ABSTRACT
In the murine testis, self-renewal of spermatogonial stem cells (SSCs) requires glial cell line-derived neurotrophic factor (GDNF) secreted from neighboring somatic cells. However, it not clear how GDNF promotes self-renewal in vivo or what downstream signaling pathways are required for SSC maintenance. We found that GDNF is normally expressed cyclically during spermatogenesis. Stage-specific ectopic expression of GDNF caused the accumulation of a GFRA1+ LIN28− Asingle population, which has enhanced SSC activity compared with wild type, suggesting that GDNF normally limits self-renewal to specific stages. Despite the increase in SSC cell number, EdU labeling during steady-stage spermatogenesis, and during recovery after busulfan-mediated spermatogonial depletion, indicated that GDNF promotes self-renewal by blocking differentiation and not by promoting proliferation. Increased GDNF signaling led to increased phosphorylation of AKT3 in undifferentiated spermatogonia, but not of AKT1 or AKT2, and was independent of RPS6 phosphorylation, suggesting that AKT3 functions in SSC self-renewal or progenitor cell expansion.
KEY WORDS: Spermatogenesis, Spermatogonial stem cells, Glial cell line-derived neurotrophic factor, GDNF family receptor α1, GFRα1
Summary: Stage-specific overexpression of GDNF in mouse spermatagonial stem cells blocks their differentiation and promotes self-renewal, rather than promoting their proliferation.
INTRODUCTION
Spermatogonial stem cells (SSCs) are characterized by their capacity to differentiate and produce the entire spermatogenic lineage while maintaining self-renewal of the stem cell pool (de Rooij and Russell, 2000). Understanding what molecular signaling pathways control the balance between spermatogonial self-renewal and differentiation remains a major issue in mammalian germ cell biology. Crucial to this balance in the testis is crosstalk between SSCs and the somatic cells that neighbor them, but how this signaling is relayed molecularly or controlled in only some spermatogonia subtypes remains unclear.
Spermatogenesis is a complex but highly regulated process that occurs cyclically within the highly organized seminiferous epithelium. The twelve stages of the mouse seminiferous epithelium delineate which cell subtypes will be present in relationship to each other within a given region of the epithelium (Russell et al., 1990). Undifferentiated Asingle (As), Apaired (Apr) and Aaligned (Aal) spermatogonia are present at all 12 stages, but proliferate primarily in stages I-IV and XI-XII (Tegelenbosch and de Rooij, 1993). In tandem, during these proliferative stages, the As SSC population undergoes self-renewal. Through stages V-X, As, Apr and Aal, cells remain relatively quiescent, ultimately differentiating to A1 spermatogonia in response to a burst of retinoic acid (Hogarth et al., 2015) during stages VII-VIII, and dividing over subsequent cycles to A2-4, Intermediate and B spermatogonia, which proceed to undergo meiosis to form spermatocytes, spermatids and, finally, spermatozoa.
As, Apr and occasional small chains of Aal cells express GFRA1, the GPI-anchored receptor for glial cell line-derived neurotrophic factor (GDNF) (Nakagawa et al., 2010; Suzuki et al., 2009). GDNF is secreted by somatic Sertoli (Meng et al., 2000) and peritubular myoid (Chen et al., 2016) cells, and is required for establishment and self-renewal of the SSC population in a dose-dependent manner (Meng et al., 2000). A decrease in GDNF levels results in germ cell loss, whereas pan-ectopic overexpression of GDNF promotes seminomatous tumors (Meng et al., 2001). Moreover, culturing of mouse germline stem cells in vitro requires GDNF (Kanatsu-Shinohara et al., 2005, 2003b; Kubota et al., 2004) and addition of GDNF in vitro promotes proliferation and self-renewal by activating the phosphoinositide-3 kinase (PI3K)/AKT pathway (Lee et al., 2007). In contrast, in vivo, ERK1/2 signaling has been shown to support SSC self-renewal, whereas PI3K/AKT signaling has been suggested to support SSC proliferation during stages when RA signaling is low and differentiation when RA signaling is high (Hasegawa et al., 2013). Here, we investigate the mode of GDNF expression throughout the spermatogenic cycle and ask how disruption of its expression promotes SSC self-renewal and AKT-mediated signaling downstream of GFRA1/RET.
