Abstract
Background
Kawasaki disease (KD), the leading cause of acquired heart disease in children, primarily affects infants and toddlers. Investigations on immune responses during KD are hampered by a limited understanding of normal immune responses in these ages. It’s well known that Infants have poorer vaccine responses and difficulty with maintaining prolonged serum immunity, but there are few studies on human infants detailing immune deficiencies. Limited studies propose an inability to maintain life-long bone marrow plasma cells. Plasmablasts are a transitional cell form of B cells that lead to long-term Plasma cells. Plasmablasts levels rise in the peripheral blood after exposure to a foreign antigen. In adult studies, these responses are both temporally and functionally well characterized. To date, there have been few studies on plasmablasts in the predominant age range of KD.
Methods
Children presenting to an urban pediatric emergency room undergoing laboratory evaluation, who had concern of KD or had fever and symptoms overlapping those of KD, were recruited. Peripheral blood mononuclear cells were isolated and evaluated utilizing flow cytometry with specific B cell markers from 18 KD subjects and 69 febrile controls.
Results
Plasmablast numbers and temporal formation are similar between infectious disease controls and KD subjects. In both groups, infants have diminished plasmablast responses compared to older children.
Conclusion
In this single-time point survey, infants have a blunted peripheral plasmablast response. Overall, similar plasmablast responses in KD and controls support an infectious disease relationship to KD. Future time-course studies of plasmablasts in infants are warranted as this phenomenon may contribute to observed immune responses in this age group.
Introduction
Kawasaki Disease (KD) is the leading cause of acquired heart disease in children [1]. Treatment with intravenous immunoglobulin (IVIG) reduces the incidence of coronary artery aneurysms from near a quarter of untreated cases to roughly 10-fold less [1–6]. The long-term effects of childhood KD on adult cardiovascular health remains unclear [7], however, numerous studies point to higher risk of cardiovascular issues and ischemic heart disease in adults [8–11]. Although, the yearly incidence in the United States is 9–19 cases per 100,000 children [12], it is nearly 10-fold higher in Japan, and evidence is that this is increasing over time [13].
KD classically presents as a minimum of five days of fever with conjunctivitis, singular lymph node swelling, oral mucous membrane inflammation, peripheral extremity swelling, and rash [1, 4, 6, 14]. Four of five criteria define classic KD, however, incomplete presentations with fewer symptoms can also result in coronary artery abnormalities [3]. Laboratory values such as leukocytosis, anemia, alanine aminotransferase (ALT) elevation, late thrombocytosis, urethritis, or hypoalbuminemia [2] can be useful to diagnose KD, particularly for incomplete forms [3]. Unfortunately, there is no specific and sensitive test to confirm either the KD diagnosis or to distinguish those that will go on to have aneurysms.
While the cause of KD is unclear [6, 15, 16], seasonality, periodic ‘outbreaks’, the peak age range in early childhood, and clinical symptoms similar to other infectious agents all support an infectious disease as a cause [14]. Diagnosis of KD prior to six months of age is rare and the normal peak in toddlers generally suggests a protective role of maternal antibodies during early development [17]. Recent studies show a lower incidence in breastfed infants further supporting protection by antibodies [18]. Other data support a role of B cell responses in the pathogenesis of KD. B cells are “activated” (CD69+) and downregulation of BCR receptor signaling occurs in Kawasaki disease [19]. Additionally, genome wide association studies implicate CD40 signaling and the involvement of the B Lymphoid Tyrosine Kinase [20, 21] in KD. If KD is caused by an acute infection, studying the acute changes to the B cell populations can be very informative.
During an acute infection, both naïve B cells and memory B cells are stimulated to form plasmablasts: B cells transitioning to plasma cells that circulate in the peripheral blood cell compartment [22, 23] recognized by surface markers of CD19 (B cell marker), downregulation of CD20, and high levels of CD27 and CD38 [1, 24]. In comparison to the general circulating B cell population, plasmablasts are enriched for B cells that contain infection-specific antibodies [25, 26]. This is variable as some studies show massive and high enrichment of plasmablasts targeting the antigen of interest [27, 28], while other studies show polyspecificity of the plasmablast population and limited enrichment [29–32]. Immunization studies in adults (tetanus [33], influenza [24], and rabies [34]) show plasmablast have more more consistent enrichment for specific antibodies, temporally peak 5–10 days after immunization, and are predictive of later sero-immunity [35]. Elevated circulating peripheral plasmablasts are not specific to infections, as they are elevated in a number of autoimmune disease and their levels correlate to disease flares [36]. Although certain infections, such as dengue virus, may set off exceedingly high plasmablast levels [37], plasmablast levels tend to be significantly higher in autoimmune conditions than levels achieved during vaccination or post-infection. This excessive circulating plasmablast response seen corresponds to flaring of the underlying inflammatory disease, and specifically correlates with CRP level in studies on ulcerative colitis [37, 38] and IGG4 related disease [39, 40].
Kawasaki disease is most prevelent, from six months to six years of age, at a time when the immune system is still developing. It is known that infants have poorer responses to infections and vaccinations [41], however, thorough study of plasmablast responses in the predominant age of KD presentation has not been performed. Post- meningococcal vaccine plasmablast responses have been evaluated, and show that infant responses [42] are diminished compared to adult levels [43]. Other infections or vaccinations have not included timecourse evaluation of Plasmablast cells. In this study, we sought to characterize the plasmablast responses of febrile children, including a subset with KD, in the age range of peak KD incidence to provide the basis for future studies on specific plasmablast responses in KD children.
Methods
Study design
This is a single site cross-sectional study of children with fever. Children nine months (chosen for safety considerations) to six years of age presenting to the Emergency Department of Women and Children’s Hospital of Buffalo from March 2014 to May 2016 were screened to determine if they met enrollment criteria. Eligible patients met the following criteria: fever of 38.3°C or above prior to presentation, had a planned blood draw as part of the evaluation of their illness, and one of the following symptoms: rash, mucous membrane changes, extremity changes, conjunctivitis or a single isolated enlarged lymph node. Children admitted or transferred from outside facilities with the specific concern for KD were also enrolled regardless of other criteria. These inclusion criteria captured KD children and a selection of febrile controls. Children with KD in this study are defined as those enrolled who were diagnosed by both primary team and Infectious disease (ID) consultant as having clinical KD and who underwent IVIG treatment after their initial blood draw. Other specific diagnoses were defined on clinical criteria alone, or considered confirmed cases if they included a positive diagnostic test result and chart review revealed clinical illness consistent with the test result. Written informed consent was obtained from parents or legal guardians. Institutional review board (IRB) approval was obtained prior to the initiation of the study.
Initial sampling coincided with admission blood collection and was drawn before IVIG treatment (if applicable). Generally, 5–10 milliliters of blood was drawn up in sodium heparin tubes and placed at room temperature on a neutator in the locked clinical laboratory in the emergency department. Attempts were also made to collect samples 48–72 hours (post- IVIG treatment when applicable) after first blood draw for those who were admitted.
Notable exclusions to prevent effects of excessive blood draws included prior study enrollment within two months, chronic or active blood borne infection (i.e. human immunodeficiency virus (HIV), hepatitis B or hepatitis C virus (HCV)), chronic anemia, excessive blood loss or other excessive blood taken for lab studies in the last eight weeks. Demographic and laboratory information collected included duration of fever, age, race, additional symptoms, laboratory values, and microbiology results. Pertinent exam findings such as patients’ vital signs, and physician’s evaluation were also documented. Access to the medical record was included in the consent to allow for review of microbiology and other laboratory data.
