Abstract
Pathophysiological, epidemiologic and genetic studies provide strong evidence that Lp(a) is a causal mediator of cardiovascular disease (CVD) and calcific aortic valve disease (CAVD). Specific therapies to address Lp(a)-mediated CVD and CAVD are in clinical development. Due to knowledge gaps, the National Heart, Lung, and Blood Institute (NHLBI) organized a Working Group (WG) that identified challenges in fully understanding the role of Lp(a) in CVD/CAVD. These included the lack of research funding, inadequate experimental models, lack of globally standardized Lp(a) assays and inadequate understanding of the mechanisms underlying current drug therapies on Lp(a) levels. Specific recommendations were provided to facilitate basic, mechanistic, preclinical, and clinical research on Lp(a), foster collaborative research and resource sharing, leverage expertise of different groups and centers with complementary skills, and utilize existing NHLBI resources. Concerted efforts to understand Lp(a) pathophysiology, together with diagnostic and therapeutic advances, are required to reduce Lp(a)-mediated risk of CVD and CAVD.
Keywords: Lipoprotein(a), pathophysiology, cardiovascular disease, aortic stenosis, metabolism, therapy
Introduction
Lipoprotein(a) [Lp(a)] is composed of an apolipoprotein B (apoB)-containing low-density lipoprotein (LDL)-like particle, covalently linked to the plasminogen-like glycoprotein apolipoprotein(a) (apo(a)) (1). Circulating plasma Lp(a) levels are primarily determined by the LPA gene locus encoding apo(a), with some influence from the APOE locus (2) and PCSK9 R46L loss of function mutations (3,4). LPA is highly polymorphic in size due to the number of kringle IV type 2 (KIV2)-encoding sequences, giving origin to >40 apo(a) isoforms varying in distribution among individuals and populations
Lp(a) is a highly prevalent, genetic risk factor for cardiovascular disease (CVD) and calcific aortic valve disease (CAVD). Lp(a) levels in the atherothrombotic range are generally accepted as >30–50 mg/dL or >75–125 nmol/L (1,5). Such levels affect 20–30% of the global population (6,7), with possibly higher incidence in patients with established CVD (8) and CAVD (9). Importantly, more than one billion people globally have elevated levels of Lp(a). Convincing evidence has emerged from pathophysiological, epidemiologic, and genetic studies on the causality of Lp(a) in contributing to CVD (myocardial infarction, stroke, peripheral arterial disease, heart failure) and CAVD (10–12).
Evidence from randomized statin and PCSK9 inhibitor trials, including 4S, AIM-HIGH, JUPITER, LIPID and FOURIER, have shown that when Lp(a) is elevated, the event rates are higher at any achieved LDL-C level, consistent with unaddressed Lp(a)-mediated risk. The Lp(a) lowering effects of PCSK9 and CETP inhibitors, mipomersen and antisense oligonucleotides have raised interest in Lp(a) from a mysterious particle of interest primarily to a small cadre of clinical lipidologists to the broader investigative, pharmaceutical and cardiology communities.
Significant knowledge gaps exist in Lp(a) biology and pathophysiology. To address these gaps, the National Heart, Lung, and Blood Institute convened a Working Group on Future Research Directions on Lipoprotein(a) and Cardiovascular Disease. The Working group brought together 14 experts from diverse backgrounds in Lp(a) basic, translational, and clinical sciences. In alignment with the Institute’s Strategic Plan Goals 1, 2, and 3 the group focused on Lp(a) metabolism, pathophysiology, including appropriate animal models for Lp(a) research, as well as Lp(a) measurements, current and emerging therapies for elevated Lp(a), identification of patients at high-risk for Lp(a)-mediated risk, understudied populations, and physician, patient and public awareness of Lp(a) as a CVD and CAVD risk factor. The salient features of the discussion and recommendations are provided below.
Lipoprotein(a) nomenclature and clinical diagnosis
A standardized nomenclature for lipoprotein(a) is not currently available and different names have been used for Lp(a) since its initial publication in 1963. The WG recommends calling this lipoprotein ‘Lipoprotein(a)” or “Lp(a)”, with no space between lipoprotein and (a). Furthermore, kringle numbers of apo(a) should be reported by Roman numerals and kringle types subscripted (e.g. KIV2 for kringle IV type 2). Subjects with elevated levels should be described as having “Elevated Lp(a)” or “Hyperlipoproteinemia(a)”. Traditional thresholds for elevated Lp(a) are >30 mg/dL or >75 nmol/L, which approximate the 75th percentile in white populations and also reflect epidemiological data of CVD risk thresholds in primary care populations (6,13). In the United States an ICD-10 code for the diagnosis of elevated Lp(a) levels does not exist. Clinicians have no way to document elevated Lp(a) levels, except with the use of a generic hypercholesterolemia code. The lack of an ICD-10 code also limits research on Lp(a) using electronic health records.
Guideline recommendations for measuring Lp(a)
The European Society of Cardiology/European Atherosclerosis Society have given a IIa recommendation with level of evidence C for measuring Lp(a) in patients with premature CVD, familial hypercholesterolemia (FH), family history of premature CVD or family history of elevated Lp(a), recurrent CVD despite optimal lipid-lowering therapy, or a ≥5% 10-year risk of fatal CVD according to the SCORE algorithm (5). They also suggest the risk of Lp(a) is significant when levels are >80th percentile, or >50 mg/dL (~100–125 nmol/L), for European populations. It is emphasized that this threshold is higher than the risk threshold in primary care populations of >20–30 mg/dL (6,13,14). The Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult had similar recommendations, but used a cutoff of Lp(a) >30 mg/dL to assign abnormal levels (15). The National Lipid Association also endorses measurement of Lp(a) in similar patient subsets (16).
What are the knowledge gaps in the Lp(a) field?
Since the publication of Lp(a) in 1963, much has been learned about Lp(a). However, significant gaps in knowledge are present as summarized below. These gaps can be organized in several themes, including defining metabolism, understanding pathophysiological mechanisms, standardizing measures of Lp(a), defining mechanisms through which current and emerging therapies affect Lp(a) levels, placing effort to elucidate the role of Lp(a) in understudied, high-risk populations and the testing of the Lp(a) hypothesis. For example, a physiological function of Lp(a) has not been determined; the sites where apo(a) and apoB are assembled to generate the Lp(a) particle, such as intra-hepatocyte, space of Disse, or vascular lumen, remain controversial; the influence of genetic and epigenetic regulatory elements, hormonal, inflammatory, co-morbid and dietary influences on LPA gene expression have not been comprehensively studied; the nature and extent of various clearance mechanisms, such as hepatic (LDLR, SR-B1, LRP-1, PLG) and non-hepatic receptors or renal mechanisms, are not well delineated; Lp(a) is composed of ~30–45% cholesterol by mass and this is reported as part of the “LDL-C” laboratory measurement (17). In the current era of very low LDL-C levels, the contribution of Lp(a) cholesterol to “LDL-C “ may be substantial. The physiological and clinical implications of this fact are unknown; the composition/functional correlation between the Lp(a) lipidome and proteome with different Lp(a) levels and apo(a) isoform sizes is under-studied.
Regarding pathophysiological mechanisms, Lp(a) may contribute to CVD and CAVD via its LDL-like component, pro-inflammatory effects of OxPL on both apoB and apo(a) (18), and anti-fibrinolytic/pro-thrombotic effects of apo(a). The contribution of the strong lysine binding site in apo(a) as a mechanism to enhance Lp(a) accumulation in vascular tissues, interfere with plasminogen activation, mediate the covalent binding of OxPL, and the in vivo roles and relative contributions of these mechanisms require further study; since Lp(a) is not present in laboratory animals, appropriate experimental models have not been optimized to study its metabolism and mechanism of action in disease processes; the relationship and contribution of Lp(a) to valvular and vascular calcification, and whether differences exist between Lp(a)-mediated processes in the arterial wall and aortic valve is not known; preliminary data suggest a proinflammatory role for the OxPL of Lp(a) on monocytes. Whether a similar property applies to endothelial cells, macrophages, as well as B and T lymphocytes is not known; Lp(a) has shown acute phase properties in some studies, particularly in acute MI and rheumatological diseases, but this requires further study to assess the mechanisms, extent and relevance of these effects.
For clinical risk prediction, Lp(a) is a risk factor for CVD in both primary and secondary prevention settings, but the risk of Lp(a) for recurrent CVD events in settings of highly aggressive secondary prevention is not well understood; whether different Lp(a) risk thresholds exist for CVD versus CAVD remains to be established; Lp(a) in high-risk populations, such as post-ACS, FH, chronic renal failure, diabetes and the elderly, has not been well studied epidemiologically. Whether such patients would benefit from Lp(a) lowering is not known; the in vivo roles of Lp(a) in mediating both venous and arterial thrombosis have not been established. Studies of how modification of Lp(a) levels affects processes of thrombosis and fibrinolysis have not been reported; for clinical risk prediction, it is not known whether absolute thresholds (i.e. >30 mg/dL or >75 nmol/L), risk percentiles (i.e. >80%) or race-specific thresholds better identify high-risk individuals.
