Abstract
Kava is a traditional beverage of various Pacific Basin countries. Kava has been introduced into the mainstream U.S. market principally as an anti-anxiety preparation. The effects of the long-term consumption of kava have not been documented adequately. Preliminary studies suggest possible serious organ system effects. The potential carcinogenicity of kava and its principal constituents are unknown. As such, kava extract was nominated for the chronic tumorigenicity bioassay conducted by the National Toxicology Program (NTP). At present toxicological evaluation of kava extract is being conducted by the NTP. The present review focuses on the recent findings on kava toxicity and the mechanisms by which kava induces hepatotoxicity.
Keywords: Kava, anti-anxiety herbal beverage, top-selling botanical, hepatotoxicity, metabolism, mechanism
Kava, prepared from the rhizome of the kava tropical shrub plant, Piper methysticum Forst F., is a traditional beverage used for many centuries, especially at ceremonial activities in the South Pacific (1–6). During recent years, kava has been used in Europe for treatment of anxiety and nervous disorders such as stress and restlessness, and in the United States as a natural alternative to anti-anxiety drugs and sleeping pills (6). Kava sales totaled more than $ 100 million in 1998, being the fifth leading seller of the North American botanicals (7).
The association between the use of kava products and hepatotoxicity has prompted many countries, including Germany, Switzerland, France, Canada, and the United Kingdom, to take regulatory actions, ranging from warning consumers to removing kava-containing products from the marketplace. On March 25, 2002, the Center for Food Safety and Applied Nutrition (CFSAN), U.S. Food and Drug Administration (FDA) issued a Consumer Advisory entitled “Kava-containing dietary supplements may be associated with severe liver injury” to the public (8, 9). Kava-containing products remain popular in the United States and continue to be sold in health food stores and ethnic markets regardless of the fact that it was banned in Germany, France, Switzerland, Australia, and Canada (10). In this review, human exposure and metabolism of kava are briefly addressed, recent findings on the toxicity of kava are reported, and possible mechanisms by which kava induces toxicity are described.
1. CHEMICAL COMPOSITION
The kava plant, belonging to the Piperacae family, is widely cultivated in the South Pacific. Kava is cultivated for its rootstock. Depending upon the amount of kavalactones contained in the resin, the rootstock shows different color, varying from white to dark yellow (2,5,6). Fresh kava rootstock contains about 80% water. Dried rootstock consists of about 43% starch, 20% fibers, 12% water, 3–4% proteins, 3% sugars, 3% minerals, and kavalactones, which range between 3 to 20%, depending on the age of the plant and the cultivar (11). The active principles of kava rootstock are mostly contained in the lipid-soluble resin (6).
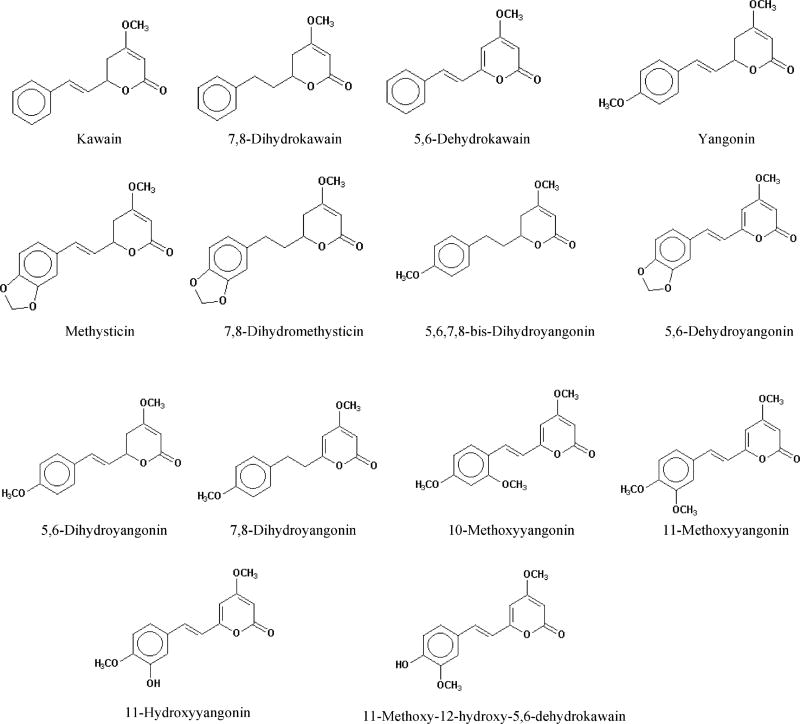
There are three chemical classes in the kava resin: (i) arylethylene-alpha-pyrones; (ii) chalcones and other flavanones; and (iii) conjugated diene ketones. The substituted 4-methoxy-5, 6-dihydro-alpha-pyrones or kava pyrones, commonly called kavalactones, possesses the highest purported pharmacological activities.
There are eighteen kavalactones that have been isolated and identified from kava root extract (6,12). Structures for the commonly studied 14 kavalactones are shown in Figure 1 (2,3,13). The six kavalactones in the highest concentrations are, accounting for approximately 95–96% of the total kavalactones in the lipid resin, yangonin, desmethoxyyangonin (5,6-dehydrokawain), methysticin, kawain, dihydromethysticin (7,8-dihydromethysticin), and dihydrokawain (7,8-dihydrokawain) (6,12). In the literature, kawain is also called kavain. Among these major kavalactones, kawain, dihydrokawain, dihydromethysticin, methysticin, 5,6-dehydromethysticin, demethoxyyangonin, 11-methoxyyangonin, 11-methoxynoryangonin, and yangonin have been prepared synthetically (2,14).
Figure 1.
Names and structures of the fourteen kavalactone constituents in kava.
2. EXPOSURE INFORMATION
In the South Pacific, the kava root beverage was prepared by mixing crushed kava root with water or coconut milk; currently in Polynesia, kava roots are pulverized into powder (15). Kava resin in Piper methysticum contains the biologically-active kavalactones, which can be extracted with organic solvents (16). Outside of South Pacific islands, the primary use of kava occurs as an herbal supplement.
In the U.S. market, kava root is supplied as a crude herb, in the form of tinctures, powdered extracts, and standardized extracts. Some European standardized products are kava extracts prepared from stem peelings and other above-ground plant parts (3). All the standardized kava products contain more than 30% kavalactones. Kava root extract in grain alcohol and vegetable glycerine and kava combined with a variety of herbs and/or vitamins are also commercially sold.
3. USE PAT TERN
Kava is a beverage with psychoactive activity, commonly used by many Pacific Island societies for social and ceremonial events (5,15). The modern pharmacologic usage of kava in Europe started around 1900 as diuretics and for treatment of gonorrhea and nervous disorders (15,17).
In the U.S., kava is promoted as a “natural” alternative to such anxiety drugs (18) as Xanax and Valium. The commonly used names for kava products in the United States include: ava, ava pepper, awa, intoxicating pepper, kava, kava kava, kava pepper, kava root, kava-kava, kawa, kawa kawa, kawa-kawa, kew, Piper methysticum, Piper methysticum Forst. f., Piper methysticum G. Forst., rauschpfeffer, Sakau, tonga, wurzelstock, and yangona (8).
4. DISPOSITION
4.1. Absorption
Kavalactones (alpha-pyrones) are lipid soluble, and thus have low absorption in the gastrointestinal tract and rapid absorption in the gut. When the uptake of dihydrokawain, kawain, desmethoxyyangonin, and yangonin was measured in mouse brain as components of kava resin, absorption was greater than when they were given as isolated materials (19).