Isolation of peripheral blood mononuclear cells (PBMCs)
Generally, we followed established published protocols [1]. Blood was collected in sodium heparin tubes and placed on a neutator until collection within 1–15 hours. Samples were processed in a BSL-2 biosafety cabinet. Blood was initially mixed 1:1 with phosphate buffered saline (PBS) (Gibco by Life technologies. Carlsbad, CA). This was layered onto a polysaccharide solution (Ficoll-paque. Sigma, St. Louis, Missouri) and centrifuged at 400 x G for 25 minutes in a containment bench top centrifuge with locking top. 1:1 PBS to Plasma samples were collected from the top layer and stored at -80 C° for future studies. PBMCs were then withdrawn from the meniscus layer, transferred to another tube and washed twice with 10% Fetal bovine serum (FBS) (Media Tech, Corning. Manassas, Virginia). Cells were then counted and cryopreserved with 5–10 million cells per one mL in 10% DMSO and 90% FBS freezing medium.
Isolation of B cells
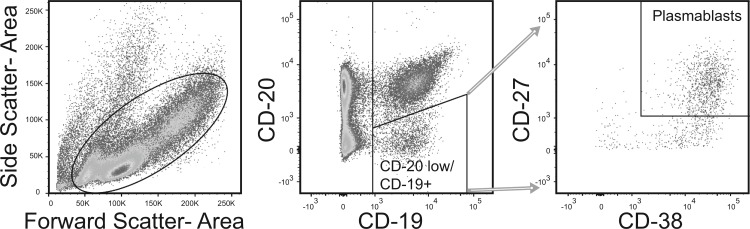
Cells were removed from cryopreservation and thawed in 37°C water bath. Cells were repetitively washed with 10% FBS in PBS. On the final dilution, cells were diluted to 1x106 cells/mL of 2% FBS in PBS. Cells were labeled with Fluorophore-conjugated goat monoclonal antibodies to the following human antigens; CD3, CD14, CD19, CD20, CD27, CD38 and IgG; purchased from Becton Dickinson Bio-sciences (San Jose, CA, U.S.A.) and with IgA+ purchased from Miltenyi (Bergisch Gladbach, Germany). Cells were labeled in 2% FBS/PBS and washed twice with 2% FBS/PBS prior to flow cytometric analysis. Flow cytometric analysis was performed with a FACSAria flow cytometer in a Biosafety Level 2+ laboratory aerosol containment accessory (Becton Dickinson, Franklin Lakes, NJ). Plasmablasts were separated similar to previous published studies [24, 44] (Fig 1).
Fig 1. Flow cytometry isolation of plasmablasts.
A single example of the flow gating and analysis is shown. After selecting for single cell events, lymphocyte gate was constructed inclusive of blasting cells, which are enriched for plasmablasts. CD14+ and CD3+ cells were excluded. CD19+/CD20low cells that were dual positive for CD27 and CD38 were defined as plasmablasts.
ELISA measurement of Interleukin-21
Methods follow those previously described [45]. Manufacturer’s protocol for the Human IL-21 ELISA kit (Ready-SET-Go! Kit, Affymetrix, San Diego) with a published sensitivity range of 8–1000 pg/mL was used in this study. Anti-Human IL-21 capture antibody was bound to 96-well ELISA plates overnight (o/n) at 4°C. IL-21 coated plates were washed three times (3x) then blocked (1 hr, room temperature (RT)), then washed once. Human IL-21 internal standards (ranging from 15.625 pg/mL to 800 pg/mL) and diluted plasma samples were layered on the plate in duplicate and incubated (o/n, 4°C). After washing (5x) plates were incubated (1 hr, RT) with anti-Human IL-21 Biotin detection antibody. Plates were washed (5x) and incubated (30 min, RT) with Avidin-HRP enzyme. After washing (7x), plates were developed with TMB solution with 2N H2SO4 acid stop and read at 450 nm in a spectrophotometer. Plasma was initially diluted with PBS during PBMC isolation (to roughly 40% of full concentrated plasma) and this was factored in to final IL-21 level reported.
Data analysis
Descriptive characteristics for study subjects were computed after detailed chart review. Categorical variables were reported as proportions in percentage, and continuous level variables were reported as means and standard deviations. Chi-square analysis was used to compare differences in level of white blood cells in urine among KD and control patients. Separate independent t-tests were used to examine differences between patients with Kawasaki Disease and controls for variables that included fever, white blood cell count, platelet count, C-reactive protein (CRP), Sedimentation rate (ESR), hemoglobin, hematocrit, Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), and serum albumin. Flow cytometry data was analyzed using FlowJo software (Ashland, Oregon). Statistical tests, as described in the text, were two-tailed with alpha of 0.05 and performed using Prism software (Graphpad, La Jolla, CA).
Results
Clinical comparison
Patient ages, gender and discharge diagnosis are listed in Table 1. A number of associations that are commonly described with KD [46] are notable: one individual on presentation fulfilled echocardiography criteria, four individuals had hydropic gallbladders, one child had a retropharyngeal phlegmon, one child had recurrent KD, and four children had imaging of their adenopathy consistent with clustering of nodes, rather than a single inflamed node. All laboratory values and samples from KD children analyzed were drawn prior to IVIG therapy unless noted in the text. Analysis of laboratory values between KD and total febrile controls showed relative thrombocytosis, hypoalbuminemia, anemia, and elevation of inflammatory markers in KD children (Table 2), which is similar to previously published studies related to KD [47–49]. Notably, control subjects had a relative elevation of Aspartate Aminotransferase (AST) in our cohort.
Table 1. Clinical characteristics of enrolled patients, classified by diagnoses.
