Abstract
Roasting is an important cocoa processing step, but has been reported to reduce the polyphenol content in the beans. We investigated the impact of whole-bean roasting on the polyphenol content, aroma-related chemistry, and in vitro pancreatic lipase (PL) inhibitory activity of cocoa under a range of roasting conditions. Total phenolics, (−)-epicatechin, and proanthocyanidin (PAC) dimer – pentamer content was reduced by roasting. By contrast, roasting at 150°C or greater increased the levels of catechin and PAC hexamers and heptamers. These compounds have greater PL inhibitory potency. Consistent with these changes in PAC composition and this previous data, we found that roasting at 170°C time-dependently increased PL inhibitory activity. Cocoa aroma-related compounds increased with roasting above 100°C, whereas deleterious sensory-related compounds formed at more severe temperatures. Our results indicate that cocoa roasting can be optimized to increase the content of larger PACs and anti-PL activity, while maintaining a favorable aroma profile.
Keywords: cocoa, roasting, proanthocyanidins, polyphenols, pancreatic lipase
1. INTRODUCTION
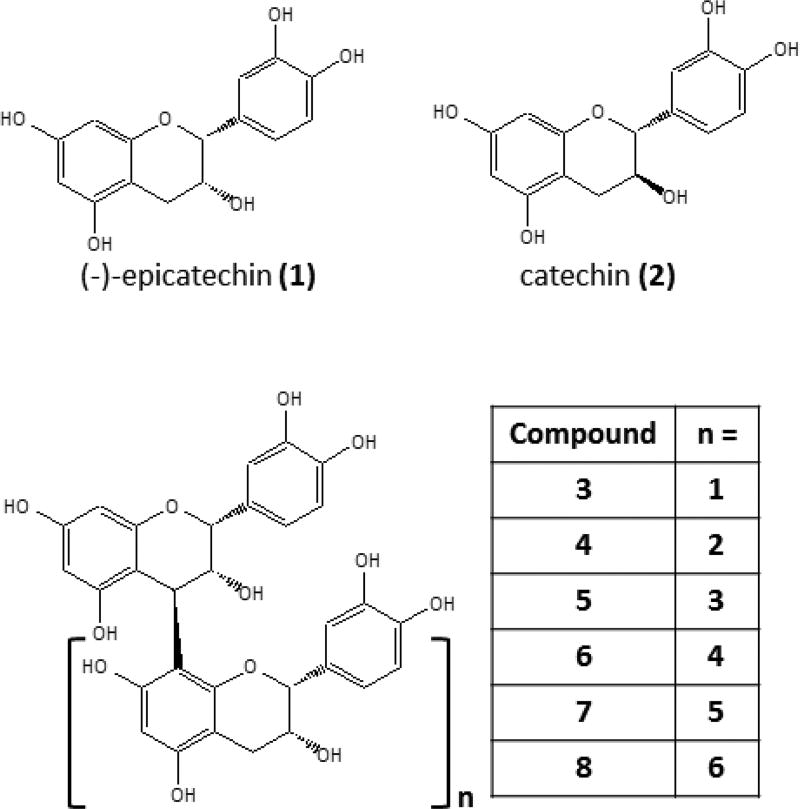
Cocoa, derived from the seeds of Theobroma cacao L. (Malvaceae), is a rich source of polyphenolic compounds and may account 12–18% of the dry mass of the beans (Miller, Hurst, Flannigan, Ou, Lee, Smith, et al., 2009; Rusconi & Conti, 2010). These compounds include the flavan-3-ols, (−)-epicatechin (1), catechin (2), and B-type proanthocyanidins (PACs, 3 – 8, Fig. 1). Laboratory and human intervention studies have reported a number of putative beneficial health effects related to consumption of cocoa or cocoa polyphenols including mitigation of inflammation, vascular dysfunction, and metabolic syndrome (Bitzer, Glisan, Dorenkott, Goodrich, Ye, O'Keefe, et al., 2015; Dorenkott, Griffin, Goodrich, Thompson-Witrick, Fundaro, Ye, et al., 2014; Gu, Yu, & Lambert, 2014; Monahan, 2012). Previous studies in our laboratory have shown that cocoa-derived PACs can inhibit pancreatic lipase (PL) and secreted phospholipase A2 (PLA2) in vitro (Gu, Hurst, Stuart, & Lambert, 2011). These effects correlated with prevention of fatty liver disease and mitigation of inflammation in high fat-fed mice (Dorenkott, et al., 2014; Gu, Yu, & Lambert, 2014; Gu, Yu, Park, Harvatine, & Lambert, 2014). The inhibitory potency of the individual cocoa PACs was directly proportional to the compound’s degree polymerization (DP) (Gu, et al., 2011).
Figure 1.
Structures of cocoa polyphenols under investigation.
