ABSTRACT
Epithelial progenitor cells are dependent upon a complex 3D niche to promote their proliferation and differentiation during development, which can be recapitulated in organoids. The specific requirements of the niche remain unclear for many cell types, including the proacinar cells that give rise to secretory acinar epithelial cells that produce saliva. Here, using ex vivo cultures of E16 primary mouse submandibular salivary gland epithelial cell clusters, we investigated the requirement for mesenchymal cells and other factors in producing salivary organoids in culture. Native E16 salivary mesenchyme, but not NIH3T3 cells or mesenchymal cell conditioned medium, supported robust protein expression of the progenitor marker Kit and the acinar/proacinar marker AQP5, with a requirement for FGF2 expression by the mesenchyme. Enriched salivary epithelial clusters that were grown in laminin-enriched basement membrane extract or laminin-111 together with exogenous FGF2, but not with EGF, underwent morphogenesis to form organoids that displayed robust expression of AQP5 in terminal buds. Knockdown of FGF2 in the mesenchyme or depletion of mesenchyme cells from the organoids significantly reduced AQP5 levels even in the presence of FGF2, suggesting a requirement for autocrine FGF2 signaling in the mesenchyme cells for AQP5 expression. We conclude that basement membrane proteins and mesenchyme cells function as niche factors in salivary organoids.
KEY WORDS: Mesenchyme, Submandibular salivary gland, FGF2, Organoid, Progenitor
Summary: FGF2-dependent primary salivary mesenchyme and laminin-111 provide niche functions in salivary organoids to promote epithelial morphogenesis and expression of the early secretory proacinar markers Kit and AQP5.
INTRODUCTION
Salivary glands, like many other organs, including the mammary gland, lacrimal gland, lung and prostate undergo branching morphogenesis to generate an arborized organ containing a large surface area within a relatively small space. Epithelial progenitor cells undergo branching morphogenesis and differentiation to produce the functional adult organ. While many studies have demonstrated that epithelial rudiments lacking mesenchyme can partially recapitulate normal developmental processes, the epithelial cells are not autonomous. Early experiments performed by Borghese in 1950 using embryonic submandibular salivary gland (SMG) organ explants (Borghese, 1950a) demonstrated that the mesenchyme is critical for the early development of the gland. Subsequent studies revealed that fibroblast growth factors (FGFs) are required for branching morphogenesis with FGFs being provided by the mesenchyme (De Moerlooze et al., 2000; Hoffman et al., 2002; Jaskoll et al., 2004, 2005; Ohuchi et al., 2000). Recent work demonstrated that while the epithelium directs early salivary gland development, FGF expression by the mesenchyme later directs bud development (Wells et al., 2013). Although epithelial rudiments can undergo branching morphogenesis in the absence of mesenchyme, under these conditions, the function of the mesenchyme must be recapitulated, which can be accomplished by growth of the epithelium in laminin-rich basement membrane extracts together with growth factors (Rebustini and Hoffman, 2009; Sequeira et al., 2010; Steinberg et al., 2005), consistent with the requirement for mesenchymal factors and a complex 3D environment for support of early morphogenesis events.
Progenitor cells are required for elaboration of mature organs. Kit (CD117), a receptor tyrosine kinase that is well known as a marker of hematopoietic progenitor cells (Kent et al., 2008), is used as a marker of salivary gland epithelial progenitor cells, although lineage tracking in the salivary gland has not been reported. Kit protein is expressed in the distal tips of cells in early embryos [i.e. embryonic day (E)14] and is expressed in cells distinct from those expressing the basal progenitor marker cytokeratin 5 (K5; cytokeratins are also known as KRT proteins) at E16 (Nelson et al., 2013). Kit+ cells are of therapeutic interest since when they are implanted in vivo, they can restore gland function (Lombaert et al., 2008; Nanduri et al., 2013; Pringle et al., 2016). In early embryos, Kit+ salivary epithelial cells are stimulated to proliferate in an FGF10- and FGFR2b-dependent manner (FGFR2b is an isoform of FGFR2). Mesenchymal FGF10 stimulates SOX9 expression in the epithelium to specify the distal epithelial cells as distinct from the proximal epithelial cells prior to the initiation of branching morphogenesis (Chatzeli et al., 2017). Combined Kit and FGFR2b signaling together positively regulate Kit+ K14+ distal progenitor epithelial cells (Patel et al., 2014). Kit ligand produced by isolated epithelium has been shown to be required for an autocrine-mediated expansion of Kit+ K14+ distal progenitor cells in early developing glands (Lombaert et al., 2013), although Kit ligand is more highly expressed at the RNA level in the E13 mesenchyme (https://sgmap.nidcr.nih.gov/). While the evolution of acinar progenitor cells is incompletely understood, AQP5 mRNA, the earliest known proacinar gene, first appears at E14 at the center of distal end buds (https://sgmap.nidcr.nih.gov/). Robust membrane-localized AQP5 protein production is detected by E15, which becomes progressively apically enriched by E16 in many cells that are Kit+ (Nelson et al., 2013). Although it is known that AQP5 mRNA expression is inhibited by Wnt signaling, which promotes K14 expression (Matsumoto et al., 2016), how Kit and/or AQP5 expression is maintained is incompletely understood.
Organoids are 3D assemblies of multiple cell types that can be used to model and decipher mechanisms required for tissue organization (Bissell, 2017; Clevers, 2016; Simian et al., 2001; Simian and Bissell, 2017). The term organoid was first used to describe branching 3D structures generated from clusters of mammary gland cells (Simian et al., 2001), but the term has now been expanded to encompass multiple techniques for forming 3D organ-like structures, ranging from primary explants of tissue fragments to epithelial–mesenchymal co-cultures to clonal derivatives of primary epithelial stem cells (Kretzschmar and Clevers, 2016; Shamir and Ewald, 2014; Simian and Bissell, 2017). Organoids self-organize in vitro when provided with niche factors that facilitate their organization using processes that in part resemble the normal developmental progression that occurs during organogenesis in vivo (Lancaster and Knoblich, 2014). We previously demonstrated that dissociated E13 primary embryonic SMG cells can self-organize to form organoid-like structures that initiate branching morphogenesis and differentiation (Wei et al., 2007). Subsequent studies demonstrated that organoids referred to as ‘organ germs’ derived from E13 embryonic salivary gland cells can undergo functional differentiation when implanted in vivo (Ogawa et al., 2013), similar to other organs (Hirayama et al., 2013; Ikeda and Tsuji, 2008; Takebe et al., 2015; Xinaris et al., 2012). Implantation of adult mouse salivary gland stem cells restored gland function when implanted into irradiated glands (Nanduri et al., 2011, 2014; Pringle et al., 2011), demonstrating the potential for future clinical application of organoids for regenerative medicine. Organoids derived from single human pluripotent stem cells can be directed to differentiate in an organ-specific manner with a stepwise application of specific combinations of growth regulators (Sato and Clevers, 2015). While directed differentiation of pluripotent stem cells is possible for many organs, knowledge of how specific niche factors facilitate formation and differentiation of salivary gland organoids is lacking.
Here, we produce complex mouse SMG organoids derived from E16 mouse primary epithelial and mesenchymal cells with the intent of defining the properties of the microenvironment that are required to stimulate and maintain proacinar differentiation. Since the percentage of epithelial cells that are Kit+ peaks at E16 in mouse submandibular glands (Lombaert et al., 2013; Nelson et al., 2013), and many cells express the proacinar marker AQP5 at this stage, we used E16 epithelial clusters to generate salivary organoids. We tested the requirement for mesenchyme in the salivary gland organoids and demonstrate that primary salivary mesenchyme can support formation of robust branching salivary organoids that we define as proacinar organoids based on expression of Kit and AQP5 proteins. FGF2 expression by the mesenchyme is critical for its niche function in these organoids, but FGF2 functions in an autocrine manner and does not stimulate the epithelium in the absence of mesenchyme. FGF2 and laminin-111 (laminin comprising α1, β1 and γ1 chains) stimulate branching and proacinar differentiation in salivary gland organoids in the presence, but not in the absence, of E16 salivary mesenchyme cells, demonstrating the importance of mesenchymal cells as a component of the submandibular salivary proacinar cell niche.
RESULTS
Primary embryonic mesenchyme supports salivary organoid formation with robust AQP5 expression in co-culture
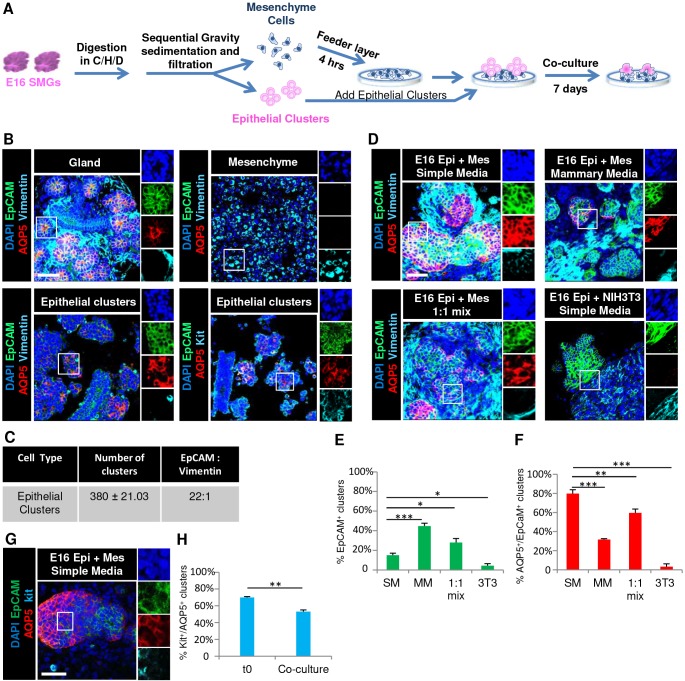
To generate mouse SMG epithelial organoids, we used E16 SMGs as a cell source since the epithelial progenitor marker Kit and the water channel protein AQP5 are both highly enriched in the developing proacini at this developmental stage (Lombaert et al., 2013; Nelson et al., 2013). We performed microdissection and enzymatic dissociation of E16 SMG followed by sequential gravity sedimentations and filtration to enrich for multicellular clusters of epithelial cells in the pellet and single mesenchymal cells in the gravity supernatant (Fig. 1A). Immunocytochemistry (ICC) of the isolated epithelial clusters demonstrated an enrichment of epithelial cell adhesion molecule (EpCAM)-positive epithelial cells, although vimentin-positive cells were also present as ∼4% of total cells in the epithelial clusters (Fig. 1B). The epithelial cells within the clusters were heterogeneous, but were enriched for cells expressing AQP5. In AQP5+ clusters, there were also cells on the periphery that were AQP5− and K14+. Non-AQP5+ clusters that contained cells that express K14 and/or the ductal marker K7, were also present as determined by ICC (Fig. S1). Of note, the AQP5+ cells were predominantly positive for the progenitor marker Kit, as expected at this stage of development (Fig. 1B) (Nelson et al., 2013). ICC of the isolated single cells in the gravity supernatant demonstrated enrichment of vimentin-positive stromal mesenchyme cells (Fig. 1B). However, the enriched mesenchyme cell fraction was a complex mixture of cells that also included the endogenous CD31+ (also known as PECAM1) endothelial cells and β3-tubulin+ nerve cells (data not shown). Schematics of the cell preparation and culture conditions as well as characterization of the starting cell populations are shown in Fig. 1, and quantification of the cell phenotypes and yields from experimental conditions are summarized in Table 1.