4.2. Metabolism
The metabolism of the three 5,6-dihydro-alpha-pyrones, dihydrokawain (7,8-dihydrokawain), kawain, and methysticin; and the two alpha-pyrones, 7,8-dihydroyangonin and yangonin in male albino rats was reported by Rasmussen et al. (20). The animals were orally dosed with the kava alpha-pyrones. After 24 hours, the metabolites and the recovered parent substrate in the urine were isolated and structurally identified and quantified. The results of each metabolism are as follows:
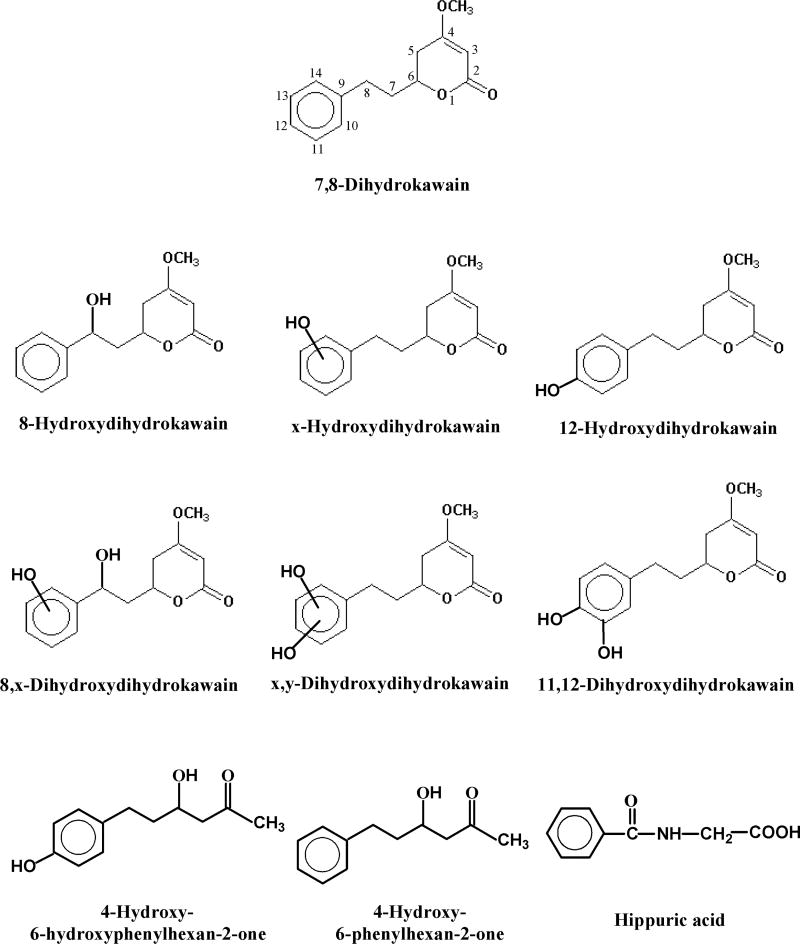
4.2.1. 7,8-Dihydrokawain
Besides the recovered substrate, nine metabolites were identified: 8-hydroxydihydrokawain, x-hydroxydihydrokawain, 12-hydroxydihydrokawain, 8,x-dihydroxydihydrokawain, x,y-dihydroxydihydrokawain, 11,12-dihydroxydihydrokawain, 4-hydroxy-6-phenylhexan-2-one, 4-hydroxy-6-hydroxyphenyl-hexan-2-one, and hippuric acid (Figure 2). 12-Hydroxydihydrokawain was the most abundant metabolite. As shown in Figure 2, there are six hydroxylated (three mono- and three di-hydroxylated) metabolites and three ring-opened metabolites, in a nearly 2:1 ratio. No metabolites were identified in feces or bile, although a small amount of unchanged 7,8-dihydrokawain was found in the feces (20).
Figure 2.
Urinary metabolites formed in male albino rats orally dosed with dihydrokawain.
4.2.2. Kawain
Compared to 7,8-dihydrokawain, lower amounts of urinary metabolites were excreted following kawain administration. Both hydroxylated and ring-opened products were formed. There were eight identified metabolites: p-hydroxybenzoic acid; 4-hydroxy-6-phenyl-5-hexen-2-one, hippuric acid, 4-hydroxy-6-hydroxyphenyl-5-hexen-2-one, p-hydroxykawain, p-hydroxydihydrokawain, hydroxykawain, and p-hydroxy-5,6-dehydrokawain. In addition, there were two unidentified metabolites. Large amounts of unchanged kawain were identified in the feces.
4.2.3. Methysticin
Metabolism of methysticin produced only two urinary metabolites, m,p-dihydroxykawain and m,p-dihydroxydihydrokawain, both in small amounts. Apparently, these metabolites were formed by demethylenation of the methylenedioxyphenyl moiety. Unchanged methysticin was identified in feces (20).
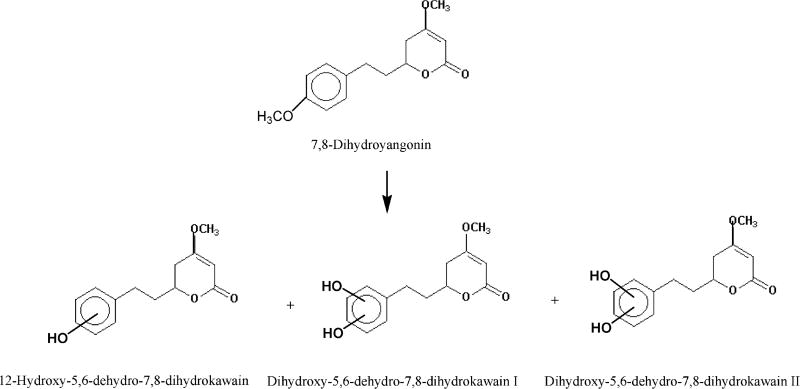
4.2.4. 7,8-Dihydroyangonin
Three urinary metabolites, p-hydroxy-5,6-dehydro-7,8-dihydrokawain and two dihydroxy-5,6-dehydro-7,8-dihydrokawains, were identified from the metabolism of 7,8-dihydroxyyangonin (Figure 3). p-Hydroxy-5,6-dehydro-7,8-dihydrokawain was the major urinary metabolite. No ring-opening products were detected.
Figure 3.
Urinary metabolites formed in male albino rats orally dosed with 7,8-dihydroyangonin.
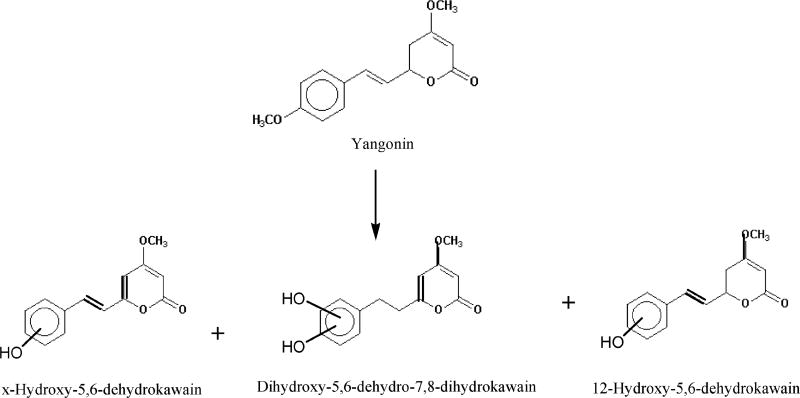
4.2.5. Yangonin
Three urinary metabolites, x-hydroxy-5,6-dehydrokawain, dihydroxy-5,6-dehydro-7,8-dihydrokawain, and p-hydroxy-5,6-dehydrokawain, were identified in the urine, of which p-hydroxy-5,6-dehydrokawain was predominant (Figure 4) (20). Apparently, all these metabolites were formed via O-demethylation. No ring-opening products were detected.
Figure 4.
Urinary metabolites formed in male albino rats orally dosed with yangonin.
Keledjian et al. (19) employed GC/MS spectral analysis to determine the uptake of dihydrokawain, kawain, desmethoxyyangonin, and yangonin into the male Balb/c mouse brain at 5, 15, 30, and 45 min following intraperitoneal (ip) administration. After 5 min, dihydrokawain and kawain attained maximum concentrations of 64.7 and 29.3 ng/mg wet brain tissue, respectively, and they were then eliminated rapidly. By comparison, both desmethoxyyangonin and yangonin reached lower maximum concentrations, but were eliminated slowly from brain tissue (19).
Duffield et al. (21) identified human urinary metabolites of kava lactones following ingestion of kava. Using methane chemical ionization gas chromatography-mass spectrometric analysis, nine kavalactone metabolites were identified, including dihydrokawain, kawain, desmethoxyyangonin, tetrahydroxyangonin, dihydromethysticin, 11-methoxytetrahydroyangonin, yangonin, methylsticin, and dehydromethylsticin. Based on the metabolites identified, the principal metabolic transformations involve: (i) reduction of the 3,4-double bond and/or demethylation of the 4-methoxyl group of the alpha-pyrone ring system; (ii) demethylation of the 12-methoxy substituent in yangonin; and (iii) hydroxylation at C-12 of desmethoxyyangonin. As compared to the metabolism patterns occurred in rat reported by Rasmussen et al. (20), no dihydroxylated metabolites of the kava lactones or products from ring opening of the alpha-pyrone ring system were identified in human urine.
Zou et al. (22) used LC/MS/MS to analyze metabolites in the urine of two human subjects following their ingestion of kava, and identified 6-phenyl-3-hexen-2-one as its mercapturic acid adduct. This metabolite was possibly formed from enzymatic demethylation of 7,8-dihydromethysticin followed by ring opening of the alpha-pyrone ring, and rearrangement.