A limited number of studies have examined the impact of processing on the biological effects of cocoa, but available data to suggests that variation in the phytochemical composition of cocoa powders can have significant impact on the biological effect of the powder (Dorenkott, et al., 2014; Gu, et al., 2011). For example, we have found that polyphenol-rich extracts of alkali-treated cocoa powder had reduced PL inhibitory potency compared to extracts from unalkalized and Lavado (unfermented) cocoa (Gu, et al., 2011). Similarly, two recent papers compared the in vitro inhibitory activity of roasted and unroasted cocoa, and fermented and unfermented cocoa against a panel of digestive enzymes (Ryan, Khoo, Stewart, O'Keefe, Lambert, & Neilson, 2017; Ryan, Khoo, Ye, Lambert, O'Keefe, & Neilson, 2016). They found that both processes impacted enzyme inhibitory potency and that the effect was not simply due to measured decreases in total phenolic content. Although these studies are interesting, the results are somewhat preliminary because a limited number of samples were examined and the approach to processing was not systematic.
Roasting is an important step in cocoa bean processing and results in the production of desirable flavor and aroma compounds, as well as color changes (Beckett, 2017). In addition, roasting can act as a pasteurization step (Beckett, 2017; Copetti, Iamanaka, Pitt, & Taniwaki, 2014; do Nascimento, Brum, Pena, Berto, & Efraim, 2012). A number of studies have examined the effects of roasting on antioxidant activity and the levels of 1 – 3 in cocoa (Arlorio, Locatelli, Travaglia, Coisson, Del Grosso, Minassi, et al., 2008; Hurst, Krake, Bergmeier, Payne, Miller, & Stuart, 2011; Kothe, Zimmermann, & Galensa, 2013). For example, it has been reported that roasting at temperatures greater than 70°C leads to substantial decreases in both 1 and 2 at temperatures greater than 70°C (Payne, Hurst, Miller, Rank, & Stuart, 2010). The authors also reported that roasting led to epimeric conversion of 1 to 2 (Payne, et al., 2010). A second study by the same group reported that roasting at 163°C for up to 25 min time-dependently reduced the levels of 1 but increased levels of 2 (Hurst, et al., 2011). To date, a limited number of studies have examined the effect of roasting on PAC levels in cocoa. One study reported that roasting at 140 – 150°C for 20 min reduced TPC by 14% and PAC dimer levels by 30 – 57% (Jolic, Redovnikovic, Markovic, Sipusic, & Delonga, 2011). More recently, the impact of roasting on PACs of higher DP was examined (Ioannone, Di Mattia, De Gregorio, Sergi, Serafini, & Sacchetti, 2015). These authors found that roasting at temperatures of up to 125 – 145°C reduced levels of PACs in a time and temperature-dependent manner. The results of this study are interesting, but the use of a relatively narrow temperature range limits the predictive values of the results.
The goal of the present study was to examine the time-temperature impact of roasting across a wide range of roasting temperatures including those relevant to industry and more extreme temperatures on the TPC and flavan-3-ols (1, 2) and PACs (3 – 8), as well as on the PL inhibitory potency of the resulting cocoa. In addition, we determined the effect of the same roasting conditions on a range of roasting-related volatile components to provide a “quality” context for the observed changes in polyphenol composition.
2. MATERIALS AND METHODS
2.1 Materials
Cocoa beans were sourced through Taza Chocolate Co. (Somerville, MA). Trinitario beans were harvested at El Vesia farm (Hato Mayor Province, Dominican Republic). Prior to shipment, beans were fermented for 5 d and dried. Beans were stored at −20°C prior to the start of experiments. All chemicals were of the highest grade commercially-available.
2.2 Roasting Conditions
Cocoa beans were selected with the following criteria: mass between 1.0 and 2.0 g, firm, not flat, and with intact shells. Cocoa beans that did not fall within these parameters were excluded in order to maintain uniformity of samples. Cocoa beans (100g) were roasted on a fine wire mesh tray in a BD-53 Binder oven (Tuttlingen, Germany) preheated for 20 min to 100, 130, 150, 170, or 190°C. Samples were roasted for 10, 20, 30, or 40 min. A full-factorial experimental design was employed and each treatment was repeated three times. Internal bean temperature was monitored during roasting. In brief, 15 beans were selected in each treatment, a one-millimeter hole was drilled into the center of each bean and an OMEGA Type T thermocouple (Stamford, CT) was fitted into the hole. The thermocouple was then sealed with fast-drying superglue. Internal bean temperature and oven temperature were monitored using a CR3000 data logger (Campbell Scientific, Logan, UT) with a 15 s sample rate. Upon removal from the oven, samples were immediately cooled in liquid nitrogen and beans were stored in heat-sealed polyester/polyethylene bags (ProAmpac, Cincinnati, OH) at −80°C until further use within 60 d of production.
2.3 Cocoa Bean Processing and Extraction
The nib and shells of roasted beans and unroasted control beans were separated using a Winn-15 Mini winnower (Bottom Line Process Technologies, Inc., Largo, FL). The winnower was cleaned between each treatment group to prevent cross-contamination. Cleaned nibs were ground using a coffee grinder (three × 10 s pulses). The lipids in the ground nibs were removed by hexane extraction. Ground nibs (2 g) were combined with 10 vol of hexane. The sample was agitated at room temperature on an orbital shaker at 400 rpm, for ten minutes. The supernatant was decanted and the extraction repeated twice. Lipid-free cocoa powder was air-dried for 24 h and the defatted mass was recorded. Lipid content was calculated as a percentage of mass loss. Phenolic extracts were prepared by combining defatted, dried, cocoa powder with 10 vol of 70% aqueous acetone and shaking the mixture for 10 min at room temperature (Stanley, Smithson, Neilson, Anantheswaran, & Lambert, 2015). The supernatant was collected following centrifugation at 1776 g for 4 min. The acetone extraction was repeated one time. The powder was then extracted twice with 50% aqueous methanol in an analogous manner. The four supernatants (2 acetone:water and 2 methanol:water) were combined and organic solvents were removed under vacuum and the remaining water was removed by lyophilization. The dried extract was stored at −80°C until later use.
