Abstract
Small saccades, known as microsaccades, occur frequently during fixation. Several recent studies have argued that a considerable fraction of these movements are present in the traces from one eye only. This claim contrasts with the findings of older reports, which concluded that microsaccades, like larger saccades, are virtually always binocular events. Here we examined the characteristics of small saccades by means of two of the most established high-resolution eye-tracking techniques available. A binocular Dual Purkinje Image eye-tracker was used to record eye movements while observers fixated, with their head immobilized, on markers displayed on a monitor. A specially designed eye-coil system was used to measure eye movements during normal head-free viewing, while subjects fixated on markers at various distances. Monocular microsaccades were virtually absent in both datasets. In the head-fixed data, not a single monocular microsaccade was observed. In the head-free data, only one event appeared to be monocular out of more than a thousand saccades. Monocular microsaccades do not seem to occur during normal head-free or head-immobilized fixation.
Keywords: microsaccades, ocular drift, fixational eye movements, visual fixation
Introduction
Much research has focused on the characteristics and functions of microsaccades, the miniature replicas of saccadic eye movements that, unlike their larger counterparts, maintain the fixated stimulus within the foveola (Kowler, 2011; Poletti & Rucci, 2013; Rolfs, 2009). While considerable progress has been made during the last decade on the resolution of classical debates, one specific issue that has remained controversial is the frequency of monocular microsaccades.
Classical studies concluded that microsaccades are conjugate movements (Ciuffreda & Tannen, 1995; Krauskopf, Cornsweet, & Riggs, 1960; Schulz, 1984). For example, by using the high-resolution optical lens lever method, Krauskopf et al. (1960) reported that more than 98% of saccades greater than 1 arcmin possess highly correlated directions and amplitudes in the two eyes. More recent studies, however, reported that monocular microsaccades occur frequently, representing a sizable fraction of the total number of microsaccades, from approximately 12% to 40% (Engbert & Kliegl, 2003a; Gautier, Bedell, Siderov, & Waugh, 2016; Kloke, Jaschinski, & Jainta, 2009; Martinez-Conde, Macknik, Troncoso, & Dyar, 2006; Valsecchi & Gegenfurtner, 2015). These high numbers of monocular microsaccades are surprising not only because they contradict earlier studies, but also in light of the recent observation that microsaccades serve a similar gaze-centering explorative function as larger saccades (Rucci & Poletti, 2015). Using new methods to determine the location of the line of sight in the scene (a vexing problem in oculomotor research), recent studies have shown that in high-acuity tasks microsaccades precisely relocate a preferred retinal locus of fixation according to the ongoing demands of the task (Poletti, Listorti, & Rucci, 2013; Stevenson & Roorda, 2005). Thus, while it is conceivable that specific alignments of stimuli in depth may trigger monocular microsaccades, one would not expect to see them in experiments in which subjects fixate stimuli in front of them, especially on flat displays.
The previous studies that reached contrasting conclusions differed in many ways, particularly on the methods for recording and analyzing eye movements. Whereas older studies employed a variety of recording techniques, more recent experiments have almost exclusively relied on video-based eye-trackers. These systems are minimally invasive and easy to operate, but they have also raised concerns on whether they are sufficiently sensitive to accurately measure the smallest eye movements (Choe, Blake, & Lee, 2014; Collewijn & Kowler, 2008; Drewes, Zhu, Hu, & Hu, 2014; Nyström, Andersson, Holmqvist, & van de Weijer, 2013; Wildenmann & Schaeffel, 2013; Wyatt, 2010). Furthermore, with some of these systems, more sophisticated algorithms for data analysis may be needed to properly handle the higher level of noise intrinsic in the oculomotor data (Mihali, van Opheusden, & Ma, 2017; Nyström, Anderson, Niehorster, & Hooge, 2017). Because of these concerns, some authors have discarded monocular events in their analyses and only relied on binocular microsaccades (Engbert & Kliegl, 2003a; Engbert & Kliegl, 2004; Otero-Millan, Troncoso, Macknik, Serrano-Pedraza, & Martinez-Conde, 2008). However, other studies did not and have, sometimes, attributed important visual functions to these events (e.g., Gautier et al., 2016), raising the question of whether they represent genuine eye movements (Collewijn & Kowler, 2008).
The purpose of this study is to reexamine the occurrence of monocular microsaccades. Using two among the most established high-resolution recording methods, a Dual Purkinje Image eye-tracker and eye coils, we analyze the characteristics of microsaccades performed while fixating on a stationary dot, either with the head immobilized or free to move normally. We report an almost complete absence of monocular microsaccades in our data.
Methods
The results presented here are based on two sets of binocular recordings collected using two different systems: a Dual Purkinje Image (DPI) eye-tracker (Crane & Steele, 1985) and the Revolving Field Monitor, a specially designed eye-coil apparatus (Epelboim et al., 1997; Steinman, Kowler, & Collewijn, 1990). Both datasets were previously collected for purposes unrelated to this study and reanalyzed here to specifically examine the binocular characteristics of microsaccades. The procedures and methods for data collection and analysis have already been described in detail in previous publications and are only briefly summarized here. Further methodological details can be found in Poletti et al. (2015) for the DPI data and in Epelboim et al. (1997) for the coil data.
Subjects and tasks
A total of seven subjects participated in two experiments. Three observers (age range 23–40) took part in the DPI recordings conducted at Boston University, and four other subjects (age range 30–70) participated in eye-coil recordings conducted at the University of Maryland. In both cases, subjects were seated in a normally illuminated room and sequentially fixated on a series of tiny high-contrast markers. In the DPI experiments, the stimuli were small crosses (27′ × 2′ bars) displayed on a CRT monitor at a refresh rate of 200 Hz. The head of the subject was immobilized by both a custom-imprint bite-bar and a headrest. In the experiments with eye coils, the fixation targets were small LEDs at distances between 50 and 60 cm from the observer, each covering a visual angle of approximately 15′. The head of the observer was free to move normally. Experiments followed the ethical procedures approved by the University of Maryland and the Charles River Campus Institutional Review Board at Boston University.
