 2017 doi: 10.1016/j.psyneuen.2017.07.486. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoover RT. Intranasal oxytocin in eighteen hundred patients. A study on its safety as used in a community hospital. Am J Obstet Gynecol. 1971;110:788–794. doi: 10.1016/0002-9378(71)90576-x. [DOI] [PubMed] [Google Scholar]
- 135.Borglin NE. Intranasal administration of oxytocin for induction and stimulation of labour. Acta Obstet Gynecol Scand. 1962;41:238–253. doi: 10.3109/00016346209158101. [DOI] [PubMed] [Google Scholar]
- 136.Okamoto Y, Ishitobi M, Wada Y, Kosaka H. The Potential of Nasal Oxytocin Administration for Remediation of Autism Spectrum Disorders. CNS & neurological disorders drug targets. 2016;15:564–577. doi: 10.2174/1871527315666160413120845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anagnostou E, et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Molecular autism. 2012;3:16. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anagnostou E, et al. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain research. 2014;1580:188–198. doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 139.Tachibana M, et al. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. Journal of child and adolescent psychopharmacology. 2013;23:123–127. doi: 10.1089/cap.2012.0048. [DOI] [PubMed] [Google Scholar]