RESULTS
Gdnf is cyclically expressed during specific stages of spermatogenesis
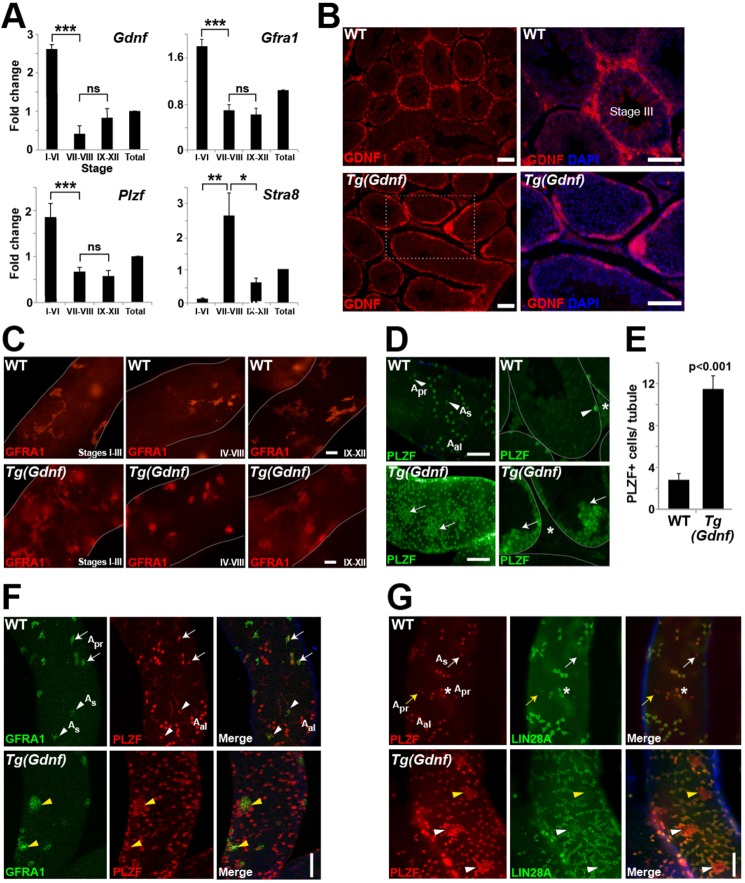
Because establishment of the SSC population and self-renewal of the stem cell pool rely on the secretion of GDNF by neighboring somatic Sertoli cells (Meng et al., 2000), we asked whether GDNF expression occurs during specific stages of the cycle of the seminiferous epithelium. Using transillumination microscopy (Fig. S1A) (Kotaja et al., 2004), we isolated stage I-VI, VII-VIII or IX-XII tubule fragments, collected RNA and performed quantitative RT-PCR (qRT-PCR) for Gdnf and markers of undifferentiated spermatogonia (Fig. 1A). Gdnf expression was significantly higher in stages I-VI compared with differentiation stages VII-VIII. This pattern was similar to that of the GDNF receptor Gfra1 and the undifferentiated spermatogonia marker Plzf. Conversely, the differentiating cell marker Stra8 showed reciprocal expression to these markers and was highest at stages VII-VIII when undifferentiated spermatogonia differentiate into A1 spermatogonia in response to retinoic acid (Hogarth et al., 2015). These results demonstrate that there is a GDNF gradient during spermatogenesis, with the highest expression levels coinciding with stages of undifferentiated spermatogonia expansion and self-renewal. This is consistent with previous studies reporting cyclical expression of Gdnf in rats, hamsters and mice (Grasso et al., 2012; Johnston et al., 2011; Sato et al., 2011; Tokue et al., 2017).
Fig. 1.
Stage-specific expression of GDNF increases the As SSC population. (A) qRT-PCR on seminiferous tubule RNA, staged by transillumination (Fig. S1A). Fold change is relative to gene expression within the total testis (arbitrary value 1). *P<0.01; **P<0.005; ***P<0.0005; ns, not significant. (B) GDNF immunostaining shows high, uniform expression in tubules of formalin-fixed sections of Tg(Gdnf) tubules. Boxed area in Tg(Gdnf) is enlarged on the right. DAPI stains nuclei. (C) GFRA1 immunostaining of whole-mount adult Tg(Gdnf) tubules shows high density of GFRA1+ cell clusters present at all stages. (D) Immunostaining in whole-mount tubules (left) or sections (right) shows large clusters of PLZF+ cells in Tg(Gdnf) mice (arrows) compared with wild type (arrowheads). Clusters are often present near interstitial spaces (asterisks). (E) Quantification of PLZF+ cells on 6-week-old testis sections (see Fig. S2A). Values represent mean PLZF+ cells±s.e.m. (n=3 per genotype). (F) In wild type, whole-mount tubule immunostaining shows co-expression of GFRA1 and PLZF in As (arrowheads) and Apr (arrows) spermatogonia. In Tg(Gdnf) testis, GFRA1+ cells cluster and co-express PLZF (yellow arrowheads). (G) Some Apr (asterisk) and all Aal chains co-express PLZF and LIN28A in wild-type whole-mount tubule immunostains. Some As (white arrows) and Apr (yellow arrows) cells do not express LIN28A. In Tg(Gdnf) tubules, the cores of PLZF-expressing clusters, show negative (yellow arrowheads) or reduced (white arrowheads) expression of LIN28A. See also Fig. S2. Scale bars: 100 µm.