2.4 TPC Determination
TPC was determined using a modification of an established colorimetric method (Singleton & Rossi, 1965). In brief, cocoa extract (1 mg/mL in DMSO) was diluted 1:100 with deionized water, combined with 1/20th volume of Folin-Ciocalteau reagent, and incubated at room temperature with shaking (260 rpm) for 5 minutes. The reaction was stopped by addition of sodium carbonate to a final concentration of 25 mg/mL solution. After a 30 min incubation at 37°C, the absorbance at λ= 765 nm was measured using an Agilent 8453 UV-Vis spectrophotometer (Santa Clara, CA) and compared to a standard curve of gallic acid.
2.5 HPLC with Electrochemical Detection
Compounds 1 – 8 were analyzed by HPLC with electrochemical detection (ECD) using our previously described method (Stanley, Smithson, Neilson, Anantheswaran, & Lambert, 2015). In brief, the HPLC system consisted of two Shimadzu LC-20AD pumps (Kyoto, Japan), a Shimadzu SIL-20AC refrigerated auto-sampler (Kyoto, Japan), and an ESA 5500 coulochem electrode array system (CEAS). The potentials of the CEAS were set at −100, 100, 300, and 500 mV. Analytes were separated using a Supelcosil LC-18 column (150 mm × 4.6 mm, 5 µm; Supelco, Bellefonte, PA). Column temperature was maintained at 35°C. The mobile phases consisted of solvent A (30 mM sodium phosphate buffer, 1.75% acetonitrile and 0.125% tetrahydrofuran, pH 3.35) and solvent B (15 mM sodium phosphate buffer, 58.5% acetonitrile and 12.5% tetrahydrofuran, pH 3.45). The gradient program has been previously described (Stanley, et al., 2015). The flow rate was maintained at 1.0 mL/min. Cocoa extracts were dissolved in DMSO to a concentration of 1 mg/mL, diluted 1:10 in water containing 2% ascorbic acid, and filtered through a 0.45 µM PTFE filter. The injection volume was 30 µL. Peak areas at specific time points were normalized to the peak area at time 0. This allowed us to focus on changes in the relative abundance of the individual flavan-3-ols as a function of time and temperature. The within- and between-day variation of the method are less than 14% and 12%, respectively, as previously reported (Stanley, et al., 2015).
2.6 GC-MS Analysis
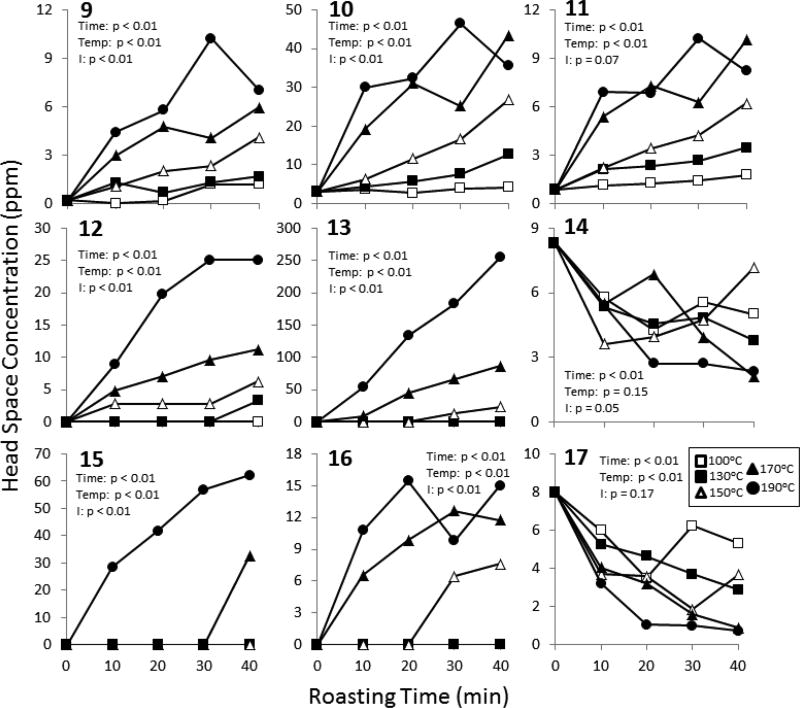
Solid-phase microextraction (SPME) was paired with gas chromatography-mass spectrometry (GC-MS) in order to extract, identify and quantify the volatile aroma-related compounds in the headspace of cocoa samples (2.0 g) using previously established methods with slight modifications (Rodriguez-Campos, Escalona-Buendia, Contreras-Ramos, Orozco-Avila, Jaramillo-Flores, & Lugo-Cervantes, 2012) A 50/30 µm divinylbenzene/carboxen/polydimethylsiloxane fiber (Supelco, Bellefonte, PA) was preconditioned at 250°C for 30 minutes before use. Samples were prepared in 10 mL clear glass vials. Prior to analysis, internal standards of 1 ppm ethyl nonanoate and 25 ppm of each 4-methyl-2-pentanol, 1,6-heptadien-4-ol and 2-isobutyl-3-methoxypyrazine (Sigma-Aldrich, St. Louis, MO) were added to each sample. All vials were sealed with silicone/polytetrafluoroethylene-lined screw caps (Gerstel, Inc., Linthicum, MD). Samples were allowed to reach equilibrium at 60°C during a 15 min incubation period at 250 rpm before a 30 min extraction period under the same conditions. A Gerstel MultiPurpose Autosampler was used for all extraction and injection procedures (Gerstel, Inc., Linthicum, MD). GC-MS analysis was carried out using an Agilent 7890A gas chromatograph with an Agilent 5975C Series mass