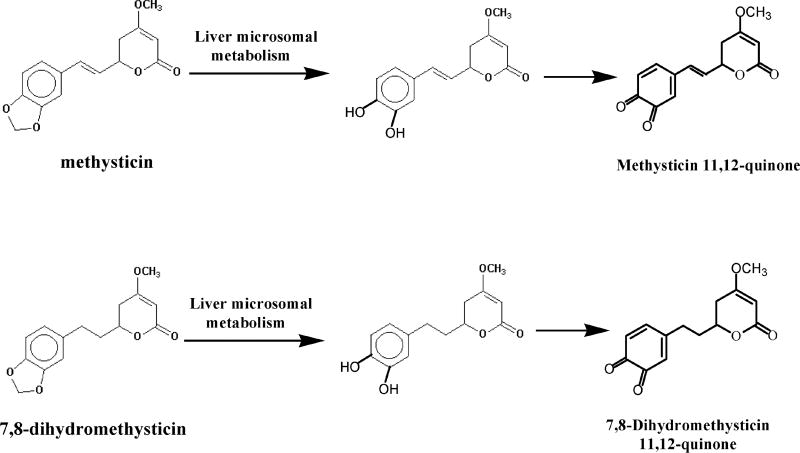
Johnson et al. (23) studied the metabolism of kava extract by dexamethasone-induced Sprague-Dawley rat liver microsomes. Employing positive ion electrospray LC-MS/MS with precursor ion scanning and product ion scanning, two novel electrophilic metabolites, kawain 11,12-quinone and 7,8-dihydrokawain 11,12-quinone were identified in their glutathione conjugate form (Figure 5). The same metabolites were formed from metabolism of kava extract by non-induced pooled human microsomes. Metabolism of kawain and 7,8-dihydrokawain by rat and human liver microsomes generated kawain 11,12-hydroquinone and 7,8-dihydrokawain 11,12-hydroquinone, respectively. The authors proposed that the microsomal formation of kawain 11,12-quinone and 7,8-dihydrokawain 11,12-quinone was mediated through the formation of kawain 11,12-hydroquinone and 7,8-dihydrokawain 11,12-hydroquinone, respectively (Figure 5).
Figure 5.
Metabolites formed from rat and human liver microsomal metabolism of kava extract.
The glucuronic acid and sulfate conjugates of kawain 11,12-hydroquinone and 7,8-dihydrokawain 11,12-hydroquinone were detected from a human volunteer who took a single dose of a dietary supplement containing kava root extract. The results suggest that these metabolites might contribute to hepatotoxicity in humans (23).
5. BIOLOGICAL EFFECTS
Kavalactones (alpha-pyrones) of the kava compounds have the highest pharmacological activity. The six major kavalactones are yangonin, methysticin, dihydromethysticin, dihydrokawain, kawain, and demethoxyyangonin. When kavalactones were tested individually, they did not exhibit biological activity similar to that found in the whole kava extract. On the other hand, the recombined kavalactones exhibited biological activity found with the entire kava extract (5,13,24).
Lipid extracts of kava produce higher pharmacological activities than aqueous kava extracts (16,25). Synergistic effect between kavalactones and other amino butyric acid-active sedatives was observed (26). Almeida and Grimsley (27) reported a 54-year-old man hospitalized in a semicomatose state may have had a possible interaction of kava and benzodiazepines (alprazolam).
Similar to methenesin, kava can cause muscle relaxation without depressing CNS function (5). Both yangonin and desmethoxy-yangonin possess weaker central nervous activity than kava extract. The other alpha-pyrones exhibit markedly enhanced activity.
Both aqueous extracts and lipid soluble extracts (kava resin) used for intoxicating beverage kava exhibit analgesic effects in mice. Among the eight purified alpha-pyrones contained in lipid soluble extracts, kawain, dihydrokawain, methysticin and dihydromethysticin were found to be very effective in producing analgesia, which was shown to occur via non-opiate pathways (24).
Kava has been used for treatment of skin disorders, asthma, lung disorders, and urologic problems in Hawaii. In Germany, before penicillin was discovered, kava was used to treat gonorrhea (15).
Wu et al. (28) reported that yangonin and methysticin showed moderate antioxidant activities in a free radical scavenging assay, but demethoxyangonin, dihydrokawain, kawain, dihydromethysticin, and methysticin did not exhibit antioxidant activities.
6. HEPATOTOXICITY
6.1. Human Data
Although the potential hepatotoxicity of kava has long been reported (8,18,29–41), no epidemiological and case studies on the association of exposure to kava and cancer risk in humans has been reported. On the other hand, kava has been implicated in a number of human liver failure cases in recent years (42–44). Whitton et al. (45) reported that standardized kava extracts produced in Europe caused many serious health problems including death.
In Europe, there have been more than 30 cases of liver damage possibly associated with kava intake, although this remains controversial. The types of liver damage include hepatitis, cirrhosis, liver failure, and death (31,46–51). It is not clear what doses were used and the period of use associated with the risk of liver damage. Both chronic and heavy use has been associated with cases of neurotoxicity, pulmonary hypertension, and dermatologic changes.
Use of kava at frequent high doses causes kava dermopathy (3,52,53). Mathews et al. (54) conducted a pilot health survey of kava users in Australian aboriginals. The survey involved 39 kava users, including 20 very heavy users (mean consumption 440 g/week), 15 heavy users (310 g/week), and 4 occasional users (100 g/week), as well as 34 non-users. The heavy use of kava appeared to be related to malnutrition and weight loss, liver damage (causing elevated serum γ-glutamyl transferase and high-density lipoprotein cholesterol levels), renal dysfunction, rashes, pulmonary hypertension, macrocytosis of red cells, lymphocytopenia, and decreasing platelet volumes. Acute kava usage caused reversible anesthesia of the mouth and skin, euphoria, sedation, muscle weakness, ataxia and, eventually, intoxication. Schelosky et al. (55) reported that four patients took kava and confronted with central dopaminergic antagonism. Kava is a spinal depressant, causing transient ataxia or uncoordinated walk (53). Pfeiffer (56) found that intake of crude root had effective antiepileptic properties.
The underground parts of the Piper methysticum shrub, which contain high quantities of kavalactones (57,58), are used to prepare the kava beverage. However, some kava herbal supplements may contain pipermethystine, which is an ingredient from aerial stem peelings. Pipermethystine was found to decrease cellular ATP levels and mitochondrial membrane potential and induce apoptosis as measured by the release of caspase-3 in cultured human hepatoma cells, HepG2 (59). These results suggest that pipermethystine, rather than kavalactones, is capable of causing cell death (59).
In the United States, kava is still commercially available, although the U.S. Food & Drug Administration (FDA) has issued warnings to consumers and physicians (8,9). The U.S. FDA stated in 2002 that “Kava-containing products have been associated with liver-related injuries—including hepatitis, cirrhosis, and liver failure—in over 25 reports of adverse events in other countries. Four patients required liver transplants. The FDA has received a report of a previously healthy young female who required liver transplantation, as well as several reports of liver-related injuries” (8).
On November 29, 2002, the U.S. Center for Disease Control and Prevention (CDC) issued a Morbidity and Mortality Weekly Reports (MMWR) entitled “Hepatic Toxicity Possibly Associated with Kava-Containing Products—United States, Germany, and Switzerland, 1999—2002” (CDC, MMWR, 51; 1065–1067, 2002). In this report, the U.S. CDC investigated two U.S. cases of liver failure associated with kava-containing dietary supplement products.
Case 1
In May 2001, a 45-year old woman who took kava-containing dietary supplement one tablet twice daily for about 8 weeks felt nausea and weakness. Several days after stopping taking the kava-containing supplement, the patient was hospitalized with jaundice and hepatitis. Liver biopsy found subfulminant hepatic necrosis. Liver transplantation was performed in July 2001, and the patient since recovered.
Case 2
During late August to mid-December 2000, a 14-year old healthy girl took two kava-containing products. Following the package directions, the girl took one product two capsules once daily intermittently for about 44 days, and the second product two capsules once daily for 7 consecutive days at the beginning of the 4-month period. In December 2000, the girl suffered from nausea, vomiting, decreased appetite, weight loss, and fatigue. One week later, she had scleral icterus and was hospitalized with acute hepatitis. At the time of hospitalization, her liver-function tests were markedly abnormal (alanine aminotransferase: 4,076 U/L, aspartate aminotransfease: 3,355 U/L, gamma-glutamyltransferase: 148 U/L, total bilirubin: 16.2 mg/dL, ammonia: 17 mg/dL, and prothrombin time: 29.4 seconds) (38). Initial liver biopsy revealed active fulminant hepatitis with extensive centrilobular necrosis, approximately 25% hepatocellular viability, and mixed inflammatory infiltrates consisting of lymphocytes, histiocytes, scattered eosinophils, and occasional neutrophils. Then, the patient received an orthotopic liver transplantation.
This CDC report also summarized the European Case Reports as follows (CDC, MMWR, 51; 1065–1067, 2002): “Eight hepatic transplant cases following hepatic failure associated with the use of kava-containing products have been reported in Europe (six in Germany and two in Switzerland). Two male patients aged 32 and 50 years and six females aged 22–61 years required liver transplants after using kava-containing products. The duration of kava use ranged from 8 weeks to 12 months. The products were used at doses ranging from 60 mg to 240 mg per day. Seven patients used kava prepared either by ethanol or acetone extraction methods; one patient used an unspecified type of kava-containing product. The patients had varying symptoms, including influenza-like symptoms and jaundice. Each patient’s condition worsened and progressed to fulminant hepatic failure. Four of these cases have been reported in medical literature (29,30,48,49).