The mechanisms through which low saturated fat diets and statins potentially increase Lp(a) or how niacin, mipomersen, CETP and PCSK9 inhibitors reduce Lp(a) levels are not fully established.
Finally, a randomized trial to test the Lp(a) hypothesis, namely that specifically reducing elevated Lp(a) levels leads to CVD or CAVD risk reduction, has not been carried out to date.
NHLBI Working Group: Challenges and Recommendations
The WG identified several important challenges and barriers that have inhibited the full understanding of the role of Lp(a) in CVD and CAVD. The main areas include the lack of significant public and private funding for basic Lp(a) research, the low number of applications, the low success rate of peer-reviewed applications, the lack of Lp(a) expertise in atherosclerosis/inflammation on grant peer-review panels, the lack of reliable in vitro cell lines and in vivo animal models, the lack of access to large bio-repositories for biomarker and clinical research and the lack of standardization of Lp(a) assays. Currently, there is also a very small cadre of active investigators in the area of Lp(a) biology. The reasons are multiple and complex, but likely reflect a combination of the lack of awareness, until recently, of investigators on the large global impact of Lp(a) in CVD and AS, the lack of mentoring programs to train Lp(a) investigators, the lack of available models to perform studies and the lack of dedicated funding on Lp(a) focused research.
The WG broadly recommended the following:
Explore mechanisms by which NHLBI could facilitate basic, mechanistic, preclinical, and clinical research on Lp(a).
Foster collaborative research and resource sharing, leverage expertise of different groups and centers with complementary skills, methods, and knowledge, and leverage existing resources such as NHLBI cohorts.
Support assignment of an ICD-10 code for the diagnosis of elevated Lp(a). An ICD-10 code will enhance appropriate and specific diagnosis for patients at risk for Lp(a)-mediated disease, will identify Lp(a) as a potential etiologic factor in patients with established CVD, and will allow assignment of familial or genetic risk, particularly in younger patients with CVD. Importantly, it will allow tracking of the prevalence of Lp(a) elevations in patient cohorts and the establishment of large databases for clinical research. Finally, with the emergence of novel Lp(a) lowering therapies, appropriate diagnosis, prognosis and potential assignment of therapeutic options will be needed for clinical care.
Organize focused working groups comprised of wide array of stakeholders, including regulatory agencies, to standardize Lp(a) measurements.
Educate the public, physicians, regulatory agencies, and funding agencies on the role of Lp(a) in CVD and CAVD.
Develop evidence-based management plans for patients.
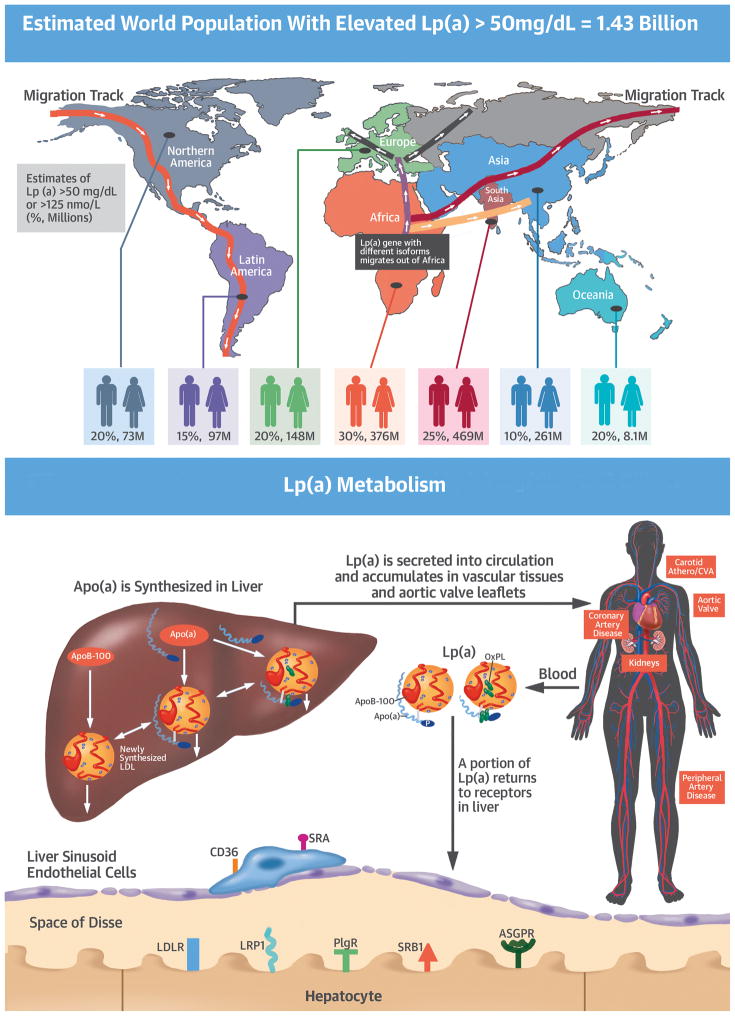
The Central Illustration summarizes the salient features of the unmet needs of Lp(a) pathophysiology.
Central Illustration. Global Prevalence of Elevated Lp(A) Levels, Metabolism of Lp(A) Ad Phenotypic Expression of Disease, and Unmet Needs In Lp(A) Pathophysiology.
The top of the figure depicts the migration of the LPA gene, and its multiple apo(a) isoforms, out of Africa to the rest of the world. Due to differences in migration of different isoforms, as well as subsequent LPA gene remodeling of single nucleotide polymorphisms, the prevalence of Lp(a) plasma levels is variable. The estimates of prevalence of elevated Lp(a) (> 50 mg/dL or >125 nmol/L is given, based on current estimates of threshold prevalence. The middle panels depict the synthesis of both alleles of apo(a) and the subsequent assembly into Lp(a) with an LDL-like particle. Lp(a) is secreted into circulation and accumulates in vascular tissues and aortic valve leaflets over a lifetime. The clearance of Lp(a) is less well understood but a large portion is cleared via hepatic receptors, and a smaller portion by renal mechanisms and other unidentified pathways. On the bottom is a very brief summary of unmet needs in the understanding of Lp(a) pathophysiology.
In order to optimize research within the Lp(a) field, the WG identified 6 key areas of research priorities and corresponding recommendations listed and discussed below.
Specific Research Priorities and Recommendations
Priority #1: To fully define mechanisms of Lp(a) synthesis, assembly, clearance and other influences on circulating levels
There is uncertainty regarding the site(s) of the assembly of Lp(a), the role of secretion in determining plasma Lp(a) levels, the stability of the bond between apo(a) and apoB in plasma, and the mechanisms of clearance and degradation of Lp(a). Specifically, there is ongoing controversy about where apo(a) and apoB-100 bind to form Lp(a); which subtypes of apoB-100 lipoproteins bind to apo(a) to form Lp(a); whether the binding of apo(a) to apoB-100 is reversible, allowing apo(a) to bind to more than one apoB-100-containing lipoprotein during its lifespan in the circulation; and if Lp(a) or apo(a) leave the circulation via the LDLR pathway or through other potential pathways. Stable isotope studies (19,20) have produced conflicting results with early studies looking at the individual component proteins providing support for intracellular assembly of Lp(a), and more recent ones reporting extracellular assembly (discussed in reference 20).
The mechanism(s) by which Lp(a) is catabolized remain remarkably poorly understood (20). Studies in preclinical model systems indicate that the liver is the major site of Lp(a) catabolism. While many in vitro studies suggest that the LDLR can function as a clearance receptor for Lp(a), the available in vivo data are not entirely consistent with this: 1) humans with homozygous FH lacking the LDLR have similar rates of Lp(a) catabolism compared with controls; 2) LDLR knockout mice have similar rates of plasma Lp(a) turnover and hepatic uptake of Lp(a) compared with wild-type mice; 3) statins upregulate the LDLR leading to increased LDL catabolism but do not reduce Lp(a). The role of the LDLR in Lp(a) catabolism has been recently explored in vivo using stable isotopes to study of the effects of PCSK9 inhibition on apoB and Lp(a) metabolism. The results indicated that other receptors, in addition to the LDLR, must be involved in the clearance of Lp(a) from the circulation (19). Additionally, that study suggested that the LDLR only plays a significant role in Lp(a) clearance when hepatic levels of the receptor are very high and LDL levels are low, as is the case in PCSK9 inhibitor therapy. The continued uncertainty regarding the clearance of Lp(a) is exemplified by two recent in vitro studies with liver cells that provided completely conflicting results for the role of LDLR levels in Lp(a) uptake (21,22).