| Enrolllee # | Gender | Age months | Febrile days before blood drawn | Clinical Diagnosis and/or associated symptoms and diagnostic result |
|---|---|---|---|---|
| Kawasaki Disease (KD) | ||||
| 4 | female | 69 | 7 | Kawasaki Disease |
| 5 | male | 32 | 7 | Kawasaki Disease, Recurrence of diagnosis 3 months prior |
| 6 | male | 28 | 6 | Kawasaki Disease, Gallbladder hydrops |
| 15 | male | 78 | 6 | Kawasaki Disease |
| 17 | male | 49 | 4 | Kawasaki Disease, Polyarthritis |
| 24 | male | 58 | 4 | Kawasaki Disease, neck ultrasound with node cluster |
| 47 | female | 81 | 5 | Kawasaki Disease, + Parainfluenza 2 Respiratory Screen |
| 53 | male | 29 | 6 | Kawasaki Disease |
| 67 | male | 10 | 6 | Kawasaki Disease |
| 68 | male | 21 | 4 | Kawasaki Disease |
| 75 | male | 20 | 7 | Kawasaki Disease, +Rapid Strep screen, + Echo (lack of tapering, LAD z score 2.5), Gallbladder hydrops, neck ultrasound with node cluster |
| 79 | male | 26 | 7 | Kawasaki Disease |
| 80 | female | 53 | 3 | Kawasaki Disease, neck CT with node cluster, retropharyngeal phlegmon |
| 93 | female | 83 | 5 | Kawasaki Disease, Gallbladder hydrops, neck ultrasound with node cluster |
| 97 | male | 54 | 6 | Kawasaki Disease, + RSV Respiratory Screen, Gallbladder hydrops |
| 99 | female | 39 | 9 | Kawasaki Disease |
| 103 | male | 23 | 5 | Kawasaki Disease |
| 119 | male | 29 | 6 | Kawasaki Disease |
| Febrile Controls | ||||
| Febrile Controls: Prolonged Fevers | ||||
| 54 | male | 71 | 14 | Septic Arthritis, pretreated |
| 66 | male | 40 | 14 | Rash, Conjunctivitis |
| 94 | female | 42 | 24 | URI, Conjunctivitis (+ Adenovirus) |
| 123 | male | 30 | 14 | URI, Adenopathy |
| Febrile Controls: Adenoviral infection | ||||
| 45 | female | 72 | 5 | URI, Rash, conjunctivitis |
| 59 | male | 36 | 6 | URI, Rash, conjunctivitis |
| 95 | female | 75 | 5 | URI, Rash, conjunctivitis |
| 131 | male | 19 | 4 | URI, conjunctivitis |
| Febrile Controls: Influenza | ||||
| 49 | male | 36 | 2 | Rapid Influenza A+, Rash |
| 101 | female | 85 | 3 | Rapid influenza A + (OSH) |
| 109 | male | 32 | 2 | Rapid influenza B + (OSH) |
| Febrile Controls: Hand-foot-and-mouth disease (HFM) | ||||
| 16 | female | 21 | 3 | Hand-foot-and-mouth disease (HFM) |
| 87 | male | 35 | 5 | Hand-foot-and-mouth disease (HFM) |
| 104 | male | 28 | 6 | Hand-foot-and-mouth disease (HFM) |
| 117 | female | 34 | 4 | Hand-foot-and-mouth disease (HFM) |
| Febrile Controls: Skin and Soft Tissue Infections (SSTI) | ||||
| 7 | male | 13 | 4 | MRSA Abscess, Cervical adenitis |
| 25 | male | 37 | 2 | Cellulitis of the leg |
| 30 | female | 13 | 3 | Periorbital cellulitis |
| 42 | male | 15 | 2 | Cervical adenitis; improved on clindamycin |
| 43 | male | 50 | 4 | Parapharyngeal abscess (S. viridans) |
| 48 | male | 14 | 11 | Cervical adenitis; improved on clindamycin |
| 60 | male | 37 | 7 | Cervical adenitis, conjunctivitis; improved on clindamycin |
| 98 | male | 14 | 5 | Cervical adenitis, Rash, Conjunctivitis; improved on ampicillin-sulbactam |
| 107 | male | 88 | 10 | Cervical adenitis; improved on clindamycin |
| 130 | male | 52 | 7 | Cervical adenitis; improved on clindamycin |
| Febrile Controls: Group A Streptococcal pharyngitis (GAS) | ||||
| 58 | male | 68 | 9 | Pharyngitis, Cervical adenopathy, Rapid strep + |
| 89 | male | 62 | 4 | Pharyngitis, Rash, Rapid strep + |
| 92 | female | 20 | 2 | Pharyngitis, Rapid Strep + |
| 113 | male | 34 | 3 | Pharyngitis, Scarlet fever, Rapid Strep + |
| Febrile Controls: not otherwise classified | ||||
| 3 | male | 19 | 4 | URI, Rash |
| 10 | male | 56 | 4 | Viral syndrome, bilateral adenopathy |
| 12 | male | 27 | 6 | Viral pneumonia |
| 13 | female | 64 | 3 | Viral pneumonia, Gastroenteritis, Conjunctivitis; + Parainfluenza 3 Respiratory Screen |
| 14 | male | 22 | 1 | Cough |
| 20 | female | 14 | 6 | URI, Rash |
| 29 | female | 52 | 6 | Pyelonephritis, Septicemia |
| 31 | female | 15 | 5 | Viral syndrome, Neutropenia |
| 34 | male | 23 | 7 | Bacteremia (Salmonella), Enteritis |
| 52 | male | 80 | 3 | Bacteremia (S. pyogenes) |
| 55 | male | 11 | 7 | Acute pyelonephritis (E. coli) |
| 61 | male | 21 | 5 | Cough, Rash, Conjunctivitis |
| 64 | female | 82 | 6 | Viral Syndrome, Peeling |
| 65 | male | 18 | 2 | Viral Syndrome |
| 69 | female | 27 | 6 | Rash |
| 70 | male | 9 | 4 | URI |
| 71 | female | 16 | 3 | Viral syndrome, Rash |
| 72 | male | 108 | 5 | Viral syndrome, Hepatitis, Rash, Conjunctivitis |
| 74 | male | 12 | 7 | Viral syndrome, + Enterovirus |
| 76 | female | 38 | 2 | Allergic reaction with fever, Rash, Hand and feet swelling |
| 77 | male | 48 | 4 | Viral syndrome, Rash, Allergic drug reaction |
| 78 | male | 67 | 2 | Viral syndrome, Adenopathy |
| 81 | male | 11 | 5 | Viral syndrome, Rash |
| 82 | male | 46 | 2 | URI, Conjunctivitis, Adenopathy |
| 83 | male | 14 | 6 | Gastroenteritis, Rash |
| 84 | male | 23 | 4 | URI, Gastroenteritis, Rash |
| 85 | female | 16 | 1 | Viral Syndrome, Rash |
| 86 | female | 49 | 4 | Viral syndrome, Gastroenteritis |
| 88 | male | 43 | 2 | URI |
| 90 | female | 43 | 6 | Viral syndrome, Gastroenteritis |
| 91 | female | 57 | 6 | Viral Pharyngitis |
| 102 | male | 10 | 1 | URI, Cough |
| 106 | male | 29 | 2 | Erythema multiforme |
| 110 | male | 59 | 1 | Staph scalded skin syndrome |
| 112 | male | 11 | 7 | Gastroenteritis |
| 115 | female | 40 | 4 | Rash, Conjunctivitis |
| 116 | male | 11 | 6 | Viral syndrome, Rash |
| 122 | male | 16 | 3 | Rash |
| 125 | male | 12 | 3 | Rash, Conjunctivitis, referred to rule-out KD |
| 129 | female | 10 | 1 | URI, Conjunctivitis |
Table 2. Demographic and laboratory comparison of Kawasaki disease to control.
| Variable | Kawasaki Disease (KD) (n = 18) |
All Controls (n = 69) |
P-value |
|---|---|---|---|
| Male Gender, n (%) | 13 (72.2) | 49 (71.0) | 0.920 |
| Age in months ±(SD) | 43.4(23.0) | 36.3 (23.6) | 0.955 |
| Febrile days prior to blood draw ±(SD) | 5.7 (1.4) | 5.0 (3.7) | 0.208 |
| White blood cell count maximum ±(SD) | 17.6 (5.4) | 13.1 (6.0) | 0.005 |
| Platelet count maximum ±(SD) | 393.4 (113.2) | 326.7 (145.9) | 0.046 |
| C-reactive protein [CRP] maximum ±(SD) | 129.4 (62.3) | 73.3 (88.9) | 0.040 |
| Sedimentation rate [ESR], prior to IVIG ±(SD) | 83.6 (30.3) | 55.4 (28.4) | 0.005 |
| Hemoglobin minimum ±(SD) | 10.4 (1.2) | 11.6 (1.0) | <0.001 |
| Hematocrit %, minimum ±(SD) | 31.2 (3.5) | 34.8 (2.9) | <0.001 |
| Aspartate Aminotransferase [AST] maximum ±(SD) | 36.8 (20.0) | 52.4 (31.2) | 0.042 |
| Alanine Aminotransferase [ALT], maximum ±(SD) | 51.2 (36.5) | 57.1 (78.3) | 0.726 |
| Serum Albumin minimum ±(SD) | 2.9 (0.5) | 3.7 (0.5) | <0.001 |
| Urine WBC, n (%) 0–5 6–100 |
6 (40) 9 (60) |
22 (78.6) 6 (21.4) |
0.011 |
Peripheral B cells
Plasmablast levels showed no significant differences between KD and our cohort of febrile controls (medians/means in our KD and control groups were 2.51%/4.53% and 2.32%/4.86% of B cells respectively with ranges in KD patients of 0.31% to 13.45%, and ranges of controls 0.18% to 34.44%) (Table 3 and Fig 2). In people without recent infection or in convalescence, circulating plasmablast levels are generally less than 1.0% and in some studies nearer to 0.40% of circulating B cells [24, 25]. In our cohort, four children had prolonged fever (>14 days) and their levels are consistent with background levels previously published (mean = 0.74 and median = 0.75, Fig 2 closed circles). Subsets of control cases with less than two weeks of fevers that qualified for specific diagnoses are also shown (open circles, Fig 2). These include five subgroups: confirmed cases of adenovirus, influenza and group A streptococcus pharyngitis (GAS) as well as groups of primarily clinical diagnoses of hand-foot-and-mouth disease and bacterial skin and soft tissue infections (SSTI). When evaluating for elevation over background, both KD and febrile control plasmablast percentage were significantly elevated when compared to our prolonged fever cohort, (p values of 0.0329 and 0.0079 respectively). The only difference of KD to any other group was the notable elevation within the adenoviral group.