Stage-specific ectopic expression of Gdnf increases the undifferentiated spermatogonia population
Previously, it was shown that pan-overexpression of GDNF in somatic cells and spermatogonia caused accumulation of undifferentiated spermatogonia in the testis (Meng et al., 2000). However, because GDNF is not endogenously expressed in germ cells, the cause of spermatogonia expansion was unclear. Because Gdnf expression is under cyclical control, we set out to express Gdnf during the stages when it is normally lowest, specifically in Sertoli cells. To do this, we designed a transgene placing the Sertoli and stage (VI-VIII)-specific rat Cathepsin L gene promoter (Charron et al., 2003) upstream of a cDNA encoding Gdnf fused to Gfp (Fig. S1B). Six independent transgenic lines were generated and confirmed for Gdnf-gfp transgene expression by RT-PCR (Fig. S1C), and a line with relatively high levels of Gdnf mRNA compared with wild type (WT) was selected for analysis (Fig. S1D).
To analyze GDNF expression in Tg(Ctsl-Gdnf)1Reb mice, referred to here as Tg(Gdnf), we immunostained formalin-fixed paraffin-embedded sections of wild-type and transgenic testes. In wild type, GDNF was expressed in a punctate pattern within Sertoli cells and at its highest levels in stage I-III tubules (Fig. 1B). In Tg(Gdnf) testes, GDNF was expressed highly and uniformly in most tubule stages (Fig. 1B).
Whole-mount immunostaining for the GDNF receptor GFRA1 revealed a greatly expanded cell population in Tg(Gdnf) tubules compared with wild type, with large clusters of tightly-packed GFRA1+ cells observed at all stages (Fig. 1C). To determine which populations of GFRA1+ spermatogonia were expanded, we immunostained sectioned and whole-mount tubules for PLZF, a protein essential for SSC maintenance and a marker of undifferentiated spermatogonia (Buaas et al., 2004; Costoya et al., 2004). At 6 weeks of age, PLZF was detected in all As, Apr and some Aal chains of spermatogonia distributed along the basal surface of wild-type whole-mount tubules (Fig. 1D, top). In Tg(Gdnf) tubules, large clusters of PLZF+ cells occupied the basal compartment (Fig. 1D, bottom left) and were found along the entire periphery of sectioned tubules (Fig. 1D, bottom right). Interestingly, regions of seminiferous tubules containing the large clusters of cells also stained strongly for GDNF (Fig. 1B, bottom right). Additionally, clusters were often close to interstitial spaces and projecting into the tubule lumen (Fig. 1D, asterisk and arrows). Quantification of PLZF+ cells on cross-sections (Fig. S2A) showed over a threefold increase in cell number in Tg(Gdnf) tubules compared with wild type (P<0.001, Fig. 1E).
As PLZF is normally expressed in the entire undifferentiated spermatogonia population (As, Apr and Aal), we asked whether specific subtypes were found in PLZF+ cell clusters. Immunostaining for GFRA1, primarily expressed in As, Apr and in smaller chains of Aal cells (Fig. 1F), showed that small GFRA1+ clusters were often localized within larger nests of cells that co-expressed PLZF, suggesting the PLZF+ nests represented a heterogeneous population of undifferentiated spermatogonia. The PLZF+ clusters were negative for SOHLH1, a differentiation marker that labels Aal and A1 spermatogonia (Fig. S2B) (Ballow et al., 2006). We also immunostained for LIN28A, a crucial regulator of the size of the undifferentiated spermatogonial pool (Chakraborty et al., 2014), and found that, in wild-type testes, LIN28A is expressed in a subset of As and Apr spermatogonia together with all Aal cells (Fig. 1G, white and yellow arrows). Interestingly, LIN28A expression was confined to cells at the periphery of Tg(Gdnf) PLZF+ clusters but rarely localized at the cores, suggesting the bulk of these clusters were made up of As cells (Fig. 1G, arrowheads). Taken together, stage-specific ectopic expression of GDNF in Sertoli cells increases the population of early GFRA1+ LIN28− As sub-types of undifferentiated spermatogonia.