Studies have suggested a role for scavenger receptor class B Type I (SR-BI), plasminogen receptors, LDLR-related protein 1 (LRP1), CD36, and other receptors in liver-mediated Lp(a) uptake; this likely reflects the ability of both the LDL and apo(a) components to act as receptor ligands. For example, SR-BI mostly leads to the selective uptake of neutral lipids, such as cholesteryl esters from the Lp(a) core, but may not significantly alter the plasma clearance of apo(a) (23). The physiologic relevance of these observations on the interaction of Lp(a) with SR-BI in humans is not clear, but patients with heterozygous loss of function mutations in SR-BI show increased plasma levels of Lp(a) (24). LRP1 may contribute to Lp(a) clearance via its affinity for apoB or Lp(a) and the different affinities for apoE isoforms. Competition of apoB-Lp(a) with apoE isoforms, particularly E4, which has strong affinity for LRP1, strongly influences Lp(a) levels (25). A member of the widely-expressed plasminogen receptor family, PlgRKT, was recently shown to mediate uptake of both Lp(a) and apo(a) in vitro (26). CD36 is as a multiligand scavenger receptor that has been shown to bind and take up OxLDL and OxPL, which may potentially take up Lp(a) via its OxPL content (27).
Recommendations for research efforts for priority #1
There is a need for studies that isolate “pure Lp(a)”, not contaminated by LDL-apoB or HDL, to allow for the kinetic examination of the metabolism of the Lp(a)-apo(a) and Lp(a)-apoB components, as well as the influence of different apo(a) isoforms on metabolism. Emerging technologies based on quantitation by mass spectrometry of specific apo(a) and apoB peptides are a promising avenue. Once validated methods of isolation are established, sophisticated modeling of the kinetic data will be crucial to enable investigators to address some of the key questions that remain, including whether: 1-) apo(a) and apoB associate inside the liver; 2- apo(a) binds to apoB lipoproteins extracellularly in the bloodstream and 3- there is recycling of Lp(a)-apo(a) or Lp(a)-apoB inside the liver after uptake of the Lp(a) particle (19).
Along with methods to purify Lp(a), the particle’s lipidome and proteome in healthy subjects and patients with CVD and CAVD should be annotated and the pathophysiologic implications of these constituents determined.
Factors that influence apo(a) transcription should be systematically examined. Epigenetic modifications that mediate gene expression or pro-inflammatory signaling should also be studied (28).
The study of Lp(a) synthesis, assembly and secretion of Lp(a) will require development of improved hepatocyte cell culture systems that express Lp(a) abundantly, since current human hepatocyte cell lines have very low apo(a) expression. Mouse primary hepatocytes need to have the proper regulatory elements of the entire LPA gene and not only cDNA constructs. Lp(a) transgenic monkey hepatocytes are not ideal for mechanistic studies because of significant differences in kringle type (e.g. some species lack KV), and all monkey Lp(a) lacks lysine binding affinity and OxPL content compared to human lines (29). The role of the LDLR in Lp(a) catabolism in the background of PCSK9 inhibition could be evaluated in vitro through systematic alterations of the ratios of LDLR, PCSK9, and Lp(a). Approaches to the study of Lp(a) catabolic pathways could include knock-downs of candidate receptors (LDLR, LRP1, CD36, SRB1, PLG-RKT) for measurement of their effect on Lp(a) uptake and degradation.
To complement candidate gene/receptor studies, unbiased genome-wide screens should be utilized to identify genes that when deleted reduce Lp(a) uptake and degradation. Modern molecular biology techniques, such as CRISPR/Cas9 libraries, are available to perform high-throughput, genome-wide screens on hepatocyte cell culture models and analyses for putative clearance receptors.
The mechanisms underlying the influence of apoE isoforms, which have very different affinities for the LDLR and LRP1 receptors that may influence Lp(a) binding, should be studied.
Once an understanding is obtained of the qualitative and quantitative role of various clearance mechanisms, studies will be required to identify the route of Lp(a) catabolism in both physiological and altered conditions. Naturally-occurring human ‘knockouts’ of candidate genes could be utilized to study the effect on Lp(a) turnover in vivo. In concert, additional in vivo metabolic studies in humans should be performed in FH patients and subjects receiving PCSK9 inhibitors.
The recent introduction of antisense therapy has demonstrated an ability to lower plasma Lp(a) levels by up to 90% or more, even in subjects with greatly elevated Lp(a) levels (30,31). Although it is known that apo(a) mRNA is reduced, it will be important to fully explore the kinetic mechanisms by which these reductions occur.
Priority #2: To understand the mechanisms underpinning Lp(a) and its associated oxidized phospholipids in mediating risk of CVD and aortic stenosis
The absence of Lp(a) in commonly used laboratory animals has resulted in limited options for the generation of relevant models to study Lp(a)-mediated mechanisms of atherogenesis and CAVD. Early studies utilized transgenic mice overexpressing a 17 kringle (17K)-containing human apo(a), which does not form a covalent Lp(a) particle with mouse apoB-100. Subsequently, mice were generated overexpressing both human apo(a) and apoB-100, with resultant circulating covalent Lp(a) particles. There is conflicting evidence whether transgenic Lp(a) mice, which generally have levels of Lp(a) in normal range, are more susceptible to atherosclerosis than the transgenic human apoB-100 mice (32). Transgenic apo(a) rabbits expressing human 17K apo(a) formed circulating Lp(a) particles with endogenous rabbit apoB-100, also in normal range, with resulting lesions reminiscent of human atheromata (32).
A compelling hypothesis of the pro-inflammatory effects of Lp(a) is the preferential accumulation on Lp(a), compared to other lipoproteins, of phosphocholine-containing OxPL in lipid phase and covalently bound to apo(a). OxPL induce pro-inflammatory signaling in endothelial cells, smooth muscle cells and macrophages and are pro-apoptotic when concentrations are high (27,33). They are relevant to the understanding of the pathobiology of Lp(a) as up to 85–90% of all OxPL found in human lipoproteins (at least those OxPL moieties detected by monoclonal antibody E06) are carried on Lp(a) (29). Further, clinical studies have demonstrated that the OxPL on Lp(a) mediates arterial wall inflammation and promotes monocyte inflammatory responses in humans (18). Finally, extensive epidemiological studies demonstrate that levels of OxPL on Lp(a) (measured as OxPL-apoB and OxPL-apo(a)) are robust predictors of CVD events and CAVD (1).
Recommendations for research efforts for priority #2
The preferred Lp(a)-transgenic model should use physiologically-relevant apo(a) isoforms, along with the native regulatory elements, and human apoB-100 to generate transgenic mice. Plasma Lp(a) levels should be in the pathogenic range, at >30 mg/dL (>~75 nmol/L) and preferably >80th percentile of human populations (>50 mg/dL or >125 nmol/L). Human “apoB-100 only variant of apoB” should be used to maximize the extent Lp(a) formation (34). Since OxPL are thought to bind specific amino acids at or near KIV10 which contains lysine binding site, point mutations eliminating critical amino acids required for OxPL and lysine-binding should be engineered in the context of a physiological apo(a) isoform size (29).
OxPL is a general term that encompasses a large number of individual species. Fundamental knowledge of mechanisms by which OxPL on Lp(a), and specifically common individual OxPL moieties, impact on cell signaling and function need to be defined. It is known that OxPL can mediate macrophage signaling via CD36, TLR2 or TLR4 and/or combinations of these (35), but little is known of the detailed cellular or molecular biology that mediates these events in vivo. OxPL present as protein adducts, such as on apo(a) or apoB, may be biologically active also when present on microvesicles or on the surface of apoptotic cells and may mediate the pathogenicity of Lp(a) (36). In parallel, the development and standardization of techniques are needed to measure OxPL species on isolated Lp(a), plasma and tissues is critical for progress in this area of investigation. Mass spectrometry is used to measure OxPL species but currently there are only a limited number of individual compounds available that can be used as standards to enable quantitative measurements. The NHLBI should facilitate development of a variety of OxPL analogues and standards by companies as was done during the LIPID MAPS program.
A high priority is the development of murine models to allow for determination of role of OxPL in vivo in a variety of inflammatory settings and in particular in the context of atherogenesis. Lp(a)-transgenic models might include models where the OxPL binding site is disabled and models with concomitant expression of antibodies that specifically bind and neutralize OxPL, or the passive transfer of such antibodies into mice with established disease. Studies of impact on atherosclerosis/CAVD should be accompanied by mechanistic studies to examine the impact on expected changes in systemic and localized cellular inflammatory signaling, macrophage uptake of OxLDL/OxPL and cholesterol accumulation.
There are also a number of murine models that have been developed to study the pathogenesis of aortic stenosis, including use of the murine apoB-100 transgenic mice on the Ldlr−/− background (37). Models with human apoB-100 can be used to determine the role of Lp(a) and OxPL as described above. Other models of CAVD, such as IGF-II transgenic mice, and new ones when the pathogenesis of CVAD is further understood may also be considered in this context.