selective detector (MSD, Agilent Technologies, Santa Clara, CA). The GC was equipped with a Thermogreen LB-2 septum and a 0.75 mm i.d. injection liner (Agilent Technologies, Santa Clara). A DB-5ms capillary column (30 m × 0.25 mm i.d. × 0.25 µm film thickness) was used for chromatographic separation (J&W Scientific, Folsom, CA). Desorption and injection were carried out for 30 seconds at 240°C. Helium was used as the carrier gas with a flow rate of 0.7 ml/min. The oven temperature was held at 40°C for 5 minutes before being ramped to 200°C at a rate of 10°C/min followed by a 30 min hold. The MSD was held at a temperature of 260°C and was operated in electron impact mode at 70 eV. Data was collected in scanning mode, using a range of 30 to 250 m/z. Compounds were identified based on retention time and m/z using MassHunter Workstation (Agilent Technologies) and concentrations were quantified relative to internal standards. Concentrations of volatile compounds were expressed as mg analyte per kg cocoa powder (ppm). We selected 25 compounds for analysis based on their reported importance to cocoa aroma profiles. The results of 9 representative compounds, as listed in Table 1, are presented in Figure 5. The data set for the remaining 16 compounds is included in Supporting Figure 1.
Table 1.
Roasting-Related Compounds Evaluated in Cocoa
| Compound No. | Name | Descriptors |
|---|---|---|
| 9 | 2,3-dimethylpyrazine | caramel, cocoa |
| 10 | 2,3,5-trimethylpyrazine | roasted, cocoa |
| 11 | 2,3,5-trimethyl-6-ethylpyrazine | sweet, candy |
| 12 | furfural | sweet, roasted |
| 13 | 2-formylpyrrole | malty, roasted nuts |
| 14 | phenylethyl acetate | honey, fruity |
| 15 | benzaldehyde | almond (odor) |
| 16 | furfuryl alcohol | bitter (flavor), burnt (odor) |
| 17 | dimethyl trisulfide | rubbery, onion |
Figure 5.
The effect of roasting time and temperature on a panel of sensory-related volatile compounds in cocoa. The levels of the aroma-related compounds were determined by GC-MS. Data represent the mean of 3 experimental replicates. Error bars were omitted for clarity. Data were analyzed by two-way ANOVA with Bonferroni’s post-test. Summary statistics are shown in the figure.
2.7 Pancreatic lipase assay
We examined the impact of roasting on the in vitro PL inhibitory effects of cocoa using our previously described methods with modifications (Gu, et al., 2011). In brief, PL was suspended in deionized water by gentle mixing (1 mg/mL) and, after centrifugation for 5 min, the supernatant was collected and diluted 1:50 in Tris-HCl buffer (20 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 1.3 mM CaCl2) to produce a PL working solution. In a 96-well microplate, 2 volumes of PL working solution was combined with 1 volume of each cocoa extract (final conc. = 0 or 100 µg/mL), and 1 volume of 4-NPB (1 mM working solution) was added to start the enzyme reaction. After incubation at 37 °C for 10 min, the absorbance was determined at 400nm.
2.8 Statistical analysis
Each treatment was repeated in three independent experiments. All statistical analyses were carried out using GraphPad Prism (La Jolla, CA). Mean ± SD were calculated for TPC, flavan-3-ol, PAC, and aroma-related compound data. Statistical analysis was performed using two-way ANOVA with Bonferroni’s post-test. The impact of time, temperature and the interaction between these factors was examined. The time-dependent effects of roasting on PL inhibitory activity were analyzed by regression analysis.