Apparatus
Head-fixed data were collected by means of a binocular Generation 6 Dual Purkinje Image (DPI) eye-tracker (Fourward Technologies) and sampled at 1 KHz. The DPI eye-tracker compares the motion of the first and fourth Purkinje images of an infrared beam. It has an internal time delay of ∼0.25 ms and root mean square noise level—measured by means of an artificial eye—of less than 20″ (Crane & Steele, 1985), yielding a resolution of approximately 1′ (Ko, Snodderly, & Poletti, 2016).
Head-free data were collected by means of the Revolving Field Monitor (RFM), a unique system developed by Dr. Steinman and collaborators at the University of Maryland (Epelboim et al., 1997). This system uses magnetic fields rotating at different frequencies on three orthogonal planes to resolve subarcminute changes in coil orientations without requiring immobilization of the head. Subjects wore 2D coils (no torsion) in both eyes. Two additional coils provided the head orientation. Eye movements relative to the head were reconstructed from both the eye and head coil signals, sampled at 488 Hz, as described in detail in Aytekin, Victor, and Rucci (2014).
Data analysis
Only periods with optimal, uninterrupted tracking and no blinks were selected for data analysis. Traces from each individual eye were first processed independently and then compared to assess binocularity. To measure velocity, horizontal and vertical eye traces were numerically differentiated by means of a third-order Savitzky-Golay filter with cut-off frequency of approximately 30 Hz (Cherici, Kuang, Poletti, & Rucci, 2012). Eye movements with peak speed higher than a predefined threshold (5°/s in Figure 3, 10°/s in Figures 2 and 5, and 15°/s in Figure 4) were selected as possible saccadic events. Because the speed of intersaccadic eye motion is considerably higher when the head is not restrained (Poletti, Aytekin, & Rucci, 2015; Skavenski, Hansen, Steinman, & Winterson, 1979), we used higher thresholds when analyzing the head-free eye-coil data.
Figure 3.
Effects of using less stringent criteria for classifying head-fixed DPI traces. The same data as in Figure 2 are here reanalyzed using a lower speed threshold: 5°/s. Note that also the three events with peak speed higher than threshold in one eye only are binocular. Graphic conventions are as in Figure 2.
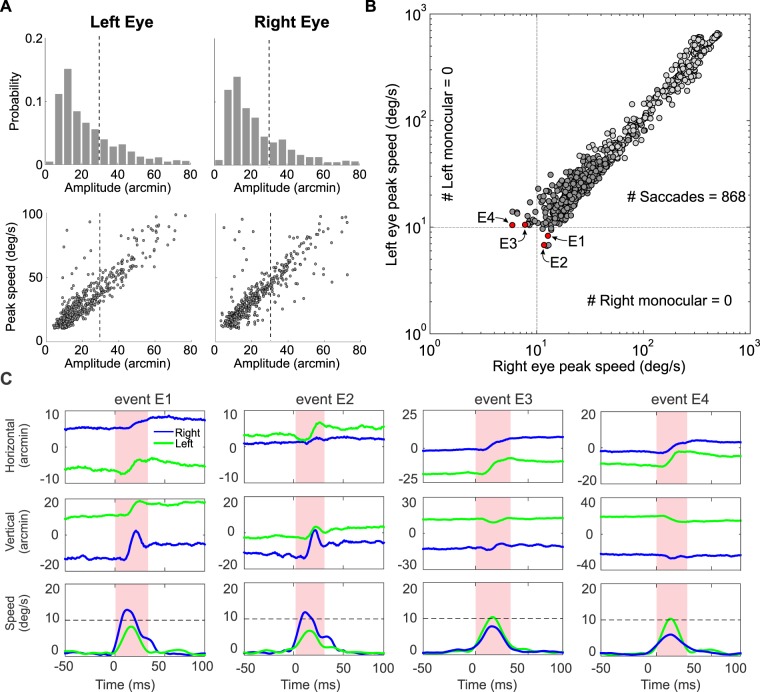
Figure 2.
Saccade characteristics during head-fixed fixation. Data were recorded by means of a binocular DPI eye-tracker. To be labeled as a saccade or microsaccade, an oculomotor event needs to be faster than 10°/s in at least one eye. (A) Distributions of saccadic amplitudes (top) and the main sequences (bottom) in both eyes (left and right columns). Panels zoom in on the range of small saccades. The dashed vertical lines mark the 30′ amplitude threshold used to define microsaccades. (B) Comparison between peak speeds in the two eyes. Every saccade and microsaccade is represented by a circle at coordinates given by the highest speed measured in the two eyes within the event's interval. Darker circles represent microsaccades. Dashed lines mark the speed threshold. (C) Examples of microsaccades with peak speed higher than 10°/s in one eye only. Different events (red circles in B) are displayed in different columns. For each event, the horizontal and vertical traces in both eyes (top graphs) are shown together with the instantaneous eye speed (bottom). The pink-shaded region marks the event's duration. The dashed horizontal line represents the saccade speed threshold.
Figure 5.
Consequences of using less stringent criteria for classifying head-free oculomotor traces. The same data as in Figure 4 are here reanalyzed using a lower speed threshold: 10°/s. Graphic conventions are as in Figure 2. Event E1 (red circle in A) is the only monocular microsaccade found in our database.
Figure 4.
Saccade characteristics during normal head-free fixation. Data collected by means of the Revolving Field Monitor are displayed as in Figure 2. To be labeled as a saccade or microsaccade, an oculomotor event needs to possess peak speed greater than 15°/s in at least one eye.