SSC-specific genes are upregulated in Tg(Gdnf) testes
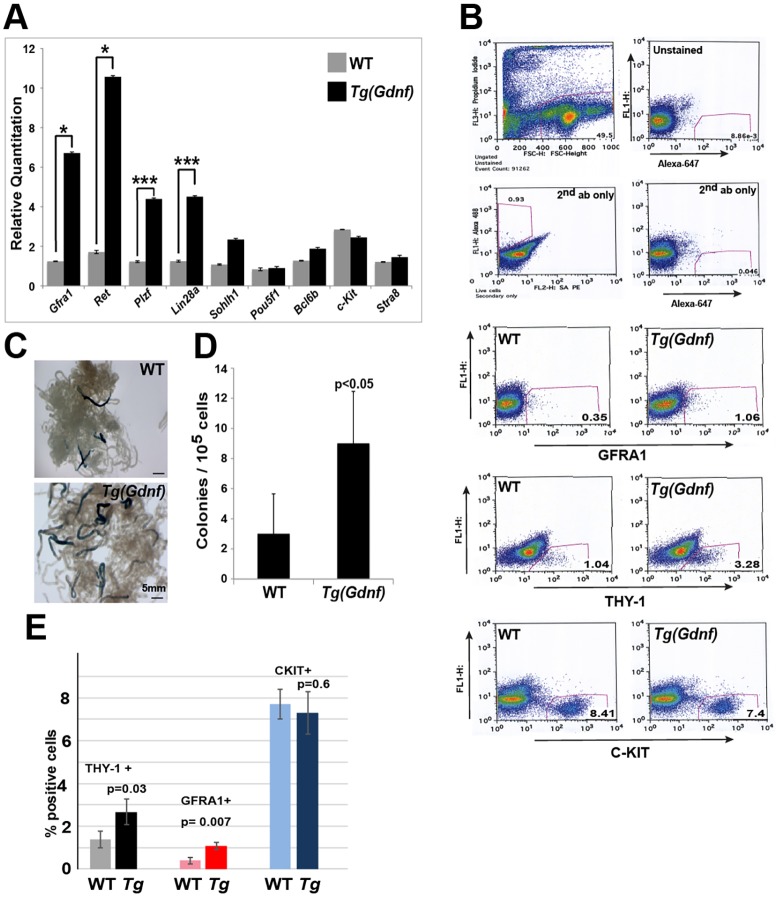
To further clarify what spermatogonia populations are affected by GDNF overexpression, we performed qRT-PCR on Tg(Gdnf) and wild-type testis RNA for a panel of undifferentiated and differentiated spermatogonia markers. Gfra1, Ret, Plzf (Zbtb16) and Lin28a were all significantly upregulated in Tg(Gdnf) testes, indicating an increase in transcripts marking the earliest subtypes of undifferentiated spermatogonia (Fig. 2A). Conversely, Sohlh1, Pou5f1, Bcl6b, Kit and Stra8 remained unchanged with GDNF overexpression, further suggesting that differentiating spermatogonia populations are unaffected (Fig. 2A). Despite the lack of changes in gene expression at 6 weeks of age, histological analysis showed that spermatogenesis eventually became impaired with age (Fig. S3).
Fig. 2.
GDNF increases SSC-specific gene expression and enhances colonization in testes transplants. (A) qRT-PCR from 6-week-old testis RNA showing a significant increase in undifferentiated SSC markers Gfra1, Ret, Plzf and Lin28a, but no changes in differentiation markers Kit or Stra8. mRNA levels were normalized to β-actin and mean±s.e.m. calculated in triplicate (n=3 per genotype). *P<0.05; ***P<0.0001. (B) FACScan analysis of Tg(Gdnf) testis shows increases in GFRA1 and THY1.2 fractions, but not the KIT fraction. (C) Seminiferous tubules in testes transplants repopulated with wild-type or Tg(Gdnf) donor cells marked by LacZ expression. (D) Quantification of LacZ-positive colonies from wild-type or Tg(Gdnf) donor cells in recipient testes 2 months post-transplantation. Values are the mean colony number per 105 donor cells±s.e.m. (three recipients per genotype). (E) Percentage of THY-1+, GFRA1+ and KIT+ cells in total testicular cell suspensions calculated from the FACS analysis in B. Scale bars: 100 µm.
To further clarify whether the undifferentiated spermatogonia population was increased in Tg(Gdnf) mice, we quantified the number of cells in 6-week-old testes expressing spermatogonia cell-surface markers by FACScan analysis. We observed a threefold increase in cell populations expressing GFRA1 and THY-1.2 in Tg(Gdnf) testes compared with wild type (Fig. 2B,E). In the same cell suspension, there was no change in the differentiated KIT population (Fig. 2B,E), suggesting that increased RNA expression of undifferentiated spermatogonia genes most likely reflects an increase in the cell population of undifferentiated spermatogonia.