Table 3. Median values of cell subsets from flow cytometry of peripheral blood mononuclear cells.
| Cell Subset | Kawasaki (n = 18) Median value 2.5–97.5th Percentile |
Control (n = 69) Median value 2.5–97.5th Percentile |
P value | |
|---|---|---|---|---|
| Percent of Lymphocytes | B cells | 17.3 15.1–22.9 |
14.8 14.1–17.5 |
0.081 |
| IgG Cells | 1.19 0.94–1.53 |
0.71 0.71–1.01 |
0.005 | |
| IgA Cells | 0.69 0.57–1.48 |
0.56 0.57–0.92 |
0.237 | |
| Plasmablast | 0.33 0.33–1.10 |
0.29 0.45–0.98 |
0.607 | |
| Percent of B cells | IgG | 7.15 5.56–9.77 |
4.74 4.85–6.44 |
0.074 |
| IgA | 4.35 3.69–7.90 |
3.59 3.81–6.10 |
0.470 | |
| Plasmablasts | 2.51 2.24–6.82 |
2.32 3.11–6.14 |
0.942 | |
| Percent of Plasmablasts | IgG | 11.93 9.96–15.34 |
9.24 9.00–12.16 |
0.177 |
| IgA | 47.4 36.6–54.2 |
43.6 38.14–45.51 |
0.441 | |
Fig 2. Plasmablast comparison between different clinical diagnoses.
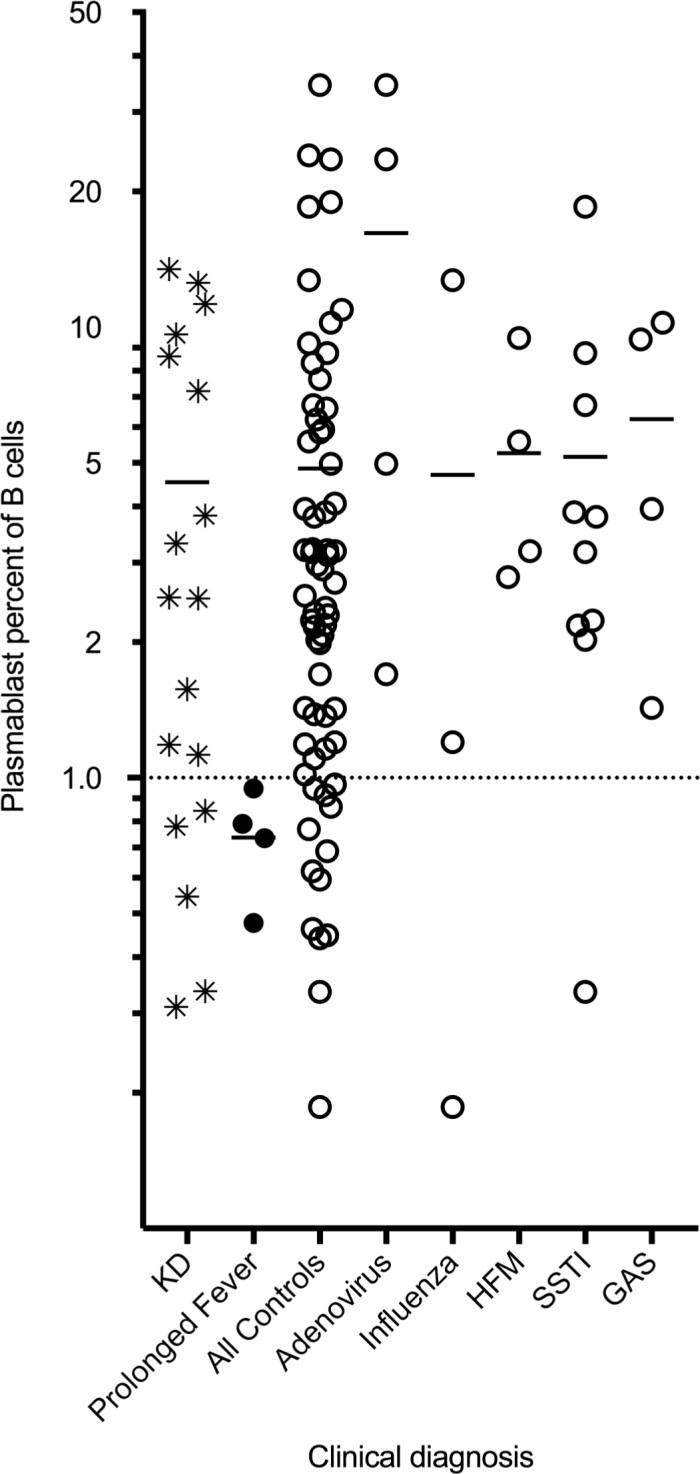
Plasmablast levels, as a percentage of overall B cell number, were compared between children with KD (star), prolonged fever (closed circle), and all controls (open circles). Mean values are marked by horizontal dash. Data is shown on a logarithmic scale to improve separation of individual points. Five subsets of all controls that qualified as specific diagnoses are shown (Adenovirus, Influenza, Hand-foot-and-mouth (HFM), Skin and Soft tissue infections (SSTI), and Group A streptococcal pharyngitis (GAS)) and also listed in Table 1.
In KD children, we also noted a similar elevation of B cells consistent with previous publications [6, 14, 19, 50], and in particular, an elevation of the IgG+ B cell subset of total lymphocytes reaches significance (p = 0.005). We also looked specifically at IgA+ B cells as these have been implicated in specific systemic immune responses with subsequent infiltration of the aneurysmal tissues [51, 52] and shown to be low in a single previous studies [1]. There was no difference in the overall numbers of IgA+ B cells between our KD and control subjects.