Fig. 1.
Co-culture of E16 salivary epithelium with E16 salivary mesenchyme promotes formation of AQP5+ Kit+ organoids. (A) Schematic of E16 SMG subjected to enzymatic treatment using collagenase/hyaluronidase/dispase (C/H/D) followed by sequential gravity sedimentation and filtration to separate the epithelial cell clusters from mesenchyme cells. The enriched epithelial clusters were co-cultured with mesenchyme feeder layers on porous polycarbonate filter for 7 days. (B) ICC and confocal imaging revealed that AQP5 is membrane-localized in intact gland pieces before enzymatic digestion, but less so in epithelial clusters that were fixed immediately after processing, as indicated by staining for the markers EpCAM (epithelium, green), AQP5 (proacinar/acinar cells, red) and vimentin (mesenchyme, cyan), with DAPI staining (nuclei, blue). The epithelial clusters also express the progenitor marker Kit (cyan, bottom right). Individual channels shown on the right are from boxed areas. (C) The number of epithelial clusters used per condition is reported ±s.e.m., and the ratio of epithelium to mesenchyme within these clusters is reported as the ratio of EpCAM to vimentin. (D) E16 epithelial clusters were grown in co-culture with primary mesenchyme or with NIH3T3 cells in simple medium (SM), mammary medium (MM) or in a 1:1 media mix. ICC and confocal imaging revealed that, after 7 days, the epithelial clusters co-cultured with primary mesenchyme preserve their epithelial phenotype and retain the proacinar marker AQP5, while epithelial clusters cultured with the NIH3T3 cells lose both the epithelial and proacinar phenotype. Quantification revealed that (E) epithelial clusters were preserved best in MM and (F) AQP5 was expressed in epithelial cells co-cultured with primary mesenchyme with the highest levels observed with culture in SM. (G) The marker Kit is retained in AQP5+ clusters after 7 days. (H) Quantitative analysis shows the percentage of AQP5+ clusters that are co-positive for the progenitor marker Kit. *P<0.05, **P<0.01 and ***P<0.001 (one-way ANOVA with Tukey post-hoc test between each condition in E and F, and a t-test was applied to H). n=3 for all experiments except n=5 for simple medium and 1:1 mix. Epi, epithelial clusters; Mes, mesenchyme. Scale bars: 50 μm.
Table 1.
Quantification of epithelial and proacinar status of organoids in specific culture conditions
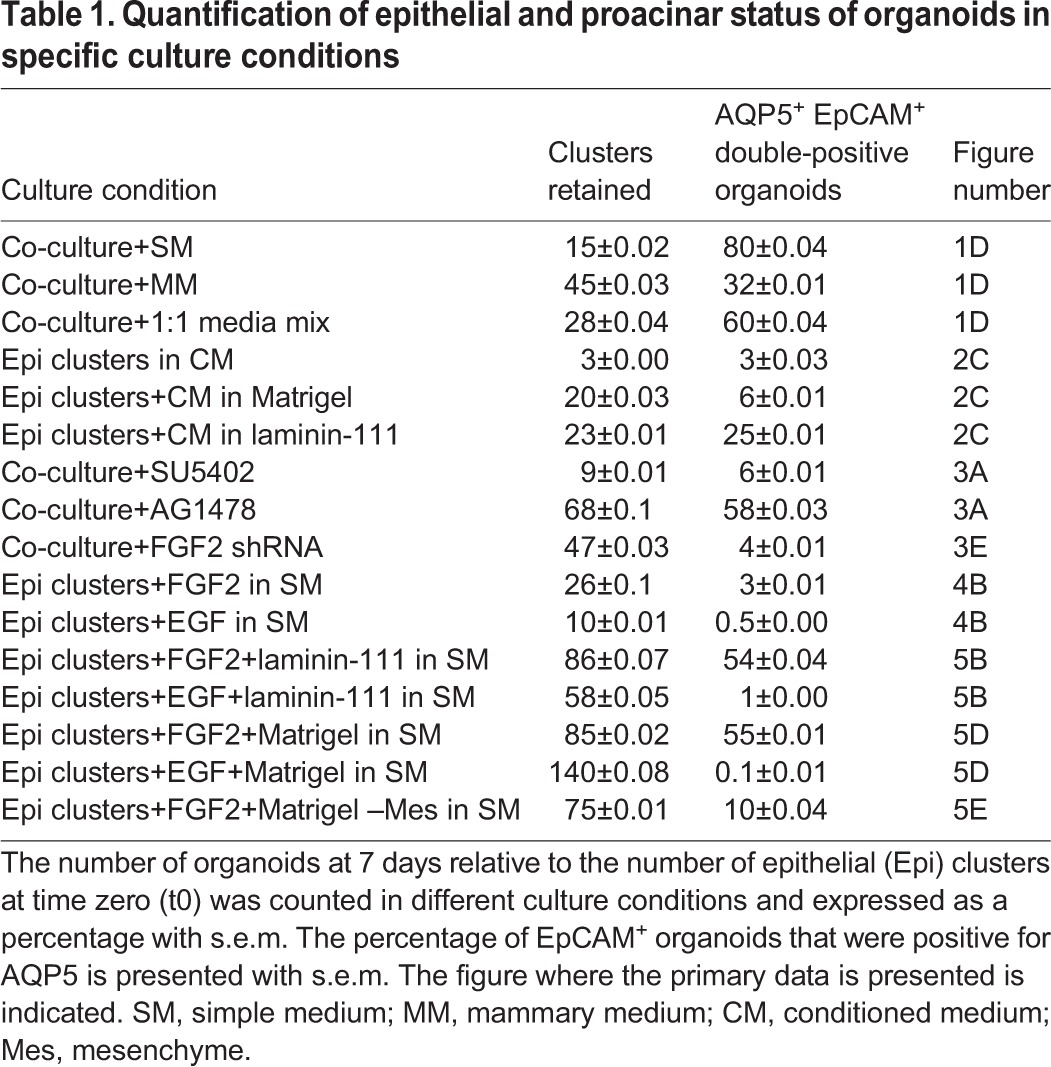
Since mesenchyme is required for branching morphogenesis during salivary gland development (Borghese, 1950b; Grobstein, 1953; Kusakabe et al., 1985; Sequeira et al., 2010; Tucker, 2007), and mesenchymal stromal cells are known components of stem cell niches, we hypothesized that co-culture with salivary mesenchymal cells would promote retention of AQP5+ secretory proacinar cells in the E16 SMG epithelial clusters in culture. To create co-cultures of the epithelial cell-enriched clusters with SMG mesenchyme, we first made feeder layers using the enriched E16 primary SMG mesenchyme in a simple 10% fetal bovine serum-containing medium grown on top of porous polycarbonate filters. After 4 h, epithelial clusters were added to the feeder layer. While the epithelial clusters cultured alone in simple medium underwent an epithelial-to-mesenchyme transition (EMT) and lost EpCAM as well as AQP5, as determined by ICC (Fig. S2), the epithelial clusters grown on a mesenchyme feeder layer were organized in branched structures having high levels of AQP5 expression, which we defined as an organoid. While only 15% of the input epithelial clusters grown in mesenchyme feeder layer co-cultures yielded 3D-organized organoid-like structures including EpCAM+ epithelium, 80% of these epithelial structures expressed AQP5 (Fig. 1D–F; Table 1). Of note, comparison with the epithelial clusters at time zero revealed that the levels of AQP5 in organoids at day 7 are comparable to levels in the input epithelial clusters and that AQP5 is highly membrane localized (Fig. 1B–D). These data suggest a function for mesenchyme in expression of AQP5 in salivary organoids.
In an attempt to increase the number of epithelial organoids produced from co-cultures, we grew co-cultures in mammary medium since this medium is known to promote retention of epithelial characteristics in isolated mammary cells (Debnath et al., 2003; Soule et al., 1990). A higher degree of organized EpCAM+ epithelium was retained in co-cultures grown in mammary medium relative to that seen in simple medium, but the percentage of epithelial structures that were AQP5+ was lower (Fig. 1D–F; Table 1). We then tested a 1:1 mixture of simple medium and mammary medium, which increased the retention of EpCAM+ epithelium, but there was less AQP5+ epithelium relative to what was seen in simple medium, resulting in fewer AQP5+ organoids (Fig. 1D–F; Table 1). Similarly, culture of isolated epithelial clusters in mammary medium alone prevented EMT, and EpCAM was retained, but not AQP5 (Fig. S2). Additionally, we observed that 53% of AQP5+ clusters were co-positive for the progenitor marker Kit (Fig. 1G,H) in co-cultures grown in simple medium. Since co-culture of epithelial cells with the NIH3T3 embryonic fibroblast cell line has been shown to maintain albumin secretion in rat hepatocytes (Lu et al., 2005; Nakazawa et al., 2011) and exocrine differentiation in the pancreas (Li et al., 2004), we tested whether co-culture with NIH3T3 cells was also sufficient to promote salivary proacinar differentiation. However, co-culture of epithelial clusters with NIH3T3 cells was ineffective in preserving the epithelium (Fig. 1D,E), and in the epithelium that did persist AQP5 levels were very low (Fig. 1D,F). These results highlight the importance of co-culture of primary salivary mesenchyme with primary salivary epithelium to promote formation of 3D cellular assemblies of epithelial cells that have robust AQP5 and Kit expression, which we define as proacinar organoids.