Priority #3: To develop a globally standardized measurement of Lp(a) applicable to commercial laboratories and to define population risk among different ethnic/racial groups
The determination of Lp(a) levels in human samples are mainly performed by immunoassays using polyclonal antibodies against apo(a). Two types of approaches are presently used to express the levels of Lp(a). The first is based on the assignment of target values to the assay calibrators in terms of total Lp(a) mass (apo(a), apoB and the lipid components). The values are expressed in mg/dL and there is no traceability of the various calibrators to any established reference material. The second approach, used in several commercial methods, is to assign the target values to assay calibrators traceable to the WHO/IFCC secondary reference material PRM-2B (38). The values are expressed in nmol/L of apo(a) thus reflecting the number of circulating particles and not the variable mass of apo(a) or the lipid component. After method calibration, the values obtained in a set of individual samples are compared to those obtained by a monoclonal-antibody based ELISA method developed at the University of Washington and considered the “gold standard” (39). Due to the size heterogeneity of apo(a), there is no conversion factor (despite an estimate of 2–2.5x conversion factor from mg/dL to nmol/L used in literature) to transform the values from one unit to the other. An extensive review of the numerous issues affecting the measurement of Lp(a) has been published recently (40).
Before even attempting any standardization of Lp(a) measurements, as previously recommended (41), the assay calibration in mg/dL of total Lp(a) mass should be discontinued considering that only apo(a) is measured by the antibodies and that the mass of apo(a) is highly variable. In general, the first step for standardization of analytical methods is the calibration of assays to be traceable to a common reference system. The second step is the verification of harmonization of results obtained by the different methods after an accuracy-based common calibration is performed. However, this classic approach is rendered more complex by the inherent lack of accuracy of methods for measuring Lp(a) generated by the very large size polymorphism of apo(a). In fact, a major effort is needed by the manufacturers to render their method less affected by apo(a) size variations.
Due to its expertise in standardization of apo AI, apo B, and lipids, Lp(a) standardization activity has been performed in the past 15 years at the University of Washington by the Northwest Lipid Metabolism and Diabetes Research Laboratory (NWLMDRL) in collaboration with some manufacturers by distributing the WHO/IFCC reference material and by verifying values obtained by different methods. However, the PRM-2B reference material is close to be depleted and a new material with high level of Lp(a) needs to be developed. To be internationally accepted by the scientific community, its value needs to be proven accurate and established by a non-antibody-based reference method. The NWLMDRL has recently developed a Liquid Chromatography Select Reaction Monitoring Tandem Mass Spectrometry (LC-SRM-MSMS) reference method accurately calibrated with a primary apo(a) reference preparation to be used to assign a target value to a secondary reference material.
However, the preparation of a secondary reference material and the numerous challenges that need to be overcome for the standardization/harmonization of Lp(a) methods, cannot be faced by a single laboratory. Everybody is in agreement that the availability of standardized methods that provide accurate and comparable results is considered essential for the performance of epidemiological studies, for future therapeutic clinical trials, for determination of risk and therapy thresholds of Lp(a). However, there is no established definition of the approaches that need to be taken to achieve the goal of method standardization and no consensus has been reached in the scientific community on how to cooperatively make decisions and implement the various standardization steps.
Along with standardized assay methodologies, the determination of what levels are considered abnormal is not fully determined. Data from subjects without prior CVD suggest the threshold for elevated CVD risk is >20–25 mg/dL or >50–75 nmol/L (13,14,42) but levels in subjects with prior CVD may be >40–50 mg/dL (1). Finally, in patients with or at risk for CAVD, levels may yet be even higher, at ~>40–60 mg/dL (9). Additional studies, particularly in the PCSK9 era of very low LDL-C are needed to fully define risk thresholds for pre-existing CVD (43).
It is also well known that population means and median levels of Lp(a) vary by race/ethnicity. Most of the data of Lp(a) risk is derived from Caucasian populations, but recent efforts have also provided data in Blacks and East and South Asians. A recent study in Blacks, Whites and Hispanics suggest that the circulating Lp(a) level is predicting risk of MACE, irrespective of race/ethnicity, LPA snps or isoforms (44,45).
Finally, it is well established that there are many LPA snps (estimate 40 – >200) that are associated with either low or high Lp(a). Interestingly, many of these snps are race specific or race prevalent, and the Lp(a) relationship of these snps is not necessarily in the same direction among races (45). For example, rs3798220 is of low prevalence (~3%) in Europeans and associated with high Lp(a), but high prevalence (>40%) in Hispanics and associated with low Lp(a) (45). This was due mainly to the concomitant presence of large isoforms in Hispanics, which are a stronger and direct driver of Lp(a) levels. It thus appears that these snps are tagging small isoforms and not directly involved with Lp(a) production. Earlier data had also suggested that small apo(a) isoforms are associated with higher risk, and although this is true, when one adjusts for plasma Lp(a), this relationship is weak or absent (45,46).
Recommendations for research efforts for priority #3
The support of the NHLBI to assist in the organization of a working group comprised of the relevant stakeholders, including scientific societies and regulatory agencies, to set standards for Lp(a) assay methodology is of paramount importance. The support of the CDC or other agencies to ensure the assays’ accuracy and comparability as a nationwide effort will be very important for interpreting epidemiological studies, providing clinical care, and performing future therapeutic trials.
It is recommended that standardized Lp(a) assays report values in apo(a) particle number, as nmol/L. Mass assays should be phased out due to their inherent limitations. Along with this, significant educational efforts will be needed in the clinical community to understand what information the test gives and how it should be interpreted.
Defining scientifically-based, risk and therapy thresholds in general populations, patients with pre-existing CVD, CAVD and in different racial/ethnic groups of Lp(a) should be carried out with existing and future databases.
To educate the public and physicians on recent guidelines on screening Lp(a) to identify high risk patients.
To promote efforts to define the role of Lp(a) risk in various racial and ethnic groups to normalize for baseline differences in population means and enhanced risk prediction.
LPA snps and isoforms should continue to be studied in a research realm to fully define their role as contributors to Lp(a)-mediated risk.
Priority #4: To understand the mechanisms through which therapeutic regimens affect Lp(a) levels
The effect of therapeutic agents on circulating levels of Lp(a) are not well understood. For example, several studies have shown that both statins and low-saturated fat diets actually raise Lp(a) levels, a seeming paradox since both are associated with CV benefit (47). In contrast, most hormones, except growth hormone, lower Lp(a) levels. In contrast, niacin, mipomersen, PCSK9 inhibitors and CETP inhibitors, and antibodies to IL-6 also decrease Lp(a) levels modestly. It is curious that all of these interventions have modest effect on Lp(a), up to ~30% in either direction, suggesting modest regulatory influences on LPA gene expression, assembly and/or clearance of Lp(a).
The FOURIER trial, with the power of 27,564 subjects, has shown a 27% reduction in Lp(a) levels with PCSK9 inhibition, which is half of that seen for LDL-C (59%) reduction, suggesting that LDLR up-regulation caused by PCSK9 inhibition also affects clearance of Lp(a). This is largely an unexpected phenomenon, since statins up-regulate the expression of the LDLR but have a neutral or Lp(a)-raising effect. The apparent paradox may be explained by the fact that PCSK9 inhibitors have a much larger effect on LDLR compared with the statins, and that Lp(a) is a poor competitor for LDLR binding in the presence of LDL. However, a recent study has suggested that the Lp(a) reduction caused by PCSK9 inhibition does not always parallel the reduction in LDL according to a 2:1 ratio, and a significant portion of treated subjects show discordance between a hefty LDL-C reduction and a minimal Lp(a) reduction (48). It is thus possible that apo(a) isoform length influences clearance behavior of Lp(a) in human plasma following up-regulation of LDLR. Finally, 40% of plasma PCSK9 compartmentalizes with LDL-sized apoB-containing lipoproteins, including LDL and Lp(a) (49,50), and the majority of lipoprotein-bound PCSK9 is in its intact form, not cleaved by furin. Since furin-cleaved PCSK9 is somewhat less effective on binding to LDLR compared with the intact PCSK9 form, it is possible that the balance between forms, as influenced by treatment with a PCSK9 inhibitor, also contributes to the degree of Lp(a) reduction on therapy.
Recommendations for research efforts for priority #4
Baseline and follow-up absolute and mean percent change levels of Lp(a) should be measured and reported routinely in clinical trials assessing lipid therapies to enhance the database of effects of therapeutic interventions.
It is now increasingly recognized that “LDL-C” is a misnomer since no clinical assay can separate the cholesterol portion on Lp(a) (Lp(a)-C) from the measure of LDL-C, which is ~30–45% of Lp(a) mass in mg/dL. Thus, LDL-C is actually “LDL-Cholesterol + Lp(a)-cholesterol” (17). In fact, all statin and PCSK9 outcomes studies have actually reported “LDL-C + Lp(a)-C” in relation to therapeutic effect. This is further supported by GWAS data showing the variation in LDL-C in response to statins is influenced by APOE and LPA loci. For example, carriers of snps associated with elevated Lp(a) have the smallest LDL-C reduction (51), findings also shown previously in JUPITER, ASCOT and CARDS. Studies should consider teasing out the proportion of “LDL-C” that is true LDL-C vs Lp(a)-C. initially this can be done by mathematical subtraction of Lp(a)-C from LDL-C and the estimated LDL-C can be reported, as done recently based on prior work (30,31). In the PCSK9 era of achieved very low LDL-C, a patient with elevated Lp(a) may have little to no circulating LDL-C and may also have circulating free apo(a). Such initial estimates are likely to have many limitations and should be complemented by rigorous biochemical studies to understand the relationship of Lp(a) mass to cholesterol, particularly in varying Lp(a) levels and isoforms. Such studies will also help to better understand the true LDL-C effects of LDL-C lowering agents.
