Abstract
Purpose
To develop a simple extraction procedure to quantify the uptake of four topical ocular pharmaceutical drugs into contact lenses (CLs).
Methods
Four silicone hydrogel (SH) CLs (balafilcon A, senofilcon A, lotrafilcon B, comfilcon B) and four conventional hydrogel (CH) CLs (nesofilcon A, hilafilcon B, nelfilcon A, etafilcon A) were evaluated. The drugs studied were natamycin, moxifloxacin, timolol maleate, and ketotifen fumarate. For drug incubation, three CLs of each type were placed in 1 mL of 1 mg/mL drug-loading solution for 24 hours. The lenses were then extracted in 2 mL methanol for 2 hours. This process was repeated to obtain a total of three extraction cycles. Detection of natamycin, moxifloxacin, ketotifen fumarate, and timolol maleate were measured by absorbance at 305, 287, 297, and 295 nm, respectively.
Results
The majority of the drugs were extracted after the first extraction cycle (P < 0.001). For moxifloxacin and timolol, CH CLs had higher drug uptake than SH CLs (P < 0.05). There were no differences in drug uptake between CH CLs and SH CLs for natamycin and ketotifen (P > 0.05).
Conclusions
This study provides a simple approach to determine drug uptake into CLs. This method can also be modified, such as changing the extraction time, extraction cycles, or extraction solvent to better suit other drugs and CL combinations.
Translational Relevance
There is considerable interest in using CLs for ocular drug delivery. Accurately quantifying drug uptake on CLs has been a challenge. Hence, this study provides a simple method to quantify drug uptake in CLs.
Keywords: contact lens, drug delivery, drug uptake, natamycin, moxifloxacin, ketotifen fumarate, timolol maleate
Introduction
Over the last decade, there has been considerable interest in using contact lenses (CLs) beyond their intended application in vision correction.1 One area of research that is rapidly gaining traction is ocular drug delivery using CLs.1–19 Drug delivery using CLs was conceptualized to address the key problems associated with conventional therapies to treat eye diseases such as glaucoma,16 infection,6,10,15 myopia,17 allergies,8 and dry eye.18,19
Currently, eye drops and ointments account for over 90% of all ophthalmic formulations for ocular drug delivery.20–23 However, the anatomy of the eye presents several barriers that prevent effective drug delivery via this approach. Factors such as tear dilution,24 blinking,24 and nonspecific absorption20,24 limits residence time and effective drug penetration through the cornea.25 To compensate for low drug bioavailability, frequent dosing can be employed with eye drops, but this practice in turn results in problems with patient compliance26 and overdosing of drugs.27
CL drug delivery provides two main advantages over ophthalmic formulations. The first benefit results from the creation of the postlens tear film generated when a CL is placed on the cornea. This physical CL barrier shields the postlens tear layer from the blinking reflex, while also restricting tear mixing and exchange.28 As a consequence, drugs released into this layer from the CL are trapped and have longer precorneal residence time.29 The second advantage is the ability to engineer the material properties of a CL in such a way that it provides sustained drug release,3,19,30 eliminating the need for multiple dosing.
Understanding the interaction between the drug and the CL material is thus essential to optimizing drug delivery. One important property is the drug partition coefficient,13 which provides an estimate of the concentration of a particular drug in the lens. This information is useful to gauge the effectiveness of a CL material for drug delivery by comparing its drug uptake with its release profile.13 However, measuring drug uptake into a lens is very difficult. So while there are numerous studies on CL drug delivery, far fewer have reported data on drug uptake.2–14,31,32
These studies typically quantify drug uptake into the lens by measuring the concentration of the drug-loading solution at the beginning and the end of the incubation period.2–13,31,32 The difference in drug concentration is then attributed to the amount of drugs sorbed by the lens.2–13,31,32 However, there are some inherent problems with this indirect measurement approach. Firstly, drugs may precipitate out of solution, collect at the bottom of the vial, or deposit onto the side of the vials. As a result, over time a decrease in drug concentration may occur that is not attributed to uptake into the CL. Secondly, the concentrations of the drug-loading solutions are many fold higher than the small amounts of drug that are actually absorbed by the lens.2–14,31,32 Consequently, there are extremely large errors associated with measuring such small changes in concentration in the drug-loading solution.
A more direct approach to measuring CL drug uptake is therefore desired, but very few studies have attempted to quantify drugs in this manner.14,15 One promising method, by Kakisu et al.,15 measured the uptake of two antibiotics, gatifloxacin and moxifloxacin, by soaking drug-containing lenses in methanol for 24 hours. This approach is similar to extraction methods commonly used to quantify protein and lipid deposits on CLs.33–37 To this end, we hypothesize that a modification of this method could be applied to quantify drug uptake on CLs. The purpose of this study is to develop a simple extraction procedure to quantify drug uptake into contemporary CL materials for four commonly used ocular pharmaceutical agents (natamycin, moxifloxacin, timolol maleate, and ketotifen fumarate).