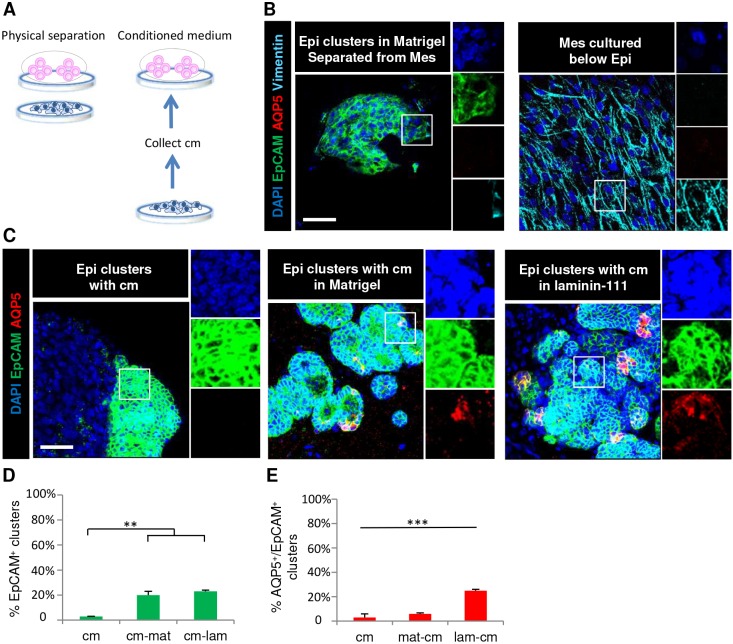
To determine whether a soluble factor from the E16 salivary mesenchyme could support proacinar differentiation, the mesenchymal feeder layer was prepared in the bottom of a glass-bottomed culture dish and grown in simple medium with the epithelial clusters grown on a porous filter floating on top of the culture medium (Fig. 2A). The epithelium was grown in Matrigel to prevent the epithelial cells from undergoing EMT. After 7 days in culture, the epithelium retained epithelial organization but lost AQP5 expression, while the mesenchyme retained vimentin expression (Fig. 2B). As putative mesenchymal soluble factors might be too dilute or unable to penetrate the membrane, conditioned medium was collected from primary E16 mesenchyme that had been grown in simple medium for 3 days and concentrated 10-fold (Fig. 2). After 7 days of culture in 1:1 fresh simple medium:mesenchymal cell conditioned medium (cm) in either Matrigel or laminin-111, the primary component of Matrigel, only a small portion of epithelial clusters were retained and the expression of AQP5 was mostly lost (Fig. 2C–E; Table 1). Thus, secreted soluble factors from the salivary mesenchyme are insufficient to form proacinar organoids from salivary epithelial clusters.
Fig. 2.
Soluble factors from primary salivary mesenchyme cannot substitute for mesenchyme cells to support AQP5 proacinar epithelial cells in culture. (A) Schematic of initial conditions for epithelial clusters cultured without direct contact with the mesenchyme when separated by a filter or when cultured with mesenchymal cell conditioned medium for 7 days. (B) E16 epithelial clusters grown in Matrigel on a polycarbonate filter were separated from primary mesenchyme cells, which were seeded on a coverslip below. ICC and confocal imaging revealed loss of AQP5 expression with partial preservation of EpCAM+ epithelium after 7 days. (C) E16 epithelial clusters were grown in Matrigel or laminin-111 in the presence of concentrated conditioned medium (cm) collected from E16 primary salivary mesenchyme. The conditioned medium (D) partially preserved the EpCAM+ epithelial phenotype in the presence of Matrigel (mat) or laminin (lam) but (E) poorly rescued AQP5 expression, as determined by staining for the markers EpCAM (epithelium, green) and AQP5 (proacinar/acinar cells, red) with DAPI (nuclei, blue). **P<0.01, ***P<0.001 (one-way ANOVA with a Tukey post-hoc test was applied to determine the statistically relevant differences between each condition). n=3 experiments. Epi, epithelial clusters; Mes, mesenchyme. Scale bars: 50 μm.
FGF2 expression by the mesenchyme is required to support salivary epithelium and proacinar differentiation in co-culture
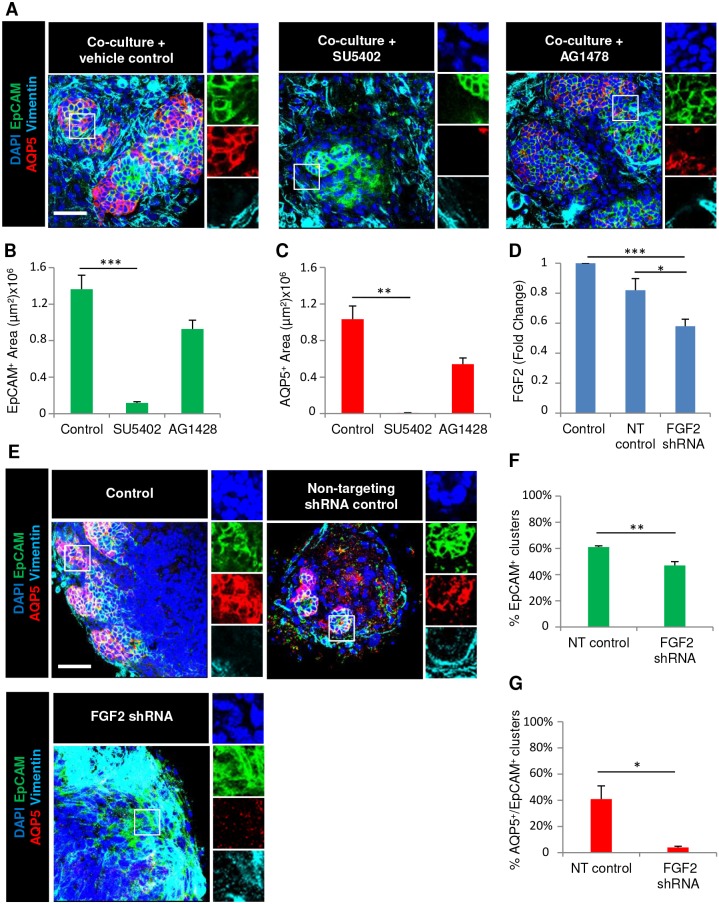
Since FGF signaling promotes SMG branching morphogenesis and the expansion of the epithelium in developing salivary glands (Hoffman et al., 2002; Lombaert et al., 2013; Matsumoto et al., 2016; Patel et al., 2006; Steinberg et al., 2005), we tested whether FGF signaling from the mesenchyme was required in the E16 co-cultures. Since FGF receptors are required for early branching morphogenesis, and inhibition with SU5402, a pharmacological inhibitor of FGF receptors, inhibits branching (Hoffman et al., 2002; Lombaert et al., 2013; Steinberg et al., 2005), we treated co-cultures with SU5402. The addition of SU5402 to SMG co-cultures led to a significant loss of epithelial structure concomitant with loss of AQP5 expression as determined by ICC (Fig. 3A–C; Table 1). Epidermal growth factor receptor (EGFR) also promotes SMG branching morphogenesis (Koyama et al., 2003), and is associated with ductal development (Gresik et al., 1997; Morita and Nogawa, 1999; Nogawa and Takahashi, 1991). Accordingly, in co-cultures treated with the EGFR inhibitor AG1478, epithelial cells were retained and were largely AQP5+ (Fig. 3A–C; Table 1). A primary ligand for FGFR1 and FGFR2 that is known to expand many progenitor cell types is FGF2 (Kobayashi et al., 2016; Kojima et al., 2011), which is primarily expressed by the SMG mesenchyme early in development (https://sgmap.nidcr.nih.gov/) (Hoffman et al., 2002). To knockdown FGF2 in the mesenchyme, we used lentiviral infection of the primary mesenchyme to deliver Fgf2 shRNA or non-targeting shRNA control. We observed a 40% decrease in FGF2 protein levels in the Fgf2 shRNA-treated mesenchyme compared to what was seen with negative control shRNA by ELISA (Fig. 3D), and greater knockdown using more lentivirus caused profound mesenchymal cell death (data not shown). To determine if Fgf2 shRNA-mediated knockdown would affect epithelial proacinar differentiation, we seeded epithelial clusters on mesenchyme feeder layers that had been pre-treated with Fgf2 shRNA lentiviral constructs or negative control shRNA. In these co-cultures, we observed a decrease in epithelium with an associated loss of AQP5 with Fgf2 shRNA relative to the controls, as determined by ICC (Fig. 3E–G; Table 1). Thus, FGF2 expression by mesenchyme is required to support epithelial survival and AQP5 expression in salivary gland co-cultures.
Fig. 3.
Mesenchyme-dependent salivary organoid formation requires FGF2 expression by the mesenchyme. (A) E16 epithelial clusters were seeded on primary mesenchyme feeder layers with the fibroblast growth factor receptor (FGFR) or epidermal growth factor (EGFR) inhibitors SU5402 and AG1478, respectively, in simple medium and were examined by ICC and confocal imaging. Quantitative analysis revealed loss of (B) epithelial EpCAM and (C) proacinar AQP5 protein expression with FGFR inhibition but not EGFR inhibition. (D) E16 mesenchyme cells were infected with lentiviral constructs expressing non-targeting (NT) shRNA or FGF2-targeting shRNA. An ELISA using cell lysates demonstrated a decline in FGF2 protein levels in mesenchymal cells infected with shRNA. (E) E16 epithelium was co-cultured with mesenchyme following lentiviral treatment with NT or FGF2 shRNA for 7 days. ICC and confocal imaging revealed (F) a decline in the epithelial population and (G) loss of proacinar differentiation, as shown by staining for the markers EpCAM (epithelium, green), AQP5 (proacinar/acinar cells, red) and vimentin (mesenchyme, cyan) with DAPI (nuclei, blue). *P<0.05, **P<0.01, ***P<0.001 (one-way ANOVA with Tukey post-hoc test between each condition for B–D; Student's t-test for F,G). n=3 experiments. Scale bars: 50 μm.