Whitton et al. (45) determined that Michael reaction between glutathione and kavalactones can destroy kavalactones by opening of the lactone ring, and thus the toxic effects of kava extracts can be reduced. As such, glutathione can provide protection against kava-induced hepatotoxicity.
6.2. Animal Data
Jamieson and Duffield (24) determined that the lipid soluble extract (kava resin) orally administered to male Balb/c mice had a LD50 of 700 mg/kg and above, with the death due to respiratory failure. The acute toxicity values LD50 (mg/kg) of the six major kavalactones in mice, dogs, cats, and rabbits were determined and the results are summarized in Table 1 (NLM, 1998).
Table 1.
LD50 (mg/kg) values of the six kavalactone components in Kava
| Species | Kawain | Dihydro- kawain |
Methysticin | Dihydromethysticin | Yangonin | Demethoxy- Yangonin |
|---|---|---|---|---|---|---|
| Mouse | ||||||
| oral | 1130 | 920 | — | 1050 | >1500 | >800 |
| ip | 420 | 325 | 530 | 420 | >1500 | >800 |
| iv | 69 | 53 | 49 | — | 41 | 55 |
| Dog | ||||||
| ip | — | >200 | — | >200 | — | — |
| Cat | ||||||
| ip | — | >250 | — | — | — | — |
| Rabbit | ||||||
| ip | — | >350 | — | 300 | — | — |
The kavalactone demethoxyyangonin reduced the cholesterol level in the first two months in Wistar rats; however, it increased the cholesterol level after three months (60). No similar effect was found in ICR mice dosed with demethoxyyangonin (60).
A lipid soluble extract of the kava beverage exhibited hypnosedative properties. Ethanol was found to enhance markedly kava-induced toxicity. Ethanol and the lipid soluble extract (kava resin) greatly increased each other’s hypnotic action in mice. Interaction of kava and alcohol has important clinical and social consequences, since kava is usually taken together with alcoholic drinks (24). However, Anke et al. (44) determined that the four kavalactones, kawain, methysticin, yangonin and desmethoxyyangonin, did not inhibit alcohol dehydrogenase in vitro, suggesting that interaction of kavalactones with alcohol does not involve alcohol dehydrogenase inhibition.
Mice dosed kava resin decreased spontaneous motility and caused a loss of muscle control (61).
6.3. Short-Term Tests
Jhoo et al. (62) investigated in vitro cytotoxicity of kavalactones-containing fractions from different parts of kava plant (Piper methysticum) to HepG2. It was found that the hexane fraction of the root exhibited stronger cytotoxic effects than the fractions of root extracted by other solvents or extracts from the leaves and stem peelings of kava. Flavokawain B in the hexane fraction was found to be the active constituent responsible for the cytotoxicity.
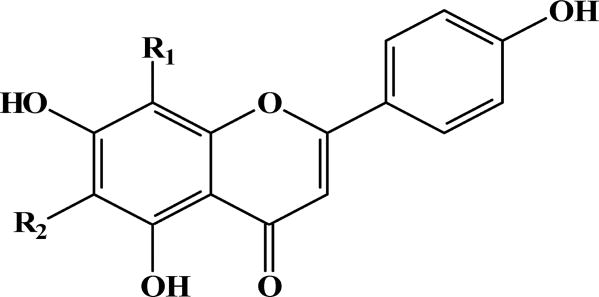
The mutagenicity bioassays of the six major kavalactones and the different solvent fractions of kava roots, leaves, and stem peelings were determined by the umu mutagenicity (63). None of the kavalactones were mutagenic. Among the different solvent fractions, the n-butanol fraction of kava leaves was found mutagenic. Further purification of this fraction indicated that two components were responsible for the mutagenicity. Based on 1H NMR, 13C NMR, HMBC, HMQC and mass spectral analyses, these two components were identified as C-glycoside flavonoids (Figure 6). However, they did not significantly increase mutant frequencies in mouse lymphoma assay, suggesting that these two C-glycoside flavonoids produce mutations mainly through a point mutagenic action (63).
Figure 6.
Molecular structures of the two mutagenic C-glycoside flavonoids extracted from kava root with n-butanol.
Lim et al. (10) determined that pipermethystine, a kava alkaloid abundant in leaves and stem peelings, induces mitochondrial toxicity in human hepatoma cells, HepG2.
7. MECHANISMS LEADING TO HEPATOTOXICITY
To date, mechanisms by which kava induces hepatotoxicity are not clearly understood. Based on the experimental data so far reported, there are at least two possible mechanisms leading to kava-induced hepatotoxicity. The first is induction of herb-drug interactions through modulation of metabolizing enzymes by which the drug metabolism can be affected. The second possible mechanism is the formation of activated metabolites that induce hepatotoxicity.
7.1. Induction of Herb-Drug Interactions
Kavalactones require hepato cytochrome P450 enzymes for clearance by the liver (45). Since both herbal medicines and therapeutic drugs require metabolizing enzymes for metabolism, the use of herbal remedies and therapeutic drugs simultaneously can raise the potential of herb-drug interactions, causing an adverse response. It is important to note that inhibition of metabolizing enzymes, such as cytochrome P450 (CYP) activities, is not a toxic effect; the inhibition can lead to modulation of drug metabolism. As such, the effect on enzyme inhibition is not expected to produce a clear dose-dependent increase in adverse events.
7.1.1. Alteration of Cytochrome P450 Metabolizing Isozymes
It has been determined that inhibition of cytochrome P450 can lead to necrosis (64). Inhibition of cytochrome P450 can lead to significant interactions with a number of drugs (65). Strahl et al. (66) observed that a Swiss woman who consumed kavalactones 210 mg/day for 3 weeks had hepatotoxicity and inhibition of cytochrome P450. Mahews et al. (54) determined that kava extracts significantly inhibited human cytochrome P450, CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4, and CYP4A9/11. Russmann et al. (32) reported the inhibition of CYP2D6 by kava extract. Russmann et al (67) conducted a study with 6 healthy subjects from New Caledonia, all regular kava extract consumed. The results suggested that kava extracts inhibited CYP1A2. Since CYP1A2 catalyzes metabolic activation of potent environmental carcinogens, such as aflatoxins, the authors proposed that through CYP1A2 inhibition, kava may play a protective effect against environmental carcinogens.
Raucy (68) used primary cultures of human hepatocytes to examine the effect of kava extracts on CYP3A4 expression in human hepatocytes and found that kava produced a 3–4 fold increase compared to control CYP3A4 mRNA. Since human CYP3A4 is responsible for approximately 60% of CYP450-mediated metabolism of drugs in therapeutic use today, with anti-cancer drugs in particular (69,70), this result suggests that kava can potentially affect drug metabolism. Upon metabolism, the kava lactones containing methylenedioxyphenyl group in kava extract inhibited multiple cytochrome P450 enzymes (71–75).
Zou et al. (76) evaluated the ability of six major kavalactones (desmethoxyyangonin, dihydrokawain, dihydromethysticin, kawain, methysticin, and yangonin) on the catalytic activity of cDNA-derived human CYP450 isozymes CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 in vitro. It was found that desmethoxyyangonin exhibited the most inhibition on CYP1A2 and dihydromethysticin inhibited CYP2C19 most effectively. Both methysticin and dihydromethysticin inhibited CYP3A4 significantly. These data suggest that kava could potentially inhibit the metabolism of co-administered medications whose primary route of elimination is via cytochrome P450.
Inhibition of CYP450 enzymes by whole kava extract and individual kavalactones (desmethoxyyangonin, dihydrokawain, dihydromethysticin, kawain, methysticin, and yangonin) was investigated in human liver microsomes (77). The extract caused significant inhibition of the activity of CYP1A2 (56% inhibition), 2C9 (92%), 2C19 (86%), 2D6 (73%), 3A4 (78%), and 4A9/11. CYP2A6, 2C8 and 2E1 activity was not affected. Both kava extract and the six kavalactones modestly stimulated P-glycoprotein ATPase activity. These results suggest that the kavalactone components, rather than other components of the extract, are responsible for the inhibition of CYP450 isozymes. Inhibition of CYP450 isozymes is believed to be the most likely factor in causing kava-mediated adverse drug reactions via inhibition of drug metabolism (77,78).
It has been determined that kava, as one of the herbal supplements, can also lead to herb-drug interactions with therapeutic drugs (6,12,79,80). Concerning interaction between kava and therapeutic drugs, inhibition of the cytochrome P450 isozymes by kavalactones can result in pharmacokinetic interaction (6). Inhibition of the CYP450 isozymes could elevate the plasma levels of the therapeutic drugs to concentrations that are toxic to various organs and tissues.