Tg(Gdnf) testes have an increased functional SSC population
Previously, it was shown that GFRA1+ and THY-1.2+ As and Apr spermatogonia have a greater potential for repopulating spermatogonia in transplant assays (Kubota et al., 2003). To determine whether the increased GFRA1 and THY-1.2 spermatogonia pool found in Tg(Gdnf) testes had a similar potential, we transplanted cells from 6-week-old wild-type or transgenic testes into the efferent duct of busulfan-treated mice. In order to mark transplanted cells, Tg(Gdnf) mice were first crossed to ROSA26-LacZ mice and β-gal-positive colonies were counted 8 weeks after transplantation (Fig. 2C). A significant increase in the number of colonies was observed in mice that received cells from Tg(Gdnf) testes (Fig. 2D, P<0.05, n=3 per genotype), suggesting that overexpression of GDNF increases the number of transplantable SSCs.
Increased GDNF signaling does not alter spermatogonia proliferation
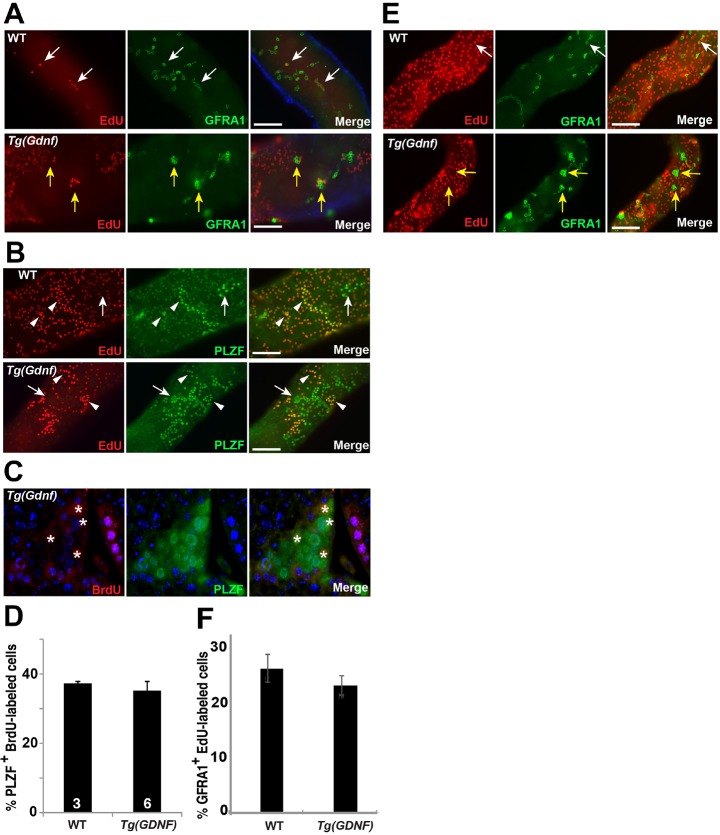
Endogenous cyclical expression of GDNF coincides with the period of greatest proliferation of Aundiff. However, it also overlaps with the stages in which Aundiff are maintained in the undifferentiated state. Conversely, GNDF levels are at their lowest when Aundiff transition into A1 spermatogonia in response to a burst of retinoic acid activity (Hogarth et al., 2015). We hypothesized that if GDNF is involved in promoting the proliferation of undifferentiated spermatogonia, then we should see an increase in the fraction of Aundiff in S phase in Tg(Gdnf) testes. To determine whether GDNF overexpression increases proliferation in vivo, we used EdU labeling to identify cells in S phase in wild-type and Tg(Gdnf) testes. Co-staining of whole-mount tubules 24 h after EdU injection showed that only a subset of GFRA1+ cells were labeled with EdU in both wild-type and in Tg(Gdnf) testes (Fig. 3A). Notably, the large clusters of GFRA1+ and PLZF+ cells found in Tg(Gdnf) tubules were rarely positive for EdU (Fig. 3A,B). Labeled cells were sometimes found on the periphery of these clusters, but not inside (Fig. 3B). In order to label and characterize a larger fraction of the proliferating population, we injected 8-week-old wild-type and Tg(Gdnf) mice with BrdU for four consecutive days and co-immunostained testis sections for PLZF (Fig. 3C). Quantification of BrdU+ PLZF+ cells revealed no significant difference between wild-type and Tg(Gdnf) tubules (Fig. 3D). We considered the possibility that the failure to see an increase in proliferation was because the density of the SSC population at the time we measured EdU incorporation had already reached a maximum autocrine-mediated set point. However, determination of the mitotic index of the GFRA1 population at P21, a time point when the population is still increasing, failed to reveal an increase in the proliferative index (Fig. 3E,F). We conclude that the increase in the As population observed in Tg(Gdnf) testes is not the result of increased cell proliferation, and that GDNF does not promote proliferation in vivo.