Materials and Methods
Lens Preparation
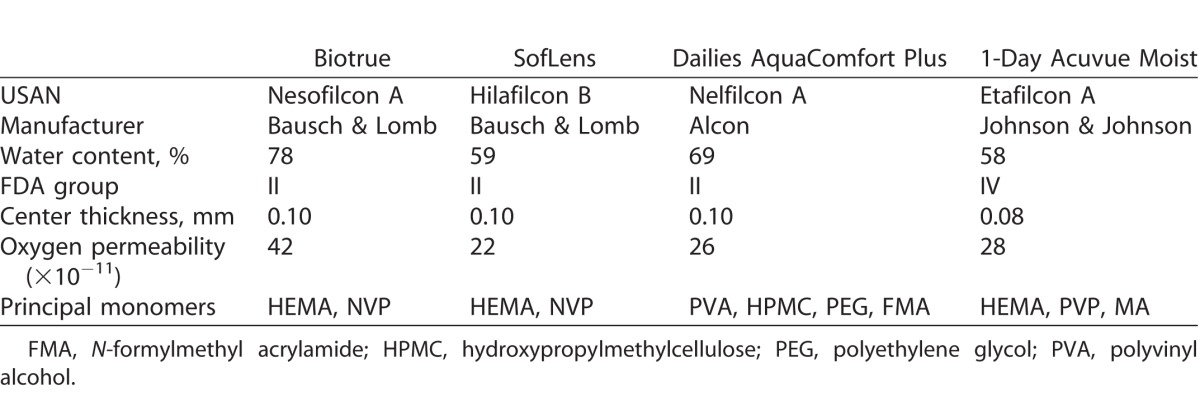
Four commercially available silicone hydrogel (SH) CLs (balafilcon A, Bausch & Lomb, Rochester, NY; senofilcon A, Johnson & Johnson, Jacksonville, FL; lotrafilcon B, Alcon, Fort Worth, TX; comfilcon B, CooperVision, Pleasanton, CA) and four conventional hydrogel (CH) CLs (nesofilcon A, Bausch & Lomb; hilafilcon B, Bausch & Lomb; nelfilcon A, Alcon; etafilcon A, Johnson & Johnson) were evaluated in the study. All lenses had an 8.6-mm base curve, and −3.00 dioptric power and were obtained from the manufacturer in the original packaging. Tables 1 and 2 detail the properties of the SH and CH CLs, respectively.
Table 1.
Properties of SHs Used in the Study
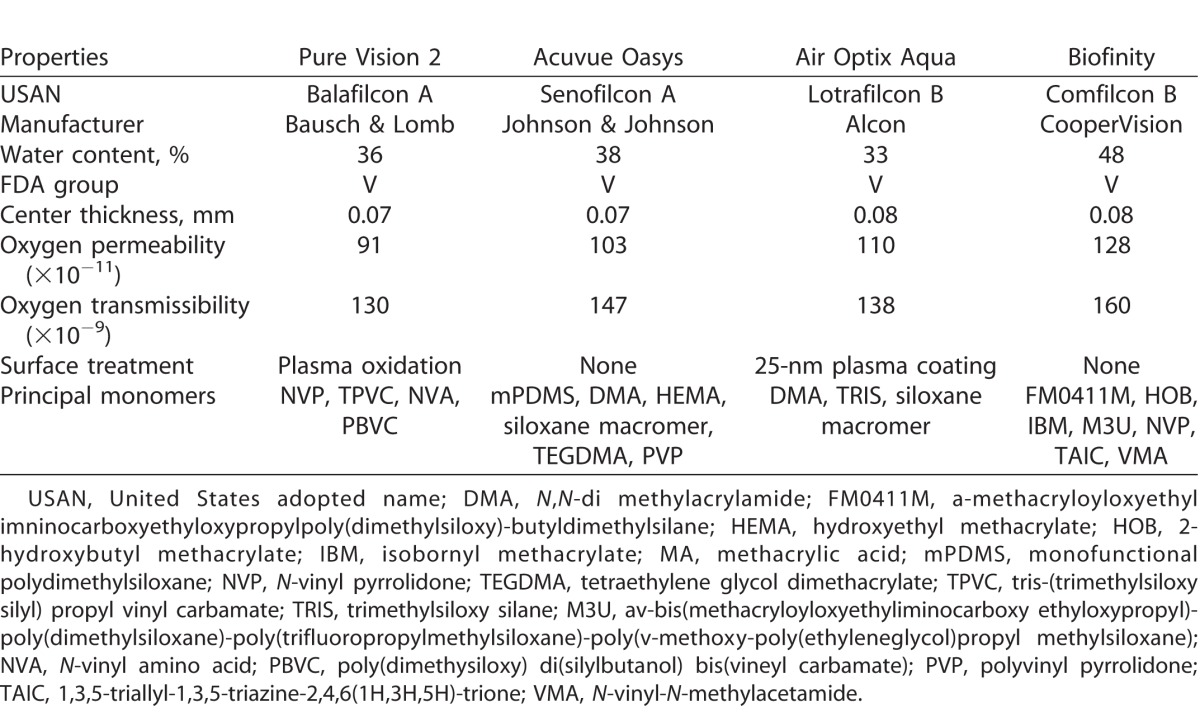
Table 2.
Properties of CHs Used in the Study
Three CLs of each type were removed from their original packaging and placed in 5 mL PBS in a 12-well clear plate (VWR International, Missauga, Ontario, Canada) and gently shaken for 24 hours at room temperature to remove any packaging solution. The lenses were then placed in 1 mL of 1 mg/mL drug-loading solution.
Drug Incubation Solution
Natamycin, ketotifen fumarate, and timolol maleate were purchased from Sigma-Aldrich Corp. (Oakville, Ontario, Canada), and moxifloxacin was purchased from Selleckchem (Houston, TX). After the initial preparation procedure, the lenses were incubated in 1 mL of 1 mg/mL drug solution in phosphate buffered saline (PBS), pH 7.4, for 24 hours. Studies with natamycin and moxifloxacin were performed in light-minimizing conditions. With the exception of natamycin, which was prepared as a suspension, all other drugs completely dissolved in solution.
Detection
Detection of natamycin,3,6 moxifloxacin,38 ketotifen fumarate,8 and timolol maleate,39 was measured at 305, 287, 297, and 295 nm, respectively, in a UV-star 96-well microplate (Greiner Bio-One, Monroe, NC) using a spectrophotometer (SpectraMax M5 UV-Vis; Molecular Devices, Sunnyvale, CA).