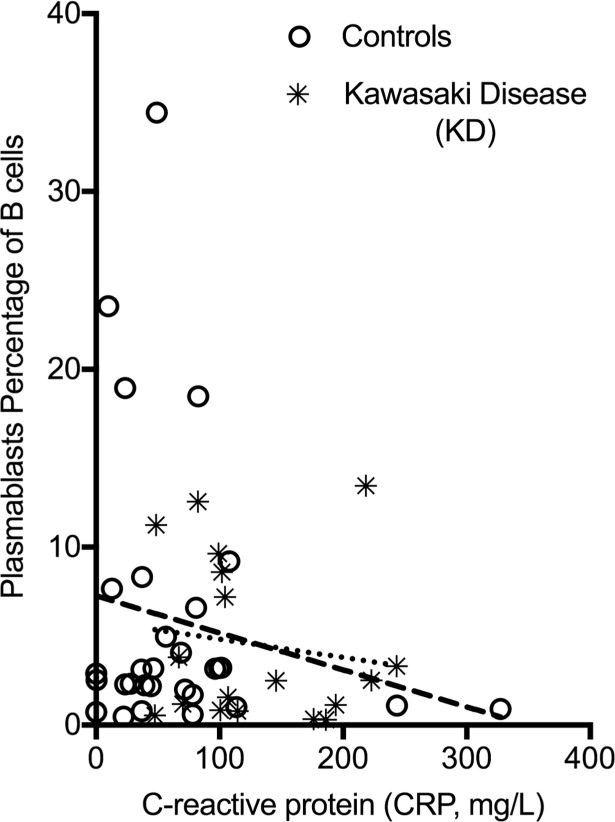
Since plasmablasts can also be highly elevated during autoimmune phenomenon, and levels are shown to correlate with disease flares in these cases [36–40], we chose to explore if there was a correlation of overall inflammation to plasmablast level in our cohort. All 18 of our KD samples and 48 of our controls had CRP data available for analysis. Unlike what has been described for a number of autoimmune conditions, there is no significant correlation of CRP and plasmablast responses in either controls or our KD samples (R2 values were <0.1). Slopes of the linear regression lines actually implied negative correlation of CRP to plasmablast level, but they were not significantly different than zero (Fig 3).
Fig 3. Plasmablast level relationship to C-reactive Protein (CRP).
Linear regression analysis of level of CRP and plasmablast level in KD (starred, small dashed line) and controls (open circles, long dashed line). Results failed to show any linear correlation.
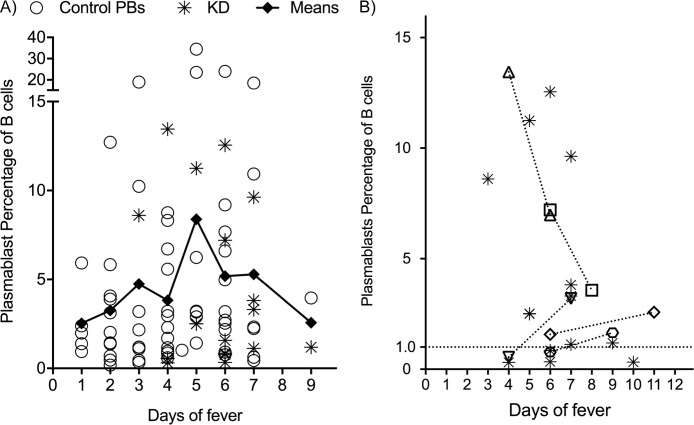
Notably, a number of KD and control children had non-stimulated plasmablast levels, which may be due to a number of factors. First, we evaluated if presentation day of fever of the samples analyzed were different in these subjects. We only had a select few individuals with multiple blood samples, so we chose to pool data by day of fever, similar to previous publications on meningococcal vaccination [26] and RSV infection [42]. Each individual underwent a review of the medical record to assign the day of fever associated with each sample. Overall, the peak in both KD and control cohorts (days 3–7) is consistent with reports of other infectious diseases [24, 26, 35] (Fig 4A). KD samples mainly had elevations on day 4–6 with peak mean was on day 5. Even in this peak time, there was a wide variability in plasmablast level. Five KD patients had paired samples taken 48–72 hours later after the initial blood draw and after initiation of IVIG therapy. No consistent trends were seen. Analyzing KD samples with multi-timepoint blood sampling (Fig 4B), shows that three initially low values rose, while two plasmablast levels that were high on day 4 and 5 receded over time.
Fig 4. Relationship of plasmablast level to day of fever.
A) Values of means of all samples graphed by day (diamonds) with connected line showing trend. KD samples (stars) and controls (open circles) are shown. B) Showing KD samples alone, single timepoint samples are again labelled with stars. Samples of five individuals with repeat samples are shown by connected (short dash) lines and distinguished by open distinct symbols.
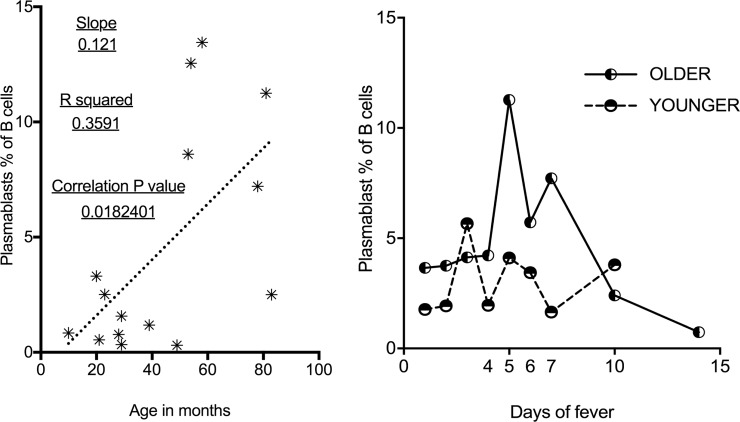
The age of each individual may affect plasmablast levels in direct or indirect fashion. Younger children are more likely to show naïve immune reponses to infection and this has been shown to potentially temporally delay the rise of plasmablasts [34]. Immune defects are described in young children that may prevent robust responses to vaccines and infections [53]. Since our cohort included both infants and toddlers, we evaluated plasmablast responses in relation to the age of the subject. Exploring the effect of age on plasmablast levels shows that overall, younger children have diminished plasmablast responses. Linear regression analysis of age in months compared to plasmablast levels of KD, controls, and all samples show slopes that are all significantly non-zero (0.018, 0.015 and 0.003 respectively) with generally higher values in older children. R2 values for these groups are 0.359, 0.149 and 0.161 respectively. Data for the KD group is illustrated in Fig 5.
Fig 5. Plasmablast level and temporal pattern relative to age of subject.
A) In KD children, Linear regression (small dashed line) shows relationship of older children to higher plasmablast levels. B) Mean values for plasmablast percentage of B cells by day of fever for those 24 months and under (long dashed line) compared to subjects over 24 months of age (solid line).
Comparing plasmablast levels in younger children (0–24 months) to older children (25 months and greater; unpaired t-test, Welch’s correction for variable Standard Deviations), indicated that younger children had significantly (p<0.024) lower circulating plasmablast numbers (means and SEM of 2.99 ± 0.63 versus 5.59 ± 0.93; Standard Deviations of 3.62 and 6.87 respectively). Comparing pooled data from days 4–7 (the described peak for a number of viruses in the literature) also shows a significant difference (p <0.007) between these age groups (up to 24 months old n = 20 and greater than 24 months of age n = 33; Mean and SEM of 2.82 ± 0.5931 and 7.097 ± 1.395, and Standard Deviations of 2.65 and 8.02 respectively). This is illustrated by comparing mean results by day in these two age groups (Fig 5B). This supports the theory that there is an inherent blunting of this response overall in young children, although other physiologic phenomenon beyond purely age may be playing a role (see discussion).