Unger et al. (74) tested several ethyl acetate extracts of kava for inhibitory potency using cDNA expressed CYP3A4. They observed a 70–80% inhibition of the enzyme by different fractions, with kawain, dihydrokawain, methysticin, dihydromethysticin, and dihydoyangonin being the main inhibitory compounds. These data collectively indicate that kava has a high potential for causing herb–drug interactions through inhibition of CYP450 enzymes responsible for the majority of the metabolism of pharmaceutical agents used currently. Co-ingestion of kava with prescription medications or over-the-counter products, including other herbal remedies that are metabolized with one or more of these enzymes, might result in elevated and potentially toxic concentrations of the co-administered agents or their metabolites (81).
Clayton et al. (82) reported the immunohistochemical analysis of hepatic cytochrome P450 expression in F344 rats following oral treatment with kava extract for 90 days. The expression of CYP1A2, 2B1, 2D1, 2E1, and 3A1 was analyzed. Protein expression of CYP enzymes in liver indicated decreased expression of CYP2D1 (human CYP2D6 homolog) in 2.0 g/kg females and increased expression of CYP1A2, 2B1, and 3A1 in 1.0 and 2.0 g/kg for both sexes.
Mathews et al. (77) conducted a study of male Fischer rats administered with 256 mg/kg of kava extract (100 mg/kg kavalactones) orally for 7 days. It was determined that total P450 was not significantly different from vehicle-treated controls. However, daily administration of 1 g/kg kava (391 mg/kg kavalactones) to male Fischer rats for 7 days achieved different results. The total hepatic P450 content increased 35%, and activities of P450 enzymes increased significantly: CYP1A2 (4200%), 2B1 (4200%), and 3A1/2 (51%). The activity of CYP2E1 was marginally increased, and activity of CYP2D1 was modestly decreased by approximately 25% (77). The results between Mathews et al. (77) and Clayton et al. (82) are similar, although the latter found that the expression of hepatic CYP2E1 was changed.
CYP2D6 is one of the most extensively studied genetically polymorphic enzymes. It is thought to cause much of the inter-individual variations seen in drug responses, adverse effects, and interactions with drugs (83). Individuals may be poor (slow), intermediate, extensive (fast), or ultra-fast metabolizers. In a Caucasian population, 7–9% individuals are homozygous deficient in CYP2D6 and thus are poor metabolizers (83). On the other hand, the incidence of CYP2D6 deficiency was almost 0% in pure Polynesian descent and about 1% in Asian populations (84). The genetic difference in CYP2D6 enzyme levels between Caucasian and the Pacific Islanders suggests that Caucasian individuals are more susceptible to kava-induced hepatotoxicity. The genetic difference may also explain why descendants of Asian migrants to the Pacific have supposedly not experienced kava hepatotoxicity.
Jhoo et al. (62) reported that kava enhanced the hypnotic effect of alcohol in mice. Liu et al. (85) determined that alteration of cytochrome P450 expression level can affect the preneoplastic changes in the early stage of hepatocarcinogenesis in Sprague-Dawley rats. Using immunohistochemical assays, it was seen that CYP2E1 and CYP2B1/2 were strongly stained around the centrolobular vein and weakly stained in the altered hepatic foci. The altered hepatic tissue bore more microsomal NADPH-dependent lipid peroxidation than normal tissue. No difference was found when CYP2E1 was inhibited. More microsomal lipid peroxidation was generated when incubated with a CYP1A inhibitor alpha-naphthoflavone. Evidently, alteration of cytochrome P450 isozyme expression levels can play important roles in the alteration of cell redox status of preneoplastic tissue in the early stage of hepatocarcinogenesis (85).
F344 rats treated with pipermethystine (10 mg/kg) for 2 weeks resulted in significant increase in hepatic glutathione, cytosolic superoxide dismutase (Cu/ZnSOD), tumor necrosis factor alpha mRNA expression, and cytochrome P450 (CYP) 2E1 and 1A2, suggesting that pipermethystine caused adaptation to oxidative stress and possible drug-drug interactions (10).
Modulation of P-glycoprotein (P-gp) and other drug transporters by herbal supplements may give rise to herb-drug interactions. Gurley et al. (86) found that kava is not a potent modulator of P-gp in humans. The inducible transcription factor nuclear, factor kappaB (NF-kappaB), regulates immune, inflammatory, and carcinogenic responses. Activation of NF-kappaB is a normal process required for cell survival and immunity. Its deregulated expression is a characteristic of inflammatory and infectious diseases. Folmer et al. (87) determined that flavokawain A, flavokawain B, kawain, and dihydrokawain inhibited NF-kappaB-driven reporter gene expression and TNF-alpha-induced binding of NF-kappaB to a consensus response element.
7.1.2. Other Enzymes Modulation
Wu et al. (28) demonstrated that kavalactones, dihydrokawain and yangonin, showed COX-2 inhibitory activity. It is known that a number of drugs that can exert hepatotoxic effects, also inhibit COX-2 enzyme activity (88). Reilly et al. (89) reported that acetaminophen induced hepatotoxicity greater in COX-2−/− and −/+ mice, and suggested that COX-2-derived mediators play an important hepato-protective role and that COX inhibition may contribute to the risk of drug-induced liver injury. Consequently, kava may exert hepatotoxicity mediated by inhibition of COX-2 enzyme activity (12).
Escher et al. (48) reported that a 50-year old patient that took kava extracts resulted in hepatotoxicity and received a liver transplant. This patient also developed changed liver enzyme levels, including a high concentration of gamaglutamyltransferase. They concluded that heavy consumption of kava can cause increased concentrations of gama glutamyltransferase, suggesting potential hepatotoxicity.
7.1.3. Idiosyncratic
Drug hepatotoxicity is the single leading cause of acute liver failure in the United States, with most drug-induced overt adverse hepatic events unpredictable. Kaplowitz (90) divided these events into categories: hypersensitivity reaction and metabolic idiosyncratic reaction. Hypersensitivity reaction associates with some combination of fever, rash, eosinophilia, and a rapid response upon rechallenge (e.g., phenytoin, amoxicillin-clavulanic acid, sulfonamides) with a latency of about one to eight weeks (shorter on rechallenge). Metabolic idiosyncratic reaction may or may not exhibit a positive rechallenge (e.g., isoniazid, troglitazone), with a very viable latency time (0.5–12 months). Each therapeutic possesses its own hepatotoxicity tends, having its characteristic signature of latency and clinical manifestations (90). Based on this classification, Clouatre (12) in his review stated that “The direct toxicity of kava extracts is quite small under any analysis, yet the potential for drug interactions and/or the potentiation of the toxicity of other compounds is large. Presently, kava toxicity appears to be idiosyncratic”.
7.2. Formation of Activated Metabolites
As described earlier, Johnson et al. (23) studied the metabolism of kava extracts by rat and human liver microsomes resulting in the identification of four metabolites, kawain 11,12-quinone, 7,8-dihydrokawain 11,12-quinone, kawain 11,12-hydroquinone, and 7,8-dihydrokawain 11,12-hydroquinone (Figure 5). The glucuronic acid and sulfate conjugates of kawain 11,12-hydroquinone and 7,8-dihydrokawain 11,12-hydroquinone were also detected from a human volunteer who took a single dose of a kava-containing dietary supplement (23).
These metabolites can potentially covalently bind to cellular DNA to form the kava-quinone methide derived DNA adducts, which might contribute to hepatotoxicity in humans, although binding of the kava-quinone methide to protein may be predominant.
8. REGULATORY STATUS
For dietary supplements on the market prior to October 15, 1994, the Dietary Supplement Health and Education Act (DSHEA) requires no proof of safety in order for them to remain on the market. The labeling requirements for supplements allow warnings and dosage recommendations as well as substantiated “structure or function” claims. All claims must prominently note that they have not been evaluated by the FDA, and they must bear the statement “This product is not intended to diagnose, treat, cure, or prevent any disease” (91). Following DSHEA, kava-containing products remain popular in the United States and continue to be sold in health food stores and ethnic markets regardless of the fact that it was banned in Western countries such as Germany, France, Switzerland, Australia, and Canada, following reports of alleged hepatotoxicity (10).
On March 25, 2002, the Center for Food Safety and Applied Nutrition (CFSAN), U.S. Food and Drug Administration (FDA) issued a Consumer Advisory entitled “Kava-containing dietary supplements may be associated with severe liver injury” to the public (8). It indicated that “Supplements containing the herbal ingredient kava are promoted for relaxation (e.g., to relieve stress, anxiety, and tension), sleeplessness, menopausal symptoms and other uses” and that “FDA has not made a determination about the ability of kava dietary supplements to provide such benefits.” It further addressed that “persons who have liver disease or liver problems, or persons who are taking drug products that can affect the liver, should consult a physician before using kava-containing supplements. Consumers who use a kava-containing dietary supplement and who experience signs of illness associated with liver disease should also consult their physician.”
On the same day, March 25, 2002, the Office of Nutritional Products, Labeling, and Dietary Supplements, CFSAN, U.S. FDA issued a “Letter to Health Care Professionals, FDA Issues Consumer Advisory That Kava Products May be Associated with Severe Liver Injury” (9).
Kava is not listed in the EPA’s Toxic Substances Control Act (TSCA) Inventory.