3.0 RESULTS AND DISCUSSION
3.1 Changes in TPC and Monomeric Flavan-3-ols
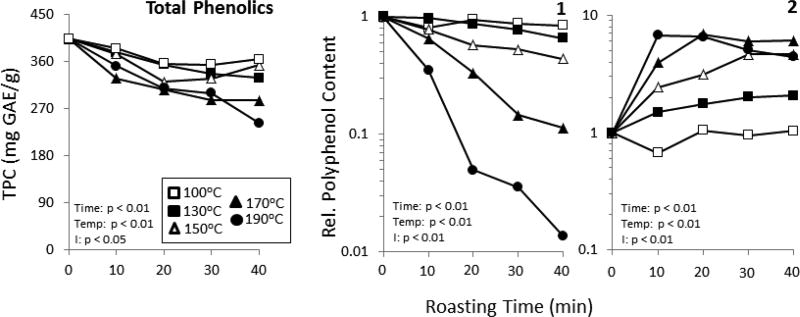
TPC in the cocoa beans was reduced by roasting (Fig. 2). Roasting for 40 min at 100°C, 130°C, 150°C, 170°C, and 190°C resulted in a decrease in total polyphenols of 9.7, 18.5, 12.5, 29.3, and 39.9%, respectively. With the exception of 190°C there were no significant effects of roasting time within temperatures. These results are consistent with previously reported trends for roasting effects on total polyphenols (Jolic, et al., 2011; Oliviero, Capuano, Cammerer, & Fogliano, 2009). The levels of 1 decreased in a time- and temperature-dependent manner (Fig. 2). Significant decreases were observed at oven temperatures of 130°C and greater. Roasting treatment at 190°C for 40 min resulted in the loss of 98.6% of EC compared to the unroasted beans. In contrast, content of 2 increased with roasting time and temperature (Fig. 2). This is consistent with previous literature demonstrating epimerization of 1 into 2 during roasting (Hurst, et al., 2011; Kothe, et al., 2013). All samples roasted at 150°C or greater developed significantly higher levels of 2 compared to unroasted beans. The greatest increase in the levels of 2 (590% compared to unroasted beans) was achieved after 20 min roast at 170°C. At 190°C, the levels of 2 were 675% higher than those in the unroasted control beans at 10 min, but levels then decreased as a function of time. This loss could be due to incorporation of 2 into PACs as has been shown in model systems (Dixon, Xie, & Sharma, 2005; Vidal, Cartalade, Souquet, Fulcrand, & Cheynier, 2002).
Figure 2.
The effect of roasting time and temperature on the total phenolic content in cocoa. Total phenolic content was estimated as gallic acid equivalents (GAE) determined using the Folin-Ciocalteu assay. Data represent the mean of 3 experimental replications. Error bars were omitted for clarity. Data were analyzed by two-way ANOVA with Bonferroni’s post-test. Summary statistics are shown in the figure.
3.2 Changes in PAC composition
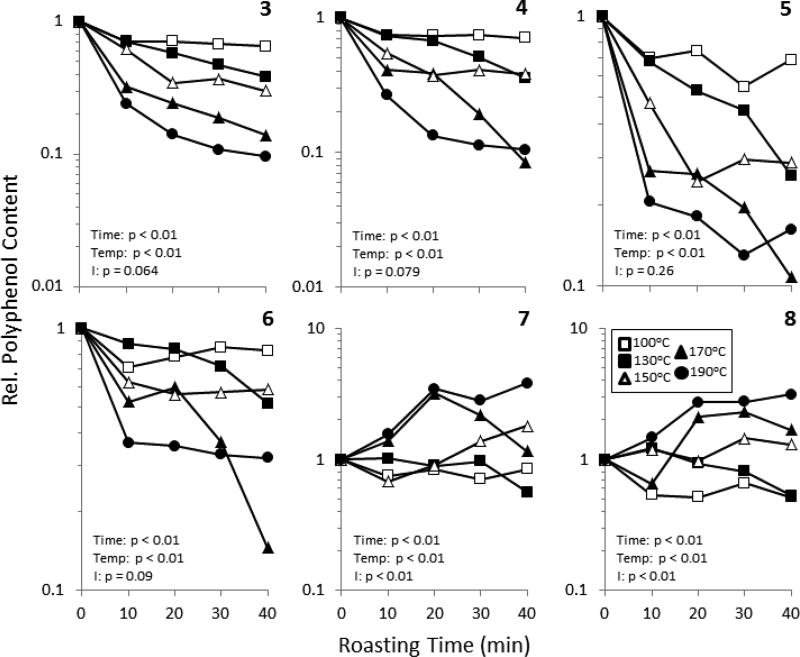
The levels of 3 – 6 were reduced in a time- and temperature-dependent fashion (Fig 3). Roasting at 150°C or greater significantly reduced the levels of these compounds compared to those in unroasted control beans, and roasting at 170°C for 40 min resulted in the greatest losses of 4 – 6 (85 – 92%). The heat-induced chemical changes in polyphenols are still not fully understood, however, the content of PACs is likely the result of the balance of degradation reactions and formation polymerization reactions (Dixon, et al., 2005). Interestingly, the levels of 7 and 8 increased as a function of roasting time and temperature (Fig. 3). These changes were significant at roasting temperatures greater than 150°C. For example, roasting at 190°C for 40 min increased 7 and 8 by 280% and 214%, respectively, compared to unroasted samples. The observed increase in 7 and 8, coupled with the observed decrease in 3 – 5, is consistent with a polymerization model in which PACs of a lower molecular weight polymerize with one another or flavan-3-ol monomers to form larger PACs.
Figure 3.
The effect of roasting time and temperature on the flavan-3-ol and proanthocyanidin content and composition of cocoa. The levels of each compound were determined by HPLC-ECD and normalized to the concentration of the content of the individual compound in unroasted cocoa. Experiments were repeated 3 times. Data represent the mean of 3 experimental replicates. Error bars were omitted for clarity. Data were analyzed by two-way ANOVA with Bonferroni’s post-test. Summary statistics are shown in the figure.