Saccade amplitude was defined as the modulus of the vector connecting the two locations at which eye speed became greater (saccade onset) and lower (saccade offset) than 3°/s in the DPI data and 5°/s in the coil data. Consecutive events closer than 15 ms were merged together into a single saccade, a method which automatically excluded postsaccadic overshoots in the DPI data (Mostofi, Boi, & Rucci, 2016). These transients result from the movement of the lens and, possibly, the damping of the eye-tracker (Deubel & Bridgeman, 1995; Stevenson & Roorda, 2005). Events with duration smaller than 10 ms and longer than 300 ms were discarded as artifacts.
For every saccade detected in the traces from one eye, we examined whether a corresponding event simultaneously occurred in the other eye. If the speed of the other eye also went over the saccade threshold within the considered interval, the saccade was directly labeled as binocular, and the earliest onset and latest offset times in the two eyes were taken as onset and offset of the binocular event, respectively (see Figure 1). If the speed of the other eye remained below threshold, the event was placed in the pool of potentially monocular microsaccades and further inspected. Its onset and offset times were those given by the traces in which the saccade was detected. Every detected saccade is plotted in Figures 2 through 7 as a point with 2D coordinates given by the highest instantaneous speeds in the onset-offset intervals in the two traces.
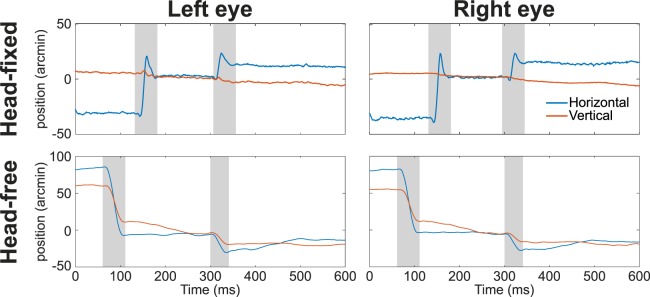
Figure 1.
Examples of oculomotor traces. (Top) Horizontal (blue line) and vertical (red) movements recorded by means of a Dual Purkinje Image eye-tracker when the head of the observer was immobilized. (Bottom) Measurements obtained with the Revolving Field Monitor, a high-resolution coil-based system that does not require immobilization of the head. Data from the left and right eyes are shown on separate columns. Shaded segments mark periods of microsaccades and small saccades.
Figure 7.
Characteristics of microsaccades detected with two different eye-trackers. (A) DPI data; (B) Data collected with a binocular EyeLink 1000 Plus. The same observer maintained fixation in both cases. The two data sets were processed identically, with a saccade speed detection threshold of 10°s.
All the events in which a saccade was detected in one eye only were carefully examined. With the exception of one single instance (see Figure 5), all these events turned out to be cases in which the saccade occurred in both eyes, but its speed remained slightly below the selected threshold in one eye (see examples in Figures 2C, 3B, 4C, and 5B). As our data show (Figure 2B, 3A, 4B, and 5A), all of them would be correctly classified as binocular if, to tolerate small differences between the peak velocities in the two eyes, we allowed for a slightly lower saccade threshold in the other eye—but still significantly above the range of values normally encountered during intersaccadic fixation— in correspondence of each detected saccade.
Figure 6 shows results obtained when the data acquired by means of the DPI eye-tracker were processed as in Gautier et al. (2016). In this method, microsaccades are defined by the intervals in which the eye speed exceeds by at least 6 SDs and for a minimum duration of 12 ms the median eye speed measured over a 2-s interval. Figure 7 compares data from the DPI eye-tracker to data from a video-based system, a binocular EyeLink 1000 Plus. The same observer repeated the fixation task described above in both cases. The EyeLink data were recorded at 1 KHz using the center of mass algorithm for pupil tracking with both heuristic filters turned off. The observer's head was immobilized by the eye-tracker's standard chin and forehead rests.
Figure 6.
Dependence on the saccade detection algorithm. The same DPI data as in Figure 2 here reanalyzed using the algorithm proposed by Gautier et al. (2016). (A) Green and blue circles represent events classified by the algorithm as monocular in the left and right eye, respectively. Binocular events are represented by red circles. (B) Examples of the events labeled by the algorithm as monocular microsaccades. Graphic conventions are as in Figure 2.
Results
We examined the characteristics of the saccades performed by human observers, as they sequentially fixated on markers at various positions. In two separate experiments, eye movements were recorded by means of different methods, both known for their precision and reliability.
In the following sections, we first report data recorded with the Dual Purkinje Image (DPI) eye-tracker, a well-tested device that compares the positions of the first and fourth Purkinje images of an infrared beam. To reach high precision, this system requires the head of the observer to be immobilized. We then analyze data collected with the Revolving Field Monitor (RFM), to our knowledge the only coil system capable of performing high-resolution measurements without requiring immobilization of the head (Epelboim et al., 1997). Examples of the oculomotor traces recorded with these two devices are shown in Figure 1. We conclude by briefly examining possible sources of discrepancy with other reports.
In keeping with our previous studies (Poletti & Rucci, 2016; Rucci & Poletti, 2015), we will restrict use of the term microsaccade to saccades with amplitude smaller than 30′. These are the movements that give more than a 50% overlap between pre- and postsaccadic images in the foveola. Note, however, that our results do not depend on this definition, and in the following we show results for all saccades independent of their amplitudes.
Head-fixed DPI data
In this experiment, subjects looked at fixation markers (small crosses composed of 27′-long and 2′-wide arms) at several locations on a CRT display. Observers were only instructed to fixate carefully on each marker and left free to select the order and duration of each fixation. The movements of both eyes were recorded by means of a binocular DPI eye-tracker, while the observer's head was immobilized by means of a head-rest and a custom dental-imprint bite bar, as it is common with this device.