The U.S. FDA issued new standards for dietary supplements in June 2007. In addition to product testing, the new standards address design and construction of manufacturing plants, record-keeping and handling of consumer complaints. Inspectors will check plants for compliance. For less serious violations, the agency may ask a company to fix a problem. Bigger problems could lead to product seizures or other action. The new rules (standards) take effect August 24, 2007, but they will be phased in so that large companies comply by June 2008, while companies with fewer than 500 employees have until June 2009 to comply. Firms with fewer than 20 employees have until June 2010 to comply.
8.1. Study by the National Toxicology Program (NTP)
Kava is brought to the attention of the Case Study Working Group (CSWG) because it is a rapidly growing, highly used dietary supplement introduced into the mainstream U.S. market relatively recently. Through this use, millions of consumers using anti-anxiety preparations are potentially exposed to kava. A traditional beverage of various Pacific Basin countries, kava clearly has psychoactive properties. The effects of its long-term consumption have not been documented adequately; preliminary studies suggest possible serious organ system effects. The potential carcinogenicity of kava and its principal constituents are unknown.
Accordingly, on December 14, 1998, the CSWG recommended that toxicological evaluation of kava, including reproductive toxicity, neurotoxicity, and genotoxicity be conducted with high priority. The rationale for recommending the studies were because (A) there is significant human exposure, (B) kava is a leading dietary supplement with growing use, (C) there are concerns that kava has been promoted as a substitute for ritilin for children, and (D) the long term effects of kava is unknown.
It is recommended that the kava extract be studied since the product has been standardized even though all 6 of the most abundant kavalactones have been synthesized. It has been shown that the kava extract exhibits greater biological effect when given in combination rather than as isolated compounds. For some unknown reasons, the 30% kavalactone mixtures are more readily absorbed than individual ones.
At present toxicological evaluation of kava extract is being conducted by the NTP. There are indications that kava extract may cause damages to the liver, kidney, brain, and the hematopoietic system. Special attentions will be paid to these organ systems in the studies. Reproductive toxicity and neurotoxicity studies will be considered after the 14-day and 90-day toxicity data are evaluated.
Acknowledgments
This article is not an official guidance or policy statement of U.S. Food and Drug Administration (FDA) or National Toxicology Program (NTP). No official support or endorsement by the U.S. FDA and NTP is intended or should be inferred.
References
- 1.Smith RM, Thakrar H, Arowolo TA, Shafi AA. High-performance liquid chromatography of kava lactones from Piper methysticum. J Chromatog. 1984;283:303–308. [Google Scholar]
- 2.Lebot V, Merlin M, Lindstrom L. Kava: The Pacific Drug. New Haven, CT: Yale University Press; 1992. p. 255. [Google Scholar]
- 3.Dentali SJ. Kava. Piper methysticum Forster f. (Piperaceae) Boulder, Co: Herb Research Foundation; 1997. Herb Safety Review; p. 29. [Google Scholar]
- 4.Schulze J, Raasch W, Siegers CP. Toxicity of kava pyrones, drug safety and precautions—a case study. Phytomedicine. 2003;10(Suppl4):68–73. doi: 10.1078/1433-187x-00300. [DOI] [PubMed] [Google Scholar]
- 5.Singh YN. Kava: an overview. J Ethnopharmacol. 1992;37(1):13–45. doi: 10.1016/0378-8741(92)90003-a. [DOI] [PubMed] [Google Scholar]
- 6.Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1–2):108–13. doi: 10.1016/j.jep.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Mirasol F. Botanicals industry posts strong growth in US. Chem. Market Rep. 1998;28(4):12–13. [Google Scholar]
- 8.CFSAN. Center for Food Safety and Applied Nutrition(CFSAN): Kava-containing dietary supplements may be associated with severe liver injury. U.S. Department of Health and Human Services, Food and Drug Administration; Rockville, Maryland: Mar 25, 2002. ( http://www.cfsan.fda.gov/~dms/ds-warn.html):. 2002. [Google Scholar]
- 9.CFSAN. Center for Food Safety and Applied Nutrition (CFSAN):Letter to health care professionals: FDA issues consumer advisory that kava products may be associated with severe liver injury (document issued March 25, 2002) Rockville, Maryland: U.S. Department of Health and Human Services, Food and Drug Administration; 2002. ( http://www.fda.gov/medwatch). 2002. [Google Scholar]
- 10.Lim ST, Dragull K, Tang CS, Bittenbender HC, Efird JT, Nerurkar PV. Effects of kava alkaloid, pipermethystine, and kavalactones on oxidative stress and cytochrome P450 in F-344 rats. Toxicol Sci. 2007;97(1):214–21. doi: 10.1093/toxsci/kfm035. [DOI] [PubMed] [Google Scholar]
- 11.He XG, Lin LZ, Lian LZ. Electrospray high performance liquid chromatography-mass spectrometry in phytochemical analysis of kava (Piper methysticum) extract. Planta Med. 1997;63(1):70–4. doi: 10.1055/s-2006-957608. [DOI] [PubMed] [Google Scholar]
- 12.Clouatre DL. Kava kava: examining new reports of toxicity. Toxicol Lett. 2004;150(1):85–96. doi: 10.1016/j.toxlet.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Shulgin AT. The narcotic pepper—the chemistry and pharmacology of Piper methysticum and related species. Bull Narc. 1973;25:59–74. [Google Scholar]
- 14.Israili ZH, Smissman EE. Synthesis of kavain, dihydrokavain, and analogues. J Org Chem. 1976;41(26):4070–74. doi: 10.1021/jo00888a004. [DOI] [PubMed] [Google Scholar]
- 15.Norton SA, Ruze P. Kava dermopathy. J Am Acad Dermatol. 1994;31(1):89–97. doi: 10.1016/s0190-9622(94)70142-3. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson DD, Duffield PH, Cheng D, Duffield AM. Comparison of the central nervous system activity of the aqueous and lipid extract of kava (Piper methysticum) Arch Int Pharmacodyn Ther. 1989;301:66–80. [PubMed] [Google Scholar]
- 17.Bilia AR, Gallon S, Vincieri FF. Kava-kava and anxiety: growing knowledge about the efficacy and safety. Life Sci. 2002;70(22):2581–97. doi: 10.1016/s0024-3205(02)01555-2. [DOI] [PubMed] [Google Scholar]
- 18.Ernst E. Herbal remedies for anxiety—a systematic review of controlled clinical trials. Phytomedicine. 2006;13(3):205–8. doi: 10.1016/j.phymed.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Keledjian J, Duffield PH, Jamieson DD, Lidgard RO, Duffield AM. Uptake into mouse brain of four compounds present in the psychoactive beverage kava. J Pharm Sci. 1988;77(12):1003–6. doi: 10.1002/jps.2600771203. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen AK, Scheline RR, Solheim E, Hansel R. Metabolism of some kava pyrones in the rat. Xenobiotica. 1979;9(1):1–16. doi: 10.3109/00498257909034699. [DOI] [PubMed] [Google Scholar]
- 21.Duffield AM, Jamieson DD, Lidgard RO, Duffield PH, Bourne DJ. Identification of some human urinary metabolites of the intoxicating beverage kava. J Chromatogr. 1989;475:273–81. doi: 10.1016/s0021-9673(01)89682-5. [DOI] [PubMed] [Google Scholar]
- 22.Zou L, Harkey MR, Henderson GL. Synthesis, in vitro, reactivity, and identification of 6-phenyl-3-hexen-2-one in human urine after kava-kava (Piper methysticum) ingestion. Planta Med. 2005;71(2):142–6. doi: 10.1055/s-2005-837781. [DOI] [PubMed] [Google Scholar]