Fig. 3.
GDNF does not change spermatogonia proliferation. (A) Whole-mount tubules labeled with EdU and immunostained for GFRA1. White arrows indicate As and Aal co-labeled cells. Yellow arrows indicate EdU− GFRA1+ clusters in Tg(Gdnf) tubules. (B) Whole-mount tubules labeled with EdU and immunostained for PLZF. Arrows indicate EdU− PLZF+ Aal and Tg(Gdnf). Arrowheads indicate EdU+ PLZF+ Aal spermatogonia. (C) Testis section labeled with BrdU and immunostained for PLZF. Asterisks indicate BrdU+ cells within a large Tg(Gdnf) cluster. (D) The percentage of BrdU+ PLZF+ cells counted on wild-type and Tg(Gdnf) testis sections as in C, after 4 consecutive days of BrdU injection. Approximately 1000 PLZF cells were counted in randomly selected tubules from each animal (n shown in bars). Data are presented as mean±s.e.m. (E) Whole-mount tubules from P21 mice labeled with EdU and immunostained for GFRA1. White arrows indicate As and Aal co-labeled cells. Yellow arrows indicate EdU− GFRA1+ clusters in Tg(Gdnf) tubules. (F) Percentage BrdU+ GFRA1+ cells counted on wild-type and Tg(Gdnf) testis sections as in E 1 day after BrdU injection. While 27% of GFRA1+ cells (∼600 cells) had incorporated EdU in wild type, only 24% of GFRA1+ were positive (∼1500 cells counted) for EdU in Tg(Gdnf) testis. Data are presented as mean±s.e.m. Scale bars: 100 μm.
Spermatogonia in Tg(Gdnf) testes regenerate more slowly after injury
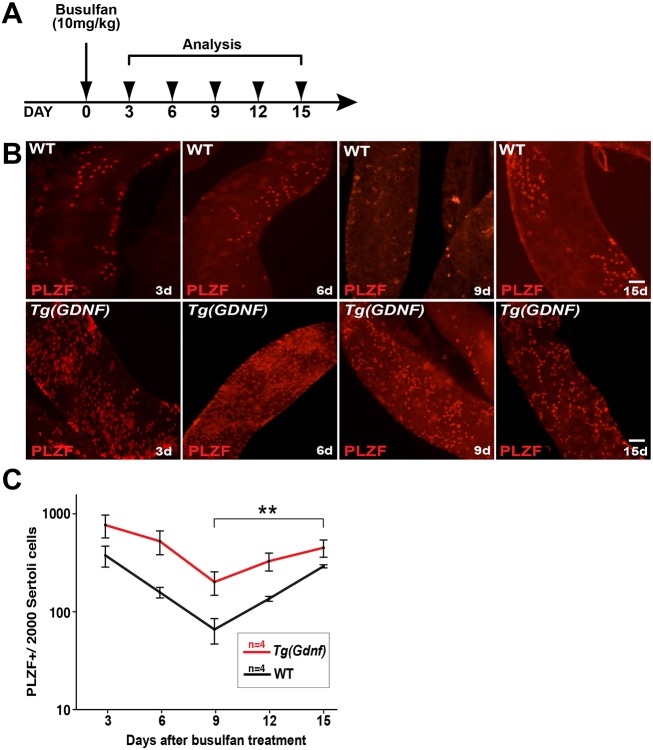
To further test our observation that overexpression of GDNF does not increase the proliferative rate of Aundiff, we used busulfan to kill proliferating spermatogonia in wild-type and Tg(Gdnf) testes, and then analyzed the kinetics of spermatogonial recovery post-injury. If GDNF promotes spermatogonial proliferation, we hypothesized that recovery of the SSC population should be increased following busulfan-induced depletion. Eight-week-old mice were injected with busulfan and whole-mount tubules harvested and immunostained for PLZF every 3 days after injection (Fig. 4A). In wild type, Aal8 and Aal16 cells were observed three days post-injection, but spermatogonia were progressively lost until day 9, when only a few As cells remained (Fig. 4B). Although it was difficult to identify specific chains in the Aal population in Tg(Gdnf) tubules due to the high number of cells, a decrease in the total PLZF+ cell population was clear (Fig. 4B).
Fig. 4.