The characteristics of the saccades recorded in this experiment are shown in Figures 2 and 3. In Figure 2, saccades were detected using a speed threshold of 10°/s, a relatively conservative value for head-fixed data, a condition in which the speed of intersaccadic fixation is normally below 1°/s. Figure 3 shows results obtained with a lower saccade threshold, 5°/s. In agreement with previous studies with tasks that do not require fine visual judgments nor prolonged fixation (Kowler, 2011), microsaccades were not too frequent in this experiment. Across the three observers, the average rate and standard deviation of saccades smaller than 30′ were 0.3 ± 0.03 events/s, yielding a total of 421 microsaccades over the three observers.
As shown by Figure 2A, most small saccades were within the range of microsaccade amplitude. Importantly, comparison between the two eyes suggests strong binocularity, as saccadic displacements were highly correlated both in amplitude (ρ = 0.95; p < 0.0001) and direction (ρ = 0.95; p < 0.0001) in the two eyes. Furthermore, the relations between saccade peak speed and amplitude (the main sequences) were also similar in the two eyes, with comparable regression slopes: 79 and 89 s–1 in the right and left eyes, respectively.
Analysis of binocularity can be graphically summarized by comparing the saccadic peak speeds in the two eyes (Figure 2B). In this graph, every detected saccade is represented by a dot at coordinates given by the highest instantaneous speeds measured during the saccade interval in the left (y axis) and right eye (x axis). As shown by these data, peak speeds were very highly correlated in the two eyes (ρ = 0.98; p < 0.00001), even for microsaccades (ρ = 0.86; p < 0.00001). In fact, all detected saccades, independent of their amplitudes, possessed corresponding events in the traces of both eyes: Whenever a saccade or microsaccades was detected in one eye, a saccadic event with similar speed was also simultaneously present in the other eye. Note that also all the events that surpassed the peak speed threshold in one eye only (27 events out of 421 microsaccades) were binocular. These were all cases in which the other eye also performed a saccade, but its speed remained slightly below the 10°/s threshold, as shown by the examples in Figure 2C. They were all correctly classified as binocular microsaccades when we used a lower speed threshold (see Figure 3A). In sum, all saccades were binocular events: Not a single monocular saccade or microsaccade was present under these standard conditions of data analysis.
The analyses in Figure 2B were conducted using a conservative method for detecting saccades: The speed threshold of 10°/s is much higher than the range of eye speeds normally encountered during fixation (typically, around 1°/s; e.g., Cherici et al., 2012), ruling out the possibility for false alarms. This threshold still enables detection of saccades with very small amplitudes, as shown by the considerable fraction of microsaccades with amplitude smaller than 20′ in Figure 2A. Thus, one would expect monocular microsaccades—if present—to be revealed by the peak-to-peak speed analysis of Figure 2B. Given the complete absence of monocular microsaccades in our data, we questioned whether these events were missed by our approach, and repeated the analyses using a lower speed threshold for detecting saccades and microsaccades.
Figure 3 shows the results obtained with a saccade speed threshold of 5°/s. As the threshold for detecting saccadic events gets closer to the range of speeds normally encountered during intersaccadic fixation, the risk for false alarms obviously increases. The speed of 5°/s is still a safe value to use with head-fixed data, as it remains considerably higher than normal drift speed. As expected, lowering the threshold resulted in larger numbers of oculomotor events being classified as microsaccades. However, these additional events followed the same rules as those already shown in Figure 2. Again, no monocular saccade or microsaccade was detected. All the previous events of Figure 2B in which one eye remained below threshold were now correctly labeled as binocular. Even the three events in which the speed of one eye was lower than 5°/s were genuine binocular microsaccades, as shown in Figure 3A. Thus, within the entire set of data collected with the DPI eye-tracker, we could not find a single instance of a monocular microsaccade.
Head-free eye coil data
To precisely measure eye movements, the data in Figures 2 and 3 were collected while the subject's head was immobilized. This is a requirement of the DPI eye-tracker, which otherwise would partially confound the motion of the Purkinje images caused by translations of the head with eye rotations. We wondered whether immobilization of the head contributed to our failure to observe monocular microsaccades. Perhaps, these eye movements tend to be more frequent when the head is allowed to move normally. For this reason, we examined data collected by means of a unique device, the Revolving Field Monitor, a system that enables high-resolution oculomotor measurements without constraints on head movements.
In this experiment, subjects sequentially looked at fixation markers (small LEDs) placed on a table in front of them. Rotations of the eyes and head were measured simultaneously by means of coils, and rotations of the eyes relative to the head were reconstructed in a Fick's reference frame via coordinate transformations, as previously described (Aytekin et al., 2014). It is known that the speed of intersaccadic fixation increases considerably when the head is left free to move normally (Skavenski, 1979), as smooth fixational eye movements partly compensate for small head movements (Poletti et al., 2015). For this reason, a higher speed threshold is necessary to reliably detect saccades during normal head-free fixation. Figures 4 and 5 show the characteristics of the saccades detected using speed thresholds of 15°/s and 10°/s, respectively.
As reported by previous studies, saccade characteristics during normal head-free fixation differed to some extent from those measured with the head immobilized. In keeping with previous reports (Malinov, Epelboim, Herst, & Steinman, 2000), the amplitude of small saccades was now slightly larger (average amplitude within the range of saccades up to 80′: 44′ ± 17′ in the RFM data vs. 24′ ± 16′ in the DPI data), so that microsaccades constituted a smaller fraction of the total number of detected saccades (only 14% in the RFM data vs. 48% in the DPI data). Furthermore, the peak speeds of microsaccades were also lower than those measured in the head-fixed experiments (cf. Figure 4A and Figure 2A). This deviation was likely caused not just by the higher ages—and, consequently, slower dynamics (Dowiasch, Marx, Einhuser, & Bremmer, 2015)—of the participants in the eye-coil group, but also by important differences in the two eye-tracking techniques. Unlike eye-coil data, signals from the DPI eye-trackers are affected by the wobbling of the lens and its nasal-temporal asymmetry (Artal & Tabernero, 2014; Crane & Steele, 1978; He, Donnelly, Stevenson, & Glasser, 2010; Schultz, Sinnott, Mutti, & Bailey, 2009). Together with the damping of the eye-tracker motors, lens movements contribute to postsaccadic overshoots (Deubel & Bridgeman, 1995; Stevenson & Roorda, 2005). Furthermore, it has been reported that eye coils affect the dynamics of eye movements (Frens & der Geest, 2002). All these effects likely contributed to the different slopes of the regression lines in Figures 2A and 4A.