- 23.Johnson BM, Qiu SX, Zhang S, Zhang F, Burdette JE, Yu L, Bolton JL, van Breemen RB. Identification of novel electrophilic metabolites of piper methysticum Forst (Kava) Chem Res Toxicol. 2003;16(6):733–40. doi: 10.1021/tx020113r. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson DD, Duffield PH. The antinociceptive actions of kava components in mice. Clin Exp Pharmacol Physiol. 1990;17(7):495–507. doi: 10.1111/j.1440-1681.1990.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 25.Kumar V. Potential medicinal plants for CNS disorders: an overview. Phytother Res. 2006;20(12):1023–35. doi: 10.1002/ptr.1970. [DOI] [PubMed] [Google Scholar]
- 26.Jussofie A, Schmiz A, Hiemke C. Kavapyrone enriched extract from Piper methysticum as modulator of the GABA binding site in different regions of rat brain. Psychopharmacology (Berl) 1994;116(4):469–74. doi: 10.1007/BF02247480. [DOI] [PubMed] [Google Scholar]
- 27.Almeida JC, Grimsley EW. Coma from the health food store: interaction between kava and alprazolam. Ann Intern Med. 1996;125(11):940–1. doi: 10.7326/0003-4819-125-11-199612010-00023. [DOI] [PubMed] [Google Scholar]
- 28.Wu D, Yu L, Nair MG, DeWitt DL, Ramsewak RS. Cyclooxygenase enzyme inhibitory compounds with antioxidant activities from Piper methysticum (kava kava) roots. Phytomedicine. 2002;9(1):41–7. doi: 10.1078/0944-7113-00068. [DOI] [PubMed] [Google Scholar]
- 29.Brauer R, Pfab R, Becker K, Berger H, Stangl M. Fulminantes leberver-sagen nach einnahme des pfalzlichen heimittels kava-kava. Z Gastroenterol. 2001;39:491. [Google Scholar]
- 30.Saß M, Schnabel S, Kröger J, Liebe S, Schareck WD. Acute liver failure from kavakava—a rare indication for liver transplantation. Z. Gastroenterol. 2001;39:491. [Google Scholar]
- 31.Humberston CL, Akhtar J, Krenzelok EP. Acute hepatitis induced by kava kava. J Toxicol Clin Toxicol. 2003;41(2):109–13. doi: 10.1081/clt-120019123. [DOI] [PubMed] [Google Scholar]
- 32.Russmann S, Lauterburg BH, Helbling A. Kava hepatotoxicity. Ann Intern Med. 2001;135(1):68–9. doi: 10.7326/0003-4819-135-1-200107030-00036. [DOI] [PubMed] [Google Scholar]
- 33.Russmann S, Barguil Y, Cabalion P, Kritsanida M, Duhet D, Lauterburg BH. Hepatic injury due to traditional aqueous extracts of kava root in New Caledonia. Eur J Gastroenterol Hepatol. 2003;15(9):1033–6. doi: 10.1097/00042737-200309000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Anon. Kava kava may cause irreversible liver damage. S. Afr. Med. J. 2002;(92):961. [PubMed] [Google Scholar]
- 35.Anon. Concerns over kava have the FDA’s attention. Mayo Clin. Health Lett. 2002;20:4. [PubMed] [Google Scholar]
- 36.Anon. Kava concerns. FDA, Botanical Council raises safety concerns. AWHONN Lifelines. 2002;6:13–15. doi: 10.1111/j.1552-6356.2002.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 37.Anon. From the Centers for Disease Control and Prevention. Hepatic toxicity possibly associated with kava-containing products-United States, Germany, and Switzerland, 1999–2002. JAMA. 2003;(289):36–37. [PubMed] [Google Scholar]
- 38.Campo JV, McNabb J, Perel JM, Mazariegos GV, Hasegawa SL, Reyes J. Kava-induced fulminant hepatic failure. J Am Acad Child Adolesc Psychiatry. 2002;41(6):631–2. doi: 10.1097/00004583-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Parkman CA. Another FDA warning: Kava supplements. Case Manager. 2002;13(4):26–8. doi: 10.1067/mcm.2002.126437. [DOI] [PubMed] [Google Scholar]
- 40.De Smet PA. Safety concerns about kava not unique. Lancet. 2002;(360):1336. doi: 10.1016/S0140-6736(02)11347-X. [DOI] [PubMed] [Google Scholar]
- 41.Clough AR, Bailie RS, Currie B. Liver function test abnormalities in users of aqueous kava extracts. J Toxicol Clin Toxicol. 2003;41(6):821–9. doi: 10.1081/clt-120025347. [DOI] [PubMed] [Google Scholar]
- 42.Anke J, Ramzan I. Kava Hepatotoxicity: Are we any closer to the truth? Planta Med. 2004;70(3):193–6. doi: 10.1055/s-2004-815533. [DOI] [PubMed] [Google Scholar]
- 43.Rietjens IM, Martena MJ, Boersma MG, Spiegelenberg W, Alink GM. Molecular mechanisms of toxicity of important food-borne phytotoxins. Mol Nutr Food Res. 2005;49(2):131–58. doi: 10.1002/mnfr.200400078. [DOI] [PubMed] [Google Scholar]
- 44.Anke J, Fu S, Ramzan I. Kavalactones fail to inhibit alcohol dehydrogenase in vitro. Phytomedicine. 2006;13(3):192–5. doi: 10.1016/j.phymed.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Whitton PA, Lau A, Salisbury A, Whitehouse J, Evans CS. Kava lactones and the kava-kava controversy. Phytochemistry. 2003;64(3):673–9. doi: 10.1016/s0031-9422(03)00381-9. [DOI] [PubMed] [Google Scholar]
- 46.Bujanda L, Palacios A, Silvarino R, Sanchez A, Munoz C. Kava-induced acute icteric hepatitis. Gastroenterol Hepatol. 2002;25(6):434–5. doi: 10.1016/s0210-5705(02)70281-1. [DOI] [PubMed] [Google Scholar]
- 47.Stickel F, Baumuller HM, Seitz K, Vasilakis D, Seitz G, Seitz HK, Schuppan D. Hepatitis induced by Kava (Piper methysticum rhizoma) J Hepatol. 2003;39(1):62–7. doi: 10.1016/s0168-8278(03)00175-2. [DOI] [PubMed] [Google Scholar]
- 48.Escher M, Desmeules J, Giostra E, Mentha G. Hepatitis associated with Kava, a herbal remedy for anxiety. Bmj. 2001;322(7279):139. doi: 10.1136/bmj.322.7279.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraft M TWS, Menzel J, Senninger N, Dietl KH, Herbst H, Domschke W, Lerch MM. Fulminant liver failure after administration of the herbal antidepressant Kava-Kava. Dtsch Med Wochensch. 2001;126:970–972. doi: 10.1055/s-2001-16966. [DOI] [PubMed] [Google Scholar]
- 50.Thomsen M, Vitetta L, Schmidt M, Sali A. Fatal fulminant hepatic failure induced by a natural therapy containing kava. Med J Aust. 2004;180(4):198–9. doi: 10.5694/j.1326-5377.2004.tb05875.x. author reply 199. [DOI] [PubMed] [Google Scholar]
- 51.Gow PJ, Connelly NJ, Hill RL, Crowley P, Angus PW. Fatal fulminant hepatic failure induced by a natural therapy containing kava. Med J Aust. 2003;178(9):442–3. doi: 10.5694/j.1326-5377.2003.tb05286.x. [DOI] [PubMed] [Google Scholar]
- 52.Ruze P. Kava-induced dermopathy: a niacin deficiency? Lancet. 1990;335(8703):1442–5. doi: 10.1016/0140-6736(90)91458-m. [DOI] [PubMed] [Google Scholar]
- 53.Norton SA. Herbal medicines in Hawaii from tradition to convention. Hawaii Med. J. 1998;(57):383–386. [PubMed] [Google Scholar]
- 54.Mathews JD, Riley MD, Fejo L, Munoz E, Milns NR, Gardner ID, Powers JR, Ganygulpa E, Gununuwawuy BJ. Effects of the heavy usage of kava on physical health: summary of a pilot survey in an aboriginal community. Med J Aust. 1988;148(11):548–55. doi: 10.5694/j.1326-5377.1988.tb93809.x. [DOI] [PubMed] [Google Scholar]
- 55.Schelosky L, Raffauf C, Jendroska K, Poewe W. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639–40. doi: 10.1136/jnnp.58.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfeiffer CC, Murphree HB, Goldstein L. Effects of kava in normal subjects and patients. In: Efron DH, Holmstedt B, Kline NS, editors. Ethnopharmacologic Search for Psychoactive Drugs; Proceedings of a symposium held in San Francisco, Ca; Jan 28–30, 1967. Public Health Service Publication 1967; No. 1645:155–161. [Google Scholar]
- 57.Dragull K, Yoshida WY, Tang CS. Piperidine alkaloids from Piper methysticum. Phytochemistry. 2003;63(2):193–8. doi: 10.1016/s0031-9422(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 58.Smith KK, Dharmaratne HR, Feltenstein MW, Broom SL, Roach JT, Nanayakkara NP, Khan IA, Sufka KJ. Anxiolytic effects of kava extract and kavalactones in the chick social separation-stress paradigm. Psychopharmacology (Berl) 2001;155(1):86–90. doi: 10.1007/s002130100686. [DOI] [PubMed] [Google Scholar]
- 59.Nerurkar PV, Dragull K, Tang CS. In vitro toxicity of kava alkaloid, pipermethystine, in HepG2 cells compared to kavalactones. Toxicol Sci. 2004;79(1):106–11. doi: 10.1093/toxsci/kfh067. [DOI] [PubMed] [Google Scholar]
- 60.Hsu SY, Lin MH, Lin LC, Chou CJ. Toxicologic studies of dihydro-5,6-dehydrokawain and 5,6-dehydrokawain. Planta Med. 1994;60(1):88–90. doi: 10.1055/s-2006-959417. [DOI] [PubMed] [Google Scholar]