Negative Control
Three lenses of each type were extracted in methanol for 48 hours. After the extraction period, an absorbance spectrum of the lens extracts for each CL was determined between 210 and 350 nm in 1-nm increments. The CL extracts were then evaporated using nitrogen purge for 1 hour. The extracts were resuspended in 250 μL methanol, and another absorbance spectrum was determined between 210 and 350 nm.
Standard Curve
A standard curve for each drug was generated by diluting the drug concentrations to a range of 0 to 1 mg/mL in methanol, and only the linear portion was used as the range of detection. The generated curves had a correlation coefficient above 99.5%. The concentration of the drugs in the samples was determined by subtracting the background absorbance of the negative control divided by the slope obtained from the standard curve.
Extraction Cycles
After the 24-hour drug incubation time, the lenses were removed from their drug-loading solution and air-dried for 10 seconds before being placed into 2 mL methanol extraction solvent. The vials were capped, covered in parafilm to avoid any evaporation, and placed on an orbital shaker at room temperature for 2 hours. After 2 hours, the lenses were removed from their vials and placed in 2 mL fresh methanol. This process was repeated for a total of three extraction cycles.
The concentration of the extracted drugs from the first extraction solution was determined by absorbance using the SpectraMax M5 UV-Vis spectrophotometer. The solution was diluted to the linear concentration ranges whenever necessary. The second and third extraction solutions were dried down using a nitrogen purge for approximately 1 hour. The extracted drugs were resuspended in 250 μL methanol. Of this solution, 200 μL was then pipetted into a 96-well UV-star microplate, and the samples were analyzed using the spectrophotometer.
Statistical Analysis
Statistical analysis was performed using statistical software (Statistica 8; StatSoft, Tulsa, OK). All data are reported as mean ± SD for n = 3. A repeated analysis of variance (ANOVA) was performed to determine the differences across extraction cycles. A 1-way ANOVA was used to determine the differences between lens types. Post-hoc Tukey multiple comparison tests were used when necessary. An unpaired t-test was used to compare the differences between the uptake of drug between CH CLs and SH CLs. In all cases, statistical significance was considered significant for a value of P < 0.05. Graphs were plotted using software (GraphPad Prism 6; GraphPad, La Jolla, CA).
Results
Tables 3–6 show the uptake of drugs on SH and conventional SH CLs incubated in a 1 mg/mL drug solution. Over 90% of total amount of extractable drug from the lenses was extracted with the first extraction cycle for most combinations of CL types and drugs tested (P < 0.001; Figs. 1–4). For moxifloxacin and timolol, CH CLs absorbed more drug than SH CLs (P < 0.05). There were no differences in drug uptake between CH CLs and SH CLs for natamycin and ketotifen (P > 0.05).
Table 3.
Uptake of Natamycin (μg) on SH and CH CLs Incubated in a 1-mg/mL Drug Solution
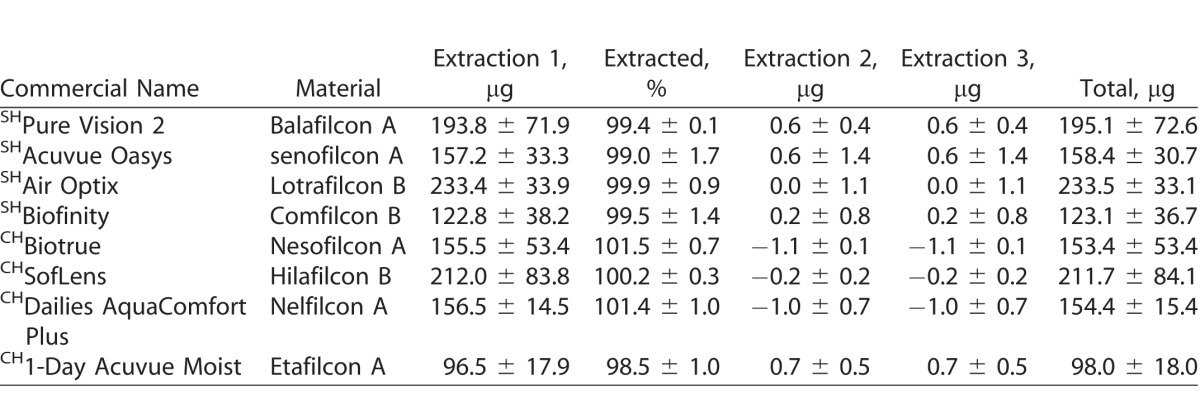
Table 6.