Despite these differences, comparison of saccadic characteristics between the two eyes again suggests strong binocularity. Both the amplitude (ρ = 0.95; p < 0.0001) and directions (ρ = 0.58; p < 0.0001) of small saccades were highly correlated in the two eyes. Furthermore, the main sequences were similar in the two eyes, with virtually identical regression slopes (39 s−1 in both eyes). Analysis of the peak speed in the two eyes at the time of saccades—the same analysis of Figure 2B for head-free data—revealed a high correlation (ρ = 0.95; p < 0.0001). Again, out of a total of 1,153 saccades, not one monocular microsaccade was found. In almost all events, whenever the instantaneous oculomotor speed exceeded the threshold in one eye, it did so also in the other eye. A few events possessed speed that remained slightly below threshold in one eye, as shown in Figure 4B. But even in these cases, a saccade occurred simultaneously in both eyes, as shown by the example traces in Figure 4C. All these events were correctly classified as binocular microsaccades using a lower speed threshold (Figure 5A).
As with the DPI data, we also examined the impact of lowering the speed threshold for detecting microsaccades. Figure 5 summarizes the results obtained with a speed threshold of 10°/s. Use of this lower threshold unveiled one oculomotor event that appeared to be correctly labeled as a monocular microsaccade (Figure 5A). The horizontal and vertical traces corresponding to this event, marked by the red circle in Figure 5A, are plotted in Figure 5B (Event E1). They show a small diagonal gaze shift in the right eye, which far exceeds the speed threshold. Although a small vertical change in trajectory also appears in the trace from the left eye, this movement is much slower and smaller, so that classification of this event as a monocular microsaccade seems appropriate. There were other rare events (seven out of 171 microsaccades) in which the instantaneous speed in one eye remained slightly below 10°/s (Figure 5A), but they were all caused by the normal variability in the saccadic speed of the two eyes. They all contained saccades in both eyes, as shown by the examples E2 and E3 in Figure 5B.
Further relaxing the speed criterion for detecting saccadic events is problematic in the conditions of this experiment, as the threshold would get very close to the normal drift speed encountered under head-free fixation (typically ∼3°/s, reaching speeds as high as 5°/s; e.g., figure 6 in Aytekin et al., 2014), thus greatly increasing the risk for false alarms in the detection of microsaccades. In fact, a saccade speed threshold of only 5°/s resulted in a very large number of events being flagged as potentially monocular microsaccades, all of which were, however, discarded as false alarms upon visual inspection of the individual traces. They were all generated by smooth changes in eye positions, which transiently exceeded the speed threshold in one eye but not in the other. Not a single event was manually confirmed as a monocular microsaccade beyond the one already reported in Figure 5.
Potential sources of discrepancy
The data in Figures 2 through 5 show that our two high-resolution oculomotor databases practically do not contain monocular microsaccades. Even the smallest saccades are binocular, like the larger ones. These results contrast with the relatively high rates of monocular microsaccades reported by other studies. Although a detailed investigation of the causes of such discrepancy is beyond the scope our study, it is worth mentioning two factors that may have contributed to these differences.
The first factor is the method for detecting saccadic events. Our results are based on a simple speed-thresholding algorithm, a method that works very effectively with low-noise signals, as the ones analyzed here. Other studies have used different approaches, some of which tune their parameters adaptively. As examined in detail by Nyström et al. (2017), one has to be particularly careful with algorithms that automatically adjust thresholds for each eye independently. In the presence of relatively high levels of noise, these algorithms could end up with significantly different thresholds in the two eyes, increasing the possibility for false alarms in the detection of monocular events.
Figure 6 shows an example of application of one of such algorithms (the method proposed by Gautier et al., 2016) to our DPI data. In striking contrast with the analyses of Figures 2 and 3, this method resulted in 74 events being flagged as monocular microsaccades. Manual inspection of each of these events confirmed that they were artifacts caused by the different speed thresholds in the two eyes. Since the algorithm processed the traces from each eye with separate parameters, it occasionally occurred that the peak speed of a binocular saccade reached threshold in one of the two eyes only (see examples in Figure 6B). Paradoxically, in some instances, a substantial difference between left and right thresholds caused a saccade to go undetected in the eye that actually moved faster (e.g., events E1 and E2).
Another important factor in the categorization of microsaccades is the level of noise in the oculomotor signals. We specifically focused on recordings obtained with methods that are known to provide high signal-to-noise ratios. All previous studies that reported high numbers of monocular microsaccades were based on recordings obtained with video-based eye-trackers. These systems appear to possess higher levels of noise, and their reliability in measuring very small eye movements has been questioned.
To provide an example, Figure 7 compares the oculomotor data recorded by a video-based system (EyeLink 1000 Plus) to those of the DPI eye-tracker. Data were collected from the same observer engaged in the same fixation task and were analyzed following identical procedures (a speed detection threshold of 10°/s). Yet results differed considerably. In contrast with the DPI data, a large fraction of the events detected in the EyeLink data were flagged as monocular. As shown by the examples in Figure 7C, these events appear to be the outcome of noise. Thus, a common video-based eyetracker seems more prone than the DPI to signaling monocular events, and further attention is required in the phase of data analysis.