PLZF+ cells recover more slowly following busulfan treatment. (A) Eight-week-old wild-type and Tg(Gdnf) mice were injected with 10 mg/kg body weight of busulfan and analyzed 3, 6, 9, 12 and 15 days post-injection. (B) Immunostaining of whole-mount tubules stained for PLZF at 3, 6, 9 and 15 days post-injection. PLZF+ Aal chains were sensitive to busulfan, and by day 9, only As cells were present in wild-type testes. (C) Quantification of PLZF+ cells post-busulfan injection in wild-type and Tg(Gdnf) mice. Values are normalized to Sertoli cells and presented as a semi-log graph. The P values of the slopes were determined using log transformation of the total number of cells to stabilize the variance (performed using JMP 7.0 program). 3-9 days slope, P=0.06; 9 days-15 days slope P=0.0017 (**P<0.005). Scale bars: 100 µm.
During the period of cell loss, from days 3 to 9, we found that the rate of PLZF+ cell loss was equivalent between wild-type and Tg(Gdnf) testes (slope P=0.06, Fig. 4C). However, analysis of stem cell recovery from days 9 to 15 showed that the number of PLZF+ cells in Tg(Gdnf) testes increased at a significantly slower rate than in controls (slope P=0.0017, Fig. 4C), supporting the conclusion that GDNF does not promote proliferation of SSCs.
AKT activation in spermatogonia
Aberrant activation of mTORC1 is detrimental to stem cell maintenance (Castilho et al., 2009) and previous studies had suggested a role for mTORC1 in SSC dynamics (Hobbs et al., 2015, 2010). Because overexpression of GDNF increases the number of undifferentiated spermatogonia and eventually causes loss of spermatogenesis (Fig. S3) (Meng et al., 2000), we asked whether mTORC1 signaling was altered in Tg(Gdnf) testes. We first compared expression of the gene family members encoding the ribosomal protein S6 kinase (RPS6) proteins Rps6ka1 and Rps6kb2 using RNA-seq from testes of 8-week old animals (Fig. 5A). Both transcripts were downregulated in Tg(Gdnf) testes, with Rps6ka1 downregulated more than threefold. To assay RPS6 phosphorylation directly, we immunostained whole-mount tubules with a phospho-specific antibody (Ser240/244). In both wild-type and Tg(Gdnf) tubules, we observed occasional GFRA1+ As cells that contained phospho-RPS6 (arrows, Fig. 5B). However, we found that none of the large GFRA1+ clusters in Tg(Gdnf) tubules was positive for phospho-RPS6 staining (asterisks, Fig. 5B). Proliferating PLZF+ As, Apr and Aal cells were also negative for active mTORC1 signaling in wild-type or Tg(Gdnf) mice within stages I-VIII (Fig. 5B, Fig. S4A), although some Aal PLZF+ differentiating spermatogonia were positive in stage X-XII tubules (Fig. S4A). The only spermatogonia in wild-type or Tg(Gdnf) tubules that were consistently labeled with phospho-RPS6 also expressed KIT, a marker of differentiated spermatogonia (Fig. S4B).
Fig. 5.
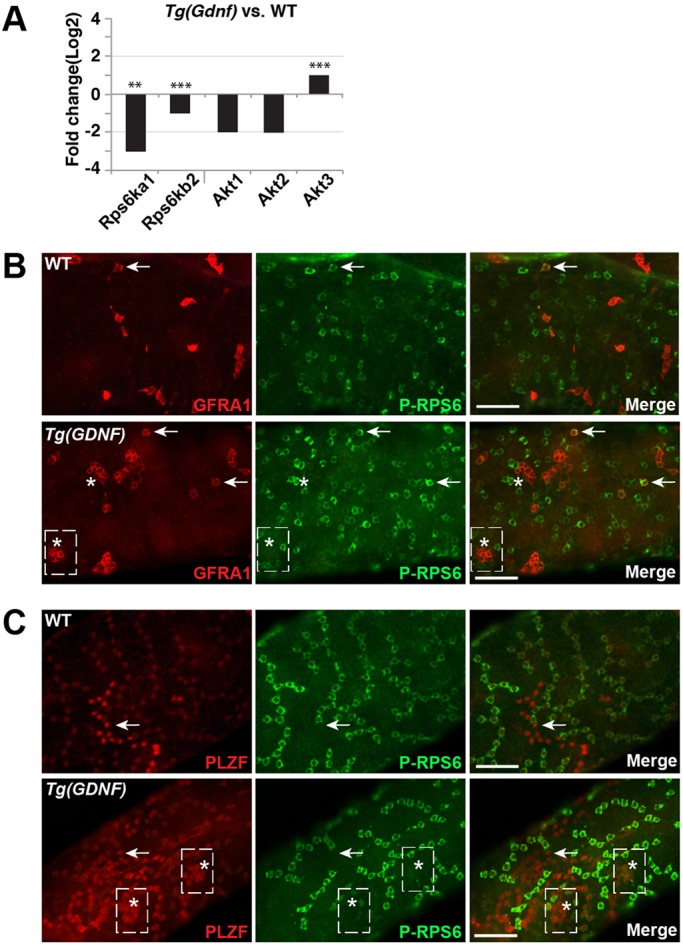
mTORC1 signaling is not activated in Tg(Gdnf) GFRA1+ PLZF+ clusters. (A) Transcript fold-change in read counts per million using RNA-seq on extracts from 4-month-old testis. Shown is the change in Tg(Gdnf) compared with wild type. (B) Whole-mount tubules stained for GFRA1 and phosphorylated RPS6, an indicator of mTORC1 activation. Arrows indicate GFRA+ P-RPS6+ spermatogonia. Asterisks indicate Tg(Gdnf) clusters that do not label for P-RPS6. (C) Whole-mount tubules stained for PLZF and phosphorylated RPS6. Arrows indicate PLZF+ Aal cells that do not label for P-RPS6. The boxes indicate clusters. The asterisks indicate cells within the clusters that do not label for P-RPS6. See also Fig. S4. Scale bars: 100 µm.