- 61.Duffield PH, Jamieson D. Development of tolerance to kava in mice. Clin Exp Pharmacol Physiol. 1991;18(8):571–8. doi: 10.1111/j.1440-1681.1991.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 62.Jhoo JW, Freeman JP, Heinze TM, Moody JD, Schnackenberg LK, Beger RD, Dragull K, Tang CS, Ang CY. In vitro cytotoxicity of nonpolar constituents from different parts of kava plant (Piper methysticum) J Agric Food Chem. 2006;54(8):3157–62. doi: 10.1021/jf051853j. [DOI] [PubMed] [Google Scholar]
- 63.Jhoo J, Ang CYW, Mei N, Chen T, Dragull K, Tang C. Kava (Piper methysticum) Safety issues and studies for cytotoxicity and mutagenicity of pipermethystine. In: Ho C, editor. American Chemistry Society Book Series: Dietary Supplements. Oxford University Press, Inc.; New York, NY: 2006. [Google Scholar]
- 64.Kaplowitz N. Hepatotoxicity of herbal remedies: insights into the intricacies of plant-animal warfare and cell death. Gastroenterology. 1997;113(4):1408–12. doi: 10.1053/gast.1997.v113.agast971131408. [DOI] [PubMed] [Google Scholar]
- 65.Eichelbaum M, Kroemer HK, Fromm MF. Impact of P450 genetic polymorphism on the first-pass extraction of cardiovascular and neuroactive drugs. Adv Drug Deliv Rev. 1997;27(2–3):171–199. doi: 10.1016/s0169-409x(97)00042-2. [DOI] [PubMed] [Google Scholar]
- 66.Strahl S, Ehret V, Dahm HH, Maier KP. Necrotizing hepatitis after taking herbal remedies. Dtsch Med Wochenschr. 1998;123(47):1410–4. doi: 10.1055/s-2007-1024196. [DOI] [PubMed] [Google Scholar]
- 67.Russmann S, Lauterburg BH, Barguil Y, Choblet E, Cabalion P, Rentsch K, Wenk M. Traditional aqueous kava extracts inhibit cytochrome P450 1A2 in humans: Protective effect against environmental carcinogens? Clin Pharmacol Ther. 2005;77(5):453–4. doi: 10.1016/j.clpt.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 68.Raucy JL. Regulation of CYP3A4 expression in human hepatocytes by pharmaceuticals and natural products. Drug Metab Dispos. 2003;31(5):533–9. doi: 10.1124/dmd.31.5.533. [DOI] [PubMed] [Google Scholar]
- 69.Gibson GG, Plant NJ, Swales KE, Ayrton A, El-Sankary W. Receptor-dependent transcriptional activation of cytochrome P4503A genes: induction mechanisms, species differences and interindividual variation in man. Xenobiotica. 2002;32(3):165–206. doi: 10.1080/00498250110102674. [DOI] [PubMed] [Google Scholar]
- 70.Meijerman I, Beijnen JH, Schellens JH. Herb-drug interactions in oncology: focus on mechanisms of induction. Oncologist. 2006;11(7):742–52. doi: 10.1634/theoncologist.11-7-742. [DOI] [PubMed] [Google Scholar]
- 71.Hodgson E, Philpot RM. Interaction of methylenedioxyphenyl (1,3-benzodioxole) compounds with enzymes and their effects on mammals. Drug Metab Rev. 1974;3(2):231–301. doi: 10.3109/03602537408993744. [DOI] [PubMed] [Google Scholar]
- 72.Murray M, Reidy GF. In vitro formation of an inhibitory complex between an isosafrole metabolite and rat hepatic cytochrome P-450 PB-B. Drug Metab Dispos. 1989;17(4):449–54. [PubMed] [Google Scholar]
- 73.Murray M, Wilkinson CF, Marcus C, Dube CE. Structure-activity relationships in the interactions of alkoxymethylenedioxybenzene derivatives with rat hepatic microsomal mixed-function oxidases in vivo. Mol Pharmacol. 1983;24(1):129–36. [PubMed] [Google Scholar]
- 74.Unger M, Holzgrabe U, Jacobsen W, Cummins C, Benet LZ. Inhibition of cytochrome P450 3A4 by extracts and kavalactones of Piper methysticum (Kava-Kava) Planta Med. 2002;68(12):1055–8. doi: 10.1055/s-2002-36360. [DOI] [PubMed] [Google Scholar]
- 75.Cote CS, Kor C, Cohen J, Auclair K. Composition and biological activity of traditional and commercial kava extracts. Biochem Biophys Res Commun. 2004;322(1):147–52. doi: 10.1016/j.bbrc.2004.07.093. [DOI] [PubMed] [Google Scholar]
- 76.Zou L, Harkey MR, Henderson GL. Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci. 2002;71(13):1579–89. doi: 10.1016/s0024-3205(02)01913-6. [DOI] [PubMed] [Google Scholar]
- 77.Mathews JM, Etheridge AS, Black SR. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab Dispos. 2002;30(11):1153–7. doi: 10.1124/dmd.30.11.1153. [DOI] [PubMed] [Google Scholar]
- 78.Mathews JM, Etheridge AS, Valentine JL, Black SR, Coleman DP, Patel P, So J, Burka LT. Pharmacokinetics and disposition of the kavalactone kawain: interaction with kava extract and kavalactones in vivo and in vitro. Drug Metab Dispos. 2005;33(10):1555–63. doi: 10.1124/dmd.105.004317. [DOI] [PubMed] [Google Scholar]
- 79.Bressler R. Herb-drug interactions: interactions between kava and prescription medications. Geriatrics. 2005;60(9):24–5. [PubMed] [Google Scholar]
- 80.Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, Duan W, Koh HL, Zhou S. Herb-drug interactions: a literature review. Drugs. 2005;65(9):1239–82. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- 81.Anke J, Ramzan I. Pharmacokinetic and pharmacodynamic drug interactions with Kava (Piper methysticum Forst. f.) J Ethnopharmacol. 2004;93(2–3):153–60. doi: 10.1016/j.jep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 82.Clayton NP, Yoshizawa K, Kissling GE, Burka LT, Chan PC, Nyska A. Immunohistochemical analysis of expressions of hepatic cytochrome P450 in F344 rats following oral treatment with kava extract. Exp Toxicol Pathol. 2007;58(4):223–36. doi: 10.1016/j.etp.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poolsup N, Li Wan Po A, Knight TL. Pharmacogenetics and psychopharmacotherapy. J Clin Pharm Ther. 2000;25(3):197–220. doi: 10.1046/j.1365-2710.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- 84.Wanwirolmuk S, Bhawan S, Coville P, Chalcroft S. Genetic polymorphism of debrisoquine (CYP2D6) and proguanil (CYP2C19) in south Pacific Polynesian populations. Euro J Clin Pharmacol. 1998;(54):431–435. doi: 10.1007/s002280050488. [DOI] [PubMed] [Google Scholar]
- 85.Liu LL, Gong LK, Qi XM, Cai Y, Wang H, Wu XF, Xiao Y, Ren J. Altered expression of cytochrome P450 and possible correlation with preneoplastic changes in early stage of rat hepatocarcinogenesis. Acta Pharmacol Sin. 2005;26(6):737–44. doi: 10.1111/j.1745-7254.2005.00737.x. [DOI] [PubMed] [Google Scholar]
- 86.Gurley BJ, Swain A, Barone GW, Williams DK, Breen P, Yates CR, Stuart LB, Hubbard MA, Tong Y, Cheboyina S. Effect of goldenseal (Hydrastis canadensis) and kava kava (Piper methysticum) supplementation on digoxin pharmacokinetics in humans. Drug Metab Dispos. 2007;35(2):240–5. doi: 10.1124/dmd.106.012708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Folmer F, Blasius R, Morceau F, Tabudravu J, Dicato M, Jaspars M, Diederich M. Inhibition of TNFalpha-induced activation of nuclear factor kappaB by kava (Piper methysticum) derivatives. Biochem Pharmacol. 2006;71(8):1206–18. doi: 10.1016/j.bcp.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 88.Knight EV, Kimball JP, Keenan CM, Smith IL, Wong FA, Barrett DS, Dempster AM, Lieuallen WG, Panigrahi D, Powers WJ, et al. Preclinical toxicity evaluation of tepoxalin, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, in Sprague-Dawley rats and beagle dogs. Fundam Appl Toxicol. 1996;33(1):38–48. doi: 10.1006/faat.1996.0141. [DOI] [PubMed] [Google Scholar]
- 89.Reilly TP, Brady JN, Marchick MR, Bourdi M, George JW, Radonovich MF, Pise-Masison CA, Pohl LR. A protective role for cyclooxygenase-2 in drug-induced liver injury in mice. Chem Res Toxicol. 2001;14(12):1620–8. doi: 10.1021/tx0155505. [DOI] [PubMed] [Google Scholar]
- 90.Kaplowitz N. Causality assessment versus guilt-by-association in drug hepatotoxicity. Hepatology. 2001;33(1):308–10. doi: 10.1053/jhep.2001.21083. [DOI] [PubMed] [Google Scholar]
- 91.Croom EMJ, Walker L. Botanicals in the pharmacy: New life for old remedies. Drug Top. 1995;139:84–93. [Google Scholar]