FGF2-dependent mesenchyme promotes elaboration of complex 3D salivary organoids containing AQP5+ secretory proacinar cells in basement membrane extracts
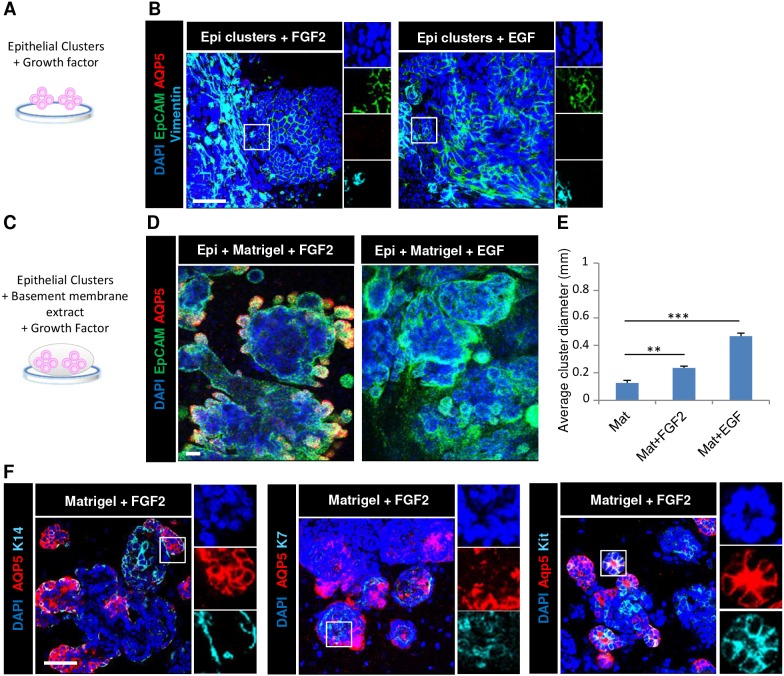
We then tested whether FGF2 is sufficient to promote salivary organoid formation with robust secretory proacinar cell differentiation (Fig. 4A). Neither addition of FGF2 nor EGF to the epithelial clusters in the absence of the mesenchyme feeder layer was sufficient to promote salivary organoid formation, and the EpCAM+ epithelium underwent an apparent EMT to form a flattened layer of largely vimentin+ cells lacking EpCAM and AQP5 expression by ICC (Fig. 4B), similar to epithelium cultured in simple medium without growth factors (Fig. S2). The laminin-rich basement membrane extract, Matrigel, is widely used for 3D epithelial cultures and organoid studies to facilitate epithelial cell self-organization (Baker et al., 2010; Lombaert et al., 2017; Maimets et al., 2016; Maria et al., 2011; Nanduri et al., 2014). To determine whether FGF2 could promote formation of such 3D salivary organoids with robust secretory proacinar cell differentiation, we seeded the E16 epithelial clusters in Matrigel either with or without growth factor supplementation in simple medium and cultured them for 7 days (Fig. 4C; Fig. S2). In epithelial clusters cultured in either Matrigel or laminin-111 in simple medium or mammary medium, but without additional growth factors, epithelial spheroid architecture was retained but AQP5 expression was lost (Fig. S2B). Importantly, epithelial clusters grown in Matrigel supplemented with FGF2, but not EGF, were able to elaborate large, complex salivary organoid structures exhibiting peripheral AQP5+ bud-like structures protruding from a central epithelial core reminiscent of native gland structure (Fig. 4D). After 7 days in culture, the organoids were polydisperse, with an increase in average diameter following 7 days culture in FGF2 and EGF relative to no growth factor supplementation, with the most enlargement detected in the presence of EGF (Fig. 4E). Some of the EpCAM+ but AQP5− epithelial cells expressed the ductal marker K7 and the progenitor/ductal marker K14 (Fig. 4F), similar to the starting population (Fig. S1) (Lombaert et al., 2013; Walker et al., 2008), illustrating the epithelial cell complexity of the salivary organoids. Some of the K14+ but AQP5− epithelial cells encircled AQP5+ cells, similar to in vivo proacinar structures, but the K7+ cells were not generally well organized into ductal-like structures (Fig. 4F). Interestingly, most of the organoid epithelium showed robust membrane-localized expression of the epithelial progenitor marker Kit, which largely, but not completely, colocalized in cells with membrane-localized AQP5 (Fig. 4F), consistent with the organoids being in a progenitor state containing distinct Kit+ and K14+ cell populations.
Fig. 4.
FGF2 promotes elaboration of complex 3D salivary organoids in basement membrane. (A) Schematic of initial condition for epithelial clusters cultured with growth factors. (B) E16 epithelial clusters were seeded on porous polycarbonate filters floating on simple medium containing FGF2 or EGF. (C) Schematic of epithelial clusters cultured in basement membrane extract with growth factors. (D) Epithelial clusters were cultured in Matrigel with FGF2 or EGF. ICC and confocal imaging revealed that after 7 days, the EpCAM+ epithelium is partially maintained with an increase in vimentin+ mesenchyme cells with growth factors, while epithelial clusters grown in Matrigel with FGF2 form complex EpCAM+ epithelial organoids with budded structures expressing AQP5. Staining was for EpCAM (epithelium, green), AQP5 (proacinar/acinar cells, red) and vimentin (mesenchyme, cyan) with DAPI (nuclei, blue). (E) Quantitative analysis shows the mean diameter for the epithelial clusters with or without growth factors in Matrigel. (F) Organoids were analyzed for the basal cell marker K14, the apical ductal cell marker K7 and the progenitor cell marker Kit in epithelial clusters after 7 days embedded in Matrigel with FGF2. Staining was for AQP5 (proacinar/acinar cells, red), K14 (basal, cyan), K7 (ductal, cyan) and Kit (progenitor, cyan) with DAPI (nuclei, blue). **P<0.01, ***P<0.001 (one-way ANOVA with Tukey post-hoc test between each condition). n=3 experiments. Scale bars: 50 μm.
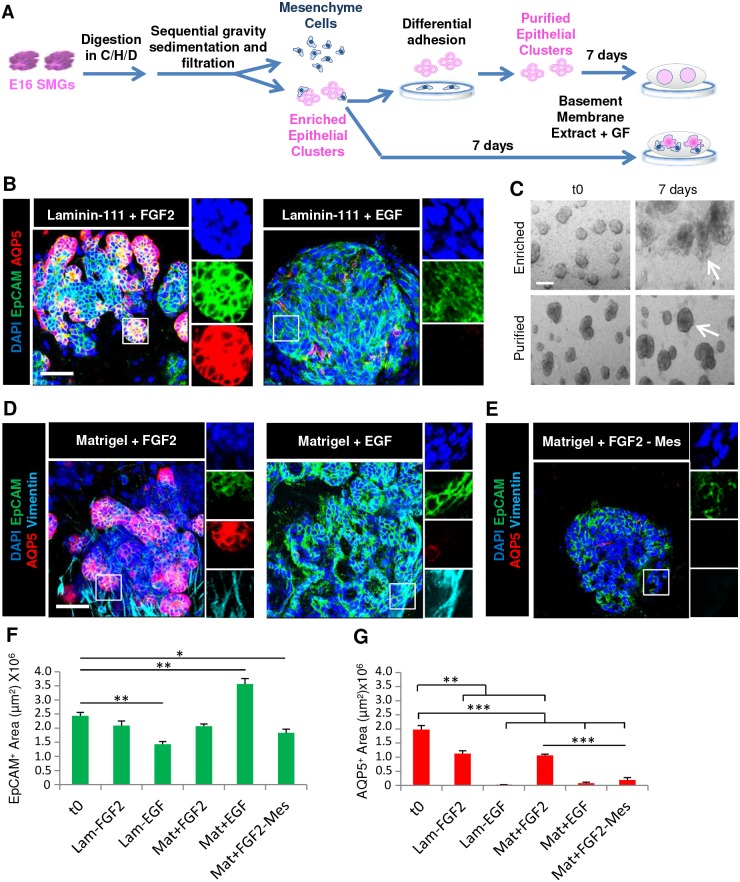
To determine whether culture in laminin-111, the primary component of Matrigel, is sufficient together with FGF2 to promote robust proacinar organoids, we isolated enriched epithelial clusters through gravity sedimentation and cultured them in laminin-111 on polycarbonate filters in simple medium containing FGF2 or EGF (Fig. 5A). High-magnification confocal imaging of the organoids revealed high levels of AQP5 expression comparable to that of the input E16 epithelium within the distal tips of protrusions, demonstrating that laminin and FGF2 are sufficient for this effect (Fig. 5B,G). Culture of enriched epithelial clusters with FGF2, but not EGF, in Matrigel was similarly effective in supporting epithelium and in promoting robust membrane-localized AQP5 expression (Fig. 5D–G). These 3D organoids cultured in laminin-rich matrix plus FGF2 were more effective than the mesenchymal co-cultures in preserving epithelial cells and architecture with 86±0.07% (Table 1) of the input epithelium retained in laminin-111 plus FGF2 versus only 15±0.02% of epithelium retained in co-culture organoids. However, co-culture was the most effective method of promoting AQP5 expression, with 55±0.01% and 54±0.04% of epithelial structures expressing AQP5 in Matrigel and laminin-111, respectively, in the presence of FGF2, but 80±0.04% of the co-culture organoids expressing AQP5 (Fig. 5F,G; Table 1).
Fig. 5.
Complex laminin and FGF2-dependent salivary proacinar organoids require mesenchymal cells. (A) Schematic demonstrating isolation and culture of enriched or purified E16 epithelial clusters. GF, growth factor. (B) E16 enriched epithelial clusters were seeded in laminin-111 on porous polycarbonate filters floating on simple medium containing either FGF2 or EGF. (C) Brightfield images show enriched epithelial clusters or purified epithelial clusters after mesenchyme depletion cultured in Matrigel with FGF2 for 7 days. (D,E) E16 enriched epithelial clusters or E16 purified epithelial clusters (-Mes) were seeded in Matrigel and grown with FGF2. Laminin-111, FGF2 and mesenchyme when together lead to AQP5 expression being retained in organoids, as shown by staining for the markers EpCAM (epithelium, green), AQP5 (proacinar/acinar cells, red) and vimentin (mesenchyme, cyan) with DAPI (nuclei, blue). Quantitative analysis demonstrates (F) EpCAM is variably preserved in all conditions, while (G) AQP5 is retained with FGF2 and basement membrane only when mesenchyme is also present. *P<0.05, **P<0.01, ***P<0.001 (one-way ANOVA with Tukey post-hoc test between each condition). n=3 experiments. Scale bars: 50 μm (B,D); 200 μm (C).
Interestingly, we noted that the vimentin-positive mesenchymal cells present in the 3D organoids expanded concomitantly with the epithelium in the presence of FGF2 (Fig. 5D), and we questioned whether these cells are required to form proacinar organoids in the presence of FGF2 and a laminin-rich matrix. To determine whether the presence of mesenchyme is required for proacinar organoid formation, we used differential adhesion-based depletion to remove the more-adherent mesenchyme from the enriched epithelial cell clusters to make purified epithelial clusters (Fig. 5A). Strikingly, mesenchyme-depleted, purified epithelial clusters grown in Matrigel and supplemented with FGF2 formed spheroids with smooth surfaces similar to those in the input clusters, and lacked the buds present in the enriched epithelial cell clusters that were grown in Matrigel with FGF2 for 7 days (Fig. 5C). The spheroids derived from purified epithelial clusters retained epithelial characteristics and were positive for EpCAM but were nearly devoid of AQP5, similar to EGF-supplemented cultures (Fig. 5E–G; Table 1), indicating that FGF2 cannot compensate for the presence of mesenchyme. Taken together, these data indicate that FGF2 functions indirectly through the mesenchyme to promote salivary organoid morphogenesis and AQP5 expression in the organoids, demonstrating a critical function for mesenchyme cells in the FGF2-dependent elaboration of complex salivary organoids.