Uptake of Timolol (μg) on SH and CH CLs Incubated in a 1-mg/mL Drug Solution
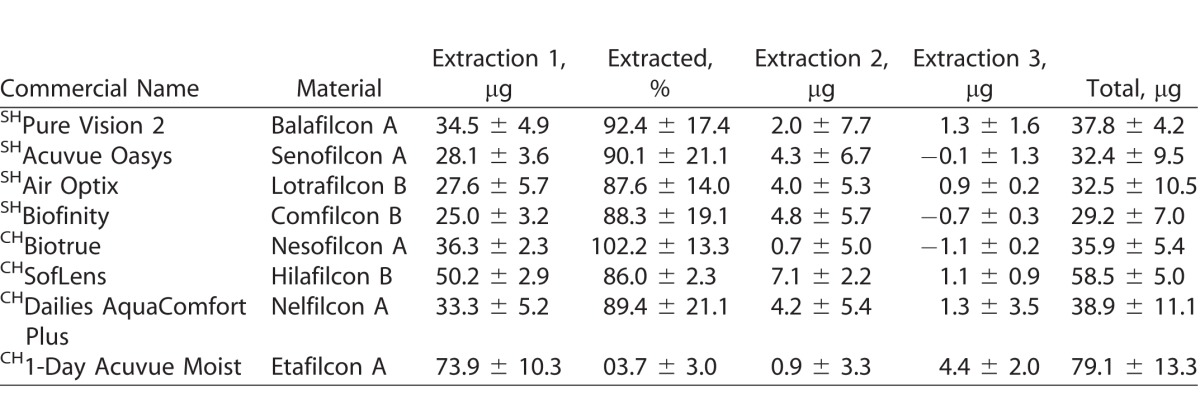
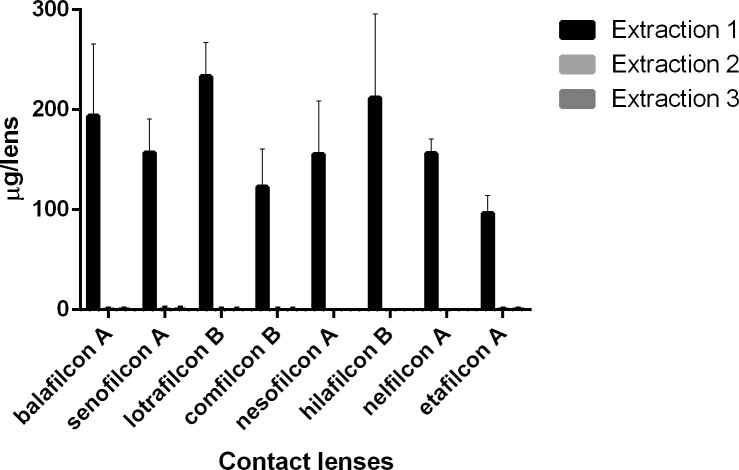
Figure 1.
Natamycin (μg) extracted from SH and CH CLs incubated in a 1-mg/mL drug solution.
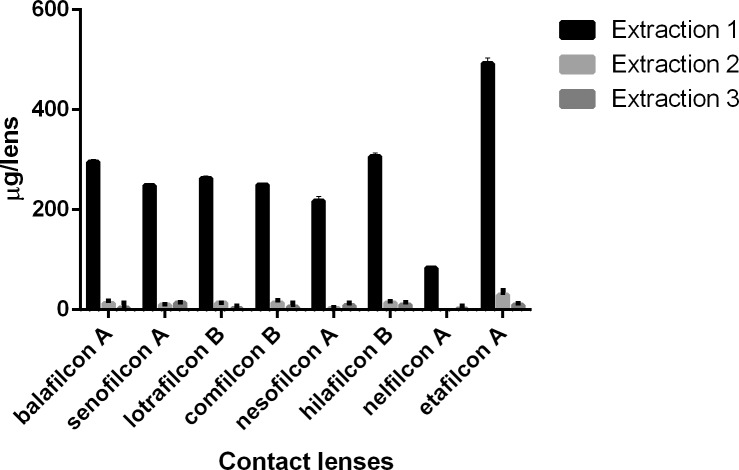
Figure 4.
Timolol (μg) extracted from SH and CH CLs incubated in a 1-mg/mL drug solution.
Table 4.
Uptake of Moxifloxacin (μg) on SH and CH CLs Incubated in a 1-mg/mL Drug Solution
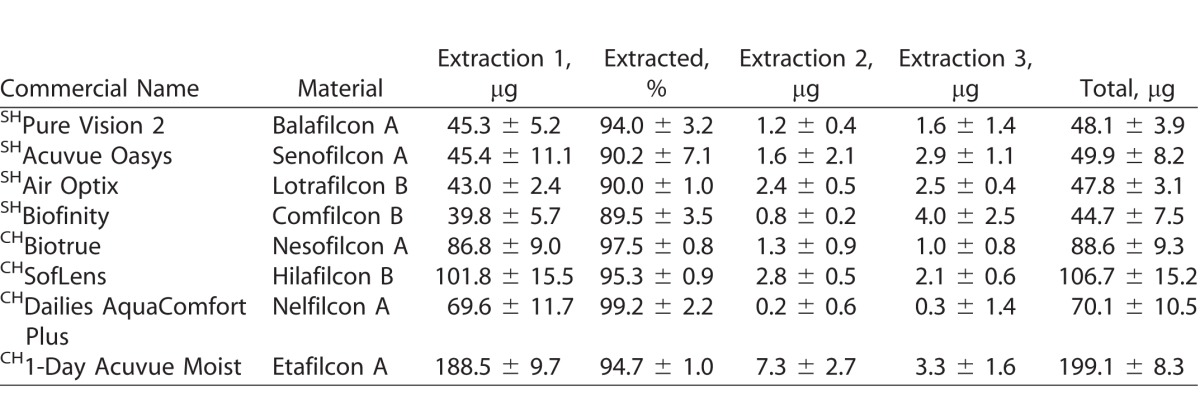
Table 5.