Discussion
Do monocular microsaccades really occur? Previous investigations of this question have reached conflicting conclusions. Here, we specifically examined the characteristics of the microsaccades recorded by means of the two most established methods for measuring very small eye movements: (a) differential tracking of the Purkinje images; and (b) magnetic induction eye-coils. In the fixation data from seven observers, over a total of 2,084 saccadic events, 607 of which smaller than 30′, we only found one monocular microsaccade. Thus, genuine monocular microsaccades seem to be exceedingly rare events.
In the last two decades, the availability of simple-to-use noninvasive eye-trackers has spurred a new wave of interest in the study of microsaccades, the small saccades that maintain the fixated stimulus within the high-acuity foveola. Several laboratories have now observed sizable fractions of monocular saccades—events only present in the traces from one eye—with rates ranging from 10% to up to 60% of the pool of saccades smaller than 1°–2°. As pointed out by Collewijn and Kowler (2008), these numbers appear suspiciously high. They not only conflict with the characteristics of larger saccades and with the results of older studies on microsaccades (Ciuffreda & Tannen, 1995; Krauskopf et al., 1960; Schulz, 1984), but they also seem to be at odds with the recent conclusions that these small movements act like their larger counterparts both in their exploratory functions (Ko, Poletti, & Rucci, 2010; Poletti et al., 2013) and adaptive changes (Havermann, Cherici, Rucci, & Lappe, 2014).
These concerns are reinforced by current knowledge on the neural substrate responsible for generating microsaccades (Hafed, 2011). Although much still needs to be learned about the mechanisms of microsaccade production, recent research has pointed at an important role of neural activity in the rostral superior colliculus in triggering microsaccades (Hafed, Goffart, & Krauzlis, 2009; Hafed & Krauzlis, 2012). The superior colliculus provides excitatory input to the saccadic burst generator and is believed to play a major role in determining conjugate saccade amplitude and direction (Iwamoto & Kaku, 2010). As is the case for larger saccades, saccadic burst neurons are active during microsaccades (Brien, Corneil, Fecteau, Bell, & Munoz, 2009; Gisbergen, Robinson, & Gielen, 1981; Horn & Cullen, 2012). It has been recently observed that premotor saccadic burst neurons encode the microsaccade size, duration, and velocity of an individual eye, thus also enabling disconjugate movements in the two eyes (Horn & Cullen, 2012). In sum, as it is the case for larger saccades, it is conceivable that specific conditions exist, with stimuli properly aligned in space, which could elicit monocular microsaccades. However, one would not expect to see them under the typical conditions of vision research experiments, in which subjects fixate on targets presented in front of them on flat displays.
The results of our study are in agreement with this general intuition. In the DPI recordings, in which subjects fixated at markers on a monitor, we did not find a single microsaccade that could be classified as monocular, not even when we lowered the speed threshold to just 5°/s. Although studies that used video-based eye-trackers have observed monocular microsaccades both with the head immobilized (Engbert & Kliegl, 2003b; Kloke et al., 2009; Martinez-Conde, 2006) and free to move (Valsecchi & Gegenfurtner, 2015), we wondered whether head immobilization contributed to our failure to find monocular microsaccades. Monocular microsaccades could, perhaps, be responsible for correcting for small vergence errors, or they may be associated with changes in accommodation during normal head-free fixation of objects in depth. For this reason, in a second experiment, we examined oculomotor data collected by means of a specially designed coil system (Steinman et al., 1990), as observers fixated on markers at various distances with their head free to move normally. Again, monocular microsaccades were virtually absent: Only one event out of 1,201 saccades and microsaccades was classified as monocular.
It is difficult to reconcile these findings with those of the recent studies arguing for high rates of monocular microsaccades. Since most events labeled as monocular microsaccades by these studies tend to have very small amplitudes (see, for example, Gautier et al., 2016), a possible source of discrepancy could be individual judgment variability among human experts in classifying eye movements data. At very low velocities and displacements, the distinction between smooth and saccadic movements becomes difficult. Use of the main sequence for guidance in categorization also loses its utility, as all slow/small movements are obviously close to the regression line. It is known that correlation in the drifts of the two eyes is far from perfect during head-free fixation and that the two eyes drift almost independently when the head is immobilized (Poletti et al., 2015). Thus, some researchers may interpret brief periods of faster drift in one eye as monocular microsaccades. However, creation of a new category of eye movements at the smallest scale, where categorization is most challenging, seems unwarranted. At slightly larger amplitudes and speeds, where no ambiguity exists between different types of eye movements, saccades and microsaccades are clearly binocular events.
Another possibility is that monocular microsaccades are actually the outcome of classification errors. Even in our own DPI data, spurious detection of monocular microsaccades occurs frequently when using one of the algorithms employed by a recent report, raising further doubts on the accuracy of this method (see Nyström et al., 2017, for a detailed analysis of this issue). Furthermore, many monocular events were present in the data acquired by a video-based eye-tracker, even though data recorded by means of the DPI eye-tracker with the same observer and task did not contain any monocular microsaccade. Visual inspection of these traces is consistent with the conclusion that monocular events are the consequence of measurement noise, which is larger in video-based eye-trackers than in the systems used in our study (Holmqvist et al., 2011).
In the presence of low levels of noise, saccades can be reliably detected by a speed thresholding algorithm. The threshold obviously needs to be safely higher than the range of intersaccadic drift speed to avoid false alarms. Thus, a higher saccade threshold is recommended during normal head-free fixation, a condition in which ocular drift tends to be considerably faster (Poletti et al., 2015; Skavenski et al., 1997). As long as the saccade threshold is sufficiently high, this simple method works accurately, yielding very few detections of potentially monocular events (three events in Figure 3 and seven in Figure 5). Note that in both our datasets, all these events are correctly classified automatically as binocular, if, in correspondence of a saccade in one eye, one allows for a slightly lower threshold in the other eye, to tolerate small differences between the peak velocities in the two eyes.