There are a number of potential immune differences that may explain this. One factor that supports plasmablast formation [54] and has been previously associated with KD [55] is IL-21. We previously attempted to confirm this association when comparing KD to febrile controls [45], however we did not explore age effect or relationship to plasmablast level previously. Here, we further analyzed IL-21 levels previously obtained in the context of age in a subset of the children in this study (13 Kawasaki children and 36 controls). On regression analysis of IL-21 related to either age in months or plasmablast % of B cells, slopes were not significantly different from zero. R2 for KD versus controls for these groups were <0.1 (0.055 and < .001 for plasmablast and IL-21 and 0.022 and 0.025 for plasmablast and age in months).
Discussion
Overall, this work reveals initial observations on overall poor plasmablast response in children at young ages. Generally, it has long been known that infants have a less ‘mature’ immune system than adults. Young children tend to have anti-inflammatory (TH2) skewing of their responses and poor humoral immunity [56]. Diminished express of the plasma cell survival factor, APRIL, has been shown previously to contribute to poor humoral immunity in both bone marrow [57]. Our results imply even before reaching the bone marrow, there is a significant issue in generation of the plasma cell precursors. This can be explained by poorer germinal center function, lower T-cell follicular helper cells and weaker B-cell receptor signaling shown predominantly from murine studies [58]. The choice of vaccine adjuvant may help overcome this anti-inflammatory skewing in children [59] and could improve these plasmblast numbers, but this specific question has not been addressed in the published studies on these alternative adjuvants. Although our results are consistent with what is physiologically known about poor germinal center responses, plasmablast responses have not been extensively explored in human studies. Only limited studies on meningococcal vaccines have collected samples at different timepoints to explore plasmablast temporal development. Overall, infants had a lower peak response [42] compared to historical data collected from adolescents utilizing the same vaccine [43].
B cells are obviously important for adequate response to infectious diseases, but a number of studies support a role for B cell responses in KD as well. Genome wide association studies have identified single polymorphisms in B-cell lymphoid kinas (BLK) and CD40 that correspond to disease risk for KD [21]. The results of our study are consistent with the majority of the literature that show B cell stimulation and increasing peripheral B cell numbers during KD [6, 14, 19, 50]. Previous post-mortem pathology studies of aneurysms have shown IgA+ plasma cell infiltrates within the coronary arteries of patients with KD [51] and have shown these are predominantly oligoclonal responses [52]. However, studies on circulating IgA+ cell numbers have shown mixed results. [1, 5]. Notably, we did not see an increase in circulating IgA+ B cells. In these studies on plasma cell infiltrates, the autopsy specimens were generally from convalescent timepoints so may not reflect acute illness changes. Potentially, the IgA+ cell infiltrates solely represent a non-specific inflammatory state, such as seen with other IgA infiltrating plasmas cells in NMDAR encephalitis [60], primary sclerosing cholangitis [61], multiple sclerosis [62], and rheumatoid pericarditis [63].
As plasmablasts are enriched for antibodies against the challenging antigen in a number of infections and in vaccinations, we are interested to explore if they can be used to identify the unknown causes of such disorders as Kawasaki disease. For this, our study raises a number of issues. We did observe a variable plasmablast response in KD children which could be seen for a number of reasons. Even on paired sampling, we show a rise in plasmablasts in three of five samples on the second blood draw. Assuming a single etiology sets off KD, potentially, the plasmablast response of the associated infection of KD may have a more unpredictable range than many other infections described [35]. Respiratory Syncytial Virus in particular shows a wide variance over time compared to other infections [26]. Another possibility would be that exposure to the infection may show differences in a naïve and a memory response, such as seen with Rabies vaccination in adults [34]. The fact that there is elevation of peripheral IgG+ cells in our cohort also implies a memory response. However, this would contradict the theory of protection by maternal immunity and that the age of KD incidence implies protection after the first “infection”. Additionally, antibodies cloned from plasmablasts during chronic HIV infection shows polyreactivity and poor enrichment [31, 32]. Polyspecificity is not unique to HIV as it has been shown in studies on acute dengue virus [25] and salmonella infections [29]. If KD is set off by a particular virus that has a lower level of plasmablast enrichment for specific antibodies, then identifying one or several KD specific antibodies may prove very challenging.
Considering that it may not be just a single infection, multiple infectious agents with different afebrile prodromes may lead to the KD state. We did not specifically enroll patients in a time course nor recruit on specific days after the beginning of fever, so our data may not overlap with the optimal window to see the plasmablast rise in all patients if this is set off by a variety of infectious agents. Also, viral infections without as much systemic viremia, such as RSV, show overall less responsive and predictable plasmablast peaks [26]. If this type of infection sets off KD, that would account for the large ranges in both groups of patients and explain many of the normal values of plasmablasts seen in both groups.
Two other notable observations are worth mentioning. First, the effect of IVIG on plamasblast responses are unknown. We only have limited data (five paired sample before and after) and with the mixed effects seen (see Fig 4B), it is unclear if a conclusion can be drawn. For future timecourses of KD children, this may be a significant confounder. Secondly, in our control group, the subjects positive for adenovirus has some of the highest levels of plasmablast percentage as a group in our cohort when compared to other described infectious diseases [28]. Studies on plasmblast responses in adenovirus in children have not been previous published, but the response appears similar to the robust responses seen during dengue infection [28]. This did not exclusively account for the highest levels as three other individuals also had peripheral circulating plasmablast percentages >15%. Although dengue virus was not tested for in these samples, there was no travel history or clinical consistency in our cohort. Future studies on a larger group of adenovirus infected children would be of interest. As mentioned, most studies have been done in adults, so children of certain ages (outside of infancy) may actually have a more robust potential to have this type of significant elevation. Although intriguing, there are too few samples to draw firm conclusions at this time.
Conclusions
This work reveals initial observations on plasmablast responses from natural infections in children at young ages. The similar plasmablast responses shown further support a role for an infectious disease setting off the inflammatory cascade of KD. Future studies evaluating plasmablasts age effects and naïve versus memory responses by repeated sampling over time are warranted to further clarify the normal responses in young children. This will assist in future studies on optimizing vaccine adjuvants in these ages. The similarity of plasmablast responses shown here implies that circulating plasmablasts during KD produce antibodies that specifically target the etiology of Kawasaki disease. Although this might prove difficult for the reasons outlined, further description of the dynamism of these cell levels and studies on the antibodies they express offers the hope of improved diagnostic tests or, potentially, a path to identify the etiology of KD.
Supporting information
This dataset contains all raw values that contributed to the analyses within this manuscript organized by diagnosis. Raw flow cytometry data and data on Interleukin-21 levels are included as well as data and calculations highlighted in Tables 1–3 and Figs 1–5.
(XLSX)
Acknowledgments
The authors are thankful to Haiping Qiao for her help with subject recruitment and enrollment. We also acknowledge Jonathon Hoffman, Meghan McLaughlin and Hakimuddin Sojar for their technical support with processing subject samples.
Data Availability
All relevant data are within the paper and its Supporting Information files. The corresponding author can be contacted for further specific information.