Uptake of Ketotifen (μg) on SH and CH CLs Incubated in a 1-mg/mL Drug Solution
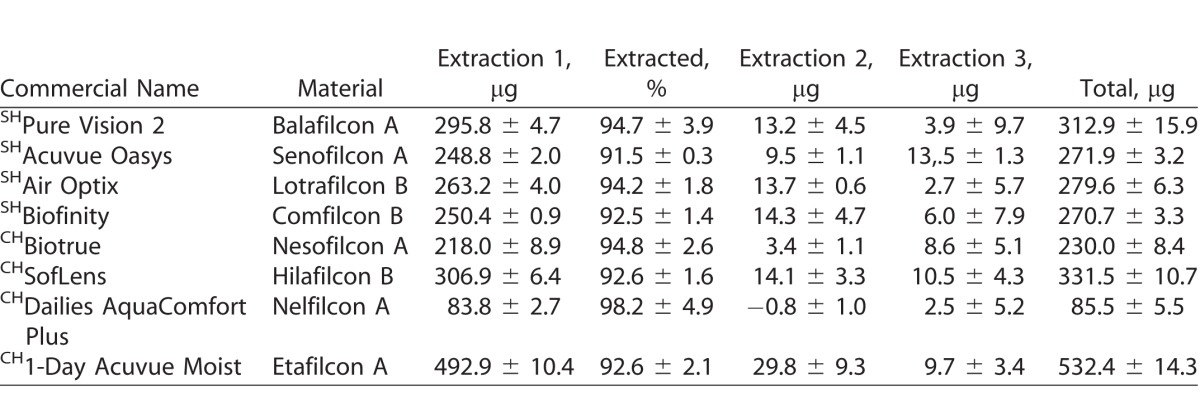
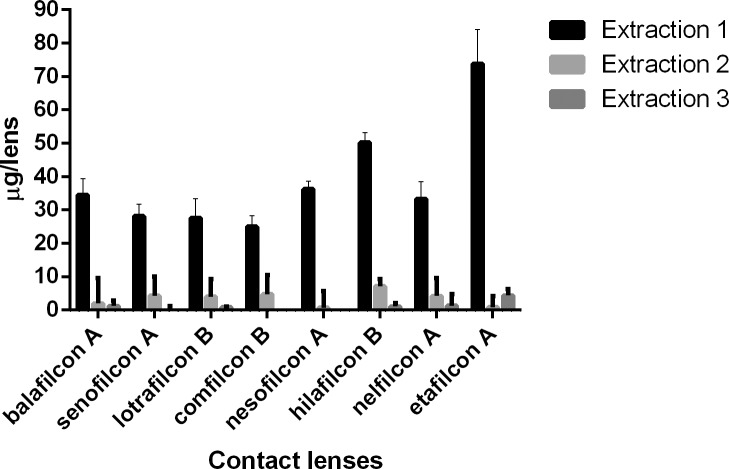
Figure 2.
Moxifloxacin (μg) extracted from SH and CH CLs incubated in a 1-mg/mL drug solution.
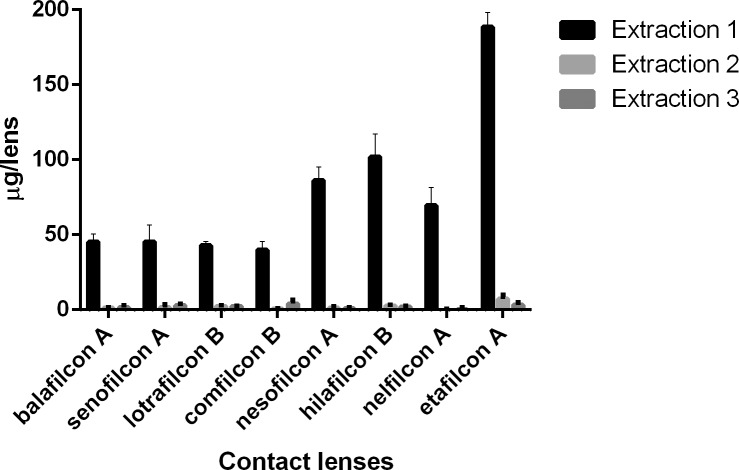
Figure 3.
Ketotifen (μg) extracted from SH and CH CLs incubated in a 1-mg/mL drug solution.
For natamycin, the CLs with the highest and lowest drug uptake were hilafilcon B (211.7 ± 84.1 μg/lens) and etafilcon A (98.0 ± 18.0 μg/lens), respectively (P < 0.001). For moxifloxacin, the CL with highest drug uptake was etafilcon A (199.1 ± 8.3 μg/lens), while comfilcon B had the lowest uptake (44.7 ± 7.5 μg/lens) (P < 0.001). The highest drug uptake for ketotifen was for etafilcon A (532.4 ± 14.3 μg/lens), and the lowest was for nelfilcon A (85.5 ± 5.5 μg/lens) (P < 0.001). Etafilcon A (79.1 ± 13.3 μg/lens) also had the highest uptake of timolol, while senofilcon A (32.4 ± 9.5 μg/lens) had the lowest uptake of the drug (P < 0.001).
All CLs swelled in methanol during the extraction phase, with the exception of nelfilcon A, which shriveled. Pure CL extracts had high absorbance at wavelengths below 270 nm, so detection of drugs with lower absorbance wavelengths is unreliable with methanol extraction.
Discussion
The objective of this study was to develop a simple direct method to quantify the amount of drug uptake in commercial CLs. Four drugs, natamycin, moxifloxacin, ketotifen, and timolol, were investigated. These drugs have been used to study a range of potential ocular therapies using CL drug delivery.3,6,8,16,38,40 A simple extraction approach was developed using methanol as the extraction solvent. The method consists of incubating CLs in methanol for 2 hours to remove the bound drugs, and this was repeated for a total of three cycles.