DISCUSSION
Despite early indications that the differentiation of the salivary gland epithelium is autonomous (Cutler, 1980; Denny et al., 1997), by using ex vivo culture of primary cells, we demonstrate a requirement for mesenchymal cells to support formation of complex salivary organoids. Co-culture of E16 mouse salivary epithelium highly enriched in Kit+ progenitors with E16 salivary mesenchyme sustained epithelial architecture and supported robust expression of the proacinar marker AQP5 that is comparable to what is seen in the native tissue and that had appropriate membrane localization. FGF2 expression by the mesenchymal cells was required for AQP5 and Kit expression in organoids. FGF2 promoted elaboration of complex organoids with robust AQP5 epithelial expression only when grown in either 3D Matrigel or polymerized laminin-111, both of which are known to preserve the 3D architecture and polarity of epithelial cells (Baker et al., 2010; Cantara et al., 2012; Daley et al., 2012; Debnath et al., 2003; Maria et al., 2011). Strikingly, FGF2-dependent 3D growth of epithelium in laminin or laminin-rich basement membrane required mesenchymal cells to support complex salivary organoid formation, suggesting that FGF2 functions in an autocrine manner on the mesenchyme itself. Although laminin-111 directly stimulated epithelial architecture, neither FGF2 nor mesenchymal soluble factors could substitute for the presence of mesenchyme cells. We conclude that basement membrane proteins and other non-soluble factors produced by FGF2-dependent mesenchyme cells function as niche factors for salivary organoids that are both AQP5+ and Kit+.
Indeed, many organoid and co-culture studies have reported variable requirements for mesenchyme signaling to support epithelial progenitor cells and differentiation ex vivo (Chou et al., 2016; Hegab et al., 2015; Wells et al., 2013). Of particular note, in some studies, soluble factors can substitute for mesenchyme to promote some epithelial characteristics (Rebustini and Hoffman, 2009; Sequeira et al., 2010; Steinberg et al., 2005) and other studies demonstrate a requirement for direct contact of the mesenchyme to support epithelial differentiation (Duss et al., 2014; Lee et al., 2014; Li et al., 2004; Park et al., 2014). FGF signaling is required for development of salivary glands and other branched organs (Hoffman et al., 2002; Lombaert et al., 2013; Miralles et al., 1999; Patel et al., 2006; Steinberg et al., 2005; Zhang et al., 2014); however, the cell types that require FGF signaling are incompletely understood. FGF2 is known to be a mitogen for human mesenchymal stem cells, and upon shRNA-mediated FGF2 knockdown in the salivary mesenchyme, we observed fewer mesenchyme cells relative to the control vector-treated cells, consistent with an autocrine function for FGF2 in the mesenchyme. FGF2 treatment of the mesenchyme is required for production of soluble factors to support epithelial human embryonic stem cells (hESCs) cultured on mouse embryonic fibroblasts (MEFs) (Greber et al., 2007; Lotz et al., 2013). However, in this study soluble factors produced by the mesenchyme incompletely supported the proacinar organoids. In many tissues, including salivary glands, the mesenchyme cooperates with the epithelium for production and assembly of the basement membrane (Ekblom et al., 1994; Keely et al., 1995; Nelson and Larsen, 2015; Yurchenco, 2011). Given that FGF2 expression by the mesenchyme is required for epithelial proacinar differentiation in the co-cultures, and that neither Matrigel nor laminin-111 together with exogenous FGF2 could substitute for the mesenchyme to promote AQP5 expression, our results suggest that FGF2 may be required for mesenchymal potency, functioning through an unknown factor that synergizes with signaling initiated by laminin-111.
In both of our assay systems, the robust AQP5+ proacinar organoids include well-organized vimentin-positive mesenchymal cells surrounding the epithelium, similar to in the native SMG architecture. Mesenchyme is an important feature of the organoids as it promotes the complex budded epithelial structure and supports the high-level membrane-localized expression of AQP5 in these buds. Indeed, many organoids have been shown to contain mesenchyme, particularly embryonic and induced pluripotent stem cell (iPSC)-derived organoids (Dye et al., 2015; Hegab et al., 2015; McQualter et al., 2010; Shamir and Ewald, 2014; Teisanu et al., 2011). Matrigel or laminin have also been used for adult SMG progenitor cell expansion in complex growth factor-containing media for formation of organoids with some level of AQP5 expression (Maimets et al., 2016; Nanduri et al., 2014). Since a mesenchymal component has not yet been reported in salivary organoids derived from adult stem or progenitor cells, this presents an important opportunity to increase the organization and secretory capacity of such organoids in future studies. Our studies revealed that E16 mesenchyme, but not NIH3T3 cells, were permissive to support epithelial survival and organization with robust AQP5 expression and organization. A recent report used hair follicle-derived mesenchyme in co-culture with adult SMG epithelium (Maruyama et al., 2015), although this mesenchymal population did not support the elaboration of well-organized proacinar epithelia as did the E16 mesenchyme in our studies. Another recent report used bone marrow mesenchymal stem cells (MSCs) or the native E13 mesenchyme to support branching morphogenesis of isolated E13 SMG epithelium; while the MSC could support minimal epithelial development it was vastly inferior to the native E13 mesenchyme (Farahat et al., 2017). These studies are consistent with our findings that native salivary mesenchyme supports maintenance of AQP5+ Kit+ cells and morphogenesis of salivary organoids.
The microenvironment provided by the native salivary mesenchyme encompasses multiple factors that could facilitate organoid formation: heterotypic cell–cell interactions, extracellular matrix (ECM), and secreted morphogens (Bhat and Bissell, 2014). Direct heterotypic cell–cell interactions in which one cell provides the receptor and the other cell provides the cognate ligand, known as juxtacrine signaling, is one potential mechanism through which the mesenchyme could support the proacinar cells (Bhatia et al., 1999). The ECM and basement membrane can alter cellular motility and control stem cell quiescence, proliferation and cell fate (Engler et al., 2007; Gattazzo et al., 2014). The ECM composition and its 3D structure largely controls the viscoelasticity of the microenvironment (Discher et al., 2009; Engler et al., 2007). Studies by our laboratory and others have demonstrated that compliance of the microenvironment is required for the development and differentiation of salivary gland organ explants (Miyajima et al., 2011; Peters et al., 2014). Although our results here show that Matrigel is insufficient to support the proacinar cells, extracellular matrix proteins elaborated by and organized by the E16 mesenchyme may provide critical signals to support the proacinar cells. Finally, paracrine-acting growth factors regulate stem cell behavior (Saraswati et al., 2012). As conditioned medium provided by mesenchyme cells was insufficient to rescue proacinar organoids in the absence of the mesenchyme cells themselves, a secreted mesenchymal morphogen is clearly insufficient. However, we cannot exclude synergistic signaling that could result from paracrine-acting morphogens together with matrix proteins and/or juxtacrine signaling as the required components of the mesenchymal niche in salivary organoids.
Organoids hold promise for applications in regenerative medicine, and bioengineered organoids have been used to partially restore salivary gland organ function in vivo in a mouse organ replacement model (Ogawa et al., 2013). Since loss of the salivary gland secretory acinar cell population is common to both autoimmune- and radiation-induced xerostomia, or dry mouth, efforts have been focused on augmenting secretory acinar cell differentiation in vitro and in vivo and characterizing signaling pathways that enhance or sustain secretory acinar cell differentiation (Kobayashi et al., 2016; Kojima et al., 2011; Lim et al., 2013; Ogawa et al., 2013; Sumita et al., 2011; Thula et al., 2005). Significantly, both MSCs and FGF2 have been shown to promote restoration of salivary function in disease models, where damage to the mesenchyme contributes to impairment of gland function (Khalili et al., 2012; Kobayashi et al., 2016; Kojima et al., 2011; Lim et al., 2013; Lombaert et al., 2017; Sumita et al., 2011; Thula et al., 2005). Thus, future studies will need to define the critical features of the mesenchyme cells that makes them competent to produce a microenvironment that stimulates or supports secretory acinar cell differentiation. With this knowledge, future regenerative therapies could be designed to augment the potency of clinically relevant mesenchyme, such as MSCs for cell therapy, or employ growth factor-mediated restoration of endogenous mesenchyme potency in salivary hypofunction conditions.
MATERIALS AND METHODS
Isolation of primary cells from mouse submandibular glands
Timed-pregnant CD-1 female mice were obtained from Charles River Laboratories. E16 mouse submandibular glands (SMGs) were collected according to the animal protocols approved by the University at Albany Institutional Animal Care and Use Committee (IACUC) by first removing the mandible slice with sharp scalpels and then removing the glands from the slice with sterile forceps under a dissecting microscope. To prepare epithelial clusters, SMGs were microdissected in 1× phosphate-buffered saline (PBS, Life Technologies) with 1× collagenase/hyaluronidase (Stem Cell Technologies, #7912) and 0.8 U/ml of dispase II (Life Technologies, #17105041) to release E16 lobules. The lobules were incubated at 37°C for 30 min with further trituration to generate both epithelial clusters and single mesenchyme cells. The enzymatic reaction was stopped by addition of 1:1 DMEM/F12 (Life Technologies, 11039047) containing 10% fetal bovine serum (FBS; Life Technologies). Cell populations were separated by gravity sedimentation for ∼5 min until the larger epithelial clusters formed a loose pellet. Epithelial clusters from this pellet were enriched with two additional gravity sedimentations. The remaining mesenchyme cell population underwent additional steps to enrich for single mesenchymal cells, including two gravity sedimentations followed by passage through cell strainers having pores of 70 µm (Falcon, #087712) and 40 µm (Fisher Scientific, #22363547) diameter.
Epithelial cell culture and media components
Epithelial clusters (derived from 2.5 glands, ∼2×104 cells in 380 clusters) were seeded on 0.1 μm pore size porous polycarbonate filters (Nuclepore, Whatman #0930051) and floated on top of 200 µl media in 50 mm glass-bottom dishes (MatTek #P50G-1.5-14F). Media used include: DMEM/F12 with 10% FBS supplemented with 100 U/ml penicillin and 100 mg/ml streptomycin (Pen-Strep, Life Technologies), which is referred to here as ‘simple medium (SM)’, a mammary epithelial medium that was developed for MCF-10A mammary epithelial cells (Debnath et al., 2003; Soule et al., 1990) that includes 5% horse serum (Invitrogen), 20 ng/ml EGF (PeproTech, #AF100-15), 1.25 μg/ml hydrocortisone (Sigma, #H0135), 100 ng/ml cholera toxin (Sigma, #C8052), 10 μg/ml insulin (Sigma, #I882) and penicillin-streptomycin (Life Technologies) and is referred to as ‘mammary medium (MM)’. Growth factors [FGF2, Peprotech #450-33, and EGF, Peprotech, AF-100-15] were solubilized in medium containing 0.2% BSA and stored at −20°C in single-use aliquots prior to use.
Co-culture of epithelial clusters with mesenchymal cells
Primary E16 mesenchyme cells (derived from five glands, ∼1.1×106 cells) were seeded on Nuclepore filters in the MatTek dishes in simple medium for 4 h to generate a feeder layer. Epithelial clusters (derived from 2.5 glands, approximately 2×104 cells in 380 clusters) were plated on top of the mesenchyme feeder layer. Media conditions included simple medium, mammary medium and a 1:1 ratio of mammary medium:simple medium. The NIH3T3 immortalized embryonic fibroblast cell line was grown in simple medium for 4 h on a Nuclepore filter (1.1×106 cells) to generate a feeder layer followed by addition of E16 primary epithelial clusters. For indirect co-culture of epithelial clusters with mesenchyme, mesenchyme cells were seeded as a feeder layer in the bottom of a 0.1% gelatin-coated MatTek dish in simple medium with the epithelial clusters embedded in Matrigel on a Nuclepore filter and cultured for up to 7 days.