3.3 Impact of Roasting on In vitro PL Inhibitory activity
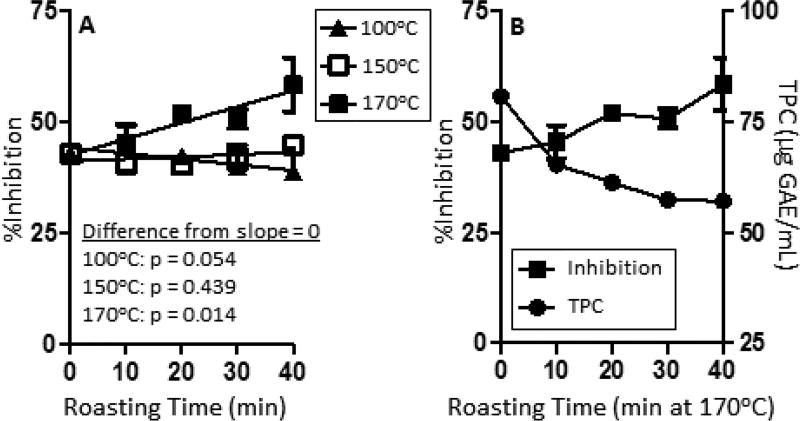
Previous research focused on monomeric flavan-3-ols, and compounds 3 and 4, have recommended roasting temperatures below 140°C to preserve the content of these compounds (Kothe, et al., 2013). Recent research, however, has reported that PACs with higher degree of polymerization (i.e. 7 and 8) may have more potent biological effects (Bitzer, et al., 2015; Bowser, Moore, McMillan, Dorenkott, Goodrich, Ye, et al., 2017; Dorenkott, et al., 2014). Given that our chemical analysis data indicate that certain roasting conditions increased compounds 7 and 8, whereas others reduced them, we examined the impact of roasting at 100°C, 150°C, and 170°C on in vitro PL inhibitory activity of cocoa extract (Fig. 4A). Roasting at 100°C tended to decreased PL inhibitory activity in a time-dependent fashion, but the effect did not reach statistical significance (p = 0.054). Roasting at 150°C had no effect on PL inhibitory activity. By contrast, PL inhibitory activity was increased by roasting at 170°C in a time-dependent fashion (35% increase in activity at roasting time = 40 min). A comparison of PL inhibitory activity and TPC content across roasting times at 170°C showed that whereas PL inhibitory activity increased with roasting time, TPC decreased (Fig. 4B). Taken together, the current data along with previous studies from our laboratory and others suggest that the identification of roasting conditions which maximize the levels of higher molecular weight PACs might impart greater biological functionality on the resulting cocoa for certain indications (Bitzer, et al., 2015; Bowser, et al., 2017; Dorenkott, et al., 2014). In addition, these results further confirm that process-induced in TPC may not accurately indicate changes in biological endpoints, therefore a more sophisticated understanding of the relationship between chemical composition and biological activity is needed.
Figure 4.
Impact of roasting on the in vitro PL inhibitory activity of cocoa extracts. (A) PL inhibitory activity of extracts (final concentration = 200 µg/mL) prepared for cocoa roasted at 100°C, 150°C, and 170°C for 0 – 40 min were determined using a commercially-available colorimetric assay. Each data point represents the mean of 3 experimental replicates. Error bars represent the SEM. The time-dependence of the data was analyzed by linear regression and the difference in the slope from 0 was determined using the F-test. (B) The time-dependent impact of roasting at 170°C on TPC and PL-inhibitory activity were plotted and revealed that whereas the former decreased the latter increased. Data represent the mean of 3 experimental replicates. Error bars were omitted for clarity.
3.4 Impact of Roasting on Aroma- and Taste-Related Compounds
The generation of aroma and flavor-related compounds is an important outcome of the roasting process. Using SPMEGC-MS, we quantified 25 volatile aroma compounds in cocoa. A representative set of 9 compounds are shown in Figure 5. The remaining 16 compounds are presented as Supporting Information (Supporting Figure 1) in order to maximize the clarity of the presentation. Compounds 9 – 13 increased as a function of roasting time and temperature (Fig. 5). These compounds are important aroma components of cocoa and been described as “nutty”, “roasted”, “caramel”, and/or “cocoa” in the literature (Afoakwa, Paterson, Fowler, & Ryan, 2009; Owusu, Petersen, & Heimdal, 2012; Rodriguez-Campos, et al., 2012). By contrast, the concentrations of 14, which has been described as “honey” or “fruity”, and 15, which is described as “almond”, decrease during roasting treatment.
Under the most extreme roasting conditions tested (170°C for 40 min and 190°C), 16 was detected. This compound has a “burnt” odor and bitter taste profile, and may have a deleterious effect on the sensory profile of the cocoa. Similarly, 17, described as “rubbery” and “onion”, was only identified in beans roasted at 150°C for 30 min or higher roasts (Owusu, et al., 2012). These findings indicate that some roasting treatments may negatively impact aroma.
Based on previously-reported odor threshold values, the changes induced by roasting at temperatures greater than 130°C should be noticeable to consumers (Frauendorfer & Schieberle, 2006). Although the results of these chemical analyses are interesting and suggest that it is possible to roast cocoa in such a way as to increase the levels of certain biologically-interesting polyphenols while maintaining a favorable aroma profile, cocoa aroma is extremely complex and contains more than 600 known volatile compounds. Consumer testing is therefore necessary to definitively characterize the consumer acceptability of the cocoa powders generated in the present studies.