For moxifloxacin and timolol, CH CLs absorbed more drugs than SH CLs (P < 0.05). These results have been observed in previous studies with moxifloxacin.38,40 There were no significant differences in uptake between CH CLs and SH CLs observed for natamycin and ketotifen fumarate (P > 0.05), which are also similar to previously published results.6,8
Natamycin is an antifungal drug used to treat superficial fungal eye infections.41 A previous study examined the uptake of natamycin in CH and SH hydrogels incubated in 20-mL suspension of 30 μg/mL of drug (600 μg/vial).3 The drug uptake, as measured indirectly by the concentration changes in the incubation solution, was between 37.9 ± 19.7 and 176.9 ± 79.0 μg/gel.3 These results are similar to those obtained in this study when corrected for the total amount of natamycin in the incubation solution (current study: 1000 μg/vial), with total drug uptake between 98.0 ± 18.0 and 211.7 ± 84.1 μg/lens.
Moxifloxacin is an important antimicrobial agent for treating a variety of ocular surface diseases.42 The drug is very hydrophilic, so the majority of the drugs bound in the lenses should also be released subsequently in an aqueous medium. The release of moxifloxacin from commercial CLs incubated in a 1 mg/mL drug solution has been previously reported.38 Nelfilcon A (Dailies AquaComfort Plus; Alcon) and etafilcon A (1-Day Acuvue Moist; Johnson & Johnson) released 37 ± 4 and 226 ± 2 μg/lens, respectively.38 These results are in fairly good agreement with the results obtained in this study for nelfilcon A (70.1 ± 10.5 μg/lens) and etafilcon A (199.1 ± 8.3 μg/lens).
Ketotifen fumarate is a drug used to alleviate the signs and symptoms of seasonal allergic conjunctivitis.43 A previous study reported the uptake of ketotifen fumarate in CLs incubated in 6 mL of 0.25 mg/mL (1250 μg/vial) ketotifen fumarate.8 Six out of the eight CLs examined in the previous study were examined in this study. In general, the results for this study (85.5 ± 5.5 to 532.4 ± 14.3 μg/lens) and the previous work (223.52 ± 14.33 to 515.85 ± 47.29 μg/lens)8 are in very good agreement when corrected for the total amount of drug in the incubation solution. The only major difference between the two studies is the amount of drug uptake reported for nelfilcon A (Dailies AquaComfort Plus). Previously, the drug uptake for nelfilcon A was reported at 223.52 ± 14.33 μg/lens, with a total release of only 40.36 ± 14.33 μg/lens, or 18.1% ± 1.4%.8 Considering that the drug release percentage for all the other lenses for that particular study was between 30.1% ± 5% and 61.7% ± 7.2%, the amount of drug uptake for nelfilcon A may have been overestimated. The amount of drug uptake for nelfilcon A in this study at 85.5 ± 5.5 μg/lens would correspond more closely to the drug release previously published.
Timolol is a drug used to lower intraocular pressure for the treatment of glaucoma.44 A previous study reported the uptake of timolol in 2 mL of 500 μg/mL (1000 μg/vial) for poly-2-hydroxyethyl methacrylate (HEMA) CLs, a CH material, at 113.58 ± 1.54 μg/lens.31 In comparison to the previous study, the amount of uptake for CH CLs in this study was slightly less, ranging between 35.9 ± 5.4 μg/lens and 79.1 ± 13.3 μg/lens of drug uptake for HEMA-containing lenses. The higher drug uptake previously reported could be attributed to the differences in the CL polymer. The commercial CLs tested in this study had a variety of other polymers in addition to HEMA, which could reduce the uptake of timolol maleate.
After the first extraction cycle for 2 hours in methanol, 90% of extractable drug was obtained for most combinations of lens types and drugs tested. Less than 10% of the total amount of extractable drugs was extracted in subsequent extraction cycles. This suggests that one extraction cycle at 2 hours with methanol is sufficient to estimate over 90% of the drug uptake in CLs. Thus, this method could provide a rapid and direct approach to quantify the amount of drug uptake in CLs. Two extraction cycles are sufficient to remove the majority of the drugs bound on the CLs. These extraction results are in consensus with other studies that quantify proteins and lipids from CLs using extraction methods.33–37
In theory, the extraction solvent should have a drug solubility equal to or higher than the release medium, should not react with the drug, should have a low boiling point so that it can be evaporated readily, and should be relatively inexpensive.45 For these reasons, methanol was chosen as the extraction solvent for this study. Furthermore, methanol also has the effect of swelling the CLs, which helps facilitate the drug extraction process. With the exception of nelfilcon A, all lenses tested in this study swelled when exposed to methanol. For drugs insoluble in methanol, an alternative solvent that has high solubility for the drug of interest can be used. The selection of the right solvent is important in ensuring a high extraction efficiency of the drug from the CL.
Indirect methods that measure the changes in the concentration of the drug-loading solution are subjected to various inaccuracies, such as drug precipitation or deposition onto vials, which can overestimate the amount of drug uptake. Furthermore, to measure small changes in a high drug-loading solution requires very sensitive instrumentation and precise handling and sample preparation, which potentially increase the errors with measurements. Unlike previous indirect approaches, this extraction method measures drug uptake directly from the CL. This method can also be modified, such as increasing the extraction time, increasing the cycles of extractions, or changing the extraction solvent as necessary to better suit other drug and CL combinations.
There are limitations that should be noted using this approach. First, methanol as an extraction solvent has an effect of also extracting other components from the CL polymer due to its swelling effect. As a result, depending on the CL properties, the lens extracts may cause spectral interferences. Consequently, a negative control with only the lens extracts for each CL type should be used as the background. The interference problem can be solved by using more advanced analytical methods based on multicomponent analysis or using high-performance liquid chromatography to separate the samples prior to UV spectrometry.
The second limitation is that, unlike the indirect approach in which the same lenses are used in both the uptake study and the release study, this approach requires two sets of lenses. The set of lenses measured for the uptake study is not the same as the one used for the release study. Consequently, experimental conditions for uptake and release studies need to be the same to ensure comparable results.