Epithelial clusters with mesenchyme conditioned medium with or without basement membrane extract
Mesenchymal conditioned medium was collected from E16 primary mesenchyme seeded on 0.1% gelatin-coated (Millipore #ES-006B) tissue culture plates (Corning) that was grown for three days. The conditioned medium was further concentrated using Amicon Ultra-4 Centrifugal Filters 3 K (Millipore #UFC800308) to a 10-fold concentrate. Concentrated conditioned medium was mixed 1:1 with simple medium (DMEM/F12+10% FBS) for culturing. Epithelial clusters (from 2.5 glands) were seeded on Nuclepore filters or embedded in either Matrigel (Corning #CB40230) or laminin-111 (Trevigen #3446-005-01) at a ratio of 1:1 with mesenchymal conditioned medium and cultured for 7 days.
Growth factor receptor inhibitors in E16 co-cultures
Primary E16 mesenchyme cells (approximately 1.1×106 cells/ml) were seeded on Nuclepore filters in MatTek dishes in simple medium for 4 h. Epithelial clusters (from 2.5 glands) were plated on top of the mesenchyme and grown for up to 7 days. FGFR and EGFR inhibitors were used as previously reported for SMG cultures (Hoffman et al., 2002; Mizukoshi et al., 2016). The FGFR inhibitor SU5402 (Sigma #SML0443) was solubilized in DMSO and used at a final concentration of 5 µM and the EGFR inhibitor AG1478 (Tocris #1276) was solubilized in DMSO and used at a 10 µM final concentration.
Lentiviral FGF2 shRNA knockdown in E16 primary mesenchyme
E16 primary mesenchyme was seeded in 96-well plates and transduced with lentiviral shRNA control (SHC-002V) or shRNA FGF2 lentivirus particles (Mission TRC-Sigma #SHCLNV-NM-008006; TRCN00000-67283), according to the manufacturer's recommendations. At 72 h after infection, cell lysates were made using RIPA buffer (Thermo Fisher Scientific, PI89900) with complete mini EDTA-free protease inhibitor cocktail (Roche, #11836170001). FGF2 ELISA (R&D Systems #MFB00) with cell lysates was performed according to the manufacturer's instructions to determine the level of FGF2 knockdown in the mesenchyme cells. For co-cultures, E16 primary mesenchyme was seeded on a Nuclepore filter and transduced with the shRNA lentiviruses. After 7 days, freshly isolated E16 epithelial clusters were added to the mesenchyme and cultured for an additional 7 days.
Culture of epithelial clusters in basement membrane extract with or without mesenchymal growth factors
For culture, enriched epithelial clusters (from 2.5 glands) were embedded in either Matrigel or laminin-111 (Trevigen #3446-005-01) at a ratio of 1:1 and placed on Nuclepore filters in MatTek dishes floating on simple medium (SM) containing FGF2 (100 ng/ml) or EGF (100 ng/ml). The organoids were grown for up to 14 days. For experiments lacking basement membrane, epithelial clusters were placed directly on the filter and grown for 7 days. To analyze marker expression at time 0 (t0, before culture), primary E16 enriched epithelial clusters (from 2.5 glands) in medium were embedded in Matrigel at a ratio of 1:1 (Matrigel:medium) and plated on Nuclepore filters in MatTek dishes and incubated for 20 min at 37°C to solidify the Matrigel before fixation.
Differential adhesion to generate purified epithelial clusters
E16 epithelial clusters were seeded in a 35 mm dish for up to 2 h to deplete mesenchyme cells from epithelial clusters. After 2 h, floating epithelial clusters were removed by performing gentle trituration of the medium, leaving the adherent mesenchyme cells attached to the dish. The mesenchyme-depleted epithelial clusters (from 2.5 glands) were embedded in Matrigel at a ratio of 1:1 and placed on Nuclepore filters in MatTek dishes in simple medium containing the growth factor FGF2 (100 ng/ml) and cultured for 7 days.
Immunocytochemistry and confocal imaging
SMG cell cultures in various configurations that were grown on Nuclepore filters were fixed with 4% paraformaldehyde (Electron Microscopy Sciences) in 1× PBS for 20 min or overnight, depending upon the culture conditions. Immunocytochemistry (ICC) was performed as described previously (Daley et al., 2009; Sequeira et al., 2012), except that 0.4% Triton X-100 (Sigma) was used for permeabilization. All of the primary and secondary antibody incubations were performed overnight at 4°C. Primary antibodies used were against: AQP5 (1:400; Alomone #AQP-005), EpCAM–FITC (1:400; eBiosciences #11-5791-82), vimentin (1:1000; Sigma #V2258), cytokeratin 14 (K14) (1:600; BioLegend #906001), cytokeratin 7 (K7) (1:200; Abcam #9021) and Kit (1:200; R&D Systems #AF1356). Secondary antibodies including Cyanine- and Alexa dye-conjugated AffiniPure F(ab′)2 fragments were all purchased from Jackson ImmunoResearch Laboratories and used at a dilution of 1:250. DAPI (Life Technologies, #D1306) was used for nuclei staining. Nuclepore filters were mounted on glass slides using Fluorogel with Tris buffer mounting medium (Electron Microscopy Sciences, #17985-11). Imaging was performed with a Leica TCS SP5 confocal microscope at 20× and 63× (oil immersion) or a Zeiss 710 confocal microscope at 40× (oil immersion) magnification using the same laser configurations for all samples within an experiment.
Image analysis and quantification
Images were acquired for quantitative analysis by using the tile scan function with 20× magnification on the Leica SP5 microscope, in order to analyze the entire area of each sample. Quantitative analysis of spheroids for marker expression was performed by using the MetaMorph (Version 6.1, MDS Analytical Technologies) program. The total number of spheroids positive for the epithelial marker EpCAM was manually counted and recorded manually. If EpCAM+ spheroids showed more than 30% (by area) of the proacinar marker AQP5 expression, they were considered to be an AQP5+ organoid. Positively stained areas (μm2) for EpCAM, AQP5 or vimentin markers were quantified by using calibrated and thresholded images using ImageJ software (Abràmofff et al., 2005), as we performed previously (Gervais et al., 2015). Organoid diameter was measured from calibrated images with ImageJ software. Data was graphed in Microsoft Excel with the standard error of mean (s.e.m.). One-way ANOVA tests and Student's two-tailed t-tests were carried out in Vassar-Stats (http://vassarstats.net/) with a Tukey post-hoc test. P<0.05 was considered to be statistically significant. At least three experiments were quantified to generate each graph with one or two samples per experiment measured unless otherwise noted.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Dr Susan LaFlamme for helpful suggestions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: M.L., Z.F.H., D.A.N.; Methodology: Z.F.H., D.A.N., N.M., L.M.S.; Validation: Z.F.H., D.A.N.; Formal analysis: Z.F.H.; Investigation: Z.F.H., N.M.; Writing - original draft: Z.F.H.; Writing - review & editing: M.L., Z.F.H., D.A.N., N.M., L.M.S., J.C.; Visualization: Z.F.H., D.A.N.; Supervision: M.L.; Project administration: M.L.; Funding acquisition: M.L., J.C.
Funding
This work was supported by National Institutes of Health [R01 DE022467, R56 DE2246706 to M.L. and J.C.; C06 RR015464 and R01 GM5154017]; the National Science Foundation [DV10922830 to J.C.]; and the University at Albany, SUNY. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.208728.supplemental
References
- Abràmofff M. D., Magalhães P. J. and Ram S. J. (2005). Image processing with ImageJ Part II. Biophotonics Int. 11, 36-43. [Google Scholar]
- Baker O. J., Ph D., Schulz D. J., Camden J. M., Liao Z., Peterson T. S., Seye C. I., Petris M. J. and Weisman G. A. (2010). Rat parotid gland cell differentiation in three-dimensional culture. Tissue Eng. Part C Methods 16, 1135-1144. 10.1089/ten.tec.2009.0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R. and Bissell M. J. (2014). Of plasticity and specificity: dialectics of the micro- and macro-environment and the organ phenotype. Wiley Interdiscip. Rev. Membr. Transp. Signal. 3, 147-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S. N., Balis U. J., Yarmush M. L. and Toner M. (1999). Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 13, 1883-1900. [DOI] [PubMed] [Google Scholar]
- Bissell M. J. (2017). Goodbye flat biology–time for the 3rd and the 4th dimensions. J. Cell Sci. 130, 3-5. 10.1242/jcs.200550 [DOI] [PubMed] [Google Scholar]
- Borghese E. (1950a). Explantation experiments on the influence of the connective tissue capsule on the development of the epithelial part of the submandibular gland of Mus musculus. J. Anat. 84, 303-318. [PMC free article] [PubMed] [Google Scholar]
- Borghese E. (1950b). The development in vitro of the submandibular and sublingual glands of Mus musculus. J. Anat. 84, 287-302. [PMC free article] [PubMed] [Google Scholar]
- Cantara S. I., Soscia D. A., Sequeira S. J., Jean-Gilles R. P., Castracane J. and Larsen M. (2012). Selective functionalization of nanofiber scaffolds to regulate salivary gland epithelial cell proliferation and polarity. Biomaterials 33, 8372-8382. 10.1016/j.biomaterials.2012.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzeli L., Gaete M. and Tucker A. S. (2017). Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development 144, 2294-2305. 10.1242/dev.146019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y.-S., Lin Y.-C., Young T.-H. and Lou P.-J. (2016). Effects of fibroblasts on the function of acinar cells from the same human parotid gland. Head Neck 38 Suppl. 1, E279-E286. 10.1002/hed.23986 [DOI] [PubMed] [Google Scholar]
- Clevers H. (2016). Modeling development and disease with organoids. Cell 165, 1586-1597. 10.1016/j.cell.2016.05.082 [DOI] [PubMed] [Google Scholar]
- Cutler L. S. (1980). The dependent and independent relationships between cytodifferentiation and morphogenesis in developing salivary gland secretory cells. Anat. Rec. 196, 341-347. 10.1002/ar.1091960310 [DOI] [PubMed] [Google Scholar]
- Daley W. P., Gulfo K. M., Sequeira S. J. and Larsen M. (2009). Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Dev. Biol. 336, 169-182. 10.1016/j.ydbio.2009.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley W. P., Gervais E. M., Centanni S. W., Gulfo K. M., Nelson D. A. and Larsen M. (2012). ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Dev. Camb. Engl. 139, 411-422. 10.1242/dev.075366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J., Muthuswamy S. K. and Brugge J. S. (2003). Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256-268. 10.1016/S1046-2023(03)00032-X [DOI] [PubMed] [Google Scholar]