4. CONCLUSIONS
Overall, our findings demonstrate that roasting at 150°C or higher results in cocoa containing elevated levels of 7 and 8 even as overall TPC and smaller PACs are significantly reduced. These changes in chemical composition were correlated to changes in an in vitro marker of obesity preventive biological activity (i.e. inhibition of PL) such that increases in 7 and 8 correlated with increased inhibitory activity. Based on these results and previous studies in our laboratory, it is desirable to optimize roasting conditions which maximize production of these compounds while preserving desirable sensory profile (Bitzer, et al., 2015; Bowser, et al., 2017; Dorenkott, et al., 2014; Gu, et al., 2011). Future studies are needed to elucidate the mechanism of PAC formation and polymerization during roasting, and to determine if cocoas containing higher levels of these compounds are acceptable to consumers.
Supplementary Material
Supplementary Figure 1. Impact of roasting on a panel of 16 additional sensory-related compounds in cocoa.
HIGHLIGHTS.
Whole bean roasting reduced total phenolic content and (−)-epicatechin in cocoa.
Roasting reduced levels of proanthocyanidin (PAC) dimers – pentamers in cocoa.
Roasting at 150°C or greater increased catechin and PAC hexamer and heptamer.
Roasting at 170°C increased pancreatic lipase (PL) inhibitory activity of cocoa.
Roasting can increase PL inhibitory activity and maintain desirable cocoa aromas.
Acknowledgments
FUNDING
This work was supported by a grant from the National Institutes of Health [grant number AT004678, by USDA Hatch Project [grant number 4565], and by the Silvio and Edith Crespo Award in Chocolate Research.
Abbreviations
- Cat
catechin
- CEAS
coulochem electrode array system
- DP
degree of polymerization
- EC
(−)-epicatechin
- ECD
electrochemical detection
- GC-MS
gas chromatography-mass spectrometry
- MSD
mass selective detector
- PAC
proanthocyanidin
- PL
pancreatic lipase
- SPME
solid phase microextraction
- TPC
total phenolic content
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
References
- Afoakwa EO, Paterson A, Fowler M, Ryan A. Matrix effects on flavour volatiles release in dark chocolates varying in particle size distribution and fat content using gc-mass spectrometry and gc-olfactometry. Food Chem. 2009;113(1):208–215. [Google Scholar]
- Arlorio M, Locatelli M, Travaglia F, Coisson JD, Del Grosso E, Minassi A, Appendino G, Martelli A. Roasting impact on the contents of clovamide (n-caffeoyl-l-dopa) and the antioxidant activity of cocoa beans (theobroma cacao l.) Food Chem. 2008;106(3):967–975. [Google Scholar]
- Beckett ST. Traditional chocolate making. In: Beckett ST, Fowler MS, Ziegler GR, editors. Industrial chocolate manufacture and use. 5. Hoboken, NJ: Wiley-Blackwell; 2017. pp. 1–9. [Google Scholar]
- Bitzer ZT, Glisan SL, Dorenkott MR, Goodrich KM, Ye L, O'Keefe SF, Lambert JD, Neilson AP. Cocoa procyanidins with different degrees of polymerization possess distinct activities in models of colonic inflammation. J Nutr Biochem. 2015;26(8):827–831. doi: 10.1016/j.jnutbio.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser SM, Moore WT, McMillan RP, Dorenkott MR, Goodrich KM, Ye L, O'Keefe SF, Hulver MW, Neilson AP. High-molecular-weight cocoa procyanidins possess enhanced insulin-enhancing and insulin mimetic activities in human primary skeletal muscle cells compared to smaller procyanidins. J Nutr Biochem. 2017;39:48–58. doi: 10.1016/j.jnutbio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Copetti MV, Iamanaka BT, Pitt JI, Taniwaki MH. Fungi and mycotoxins in cocoa: From farm to chocolate. Int J Food Microbiol. 2014;178:13–20. doi: 10.1016/j.ijfoodmicro.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Xie DY, Sharma SB. Proanthocyanidins--a final frontier in flavonoid research? New Phytol. 2005;165(1):9–28. doi: 10.1111/j.1469-8137.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- do Nascimento MD, Brum DM, Pena PO, Berto MI, Efraim P. Inactivation of salmonella during cocoa roasting and chocolate conching. Int J Food Microbiol. 2012;159(3):225–229. doi: 10.1016/j.ijfoodmicro.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Dorenkott MR, Griffin LE, Goodrich KM, Thompson-Witrick KA, Fundaro G, Ye L, Stevens JR, Ali M, O'Keefe SF, Hulver MW, Neilson AP. Oligomeric cocoa procyanidins possess enhanced bioactivity compared to monomeric and polymeric cocoa procyanidins for preventing the development of obesity, insulin resistance, and impaired glucose tolerance during high-fat feeding. J Agric Food Chem. 2014;62(10):2216–2227. doi: 10.1021/jf500333y. [DOI] [PubMed] [Google Scholar]
- Frauendorfer F, Schieberle P. Identification of the key aroma compounds in cocoa powder based on molecular sensory correlations. J Agric Food Chem. 2006;54(15):5521–5529. doi: 10.1021/jf060728k. [DOI] [PubMed] [Google Scholar]