Funding efforts should be focused on delineating the basic, underlying mechanisms of diet, hormonal/post-menopausal status, statin therapy, mipomersen, and both PCSK9 and CETP inhibition on plasma Lp(a). This will not only provide insights into Lp(a) regulation, but also help to understand the complex mechanisms of these treatments on Lp(a) biology. Understanding why low-fat diets and statins can raise Lp(a) in some patients will allow an assessment if this is part of the “residual risk” noted despite optimal and diet management.
Priority #5: Focused populations at risk for CVD with high Lp(a) requiring special research emphasis
Familial hypercholesterolemia: FH is associated with an elevated lifetime risk of CVD and higher Lp(a) levels increase this risk as shown in prospective studies (52). Lp(a) may also predispose to aortic valve calcification in FH patients (53). There is controversy whether genetic defects that cause the FH phenotype, mainly defects on LDLR expression, increase Lp(a) levels.
Recommendations for research efforts for FH
FH is the natural model to test the role of the LDLR on Lp(a) catabolism. Kinetic studies in both homozygous and heterozygous FH caused by the LDLR as well as other molecular defects in APOB or PCSK9 genes should be performed.
Co-segregation of molecular defects on the LPA gene that raise Lp(a) levels should be tested in individuals with proven FH-causing defects and in non-affected relatives to test if effects on plasma Lp(a) are additive or synergistic.
Coronary computed tomography studies should also be performed to test if Lp(a) predisposes to coronary plaques in FH.
Lp(a) levels and genotypes should be tested in FH individuals prospectively to prove the association with CAVD in both homozygous and heterozygous FH.
Studies should examine whether more intensive LDL-C reduction with different treatments (PCSK9 inhibitors, lipoprotein apheresis) be instituted in FH individuals with higher Lp(a) levels.
Calcific Aortic Valve Disease: CAVD remains the leading cause of aortic valve replacement in the developed world, yet no drug therapies exist to slow disease progression. A genome-wide association study reported an association of LPA snps with computed tomographic (CT) assessment of aortic valve calcium (54), which has been validated in several other cohorts (55–57). In the ASTRONOMER trial, elevated Lp(a) levels were linked to echocardiographically-measured progression, as well as the need for aortic valve replacement (AVR) (9). Finally, 4 studies have linked the role of OxPL and/or their downstream metabolites with a higher risk of developing CAVD (9,58–61). These studies are consistent with the hypothesis that Lp(a) may deliver OxPL to aortic valve leaflets, and that OxPL from Lp(a) and other sources (LDL, VLDL, apoptotic cells, cell membrane lipids) can be converted to pro-calcifying lysophosphatidic acid by the enzyme autotaxin. These findings point to a potential novel therapeutic strategy to reduce CAVD, by targeting Lp(a) and/or inactivating OxPL. It is estimated that 1 in 7 cases of aortic stenosis could be prevented by marked Lp(a) lowering (62).
Recommendations for research efforts for CAVD
After development of appropriate Lp(a)-transgenic CAVD models as noted above, therapeutic studies to assess effect of reduction in the progression rate can be conducted by Lp(a) lowering agents, or inactivating OxPL with antibodies directed to OxPL.
Clinical studies in CAVD, including valve surgery and TAVR, should evaluate the role of Lp(a) and OxPL biomarker studies in calcification with established techniques or with emerging techniques, such as Na18F PET imaging (63).
Although limited options currently exist to lower Lp(a), recent novel therapies such as antisense oligonucleotides to apo(a), which lower Lp(a) >90%, could be used in randomized trials to assess the effects on progression of CAVD (1,31). Several key questions remain to be addressed including which stage of disease should be targeted (sclerosis, mild, moderate or severe aortic stenosis); what Lp(a) threshold should be used (e.g. >50 mg/dL or >125 nmol/L; which agents and targets (Lp(a), OxPL, calcium) should be addressed; what outcomes should be monitored, (subclinical outcomes such as aortic valve calcium, Na18F, valve velocity or gradients vs clinical outcomes such as valve replacement and death, or a combination). Typical endpoints in CVD outcomes trials, such as CV death and MI, will be low frequency in CAVD trials where the disease is known for many years and where death can be prevented by AVR, thus they will not be useful as primary endpoints. Since the progression rate of CAVD on echocardiography measured by aortic valve velocity is a high-fidelity reflector of clinical complications, this may be the optimal primary endpoint in trials, with AVR a secondary endpoint depending on the stage of the disease at entry into the trial.
Renal disease
It is known that Lp(a) levels are substantially increased in persons with end-stage renal disease (ESRD). Lp(a) catabolism is markedly impaired in patients with ESRD on dialysis. Lp(a) levels do not change with the initiation of dialysis, but do decrease upon renal transplantation. More recent evidence indicates that Lp(a) levels are elevated in chronic kidney disease (CKD) and increase with worsening of renal function over time. The mechanisms by which CKD and ESRD increase Lp(a) levels and impair Lp(a) catabolism are not understood. A key question has been whether elevated Lp(a) levels contribute to the increased cardiovascular risk in CKD. Recent data indicate that Lp(a) is an independent predictor of incident CHD events and mortality in CKD (64). Thus, reducing Lp(a) in patients with CKD could be an important approach to reducing CV risk in this population.
Recommendations for research efforts for renal disease
The mechanisms by which Lp(a) is elevated in CKD requires intense investigation and could provide insights into the normal pathways regulating Lp(a) metabolism.
The CKD population could be a valuable group for testing the impact of Lp(a) lowering interventions on CV outcomes.
High thrombosis risk
Since the similarity between the apo(a) component of Lp(a) and the fibrinolytic proenzyme plasminogen was reported in 1987, a role for Lp(a) in thrombotic and fibrinolytic events in the vasculature has been a research focus (10). Mechanisms supporting an effect of Lp(a) in both promotion of clot formation and inhibition of fibrinolysis have been identified using in vitro systems and animal models. However, large Mendelian randomization studies have failed to demonstrate a role for elevated Lp(a) levels in venous thrombosis (65). The role of Lp(a) in arterial thrombosis is not fully defined, particularly since it is difficult to separate atherosclerosis from thrombosis clinically.
Recommendations for research efforts for high thrombosis risk
Direct assessment of a role of Lp(a) in fibrinolysis/thrombosis can be accomplished using fat-fed transgenic Lp(a) mice. Thrombosis can be triggered by a variety of techniques, including chemical or mechanical irritants.
The role of Lp(a) in ongoing or past imaging studies, particularly plaque specific techniques such as IVUS, NIRS, OCT, FDG-PET, where blood samples are available should be analyzed to assess if Lp(a) is preferentially associated with plaque burden or features of plaque instability (thin fibrous cap, lipid pool, cholesteryl ester and macrophage content, necrotic core), or both.
Gene-gene interactions between the LPA locus and thrombophilia susceptibility genes such as Factor V Leiden should be examined. This may be of particular importance in the pediatric population where Lp(a) has been reported to be a risk factor for recurrent stroke in the absence of atherosclerosis (66).
Patients who have recurrent CVD events, progressive CAD or refractory angina
Lipoprotein apheresis (LA) is increasingly being applied in patients with elevated Lp(a) and ongoing CVD, irrespective of LDL-C levels. In the United States, very few patients (<50) with ongoing CVD and isolated increased Lp(a) are currently receiving LA therapy, whereas in Germany there are >1500 such patients. Three prospective/retrospective trials from Germany demonstrated reduction of CVD events by ~80% (MACE rate per year range: pre apheresis (0.41–2.80), post apheresis (0.08–0.14)) with LA therapy in CVD patients with Lp(a) >60 mg/dL and LDL-C ~100 mg/dL on maximally-tolerated therapy (reviewed in (67). Recently, in patients with refractory angina and elevated Lp(a) >60 mg/dL, LA therapy was shown to improve coronary blood flow by MRI and reduce the frequency of angina (68). A second study showed that the best response of gene therapy with a AdVEGF-D vector in patients with refractory angina was in the highest Lp(a) tertile at baseline (69).
Recommendations for research efforts for lipoprotein apheresis
The mechanisms through which LA for elevated Lp(a) improves clinical benefit are not well defined. Comparing LA, which affects multiple lipid and viscosity and other parameters and lowers levels of PCSK9, to Lp(a) lowering therapies that only affect Lp(a) levels may be informative. These studies can be complimented by biomarker studies that assess endothelial integrity, oxidation, inflammation and viscosity, changes in OxPL and monocyte pro-inflammatory activity, which can then be linked to clinical findings such as refractory angina and MACE.