- De Moerlooze L., Spencer-Dene B., Revest J. M., Hajihosseini M., Rosewell I. and Dickson C. (2000). An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Dev. Camb. Engl. 127, 483-492. [DOI] [PubMed] [Google Scholar]
- Denny P., Ball W. and Redman R. (1997). Salivary glands: a paradigm for diversity of gland development. Crit. Rev. Oral Biol. Med. 8, 51-75. 10.1177/10454411970080010301 [DOI] [PubMed] [Google Scholar]
- Discher D., Dong C., Fredberg J. J., Guilak F., Ingber D., Janmey P., Kamm R. D., Schmid-Schönbein G. W. and Weinbaum S. (2009). Biomechanics: cell research and applications for the next decade. Ann. Biomed. Eng. 37, 847-859. 10.1007/s10439-009-9661-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duss S., Brinkhaus H., Britschgi A., Cabuy E., Frey D. M., Schaefer D. J. and Bentires-Alj M. (2014). Mesenchymal precursor cells maintain the differentiation and proliferation potentials of breast epithelial cells. Breast Cancer Res. 16, R60 10.1186/bcr3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye B. R., Hill D. R., Ferguson M. A., Tsai Y.-H., Nagy M. S., Dyal R., Wells J. M., Mayhew C. N., Nattiv R., Klein O. D. et al. (2015). In vitro generation of human pluripotent stem cell derived lung organoids. eLife 4, 1-25. 10.7554/eLife.05098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P., Ekblom M., Fecker L., Klein G., Zhang H. Y., Kadoya Y., Chu M. L., Mayer U. and Timpl R. (1994). Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Dev. Camb. Engl. 120, 2003-2014. [DOI] [PubMed] [Google Scholar]
- Engler A. J., Sweeney H. L., Discher D. E. and Schwarzbauer J. E. (2007). Extracellular matrix elasticity directs stem cell differentiation. J. Musculoskelet. Neuronal Interact. 7, 335. [PubMed] [Google Scholar]
- Farahat M., Sathi G. A., Hara E. S., Taketa H., Kuboki T. and Matsumoto T. (2017). MSCs feeder layers induce SMG self-organization and branching morphogenesis. PLOS ONE 12, e0176453 10.1371/journal.pone.0176453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattazzo F., Urciuolo A. and Bonaldo P. (2014). Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 1840, 2506-2519. 10.1016/j.bbagen.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais E. M., Desantis K. A., Pagendarm N., Nelson D. A., Enger T., Skarstein K., Liaaen Jensen J. and Larsen M. (2015). Changes in the submandibular salivary gland epithelial cell subpopulations during progression of Sjögren's syndrome-like disease in the NOD/ShiLtJ mouse model. Anat. Rec. 298, 1622-1634. 10.1002/ar.23190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber B., Lehrach H. and Adjaye J. (2007). Fibroblast growth factor 2 modulates transforming growth factor β signaling in mouse embryonic fibroblasts and human ESCs (hESCs) to support hESC self-renewal. Stem Cells 25, 455-464. 10.1634/stemcells.2006-0476 [DOI] [PubMed] [Google Scholar]
- Gresik E. W., Kashimata M., Kadoya Y., Mathews R., Minami N. and Yamashina S. (1997). Expression of epidermal growth factor receptor in fetal mouse submandibular gland detected by a biotinyltyramide-based catalyzed signal amplification method. J. Histochem. Cytochem. Off. J. Histochem. Soc. 45, 1651-1657. 10.1177/002215549704501208 [DOI] [PubMed] [Google Scholar]
- Grobstein C. (1953). Inductive epithelio-mesenchymal interaction in cultured organ rudiments of the mouse. Science 118, 52-55. 10.1126/science.118.3054.52 [DOI] [PubMed] [Google Scholar]
- Hegab A. E., Arai D., Gao J., Kuroda A., Yasuda H., Ishii M., Naoki K., Soejima K. and Betsuyaku T. (2015). Mimicking the niche of lung epithelial stem cells and characterization of several effectors of their in vitro behavior. Stem Cell Res. 15, 109-121. 10.1016/j.scr.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Hirayama M., Ogawa M., Oshima M., Sekine Y., Ishida K., Yamashita K., Ikeda K., Shimmura S., Kawakita T., Tsubota K et al. et al. (2013). Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat. Commun. 4, 2497 10.1038/ncomms3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M. P., Kidder B. L., Steinberg Z. L., Lakhani S., Ho S., Kleinman H. K. and Larsen M. (2002). Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development 129, 5767-5778. 10.1242/dev.00172 [DOI] [PubMed] [Google Scholar]
- Ikeda E. and Tsuji T. (2008). Growing bioengineered teeth from single cells: potential for dental regenerative medicine. Expert Opin. Biol. Ther. 8, 735-744. 10.1517/14712598.8.6.735 [DOI] [PubMed] [Google Scholar]
- Jaskoll T., Witcher D., Toreno L., Bringas P., Moon A. M. and Melnick M. (2004). FGF8 dose-dependent regulation of embryonic submandibular salivary gland morphogenesis. Dev. Biol. 268, 457-469. 10.1016/j.ydbio.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Jaskoll T., Abichaker G., Witcher D., Sala F. G., Bellusci S., Hajihosseini M. K. and Melnick M. (2005). FGF10/FGFR2b signaling plays essential roles during in vivo embryonic submandibular salivary gland morphogenesis. BMC Dev. Biol. 5, 11 10.1186/1471-213X-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely P. J., Wu J. E. and Santoro S. A. (1995). The spatial and temporal expression of the alpha2beta1 integrin and its ligands, collagen I, collagen IV, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation 59, 1-13. 10.1046/j.1432-0436.1995.5910001.x [DOI] [PubMed] [Google Scholar]
- Kent D., Copley M., Benz C., Dykstra B., Bowie M. and Eaves C. (2008). Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 14, 1926-1930. 10.1158/1078-0432.CCR-07-5134 [DOI] [PubMed] [Google Scholar]
- Khalili S., Liu Y., Kornete M., Roescher N., Kodama S., Peterson A., Piccirillo C. A. and Tran S. D. (2012). Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with Sjogren's-like disease. PLoS ONE 7, e38615 10.1371/journal.pone.0038615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi F., Matsuzaka K. and Inoue T. (2016). The effect of basic fibroblast growth factor on regeneration in a surgical wound model of rat submandibular glands. Int. J. Oral Sci. 8, 16-23. 10.1038/ijos.2015.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T., Kanemaru S., Hirano S., Tateya I., Suehiro A., Kitani Y., Kishimoto Y., Ohno S., Nakamura T. and Ito J. (2011). The protective efficacy of basic fibroblast growth factor in radiation-induced salivary gland dysfunction in mice. Laryngoscope 121, 1870-1875. 10.1002/lary.21873 [DOI] [PubMed] [Google Scholar]
- Koyama N., Kashimata M., Sakashita H., Sakagami H. and Gresik E. W. (2003). EGF-stimulated signaling by means of PI3K, PLCgamma1, and PKC isozymes regulates branching morphogenesis of the fetal mouse submandibular gland. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 227, 216-226. [DOI] [PubMed] [Google Scholar]
- Kretzschmar K. and Clevers H. (2016). Organoids: modeling development and the stem cell niche in a dish. Dev. Cell 38, 590-600. 10.1016/j.devcel.2016.08.014 [DOI] [PubMed] [Google Scholar]
- Kusakabe M., Sakakura T., Sano M. and Nishizuka Y. (1985). A pituitary-salivary mixed gland induced by tissue recombination of embryonic pituitary epithelium and embryonic submandibular gland mesenchyme in mice. Dev. Biol. 110, 382-391. 10.1016/0012-1606(85)90097-1 [DOI] [PubMed] [Google Scholar]
- Lancaster M. A. and Knoblich J. A. (2014). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125-1247125 10.1126/science.1247125 [DOI] [PubMed] [Google Scholar]
- Lee J.-H., Bhang D. H., Beede A., Huang T. L., Stripp B. R., Bloch K. D., Wagers A. J., Tseng Y.-H., Ryeom S. and Kim C. F. (2014). Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 156, 440-455. 10.1016/j.cell.2013.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Manna P., Kobayashi H., Spilde T., Bhatia A., Preuett B., Prasadan K., Hembree M. and Gittes G. K. (2004). Multifaceted pancreatic mesenchymal control of epithelial lineage selection. Dev. Biol. 269, 252-263. 10.1016/j.ydbio.2004.01.043 [DOI] [PubMed] [Google Scholar]
- Lim J.-Y., Ra J. C., Shin I. S., Jang Y. H., An H.-Y., Choi J.-S., Kim W. C. and Kim Y.-M. (2013). Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PLoS ONE 8, e71167 10.1371/journal.pone.0071167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I. M. A., Brunsting J. F., Wierenga P. K., Faber H., Stokman M. A., Kok T., Visser W. H., Kampinga H. H., de Haan G. and Coppes R. P. (2008). Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE 3, e2063 10.1371/journal.pone.0002063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I. M. A., Abrams S. R., Li L., Eswarakumar V. P., Sethi A. J., Witt R. L. and Hoffman M. P. (2013). Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organogenesis. Stem Cell Rep. 1, 604-619. 10.1016/j.stemcr.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I., Movahednia M. M., Adine C. and Ferreira J. N. (2017). Concise review: salivary gland regeneration: therapeutic approaches from stem cells to tissue organoids. Stem Cells 35, 97-105. 10.1002/stem.2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz S., Goderie S., Tokas N., Hirsch S. E., Ahmad F., Corneo B., Le S., Banerjee A., Kane R. S., Stern J. H. et al. (2013). Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS ONE 8, e56289 10.1371/journal.pone.0056289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.-F., Chua K.-N., Zhang P.-C., Lim W.-S., Ramakrishna S., Leong K. W. and Mao H.-Q. (2005). Three-dimensional co-culture of rat hepatocyte spheroids and NIH/3T3 fibroblasts enhances hepatocyte functional maintenance. Acta Biomater. 1, 399-410. 10.1016/j.actbio.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Maimets M., Rocchi C., Bron R., Pringle S., Kuipers J., Giepmans B. N. G., Vries R. G. J., Clevers H., De Haan G., Van Os R et al. et al. (2016). Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Rep. 6, 150-162. 10.1016/j.stemcr.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria O. M., Maria O., Liu Y., Komarova S. V. and Tran S. D. (2011). Matrigel improves functional properties of human submandibular salivary gland cell line. Int. J. Biochem. Cell Biol. 43, 622-631. 10.1016/j.biocel.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Maruyama C. L. M., Leigh N. J., Nelson J. W., McCall A. D., Mellas R. E., Lei P., Andreadis S. T. and Baker O. J. (2015). Stem cell-soluble signals enhance multilumen formation in SMG cell clusters. J. Dent. Res. 94, 1610-1617. 10.1177/0022034515600157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Kurimoto T., Taketo M. M., Fujii S. and Kikuchi A. (2016). The WNT/MYB pathway suppresses KIT expression to control the timing of salivary proacinar differentiation and duct formation. Development 143, 2311-2324. 10.1242/dev.134486 [DOI] [PubMed] [Google Scholar]