- Gu Y, Hurst WJ, Stuart DA, Lambert JD. Inhibition of key digestive enzymes by cocoa extracts and procyanidins. J Agric Food Chem. 2011;59(10):5305–5311. doi: 10.1021/jf200180n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Yu S, Lambert JD. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. Eur J Nutr. 2014;53(1):149–158. doi: 10.1007/s00394-013-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Yu S, Park JY, Harvatine K, Lambert JD. Dietary cocoa reduces metabolic endotoxemia and adipose tissue inflammation in high-fat fed mice. J Nutr Biochem. 2014;25(4):439–445. doi: 10.1016/j.jnutbio.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst WJ, Krake SH, Bergmeier SC, Payne MJ, Miller KB, Stuart DA. Impact of fermentation, drying, roasting and dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chem Cent J. 2011;5:53. doi: 10.1186/1752-153X-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannone F, Di Mattia CD, De Gregorio M, Sergi M, Serafini M, Sacchetti G. Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (theobroma cacao l.) roasting as affected by temperature and time of processing. Food Chem. 2015;174:256–262. doi: 10.1016/j.foodchem.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Jolic SM, Redovnikovic IR, Markovic K, Sipusic DI, Delonga K. Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. Int J Food Sci Technol. 2011;46(9):1793–1800. [Google Scholar]
- Kothe L, Zimmermann BF, Galensa R. Temperature influences epimerization and composition of flavanol monomers, dimers and trimers during cocoa bean roasting. Food Chem. 2013;141(4):3656–3663. doi: 10.1016/j.foodchem.2013.06.049. [DOI] [PubMed] [Google Scholar]
- Miller KB, Hurst WJ, Flannigan N, Ou B, Lee CY, Smith N, Stuart DA. Survey of commercially available chocolate- and cocoa-containing products in the united states. 2. Comparison of flavan-3-ol content with nonfat cocoa solids, total polyphenols, and percent cacao. J Agric Food Chem. 2009;57(19):9169–9180. doi: 10.1021/jf901821x. [DOI] [PubMed] [Google Scholar]
- Monahan KD. Effect of cocoa/chocolate ingestion on brachial artery flow-mediated dilation and its relevance to cardiovascular health and disease in humans. Arch Biochem Biophys. 2012;527(2):90–94. doi: 10.1016/j.abb.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Oliviero T, Capuano E, Cammerer B, Fogliano V. Influence of roasting on the antioxidant activity and hmf formation of a cocoa bean model systems. J Agric Food Chem. 2009;57(1):147–152. doi: 10.1021/jf802250j. [DOI] [PubMed] [Google Scholar]
- Owusu M, Petersen MA, Heimdal H. Effect of fermentation method, roasting and conching conditions on the aroma volatiles of dark chocolate. J Food Process Preserv. 2012;36(5):446–456. doi: 10.1007/s13197-011-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne MJ, Hurst WJ, Miller KB, Rank C, Stuart DA. Impact of fermentation, drying, roasting, and dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients. J Agric Food Chem. 2010;58(19):10518–10527. doi: 10.1021/jf102391q. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Campos J, Escalona-Buendia HB, Contreras-Ramos SM, Orozco-Avila I, Jaramillo-Flores E, Lugo-Cervantes E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012;132(1):277–288. doi: 10.1016/j.foodchem.2011.10.078. [DOI] [PubMed] [Google Scholar]
- Rusconi M, Conti A. Theobroma cacao l., the food of the gods: A scientific approach beyond myths and claims. Pharmacol Res. 2010;61(1):5–13. doi: 10.1016/j.phrs.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Ryan CM, Khoo W, Stewart AC, O'Keefe SF, Lambert JD, Neilson AP. Flavanol concentrations do not predict dipeptidyl peptidase-iv inhibitory activities of four cocoas with different processing histories. Food Funct. 2017;8(2):746–756. doi: 10.1039/c6fo01730d. [DOI] [PubMed] [Google Scholar]
- Ryan CM, Khoo W, Ye L, Lambert JD, O'Keefe SF, Neilson AP. Loss of native flavanols during fermentation and roasting does not necessarily reduce digestive enzyme-inhibiting bioactivities of cocoa. J Agric Food Chem. 2016;64(18):3616–3625. doi: 10.1021/acs.jafc.6b01725. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticul. 1965;16:144–158. [Google Scholar]
- Stanley TH, Smithson AT, Neilson AP, Anantheswaran RC, Lambert JD. Analysis of cocoa proanthocyanidins using reversed phase high-performance liquid chromatography and electrochemical detection: Application to studies on the effect of alkaline processing. J Agric Food Chem. 2015;63(25):5970–5975. doi: 10.1021/acs.jafc.5b02661. [DOI] [PubMed] [Google Scholar]
- Vidal S, Cartalade D, Souquet JM, Fulcrand H, Cheynier V. Changes in proanthocyanidin chain length in winelike model solutions. J Agric Food Chem. 2002;50(8):2261–2266. doi: 10.1021/jf011180e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Impact of roasting on a panel of 16 additional sensory-related compounds in cocoa.