Prospective, randomized trials in patients eligible for LA according to established criteria (controlled LDL-C, recurrent events or progression of CVD, Lp(a) >60 mg/dL) with elevated Lp(a) should be supported. A creative trial in that regard is now ongoing in Germany and Europe (Effect of Lipoprotein(a) Elimination by Lipoprotein Apheresis on Cardiovascular Outcomes (MultiSELECt, NCT02791802). Despite approval by the American Society For Apheresis (ASFA) and the International Society For Apheresis (ISFA), LA apheresis is approved only in Germany for such patients and it is felt to be unethical to perform such a trial in Germany. Thus, the German sites will all be randomized to apheresis and the non-German sites to “usual care”. The study has an estimated enrollment of 1000 patients and is powered to assess a MACE endpoint. In the United States where LA apheresis for such patients is exceedingly rare and only performed ad hoc and not FDA approved, there are no impediments to a randomized clinical apheresis trial.
Ongoing studies of refractory angina should consider measuring Lp(a) in their cohorts to assess its relationship to both baseline symptoms and treatment effects. Consideration should also be given to stratify inclusion criteria based on baseline Lp(a) to insure balanced baseline characteristics and allow an improved statistical assessment of any benefit.
Cerebrovascular accident (CVA) in children and young adults
The etiology of pediatric acute ischemic stroke (AIS) is complex, but evidence suggests elevated Lp(a) may play a role (70). It is not clear if Lp(a) is a primary determinant, as part of a double-hit with other risk factors, or only plays a role in the extension of thrombus versus initiation of thrombus. Pediatric AIS is rare but when it occurs almost 40% is diagnosed as cryptogenic. For adults, most strokes are associated with atherosclerosis compared to only 2% in pediatric patients. Children with an elevated Lp(a) level have a fourfold increased risk of AIS and the risk of recurrent ischemic strokes is increased by more than 10X in patients with an elevated Lp(a) >90th percentile (66). These studies are limited by lack of prospective methodology and are hypothesis generating at present.
Recommendations for research efforts for CVA
Epidemiologic studies on the role of Lp(a) in pediatric stroke should be supported to define incidence and prevalence of elevated Lp(a).
The association of Lp(a) with anti-fibrinolytic effects may be most evident in this population as they generally do not have atherosclerosis, and therefore should be a focus of investigation.
Interactions between Lp(a) and other pro-thrombotic risk factors may be a useful avenue of research to tease out if Lp(a) has a primary, additive or synergistic role in pediatric AIS.
Dedicated research studies to acutely lower Lp(a) in pediatric AIS, with apheresis or novel technologies, would be reasonable in assessing diminished risk of recurrence.
Priority #6: Testing of the “Lp(a) Hypothesis”
The Lp(a) hypothesis, namely the randomization of patients with elevated Lp(a) levels to Lp(a) lowering agents versus placebo in setting of otherwise guideline recommended preventative therapies, has not been tested to date due to lack of therapeutic agents that specifically lower Lp(a). Although Mendelian randomization and genome-wide data provide strong evidence of causality, definitive proof will likely require an outcomes trial prior to clinical approval of any targeted drug therapy. The arrival of potent and specific therapies to lower Lp(a), such as antisense therapy (30,31), convey the beginning of the era of testing of the Lp(a) hypothesis.
The testing of the Lp(a) hypothesis in primary care populations is strongly supported by the putative causality derived from epidemiological and genetic studies. A cardiovascular outcomes trial in subjects with elevated Lp(a) but without prior CVD can be daunting in terms of power, size, cost, and duration of follow-up. However, the Lp(a) threshold in such patients where benefit may accrue is fairly low (i.e. >30 mg/dL) so that both ease of recruitment and efficacy would be favorable. The Lp(a) hypothesis can also be tested in secondary prevention cohorts, i.e. in patients with prior CAD/MI, TIA/stroke and PAD on maximally tolerated lipid therapies. In such patients, who are at higher risk and also accrue higher rates of recurrent events but are also on multiple prevention therapies for all risk factors, the strength of the association is less established in settings where LDL-C is <70 mg/dL (43,71). For example, in secondary prevention settings in randomized trials, LIPID, AIM-HIGH, and JUPITER (1), 4S (72), and TNT (73), showed that baseline and/or on-treatment Lp(a) predicts higher events rates even in settings with achieved LDL-C as low as 55 mg/dL. However, the epidemiological cohorts LURIC/GENIUS-CHD showed a higher association of elevated Lp(a) and CAD for the initial event but not subsequent mortality,. The dal-Outcomes trial of CETP inhibition in a post ACS setting, where Lp(a) can act as an acute phase reactant and potentially confound associations, showed no relationship to subsequent events (74). The SATURN IVUS trial showed no relationship of Lp(a) to atheroma progression (75), however since Lp(a) is both an atherogenic and thrombotic risk factor and most strongly associated with clinical myocardial infarction, change sin atheroma volume may not fully reflect Lp(a) risk. A major disadvantage of all such studies is that since subjects were not recruited for elevated Lp(a) and baseline levels are in the normal range (10–15 mg/dL), thus uniformly lacking adequate power for hypothesis testing hypotheses.
We propose that existing and new clinical CVD outcomes studies and trials measure Lp(a) and report their Lp(a)-related outcomes. Importantly, collaborative, patient-level meta-analysis should be performed to define the role of baseline and on-treatment Lp(a) and CVD risk in secondary prevention settings. Additionally, reporting of high-risk patient subgroups (patients with recurrent events, younger patients with generically driven disease, FH, multiple revascularizations, diabetics), and using a variety of Lp(a) cutoffs, such as >30, >50–60 mg/dL and >100 mg/dL may allow identification of patients most likely to benefit from Lp(a) lowering.
Finally, the Lp(a) hypothesis should ideally be tested in patients with optimal LDL-C reduction, but not necessarily based on LDL-C goal, since many high-risk patients cannot achieve target LDL-C (FH, polygenic hypercholesterolemia, statin intolerant). It is also crucial to emphasize that since the Lp(a)-C content is present in the laboratory measurement of “LDL-C” (17), and that statins and ezetimibe tend to increase Lp(a) mass and Lp(a)-C levels (76), thus patients with elevated Lp(a) are less likely to achieve target LDL-C. This was recently suggested in the FOURIER trial (77), where in quartile 1 of achieved LDL-C (<0.5 mmol/L or ~20 mg/dL), the mean level of Lp(a) was 22 nmol/L (IQR 9–53 nmol/L. In contrast, in quartile 5 (>2.6 mmol/L or ~100 mg/dL) the mean Lp(a) was 49 (IQR 9–188) nmol/L, further suggesting that the patients achieving very low LDL-C are primarily the patients with low Lp(a). Thus, using a low LDL-C cutoff in such a trial will inadvertently exclude many patients with elevated Lp(a) who are at the highest risk spectrum.
Conclusions
To improve public health in subjects with elevated Lp(a), all stakeholders need to be incentivized to work together to achieve these goals. The newly formed Lp(a) Foundation provides awareness and educational materials. Greater knowledge of Lp(a) biology will likely bring forward new insights that will enhance diagnostic and therapeutic options in patients at risk for Lp(a)-mediated vascular and valvular disease.
Acknowledgments
We would like to thank Sandra Revill Tremulis for helpful discussions during the National Heart, Lung, and Blood Institute Working Group meeting entitled, “Future Research Directions on Lipoprotein(a) and Cardiovascular Disease” held on March 20–21, 2017, in Bethesda, MD.