Funding Statement
This work was supported from the Wildermuth Foundation via the Variety Club of Buffalo to MH. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Shingadia D, O'Gorman M, Rowley AH, Shulman ST. Surface and cytoplasmic immunoglobulin expression in circulating B-lymphocytes in acute Kawasaki disease. Pediatr Res. 2001;50(4):538–43. doi: 10.1203/00006450-200110000-00019 [DOI] [PubMed] [Google Scholar]
- 2.Terai M, Honda T, Yasukawa K, Higashi K, Hamada H, Kohno Y. Prognostic impact of vascular leakage in acute Kawasaki disease. Circulation. 2003;108(3):325–30. doi: 10.1161/01.CIR.0000079166.93475.5F [DOI] [PubMed] [Google Scholar]
- 3.Jun HO, Yu JJ, Kang SY, Seo CD, Baek JS, Kim YH, et al. Diagnostic characteristics of supplemental laboratory criteria for incomplete Kawasaki disease in children with complete Kawasaki disease. Korean J Pediatr. 2015;58(10):369–73. doi: 10.3345/kjp.2015.58.10.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo HC, Lo MH, Hsieh KS, Guo MM, Huang YH. High-Dose Aspirin Is Associated with Anemia and Does Not Confer Benefit to Disease Outcomes in Kawasaki Disease. PLoS One. 2015;10(12):e0144603 doi: 10.1371/journal.pone.0144603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohshio G, Furukawa F, Khine M, Yoshioka H, Kudo H, Hamashima Y. High levels of IgA-containing circulating immune complex and secretory IgA in Kawasaki disease. Microbiol Immunol. 1987;31(9):891–8. [DOI] [PubMed] [Google Scholar]
- 6.Chang CJ, Kuo HC, Chang JS, Lee JK, Tsai FJ, Khor CC, et al. Replication and meta-analysis of GWAS identified susceptibility loci in Kawasaki disease confirm the importance of B lymphoid tyrosine kinase (BLK) in disease susceptibility. PLoS One. 2013;8(8):e72037 doi: 10.1371/journal.pone.0072037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holve TJ, Patel A, Chau Q, Marks AR, Meadows A, Zaroff JG. Long-term cardiovascular outcomes in survivors of Kawasaki disease. Pediatrics. 2014;133(2):e305–11. doi: 10.1542/peds.2013-1638 [DOI] [PubMed] [Google Scholar]
- 8.Pinna GS, Kafetzis DA, Tselkas OI, Skevaki CL. Kawasaki disease: an overview. Curr Opin Infect Dis. 2008;21(3):263–70. doi: 10.1097/QCO.0b013e3282fbf9cd [DOI] [PubMed] [Google Scholar]
- 9.Suda K, Iemura M, Nishiono H, Teramachi Y, Koteda Y, Kishimoto S, et al. Long-term prognosis of patients with Kawasaki disease complicated by giant coronary aneurysms: a single-institution experience. Circulation. 2011;123(17):1836–42. doi: 10.1161/CIRCULATIONAHA.110.978213 [DOI] [PubMed] [Google Scholar]
- 10.Tsuda E, Abe T, Tamaki W. Acute coronary syndrome in adult patients with coronary artery lesions caused by Kawasaki disease: review of case reports. Cardiol Young. 2011;21(1):74–82. doi: 10.1017/S1047951110001502 [DOI] [PubMed] [Google Scholar]
- 11.Daniels LB, Tjajadi MS, Walford HH, Jimenez-Fernandez S, Trofimenko V, Fick DB Jr., et al. Prevalence of Kawasaki disease in young adults with suspected myocardial ischemia. Circulation. 2012;125(20):2447–53. doi: 10.1161/CIRCULATIONAHA.111.082107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997–2007. Pediatr Infect Dis J. 2010;29(6):483–8. doi: 10.1097/INF.0b013e3181cf8705 [DOI] [PubMed] [Google Scholar]
- 13.Makino N, Nakamura Y, Yashiro M, Ae R, Tsuboi S, Aoyama Y, et al. Descriptive epidemiology of Kawasaki disease in Japan, 2011–2012: from the results of the 22nd nationwide survey. J Epidemiol. 2015;25(3):239–45. doi: 10.2188/jea.JE20140089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HH, Park IH, Shin JS, Kim DS. Immunoglobulin V(H) chain gene analysis of peripheral blood IgM-producing B cells in patients with Kawasaki disease. Yonsei Med J. 2009;50(4):493–504. doi: 10.3349/ymj.2009.50.4.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodo X, Ballester J, Cayan D, Melish ME, Nakamura Y, Uehara R, et al. Association of Kawasaki disease with tropospheric wind patterns. Sci Rep. 2011;1:152 doi: 10.1038/srep00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns JC, Cayan DR, Tong G, Bainto EV, Turner CL, Shike H, et al. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005;16(2):220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newburger JW, Taubert KA, Shulman ST, Rowley AH, Gewitz MH, Takahashi M, et al. Summary and abstracts of the Seventh International Kawasaki Disease Symposium: December 4–7, 2001, Hakone, Japan. Pediatr Res. 2003;53(1):153–7. doi: 10.1203/00006450-200301000-00026 [DOI] [PubMed] [Google Scholar]
- 18.Yorifuji T, Tsukahara H, Doi H. Breastfeeding and Risk of Kawasaki Disease: A Nationwide Longitudinal Survey in Japan. Pediatrics. 2016;137(6). [DOI] [PubMed] [Google Scholar]
- 19.Ikeda K, Yamaguchi K, Tanaka T, Mizuno Y, Hijikata A, Ohara O, et al. Unique activation status of peripheral blood mononuclear cells at acute phase of Kawasaki disease. Clin Exp Immunol. 2010;160(2):246–55. doi: 10.1111/j.1365-2249.2009.04073.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YC, Kuo HC, Chang JS, Chang LY, Huang LM, Chen MR, et al. Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nature genetics. 2012;44(5):522–5. doi: 10.1038/ng.2227 [DOI] [PubMed] [Google Scholar]
- 21.Onouchi Y, Ozaki K, Burns JC, Shimizu C, Terai M, Hamada H, et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet. 2012;44(5):517–21. doi: 10.1038/ng.2220 [DOI] [PubMed] [Google Scholar]
- 22.van Zelm MC, van der Burg M, van Dongen JJ. Homeostatic and maturation-associated proliferation in the peripheral B-cell compartment. Cell Cycle. 2007;6(23):2890–5. doi: 10.4161/cc.6.23.4952 [DOI] [PubMed] [Google Scholar]
- 23.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nature reviews Immunology. 2015;15(3):160–71. doi: 10.1038/nri3795 [DOI] [PubMed] [Google Scholar]
- 24.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453(7195):667–71. doi: 10.1038/nature06890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balakrishnan T, Bela-Ong DB, Toh YX, Flamand M, Devi S, Koh MB, et al. Dengue virus activates polyreactive, natural IgG B cells after primary and secondary infection. PLoS One. 2011;6(12):e29430 doi: 10.1371/journal.pone.0029430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee FE, Falsey AR, Halliley JL, Sanz I, Walsh EE. Circulating antibody-secreting cells during acute respiratory syncytial virus infection in adults. J Infect Dis. 2010;202(11):1659–66. doi: 10.1086/657158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia M, Iglesias A, Landoni VI, Bellomo C, Bruno A, Cordoba MT, et al. Massive plasmablast response elicited in the acute phase of hantavirus pulmonary syndrome. Immunology. 2017;151(1):122–35. doi: 10.1111/imm.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, et al. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J Virol. 2012;86(6):2911–8. doi: 10.1128/JVI.06075-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Niro R, Lee SJ, Vander Heiden JA, Elsner RA, Trivedi N, Bannock JM, et al. Salmonella Infection Drives Promiscuous B Cell Activation Followed by Extrafollicular Affinity Maturation. Immunity. 2015;43(1):120–31. doi: 10.1016/j.immuni.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kauffman RC, Bhuiyan TR, Nakajima R, Mayo-Smith LM, Rashu R, Hoq MR, et al. Single-Cell Analysis of the Plasmablast Response to Vibrio cholerae Demonstrates Expansion of Cross-Reactive Memory B Cells. MBio. 2016;7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao HX, Chen X, Munshaw S, Zhang R, Marshall DJ, Vandergrift N, et al. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med. 2011;208(11):2237–49. doi: 10.1084/jem.20110363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao H, Yu Y, Li S, Yue Y, Tao C, Su K, et al. Circulating Plasmablasts from Chronically Human Immunodeficiency Virus-Infected Individuals Predominantly Produce Polyreactive/Autoreactive Antibodies. Front Immunol. 2017;8:1691 doi: 10.3389/fimmu.2017.01691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105(4):1614–21. doi: 10.1182/blood-2004-07-2507 [DOI] [PubMed] [Google Scholar]