- McQualter J. L., Yuen K., Williams B. and Bertoncello I. (2010). Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc. Natl. Acad. Sci. USA 107, 1414-1419. 10.1073/pnas.0909207107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F., Czernichow P., Ozaki K., Itoh N. and Scharfmann R. (1999). Signaling through fibroblast growth factor receptor 2b plays a key role in the development of the exocrine pancreas. Proc. Natl. Acad. Sci. USA 96, 6267-6272. 10.1073/pnas.96.11.6267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima H., Matsumoto T., Sakai T., Yamaguchi S., An S. H., Abe M., Wakisaka S., Lee K. Y., Egusa H. and Imazato S., (2011). Hydrogel-based biomimetic environment for in vitro modulation of branching morphogenesis. Biomaterials 32, 6754-6763. 10.1016/j.biomaterials.2011.05.072 [DOI] [PubMed] [Google Scholar]
- Mizukoshi K., Koyama N., Hayashi T., Zheng L., Matsuura S. and Kashimata M. (2016). Shh/Ptch and EGF/ErbB cooperatively regulate branching morphogenesis of fetal mouse submandibular glands. Dev. Biol. 412, 278-287. 10.1016/j.ydbio.2016.02.018 [DOI] [PubMed] [Google Scholar]
- Morita K. and Nogawa H. (1999). EGF-dependent lobule formation and FGF7-dependent stalk elongation in branching morphogenesis of mouse salivary epithelium in vitro. Dev. Dyn. 215, 148-154. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Shinmura Y., Higuchi A. and Sakai Y. (2011). Effects of culture conditions on a micropatterned co-culture of rat hepatocytes with 3T3 cells. J. Bioprocess. Biotech. S3, 002. 10.4172/2155-9821.S3-002 [DOI] [Google Scholar]
- Nanduri L. S. Y., Maimets M., Pringle S. A., van der Zwaag M., van Os R. P. and Coppes R. P. (2011). Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 99, 367-372. 10.1016/j.radonc.2011.05.085 [DOI] [PubMed] [Google Scholar]
- Nanduri L. S. Y., Lombaert I. M. A., van der Zwaag M., Faber H., Brunsting J. F., van Os R. P. and Coppes R. P. (2013). Salisphere derived c-Kit+ cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 108, 458-463. 10.1016/j.radonc.2013.05.020 [DOI] [PubMed] [Google Scholar]
- Nanduri L. S. Y., Baanstra M., Faber H., Rocchi C., Zwart E., De Haan G., Van Os R. and Coppes R. P. (2014). Purification and Ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep. 3, 957-964. 10.1016/j.stemcr.2014.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. A. and Larsen M. (2015). Heterotypic control of basement membrane dynamics during branching morphogenesis. Dev. Biol. 401, 103-109. 10.1016/j.ydbio.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. A., Manhardt C., Kamath V., Sui Y., Santamaria-Pang A., Can A., Bello M., Corwin A., Dinn S. R., Lazare M. et al. (2013). Quantitative single cell analysis of cell population dynamics during submandibular salivary gland development and differentiation. Biol. Open 2, 439-447. 10.1242/bio.20134309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa H. and Takahashi Y. U. (1991). Substitution for mesenchyme by basement-membrane-like substratum and epidermal growth factor in inducing branching morphogenesis of mouse salivary epithelium. Development 112, 855-861. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Oshima M., Imamura A., Sekine Y., Ishida K., Yamashita K., Nakajima K., Hirayama M., Tachikawa T. and Tsuji T. (2013). Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat. Commun. 4, 2498 10.1038/ncomms3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H., Hori Y., Yamasaki M., Harada H., Sekine K., Kato S. and Itoh N. (2000). FGF10 acts as a major ligand for FGF receptor 2 IIIB in mouse multi-organ development. Biochem. Biophys. Res. Commun. 277, 643-649. 10.1006/bbrc.2000.3721 [DOI] [PubMed] [Google Scholar]
- Park Y.-J., Koh J., Gauna A. E., Chen S. and Cha S. (2014). Identification of regulatory factors for mesenchymal stem cell-derived salivary epithelial cells in a co-culture system. PLoS ONE 9, e112158 10.1371/journal.pone.0112158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. N., Rebustini I. T. and Hoffman M. P. (2006). Salivary gland branching morphogenesis. Differentiation 74, 349-364. 10.1111/j.1432-0436.2006.00088.x [DOI] [PubMed] [Google Scholar]
- Patel V. N., Lombaert I. M. A., Cowherd S. N., Shworak N. W., Xu Y., Liu J. and Hoffman M. P. (2014). Hs3st3-modified heparan sulfate controls KIT+ progenitor expansion by regulating 3-O-sulfotransferases. Dev. Cell 29, 662-673. 10.1016/j.devcel.2014.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S. B., Naim N., Nelson D. A., Mosier A. P., Cady N. C. and Larsen M. (2014). Biocompatible tissue scaffold compliance promotes salivary gland morphogenesis and differentiation. Tissue Eng. Part A 20, 1632-1642. 10.1089/ten.tea.2013.0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle S., Nanduri L. S. Y., van der Zwaag M., van Os R., Coppes R. P. (2011). Isolation of mouse salivary gland stem cells. J. Vis. Exp. 8, 2484. 10.3791/2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle S., Maimets M., van der Zwaag M., Stokman M. A., van Gosliga D., Zwart E., Witjes M. J. H., de Haan G., van Os R. and Coppes R. P. (2016). Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 34, 640-652. 10.1002/stem.2278 [DOI] [PubMed] [Google Scholar]
- Rebustini I. T. and Hoffman M. P. (2009). Extracellular matrix protocols. Methods Mol. Biol. 522, 319-330. 10.1007/978-1-59745-413-1_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswati S., Bastakoty D. and Young P. P. (2012). Molecular and signaling pathways that modulate mesenchymal stem cell self-renewal. Stem Cells and Cancer Stem Cells 6, 131-141. 10.1007/978-94-007-2993-3_12 [DOI] [Google Scholar]
- Sato T. and Clevers H. (2015). SnapShot: growing organoids from stem cells. Cell 161, 1700-1700.e1. 10.1016/j.cell.2015.06.028 [DOI] [PubMed] [Google Scholar]
- Sequeira S. J., Larsen M. and DeVine T. (2010). Extracellular matrix and growth factors in salivary gland development. Front. Oral Biol. 14, 48-77. 10.1159/000313707 [DOI] [PubMed] [Google Scholar]
- Sequeira S. J., Soscia D. A., Oztan B., Mosier A. P., Jean-Gilles R., Gadre A., Cady N. C., Yener B., Castracane J. and Larsen M. (2012). The regulation of focal adhesion complex formation and salivary gland epithelial cell organization by nanofibrous PLGA scaffolds. Biomaterials 33, 3175-3186. 10.1016/j.biomaterials.2012.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamir E. R. and Ewald A. J. (2014). Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 15, 647-664. 10.1038/nrm3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simian M. and Bissell M. J. (2017). Organoids: a historical perspective of thinking in three dimensions. J. Cell Biol. 216, 31-40. 10.1083/jcb.201610056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simian M., Hirai Y., Navre M., Werb Z., Lochter A. and Bissell M. J. (2001). The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Dev. Camb. Engl. 128, 3117-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. D., Maloney T. M., Wolman S. R., Peterson W. D., Brenz R., McGrath C. M., Russo J., Pauley R. J., Jones R. F. and Brooks S. C. (1990). Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 50, 6075-6086. [PubMed] [Google Scholar]
- Steinberg Z., Myers C., Heim V. M., Lathrop C. A., Rebustini I. T., Stewart J. S., Larsen M. and Hoffman M. P. (2005). FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Dev. Camb. Engl. 132, 1223-1234. [DOI] [PubMed] [Google Scholar]
- Sumita Y., Liu Y., Khalili S., Maria O. M., Xia D., Key S., Cotrim A. P., Mezey E. and Tran S. D. (2011). Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int. J. Biochem. Cell Biol. 43, 80-87. 10.1016/j.biocel.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe T., Enomura M., Yoshizawa E., Kimura M., Koike H., Ueno Y., Matsuzaki T., Yamazaki T., Toyohara T., Osafune K. et al. (2015). Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 16, 556-565. 10.1016/j.stem.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Teisanu R. M., Chen H., Matsumoto K., McQualter J. L., Potts E., Foster W. M., Bertoncello I. and Stripp B. R. (2011). Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am. J. Respir. Cell Mol. Biol. 44, 794-803. 10.1165/rcmb.2010-0098OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thula T. T., Schultz G., Tran-Son-Tay R. and Batich C. (2005). Effects of EGF and bFGF on irradiated parotid glands. Ann. Biomed. Eng. 33, 685-695. 10.1007/s10956-005-1853-z [DOI] [PubMed] [Google Scholar]
- Tucker A. S. (2007). Salivary gland development. Semin. Cell Dev. Biol. 18, 237-244. 10.1016/j.semcdb.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Walker J. L., Menko A. S., Khalil S., Rebustini I., Hoffman M. P., Kreidberg J. A. and Kukuruzinska M. A. (2008). Diverse roles of E-cadherin in the morphogenesis of the submandibular gland: Insights into the formation of acinar and ductal structures. Dev. Dyn. 237, 3128-3141. 10.1002/dvdy.21717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Larsen M., Hoffman M. P. and Yamada K. M. (2007). Self-Organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 13, 721-735. 10.1089/ten.2006.0123 [DOI] [PubMed] [Google Scholar]
- Wells K. L., Gaete M., Matalova E., Deutsch D., Rice D. and Tucker A. S. (2013). Dynamic relationship of the epithelium and mesenchyme during salivary gland initiation: the role of Fgf10. Biol. Open 2, 981-989. 10.1242/bio.20135306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xinaris C., Benedetti V., Rizzo P., Abbate M., Corna D., Azzollini N., Conti S., Unbekandt M., Davies J. A., Morigi M. et al. (2012). In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J. Am. Soc. Nephrol. JASN 23, 1857-1868. 10.1681/ASN.2012050505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P. D. (2011). Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 3, 1-28. 10.1101/cshperspect.a004911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Martinez D., Koledova Z., Qiao G., Streuli C. H. and Lu P. (2014). FGF ligands of the postnatal mammary stroma regulate distinct aspects of epithelial morphogenesis. Development 141, 3352-3362. 10.1242/dev.106732 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.