Funding sources: S. Fazio has served as an advisor/consultant for Merck, Kowa, Amarin, Amgen, Akcea, Pfizer and research support from NIH NHLBI grant R01132985. K.C. Ferdinand has served as a consultant with Amgen, Sanofi Aventis, Novartis, Quantum Genomics and Boehringer Ingelheim. M.L. Koschinsky has served as a consultant for Ionis Pharmaceuticals, Sanofi, and Eli Lilly and research support from the Canadian Institutes of Health Research (MOP# 126076), the Heart and Stroke Foundation of Ontario (G-13-0003091), the Natural Sciences and Engineering Research Council (RGPIN/5006-2015), ASPIRE Cardiovascular, Ionis Pharmaceuticals, Regeneron Pharmaceuticals, Eli Lilly and Pfizer. S.M. Marcovina has served as an advisor/consultant to Denka Seiken, MedTest and Amgen. G. Reyes-Soffer has served as an advisor/consultant for Merck, Inc. and research support from Merck, Sanofi/Regeneron, Ionis Pharmaceuticals, Pfizer Cardiovascular Research and NIH UL1TR001873 and KL2TR001874. H.N. Ginsberg has received research support from Merck, Sanofi/Regeneron, Ionis Pharmaceuticals and Pfizer Cardiovascular Research and NIH 1R35HL135833. P.M. Moriarty has served as an advisor/consultant to Amgen, Regeneron, Sanofi, RegenXBio, Duke Clinical Research, Alexion, Esperion, Eliaz Therapeutics and Ionis, speaker bureau for Amgen, Sanofi, and Regeneron and research support from Regeneron, Sanofi, Amgen, Ionis, Catabasis, Pfizer, Novartis, Kaneka and Stage 2 Innovations. R.D. Santos has served as an advisor/consultant to Amgen, Astra Zeneca, Boehringer Ingelheim, Genzyme, Merck, Novo Nordisk, Pfizer, Eli Lilly, Kowa, Unilever, Sanofi/Regeneron, Torrent and Procaps. G. Thanassoulis has served as an advisor/consultant Servier Canada, Amgen, Ionis, speakers bureau of Servier Canada and Sanofi, and research support from Ionis, the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, and NIH R01 HL128550. S. Tsimikas has research support from the Fondation Leducq and NIH grants R01-HL119828, R01-HL078610, R01 HL106579, R01 HL128550, R01 HL136098, P01 HL136275 and R35 HL135737, currently has a dual appointment at the University of California San Diego and Ionis Pharmaceuticals and is a co-inventor and receive royalties from patents owned by the University of California San Diego on oxidation-specific antibodies. J.L. Witztum has served as a consultant to Ionis Pharmaceuticals and is supported by NIH grants P01 HL088093, R01 HL119828, R35 HL135737, P01 HL1136275 and is co-inventor and receive royalties from patents owned by the University of California San Diego on oxidation-specific antibodies.
Abbreviations
- CAVD
Calcific aortic valve disease
- ICD-10
International classification of diseases-10
- apoB
apolipoprotein B-100
- LRP1
LDLR-related protein 1
- SR-BI
Scavenger receptor B-I
- CD36
Cluster of differentiation 36 receptor
- PCSK9
Proprotein convertase subtilisin kexin type 9
- E06
monoclonal antibody E06
- OxPL
Oxidized phospholipids
- WHO/IFCC
World Health Organization/International Federation of Clinical Chemistry and Laboratory Medicine
Footnotes
Disclaimer: The contents of this manuscript are solely the responsibility of the authors and do not necessarily reflect the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the US government.
Disclosures
The other authors report no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsimikas S. A test in context: Lipoprotein(a): Diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 2.Moriarty PM, Varvel SA, Gordts PL, McConnell JP, Tsimikas S. Lipoprotein(a) mass levels increase significantly according to APOE genotype: An analysis of 431 239 patients. Arterioscler Thromb Vasc Biol. 2017;37:580–588. doi: 10.1161/ATVBAHA.116.308704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langsted A, Nordestgaard BG, Benn M, Tybjaerg-Hansen A, Kamstrup PR. PCSK9 R46L Loss-of-Function Mutation Reduces Lipoprotein(a), LDL Cholesterol, and Risk of Aortic Valve Stenosis. J Clin Endocrinol Metab. 2016;101:3281–7. doi: 10.1210/jc.2016-1206. [DOI] [PubMed] [Google Scholar]
- 4.Verbeek R, Boyer M, Boekholdt SM, et al. Carriers of the PCSK9 R46L Variant Are Characterized by an Antiatherogenic Lipoprotein Profile Assessed by Nuclear Magnetic Resonance Spectroscopy-Brief Report. Arterioscler Thromb Vasc Biol. 2017;37:43–48. doi: 10.1161/ATVBAHA.116.307995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 6.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–53. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36:2239–2245. doi: 10.1161/ATVBAHA.116.308011. [DOI] [PubMed] [Google Scholar]
- 8.Afshar M, Pilote L, Dufresne L, Engert JC, Thanassoulis G. Lipoprotein(a) interactions with low-density lipoprotein cholesterol and other cardiovascular risk factors in premature acute coronary syndrome (ACS) Journal of the American Heart Association. 2016;5:e003012. doi: 10.1161/JAHA.115.003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capoulade R, Chan KL, Yeang C, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66:1236–46. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Boffa MB, Koschinsky ML. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res. 2016;57:745–757. doi: 10.1194/jlr.R060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a) J Lipid Res. 2016;57:1339–59. doi: 10.1194/jlr.R067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57:1953–1975. doi: 10.1194/jlr.R071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Emerging Risk Factors Collaboration. Erqou S, Kaptoge S, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khera AV, Everett BM, Caulfield MP, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) Circulation. 2014;129:635–42. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263–1282. doi: 10.1016/j.cjca.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 16.Davidson MH, Ballantyne CM, Jacobson TA, et al. Clinical utility of inflammatory markers and advanced lipoprotein testing: Advice from an expert panel of lipid specialists. J Clin Lipidol. 2011;5:338–67. doi: 10.1016/j.jacl.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Yeang C, Witztum JL, Tsimikas S. ‘LDL-C’ = LDL-C + Lp(a)-C: implications of achieved ultra-low LDL-C levels in the proprotein convertase subtilisin/kexin type 9 era of potent LDL-C lowering. Curr Opin Lipidol. 2015;26:169–178. doi: 10.1097/MOL.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 18.van der Valk FM, Bekkering S, Kroon J, et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134:611–24. doi: 10.1161/CIRCULATIONAHA.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes-Soffer G, Pavlyha M, Ngai C, et al. Effects of PCSK9 Inhibition with alirocumab on lipoprotein metabolism in healthy humans. Circulation. 2017;135:352–362. doi: 10.1161/CIRCULATIONAHA.116.025253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reyes-Soffer G, Ginsberg HN, Ramakrishnan R. The metabolism of lipoprotein (a): an ever-evolving story. J Lipid Res. 2017;58:1756–1764. doi: 10.1194/jlr.R077693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villard EF, Thedrez A, Blankenstein J, et al. PCSK9 modulates the secretion but not the cellular uptake of lipoprotein(a) ex vivo. JACC: Basic to Translational Science. 2016;1:419–427. doi: 10.1016/j.jacbts.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romagnuolo R, Scipione CA, Boffa MB, Marcovina SM, Seidah NG, Koschinsky ML. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J Biol Chem. 2015;290:11649–62. doi: 10.1074/jbc.M114.611988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X-P, Amar MJ, Vaisman B, et al. Scavenger receptor-BI is a receptor for lipoprotein(a) J Lipid Res. 2013;54:2450–2457. doi: 10.1194/jlr.M038877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Sethi A, Yanek LR, et al. SCARB1 gene variants are associated with the phenotype of combined high high-density lipoprotein cholesterol and high lipoprotein (a) Circ Cardiovasc Genet. 2016;9:408–418. doi: 10.1161/CIRCGENETICS.116.001402. [DOI] [PubMed] [Google Scholar]
- 25.Yeang C, Gordts PL, Tsimikas S. Novel lipoprotein(a) catabolism pathway via apolipoprotein(a) recycling: adding the plasminogen receptor PlgRKT to the list. Circ Res. 2017;120:1050–1052. doi: 10.1161/CIRCRESAHA.117.310700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma M, Redpath GM, Williams MJ, McCormick SP. Recycling of apolipoprotein(a) after PlgRKT-mediated endocytosis of lipoprotein(a) Circ Res. 2017;120:1091–1102. doi: 10.1161/CIRCRESAHA.116.310272. [DOI] [PubMed] [Google Scholar]
- 27.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 2009;50(Suppl):S207–12. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoeksema MA, Gijbels MJ, Van den Bossche J, et al. Targeting macrophage histone deacetylase 3 stabilizes atherosclerotic lesions. EMBO Mol Med. 2014;6:1124–32. doi: 10.15252/emmm.201404170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leibundgut G, Scipione C, Yin H, et al. Determinants of binding of oxidized phospholipids on apolipoprotein(a) and lipoprotein(a) J Lipid Res. 2013;54:2815–30. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsimikas S, Viney NJ, Hughes SG, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–83. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 31.Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 32.Yeang C, Cotter B, Tsimikas S. Experimental animal models evaluating the causal role of lipoprotein(a) in atherosclerosis and aortic stenosis. Cardiovasc Drugs Ther. 2016;30:75–85. doi: 10.1007/s10557-015-6634-1. [DOI] [PubMed] [Google Scholar]