- 34.Blanchard-Rohner G, Pulickal AS, Jol-van der Zijde CM, Snape MD, Pollard AJ. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood. 2009;114(24):4998–5002. doi: 10.1182/blood-2009-03-211052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fink K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Front Immunol. 2012;3:78 doi: 10.3389/fimmu.2012.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivas JR, Ireland SJ, Chkheidze R, Rounds WH, Lim J, Johnson J, et al. Peripheral VH4+ plasmablasts demonstrate autoreactive B cell expansion toward brain antigens in early multiple sclerosis patients. Acta Neuropathol. 2017;133(1):43–60. doi: 10.1007/s00401-016-1627-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarlton NJ, Green CM, Lazarus NH, Rott L, Wong AP, Abramson ON, et al. Plasmablast frequency and trafficking receptor expression are altered in pediatric ulcerative colitis. Inflamm Bowel Dis. 2012;18(12):2381–91. doi: 10.1002/ibd.22962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosomi S, Oshitani N, Kamata N, Sogawa M, Okazaki H, Tanigawa T, et al. Increased numbers of immature plasma cells in peripheral blood specifically overexpress chemokine receptor CXCR3 and CXCR4 in patients with ulcerative colitis. Clin Exp Immunol. 2011;163(2):215–24. doi: 10.1111/j.1365-2249.2010.04290.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattoo H, Mahajan VS, Della-Torre E, Sekigami Y, Carruthers M, Wallace ZS, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol. 2014;134(3):679–87. doi: 10.1016/j.jaci.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace ZS, Mattoo H, Carruthers M, Mahajan VS, Della Torre E, Lee H, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190–5. doi: 10.1136/annrheumdis-2014-205233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegrist CA. The challenges of vaccine responses in early life: selected examples. J Comp Pathol. 2007;137 Suppl 1:S4–9. [DOI] [PubMed] [Google Scholar]
- 42.Kelly DF, Snape MD, Perrett KP, Clutterbuck EA, Lewis S, Blanchard Rohner G, et al. Plasma and memory B-cell kinetics in infants following a primary schedule of CRM 197-conjugated serogroup C meningococcal polysaccharide vaccine. Immunology. 2009;127(1):134–43. doi: 10.1111/j.1365-2567.2008.02934.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly DF, Snape MD, Clutterbuck EA, Green S, Snowden C, Diggle L, et al. CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells. Blood. 2006;108(8):2642–7. doi: 10.1182/blood-2006-01-009282 [DOI] [PubMed] [Google Scholar]
- 44.Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, et al. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc. 2009;4(3):372–84. doi: 10.1038/nprot.2009.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelberg R, Martin M, Wrotniak BH, Hicar MD. Observational study of Interleukin-21 (IL-21) does not distinguish Kawasaki disease from other causes of fever in children. Pediatr Rheumatol Online J. 2017;15(1):32 doi: 10.1186/s12969-017-0163-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017;135(17):e927–e99. doi: 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 47.Tremoulet AH, Jain S, Chandrasekar D, Sun X, Sato Y, Burns JC. Evolution of laboratory values in patients with Kawasaki disease. Pediatr Infect Dis J. 2011;30(12):1022–6. doi: 10.1097/INF.0b013e31822d4f56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuwabara M, Yashiro M, Kotani K, Tsuboi S, Ae R, Nakamura Y, et al. Cardiac lesions and initial laboratory data in Kawasaki disease: a nationwide survey in Japan. J Epidemiol. 2015;25(3):189–93. doi: 10.2188/jea.JE20140128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yellen ES, Gauvreau K, Takahashi M, Burns JC, Shulman S, Baker AL, et al. Performance of 2004 American Heart Association recommendations for treatment of Kawasaki disease. Pediatrics. 2010;125(2):e234–41. doi: 10.1542/peds.2009-0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giordani L, Quaranta MG, Marchesi A, Straface E, Pietraforte D, Villani A, et al. Increased frequency of immunoglobulin (Ig)A-secreting cells following Toll-like receptor (TLR)-9 engagement in patients with Kawasaki disease. Clin Exp Immunol. 2011;163(3):346–53. doi: 10.1111/j.1365-2249.2010.04297.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowley AH, Eckerley CA, Jack HM, Shulman ST, Baker SC. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol. 1997;159(12):5946–55. [PubMed] [Google Scholar]
- 52.Rowley AH, Shulman ST, Spike BT, Mask CA, Baker SC. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol. 2001;166(2):1334–43. [DOI] [PubMed] [Google Scholar]
- 53.Gans HA, Arvin AM, Galinus J, Logan L, DeHovitz R, Maldonado Y. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA. 1998;280(6):527–32. [DOI] [PubMed] [Google Scholar]
- 54.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175(12):7867–79. [DOI] [PubMed] [Google Scholar]
- 55.Bae YJ, Kim MH, Lee HY, Uh Y, Namgoong MK, Cha BH, et al. Elevated Serum Levels of IL-21 in Kawasaki Disease. Allergy Asthma Immunol Res. 2012;4(6):351–6. doi: 10.4168/aair.2012.4.6.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saso A, Kampmann B. Vaccine responses in newborns. Semin Immunopathol. 2017;39(6):627–42. doi: 10.1007/s00281-017-0654-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111(5):2755–64. doi: 10.1182/blood-2007-09-110858 [DOI] [PubMed] [Google Scholar]
- 58.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9(3):185–94. doi: 10.1038/nri2508 [DOI] [PubMed] [Google Scholar]
- 59.Mohr E, Siegrist CA. Vaccination in early life: standing up to the challenges. Curr Opin Immunol. 2016;41:1–8. doi: 10.1016/j.coi.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 60.Martinez-Hernandez E, Horvath J, Shiloh-Malawsky Y, Sangha N, Martinez-Lage M, Dalmau J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77(6):589–93. doi: 10.1212/WNL.0b013e318228c136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takuma K, Kamisawa T, Igarashi Y. Autoimmune pancreatitis and IgG4-related sclerosing cholangitis. Curr Opin Rheumatol. 2011;23(1):80–7. doi: 10.1097/BOR.0b013e3283412f60 [DOI] [PubMed] [Google Scholar]
- 62.Krumbholz M, Derfuss T, Hohlfeld R, Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol. 2012;8(11):613–23. doi: 10.1038/nrneurol.2012.203 [DOI] [PubMed] [Google Scholar]
- 63.Butman S, Espinoza LR, Del Carpio J, Osterland CK. Rheumatoid pericarditis. Rapid deterioration with evidence of local vasculitis. JAMA. 1977;238(22):2394–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This dataset contains all raw values that contributed to the analyses within this manuscript organized by diagnosis. Raw flow cytometry data and data on Interleukin-21 levels are included as well as data and calculations highlighted in Tables 1–3 and Figs 1–5.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. The corresponding author can be contacted for further specific information.