The results of this study suggest that both indirect and direct approaches to measuring drug uptake in CLs provide comparable results. However, in cases where the concentrations of the drug-loading solution are very high or when the amounts of drugs released from the lens are relatively low, the direct approach may provide a better estimate of drug uptake. Furthermore, the direct approach is less sensitive to errors with instrumentation, sample preparation, and handling. For a simple and rapid estimate of the drug uptake in a CL in this study, a 2-hour methanol extraction of the CL after the drug incubation period was sufficient. Future studies will examine alternative extraction solvents to measure the uptake of other drugs on CLs.
Acknowledgments
Disclosure: C.-M. Phan, None; S. Weber, None; J. Mueller, None; A. Yee, None; L. Jones, None
References
- 1. Jones LW, Byrne M, Ciolino JB,et al. . Revolutionary future uses of contact lenses. Optom Vis Sci. 2016; 93: 325– 327. [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Millan E, Koprivnik S, Otero-Espinar FJ. . Drug loading optimization and extended drug delivery of corticoids from pHEMA based soft contact lenses hydrogels via chemical and microstructural modifications. Int J Pharm. 2015; 487: 260– 269. [DOI] [PubMed] [Google Scholar]
- 3. Phan CM, Subbaraman L, Liu S, Gu F, Jones L. . In vitro uptake and release of natamycin Dex-b-PLA nanoparticles from model contact lens materials. J Biomater Sci Polym Ed. 2014; 25: 18– 31. [DOI] [PubMed] [Google Scholar]
- 4. Phan CM, Bajgrowicz M, Gao H, Subbaraman LN, Jones LW. . Release of fluconazole from contact lenses using a novel in vitro eye model. Optom Vis Sci. 2016; 93: 387– 394. [DOI] [PubMed] [Google Scholar]
- 5. Guidi G, Hughes TC, Whinton M, Brook MA, Sheardown H. . The effect of silicone hydrogel contact lens composition on dexamethasone release. J Biomater Appl. 2014; 29: 222– 233. [DOI] [PubMed] [Google Scholar]
- 6. Phan CM, Subbaraman LN, Jones L. . In vitro uptake and release of natamycin from conventional and silicone hydrogel contact lens materials. Eye Contact Lens. 2013; 39: 162– 168. [DOI] [PubMed] [Google Scholar]
- 7. Jones L, Powell CH. . Uptake and release phenomena in contact lens care by silicone hydrogel lenses. Eye Contact Lens. 2013; 39: 29– 36. [DOI] [PubMed] [Google Scholar]
- 8. Soluri A, Hui A, Jones L. . Delivery of ketotifen fumarate by commercial contact lens materials. Optom Vis Sci. 2012; 89: 1140– 1149. [DOI] [PubMed] [Google Scholar]
- 9. Schultz CL, Morck DW. . Contact lenses as a drug delivery device for epidermal growth factor in the treatment of ocular wounds. Clin Exp Optom. 2010; 93: 61– 65. [DOI] [PubMed] [Google Scholar]
- 10. Hui A, Boone A, Jones L. . Uptake and release of ciprofloxacin-HCl from conventional and silicone hydrogel contact lens materials. Eye Contact Lens. 2008; 34: 266– 271. [DOI] [PubMed] [Google Scholar]
- 11. Boone A, Hui A, Jones L. . Uptake and release of dexamethasone phosphate from silicone hydrogel and group I, II, and IV hydrogel contact lenses. Eye Contact Lens. 2009; 35: 260– 267. [DOI] [PubMed] [Google Scholar]
- 12. Tian X, Iwatsu M, Sado K, Kanai A. . Studies on the uptake and release of fluoroquinolones by disposable contact lenses. CLAO J. 2001; 27: 216– 220. [PubMed] [Google Scholar]
- 13. Pimenta AF, Ascenso J, Fernandes JC, Colaco R, Serro AP, Saramago B. . Controlled drug release from hydrogels for contact lenses: drug partitioning and diffusion. Int J Pharm. 2016; 515: 467– 475. [DOI] [PubMed] [Google Scholar]
- 14. Mahomed A, Tighe BJ. . The design of contact lens based ocular drug delivery systems for single-day use: structural factors, surrogate ophthalmic dyes and passive diffusion studies. J Biomater Appl. 2014; 29 1, pt 3: 341– 353. [DOI] [PubMed] [Google Scholar]
- 15. Kakisu K, Matsunaga T, Kobayakawa S, Sato T, Tochikubo T. . Development and efficacy of a drug-releasing soft contact lens. Invest Ophthalmol Vis Sci. 2013; 54: 2551– 2561. [DOI] [PubMed] [Google Scholar]
- 16. Peng CC, Ben-Shlomo A, Mackay EO, Plummer CE, Chauhan A. . Drug delivery by contact lens in spontaneously glaucomatous dogs. Curr Eye Res. 2012; 37: 204– 211. [DOI] [PubMed] [Google Scholar]
- 17. Lasowski F, Sheardown H. . Atropine and roscovitine release from model silicone hydrogels. Optom Vis Sci. 2016; 93: 404– 411. [DOI] [PubMed] [Google Scholar]
- 18. White CJ, DiPasquale SA, Byrne ME. . Controlled release of multiple therapeutics from silicone hydrogel contact lenses. Optom Vis Sci. 2016; 93: 377– 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu KH, de la Jara PL, Ariyavidana A,et al. . Release of betaine and dexpanthenol from vitamin E modified silicone-hydrogel contact lenses. Curr Eye Res. 2015; 40: 267– 273. [DOI] [PubMed] [Google Scholar]
- 20. Le Bourlais C, Acar L, Zia H, Sado PA, Needham T, Leverge R. . Ophthalmic drug delivery systems–recent advances. Prog Retin Eye Res. 1998; 17: 33– 58. [DOI] [PubMed] [Google Scholar]
- 21. Saettone MF. . Progress and problems in ophthalmic drug delivery. Pharmatech. 2002: 1– 6.