- 33.Boullier A, Friedman P, Harkewicz R, et al. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46:969–76. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Schneider M, Witztum JL, Young SG, et al. High-level lipoprotein [a] expression in transgenic mice: evidence for oxidized phospholipids in lipoprotein [a] but not in low density lipoproteins. J Lipid Res. 2005;46:769–78. doi: 10.1194/jlr.M400467-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–82. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsiantoulas D, Perkmann T, Afonyushkin T, et al. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J Lipid Res. 2015;56:440–8. doi: 10.1194/jlr.P054569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res. 2011;108:1392–412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcovina SM, Albers JJ, Scanu AM, et al. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a) Clin Chem. 2000;46:1956–67. [PubMed] [Google Scholar]
- 39.Marcovina SM, Albers JJ, Gabel B, Koschinsky ML, Gaur VP. Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein(a) Clin Chem. 1995;41:246–55. [PubMed] [Google Scholar]
- 40.Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. 2016;57:526–37. doi: 10.1194/jlr.R061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the National Heart, Lung, and Blood Institute workshop on lipoprotein(a) and cardiovascular disease: recent advances and future directions. Clin Chem. 2003;49:1785–96. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- 42.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 43.Ray KK, Ginsberg HN, Davidson MH, et al. Abstract 18484: Modest potential association between reductions in lipoprotein(a) and major adverse cardiovascular events in the phase 3 trials of alirocumab versus control. Circulation. 2016;134:A18484–A18484. [Google Scholar]
- 44.Virani SS, Brautbar A, Davis BC, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in Black and White subjects: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125:241–9. doi: 10.1161/CIRCULATIONAHA.111.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SR, Prasad A, Choi YS, et al. The LPA gene, ethnicity, and cardiovascular events. Circulation. 2016;135:251–263. doi: 10.1161/CIRCULATIONAHA.116.024611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willeit P, Kiechl S, Kronenberg F, et al. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;64:851–60. doi: 10.1016/j.jacc.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 47.Yeang C, Hung MY, Byun YS, et al. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a) J Clin Lipidol. 2016;10:594–603. doi: 10.1016/j.jacl.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Edmiston JB, Brooks N, Tavori H, et al. Discordant response of low-density lipoprotein cholesterol and lipoprotein(a) levels to monoclonal antibodies targeting proprotein convertase subtilisin/kexin type 9. J Clin Lipidol. 2017;11:667–673. doi: 10.1016/j.jacl.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Tavori H, Giunzioni I, Linton MF, Fazio S. Loss of plasma proprotein convertase subtilisin/kexin 9 (PCSK9) after lipoprotein apheresis. Circ Res. 2013;113:1290–5. doi: 10.1161/CIRCRESAHA.113.302655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tavori H, Christian D, Minnier J, et al. PCSK9 association with lipoprotein(a) Circ Res. 2016;119:29–35. doi: 10.1161/CIRCRESAHA.116.308811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Postmus I, Trompet S, Deshmukh HA, et al. Pharmacogenetic meta-analysis of genome-wide association studies of LDL cholesterol response to statins. Nature communications. 2014;5:5068. doi: 10.1038/ncomms6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santos RD, Gidding SS, Hegele RA, et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. The lancet Diabetes & endocrinology. 2016;4:850–61. doi: 10.1016/S2213-8587(16)30041-9. [DOI] [PubMed] [Google Scholar]
- 53.Vongpromek R, Bos S, Ten Kate GJ, et al. Lipoprotein(a) levels are associated with aortic valve calcification in asymptomatic patients with familial hypercholesterolaemia. J Intern Med. 2015;278:166–73. doi: 10.1111/joim.12335. [DOI] [PubMed] [Google Scholar]
- 54.Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–12. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arsenault BJ, Boekholdt SM, Dube MP, et al. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7:304–10. doi: 10.1161/CIRCGENETICS.113.000400. [DOI] [PubMed] [Google Scholar]
- 56.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–7. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 57.Cairns BJ, Coffey S, Travis RC, et al. A Replicated, genome-wide significant association of aortic stenosis with a genetic variant for lipoprotein(a): meta-analysis of published and novel data. Circulation. 2017;135:1181–1183. doi: 10.1161/CIRCULATIONAHA.116.026103. [DOI] [PubMed] [Google Scholar]
- 58.Bouchareb R, Mahmut A, Nsaibia MJ, et al. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 2015;132:677–690. doi: 10.1161/CIRCULATIONAHA.115.016757. [DOI] [PubMed] [Google Scholar]
- 59.Nsaibia MJ, Mahmut A, Boulanger MC, et al. Autotaxin interacts with lipoprotein(a) and oxidized phospholipids in predicting the risk of calcific aortic valve stenosis in patients with coronary artery disease. J Intern Med. 2016;280:509–517. doi: 10.1111/joim.12519. [DOI] [PubMed] [Google Scholar]
- 60.Torzewski M, Ravandi A, Yeang C, et al. Lipoprotein(a)-Associated Molecules Are Prominent Components in Plasma and Valve Leaflets in Calcific Aortic Valve Stenosis. JACC: Basic to Translational Science. 2017;2:229–240. doi: 10.1016/j.jacbts.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamstrup PR, Hung MY, Witztum JL, Tsimikas S, Nordestgaard BG. Oxidized phospholipids and risk of calcific aortic valve disease: The Copenhagen General Population Study. Arterioscler Thromb Vasc Biol. 2017;37:1570–1578. doi: 10.1161/ATVBAHA.116.308761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Afshar M, Kamstrup PR, Williams K, Sniderman AD, Nordestgaard BG, Thanassoulis G. Estimating the population impact of lp(a) lowering on the incidence of myocardial infarction and aortic stenosis-brief report. Arterioscler Thromb Vasc Biol. 2016;36:2421–2423. doi: 10.1161/ATVBAHA.116.308271. [DOI] [PubMed] [Google Scholar]
- 63.Jenkins WS, Vesey AT, Shah AS, et al. Valvular (18)F-Fluoride and (18)F-fluorodeoxyglucose uptake predict disease progression and clinical outcome in patients with aortic stenosis. J Am Coll Cardiol. 2015;66:1200–1. doi: 10.1016/j.jacc.2015.06.1325. [DOI] [PubMed] [Google Scholar]
- 64.Bajaj A, Damrauer SM, Anderson AH, et al. Lipoprotein(a) and risk of myocardial infarction and death in chronic kidney disease: findings from the CRIC Study (Chronic Renal Insufficiency Cohort) Arterioscler Thromb Vasc Biol. 2017 doi: 10.1161/ATVBAHA.117.309920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Emdin CA, Khera AV, Natarajan P, et al. Phenotypic characterization of genetically lowered human lipoprotein(a) levels. J Am Coll Cardiol. 2016;68:2761–2772. doi: 10.1016/j.jacc.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldenberg NA, Bernard TJ, Hillhouse J, et al. Elevated lipoprotein (a), small apolipoprotein (a), and the risk of arterial ischemic stroke in North American children. Haematologica. 2013;98:802–7. doi: 10.3324/haematol.2012.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moriarty PM, Hemphill L. Lipoprotein apheresis. Endocrinol Metab Clin North Am. 2016;45:39–54. doi: 10.1016/j.ecl.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Khan TZ, Hsu LY, Arai AE, et al. Apheresis as novel treatment for refractory angina with raised lipoprotein(a): a randomized controlled cross-over trial. Eur Heart J. 2017;38:1561–1569. doi: 10.1093/eurheartj/ehx178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hartikainen J, Hassinen I, Hedman A, et al. Adenoviral intramyocardial VEGF-D N C gene transfer increases myocardial perfusion reserve in refractory angina patients: a phase I/IIa study with 1-year follow-up. European Heart Journal. 2017;38:2547–2555. doi: 10.1093/eurheartj/ehx352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McNeal CJ. Lipoprotein(a): Its relevance to the pediatric population. J Clin Lipidol. 2015;9:S57–66. doi: 10.1016/j.jacl.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 71.O’Donoghue ML, Morrow DA, Tsimikas S, et al. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J Am Coll Cardiol. 2014;63:520–7. doi: 10.1016/j.jacc.2013.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berg K, Dahlen G, Christophersen B, Cook T, Kjekshus J, Pedersen T. Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian Simvastatin Survival Study. Clin Genet. 1997;52:254–61. doi: 10.1111/j.1399-0004.1997.tb04342.x. [DOI] [PubMed] [Google Scholar]
- 73.Arsenault BJ, Barter P, DeMicco DA, et al. Prediction of cardiovascular events in statin-treated stable coronary patients of the treating to new targets randomized controlled trial by lipid and non-lipid biomarkers. PLoS One. 2014;9:e114519. doi: 10.1371/journal.pone.0114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwartz GG, Ballantyne CM, Barter PJ, et al. Association of Lipoprotein(a) With Risk of Recurrent Ischemic Events Following Acute Coronary Syndrome: Analysis of the dal-Outcomes Randomized Clinical Trial. JAMA Cardiol. 2017 doi: 10.1001/jamacardio.2017.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Puri R, Ballantyne CM, Hoogeveen RC, et al. Lipoprotein(a) and coronary atheroma progression rates during long-term high-intensity statin therapy: Insights from SATURN. Atherosclerosis. 2017;263:137–144. doi: 10.1016/j.atherosclerosis.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 76.Yeang C, Wilkinson MJ, Tsimikas S. Lipoprotein(a) and oxidized phospholipids in calcific aortic valve stenosis. Current opinion in cardiology. 2016;31:440–50. doi: 10.1097/HCO.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017 doi: 10.1016/S0140-6736(17)32290-0. [DOI] [PubMed] [Google Scholar]