In sum, our results support the conclusion that microsaccades are binocular events. We do not argue against the possibility that conditions may exist, with proper alignment of objects in depth, which could lead to small saccadic displacements in one eye only. However, monocular microsaccades do not seem to occur during normal fixation with or without head immobilization.
Acknowledgments
This work was supported by National Institutes of Health grant EY18363 and National Science Foundation grants 1457238, 1420212, and 1534932. We thank Robert M. Steinman for making data collected with the Maryland Revolving Field Monitor, which was supported by the Air Force Office of Scientific Research, available for analysis.
Commercial relationships: none.
Corresponding author: Michele Rucci.
Email: mrucci@bu.edu.
Address: Center for Visual Science, and Department of Brain and Cognitive Sciences, University of Rochester, Rochester, NY, USA.
References
- Artal, P., Tabernero, J.. (2014). Lens oscillations in the human eye. Implications for post-saccadic suppression of vision. PLoS One, 9 4, e95764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytekin, M., Victor, J. D., Rucci, M.. (2014). The visual input to the retina during natural head-free fixation. The Journal of Neuroscience, 34 38, 12701– 12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien, D., Corneil, B., Fecteau, J., Bell, A., Munoz, D.. (2009). The behavioural and neurophysiological modulation of microsaccades in monkeys. Journal of Eye Movement Research, 3 2, 1– 12. [Google Scholar]
- Cherici, C., Kuang, X., Poletti, M., Rucci, M.. (2012). Precision of sustained fixation in trained and untrained observers. Journal of Vision, 12 6: 31, 1– 16, https://doi.org/10.1167/12.6.31. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe, K. W., Blake, R., Lee, S.-H.. (2014). Correcting video-based eye tracking signals for pupil size artifacts. Journal of Vision, 14 10: 754, https://doi.org/10.1167/14.10.754. [Abstract] [Google Scholar]
- Ciuffreda, K. L., Tannen, B.. (1995). Eye movement basics for the clinician. St. Louis, MO: Mosby. [Google Scholar]
- Collewijn, H., Kowler, E.. (2008). The significance of microsaccades for vision and oculomotor control. Journal of Vision, 8 14: 20, 1– 21, https://doi.org/10.1167/8.14.20. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane, H., Steele, C.. (1978). Accurate 3-dimensional eye tracker. Applied Optics, 17, 691– 705. [DOI] [PubMed] [Google Scholar]
- Crane, H. D., Steele, C.. (1985). Generation V Dual Purkinje-Image eyetracker. Applied Optics, 24 4, 527– 537. [DOI] [PubMed] [Google Scholar]
- Deubel, H., Bridgeman, B.. (1995). Fourth purkinje image signals reveal eye-lens deviations and retinal image distortions during saccades. Vision Research, 35, 529– 538. [DOI] [PubMed] [Google Scholar]
- Dowiasch, S., Marx, S., Einhuser, W., Bremmer, F.. (2015). Effects of aging on eye movements in the real world. Frontiers in Human Neuroscience, 9, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes, J., Zhu, W., Hu, Y., Hu, X.. (2014). Smaller is better: Drift in gaze measurements due to pupil dynamics. PLoS One, 9, e111197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert, R., Kliegl, R.. (2003a). Binocular coordination in microsaccades. Radach, R., Hyona, J., Deubel, H., Bouma, H., Bouwhuis, D.. (Eds.), The mind's eye: Cognitive and applied aspects of eye movement research. Amsterdam, the Netherlands: Elsevier Science BV. [Google Scholar]
- Engbert, R., Kliegl, R.. (2003b). Microsaccades uncover the orientation of covert attention. Vision Research, 43 9, 1035– 1045. [DOI] [PubMed] [Google Scholar]
- Engbert, R., Kliegl, R.. (2004). Microsaccades keep the eyes' balance during fixation. Psychological Science, 15, 431– 436. [DOI] [PubMed] [Google Scholar]
- Epelboim, J., Steinman, R. M., Kowler, E., Pizlo, Z., Erkelens, C. J., Collewijn, H.. (1997). Gaze-shift dynamics in two kinds of sequential looking tasks. Vision Research, 37, 2597– 2607. [DOI] [PubMed] [Google Scholar]
- Frens, M., der Geest, J. V.. (2002). Scleral search coils influence saccade dynamics. Journal of Neurophysiology, 88, 692– 698. [DOI] [PubMed] [Google Scholar]
- Gautier, J., Bedell, H. E., Siderov, J., Waugh, S. J.. (2016). Monocular microsaccades are visual-task related. Journal of Vision, 16 3: 37, 1– 16, https://doi.org/10.1167/16.3.37. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Gisbergen, J. V., Robinson, D., Gielen, S.. (1981). A quantitative analysis of generation of saccadic eye movements by burst neurons. Journal of Neurophysiology, 45 3, 417– 442. [DOI] [PubMed] [Google Scholar]
- Hafed, Z. (2011). Mechanisms for generating and compensating for the smallest possible saccades. The Journal of Neuroscience, 35, 2101– 2113. [DOI] [PubMed] [Google Scholar]
- Hafed, Z. M., Goffart, L., Krauzlis, R. J.. (2009, February 13) A neural mechanism for microsaccade generation in the primate superior colliculus. Science, 323 5916, 940– 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed, Z. M., Krauzlis, R. J.. (2012). Similarity of superior colliculus involvement in microsaccade and saccade generation. Journal of Neurophysiology, 107 7, 1904– 1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havermann, K., Cherici, C., Rucci, M., Lappe, M.. (2014). Fine-scale plasticity of microscopic saccades. The Journal of Neuroscience, 34 35, 11665– 11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, L., Donnelly, W., Stevenson, S., Glasser, A.. (2010). Saccadic lens instability increases with accommodative stimulus in presbyopes. Journal of Vision, 10 4: 14, 1– 16, https://doi.org/10.1167/10.4.14. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist, K., Nystrom, M., Andersson, R., Dewhurst, R., Jarodzka, H., de Weijer, J. V.. (2011). Eye tracking: A comprehensive guide to methods and measures. Oxford, UK: Oxford University Press. [Google Scholar]