- 22. Urtti A. . Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006; 58: 1131– 1135. [DOI] [PubMed] [Google Scholar]
- 23. Kumar A, Malviya R, Sharma PK. . Recent trends in ocular drug delivery: a short review. Eur J Appl Sci. 2011; 3: 86– 92. [Google Scholar]
- 24. Chrai SS, Makoid MC, Eriksen SP, Robinson JR. . Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci. 1974; 63: 333– 338. [DOI] [PubMed] [Google Scholar]
- 25. Ghate D, Edelhauser HF. . Barriers to glaucoma drug delivery. J Glaucoma. 2008; 17: 147– 156. [DOI] [PubMed] [Google Scholar]
- 26. Gurwitz JH, Glynn RJ, Monane M,et al. . Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993; 83: 711– 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trawick AB. . Potential systemic and ocular side effects associated with topical administration of timolol maleate. J Am Optom Assoc. 1985; 56: 108– 112. [PubMed] [Google Scholar]
- 28. Lin MC, Soliman GN, Lim VA,et al. . Scalloped channels enhance tear mixing under hydrogel contact lenses. Optom Vis Sci. 2006; 83: 874– 878. [DOI] [PubMed] [Google Scholar]
- 29. Li CC, Chauhan A. . Modeling ophthalmic drug delivery by soaked contact lenses. Ind Eng Chem Res. 2006; 45: 3718– 3734. [Google Scholar]
- 30. Hui A, Willcox M, Jones L. . In vitro and in vivo evaluation of novel ciprofloxacin-releasing silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2014; 55: 4896– 4904. [DOI] [PubMed] [Google Scholar]
- 31. Maulvi FA, Soni TG, Shah DO. . Effect of timolol maleate concentration on uptake and release from hydrogel contact lenses using soaking method. J Pharm Appl Sci. 2014; 1: 17– 23. [Google Scholar]
- 32. Hiratani H, Alvarez-Lorenzo C. . Timolol uptake and release by imprinted soft contact lenses made of N, N-diethylacrylamide and methacrylic acid. J Control Release. 2002; 83: 223– 230. [DOI] [PubMed] [Google Scholar]
- 33. Pucker AD, Thangavelu M, Nichols JJ. . In vitro lipid deposition on hydrogel and silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010; 51: 6334– 6340. [DOI] [PubMed] [Google Scholar]
- 34. Babaei Omali N, Zhu H, Zhao Z,et al. . Effect of cholesterol deposition on bacterial adhesion to contact lenses. Optom Vis Sci. 2011; 88: 950– 958. [DOI] [PubMed] [Google Scholar]
- 35. Heynen M, Lorentz H, Srinivasan S, Jones L. . Quantification of non-polar lipid deposits on senofilcon a contact lenses. Optom Vis Sci. 2011; 88: 1172– 1179. [DOI] [PubMed] [Google Scholar]
- 36. Bontempo AR, Rapp J. . Protein and lipid deposition onto hydrophilic contact lenses in vivo. CLAO J. 2001; 27: 75– 80. [PubMed] [Google Scholar]
- 37. Jones L, Senchyna M, Glasier MA,et al. . Lysozyme and lipid deposition on silicone hydrogel contact lens materials. Eye Contact Lens. 2003; 29: S75– 79; discussion S83,– 84, S192– 194. [DOI] [PubMed] [Google Scholar]
- 38. Bajgrowicz M, Phan CM, Subbaraman LN, Jones L. . Release of ciprofloxacin and moxifloxacin from daily disposable contact lenses from an in vitro eye model. Invest Ophthalmol Vis Sci. 2015; 56: 2234– 2242. [DOI] [PubMed] [Google Scholar]
- 39. Korogiannaki M, Guidi G, Jones L, Sheardown H. . Timolol maleate release from hyaluronic acid-containing model silicone hydrogel contact lens materials. J Biomater Appl. 2015; 30: 361– 376. [DOI] [PubMed] [Google Scholar]
- 40. Phan CM, Bajgrowicz-Cieslak M, Subbaraman LN, Jones L. . Release of moxifloxacin from contact lenses using an in vitro eye model: impact of artificial tear fluid composition and mechanical rubbing. Transl Vis Sci Technol. 2016; 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaur IP, Rana C, Singh H. . Development of effective ocular preparations of antifungal agents. J Ocul Pharmacol Ther. 2008; 24: 481– 493. [DOI] [PubMed] [Google Scholar]
- 42. Smith A, Pennefather PM, Kaye SB, Hart CA. . Fluoroquinolones: place in ocular therapy. Drugs. 2001; 61: 747– 761. [DOI] [PubMed] [Google Scholar]
- 43. Greiner JV, Mundorf T, Dubiner H,et al. . Efficacy and safety of ketotifen fumarate 0.025% in the conjunctival antigen challenge model of ocular allergic conjunctivitis. Am J Ophthalmol. 2003; 136: 1097– 1105. [DOI] [PubMed] [Google Scholar]
- 44. Brooks AM, Gillies WE. . Ocular beta-blockers in glaucoma management. Clinical pharmacological aspects. Drugs Aging. 1992; 2: 208– 221. [DOI] [PubMed] [Google Scholar]
- 45. Williamson KL, Masters KM. . Macroscale and Microscale Organic Experiments. Boston, MA: Cengage Learning; 2010. [Google Scholar]