- Horn, M. R. V., Cullen, K. E.. (2012). Coding of microsaccades in three-dimensional space by premotor saccadic neurons. The Journal of Neuroscience, 32 6, 1974– 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto, Y., Kaku, Y.. (2010). Saccade adaptation as a model of learning in voluntary movements. Experimental Brain Research, 204 2, 145– 162. [DOI] [PubMed] [Google Scholar]
- Kloke, W. B., Jaschinski, W., Jainta, S.. (2009). Microsaccades under monocular viewing conditions. Journal of Eye Movement Research, 3 1, 1– 7. [Google Scholar]
- Ko, H.-K., Poletti, M., Rucci, M.. (2010). Microsaccades precisely relocate gaze in a high visual acuity task. Nature Neuroscience, 13 12, 1549– 1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, H.-K., Snodderly, D. M., Poletti, M.. (2016). Eye movements between saccades: Measuring ocular drift and tremor. Vision Research, 122, 93– 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowler, E. (2011). Eye movements: The past 25 years. Vision Research, 51 13, 1457– 1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauskopf, J., Cornsweet, T. N., Riggs, L. A.. (1960). Analysis of eye movements during monocular and binocular fixation. Journal of the Optical Society of America, 50 6, 572– 578. [DOI] [PubMed] [Google Scholar]
- Malinov, I. V., Epelboim, J., Herst, A. N., Steinman, R. M.. (2000). Characteristics of saccades and vergence in two kinds of sequential looking tasks. Vision Research, 40 16, 2083– 2090. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde, S., Macknik, S. L., Troncoso, X. G., Dyar, T. A.. (2006). Microsaccades counteract fading during fixation. Neuron, 49 2, 297– 305. [DOI] [PubMed] [Google Scholar]
- Mihali, A., van Opheusden, B., Ma, W.. (2017). Bayesian microsaccade detection. Journal of Vision, 17 1: 13, 1– 23, https://doi.org/10.1167/17.1.13. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofi, N., Boi, M., Rucci, M.. (2016). Are the visual transients from microsaccades helpful? Measuring the influences of small saccades on contrast sensitivity. Vision Research, 118 Suppl. C, 60– 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström, M., Anderson, R., Niehorster, D., Hooge, I.. (2017). Searching for monocular microsaccades: A red herring of modern eye trackers? Vision Research, 140, 44– 54. [DOI] [PubMed] [Google Scholar]
- Nyström, M., Andersson, R., Holmqvist, K., van de Weijer, J.. (2013). The influence of calibration method and eye physiology on eyetracking data quality. Behavior Research Methods, 45 1, 272– 288. [DOI] [PubMed] [Google Scholar]
- Otero-Millan, J., Troncoso, X. G., Macknik, S. L., Serrano-Pedraza, I., Martinez-Conde, S.. (2008). Saccades and microsaccades during visual fixation, exploration, and search: Foundations for a common saccadic generator. Journal of Vision, 8 14: 21, 1– 18, https://doi.org/10.1167/8.14.21. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Poletti, M., Aytekin, M., Rucci, M.. (2015). Head/eye coordination at the microscopic scale. Current Biology, 25, 3253– 3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti, M., Listorti, C., Rucci, M.. (2013). Microscopic eye movements compensate for nonhomogeneous vision within the fovea. Current Biology, 23 17, 1691– 1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti, M., Rucci, M.. (2016). A compact field guide to the study of microsaccades: Challenges and functions. Vision Research, 118, 83– 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs, M. (2009). Microsaccades: Small steps on a long way. Vision Research, 49 20, 2415– 2441. [DOI] [PubMed] [Google Scholar]
- Rucci, M., Poletti, M.. (2015). Control and function of fixational eye movements. Annual Review of Vision Science, 1, 499– 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, K. E., Sinnott, L. T., Mutti, D. O., Bailey, M. D.. (2009). Accommodative fluctuations, lens tension, and ciliary body thickness in children. Optometry and Vision Science, 86, 677– 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, E. (1984). Binocular micromovements in normal persons. Graefe's Archive for Clinical and Experimental Ophthalmology, 222, 95– 100. [DOI] [PubMed] [Google Scholar]
- Skavenski, A. A., Hansen, R. M., Steinman, R. M., Winterson, B. J.. (1979). Quality of retinal image stabilization during small natural and artificial body rotations in man. Vision Research, 19 6, 675– 683. [DOI] [PubMed] [Google Scholar]
- Steinman, R. M., Kowler, E., Collewijn, H.. (1990). New directions for oculomotor research. Vision Research, 30 11, 1845– 1864. [DOI] [PubMed] [Google Scholar]
- Stevenson, S., Roorda, A.. (2005). Correcting for miniature eye movements in high resolution scanning laser ophthalmoscopy. Proceedings of SPIE (The International Society for Optical Engineering), 5688, 145– 151. [Google Scholar]
- Valsecchi, M., Gegenfurtner, K. R.. (2015). Control of binocular gaze in a high-precision manual task. Vision Research, 110, 203– 214. [DOI] [PubMed] [Google Scholar]
- Wildenmann, U., Schaeffel, F.. (2013). Variations of pupil centration and their effects on video eye tracking. Ophthalmic & Physiological Optics, 33 6, 634– 641. [DOI] [PubMed] [Google Scholar]
- Wyatt, H. J. (2010). The human pupil and the use of video-based eyetrackers. Vision Research, 50 19, 1982– 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]