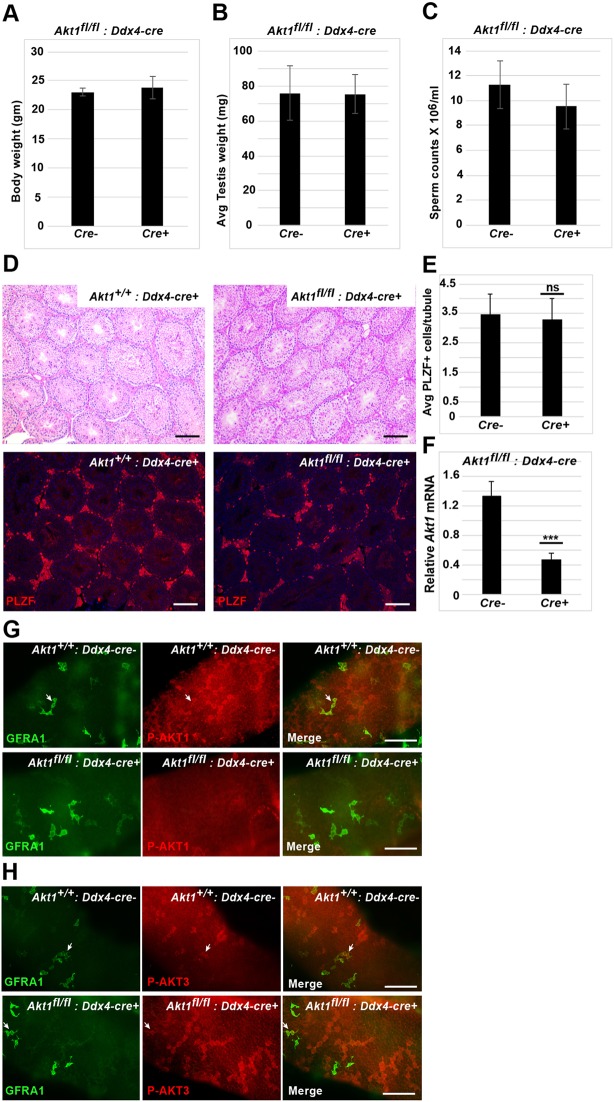
Previous studies have implicated PI3K/AKT activation downstream of GDNF signaling in SSC self-renewal in vitro (Lee et al., 2007) and in vivo (Goertz et al., 2011). To determine whether GDNF controls AKT1 phosphorylation in vivo, we probed wild-type and Tg(Gdnf) testes with an antibody specific for P-AKT1. We did not observe phosphorylation of AKT1 in most GFRA1+ and PLZF+ cells in wild-type or Tg(Gdnf) mice, nor did we see P-AKT1 in Tg(Gdnf) clusters (Fig. 6A, Fig. S5A). Rather, the bulk of P-AKT1 was observed in differentiating spermatogonia expressing KIT, similar to phospho-RPS6 (Fig. S5B), and consistent with AKT activation playing a role in spermatogonial differentiation (Blume-Jensen et al., 2000). Because genetic studies have suggested a role for AKT1 in the testis (Chen et al., 2001), we conditionally deleted Akt1 by generating Akt1fl/lf; Tg(Ddx4-Cre) animals. Mice lacking AKT1 in the germ line had normal testis weight, normal histology and no change in the PLZF-positive population (Fig. 7A-G). Taken together, we conclude that overexpression of GDNF does not activate mTORC1 signaling in the self-renewing spermatogonia population through P-AKT1, and that AKT1 itself is not required for spermatogenesis under steady-state conditions.
Fig. 6.
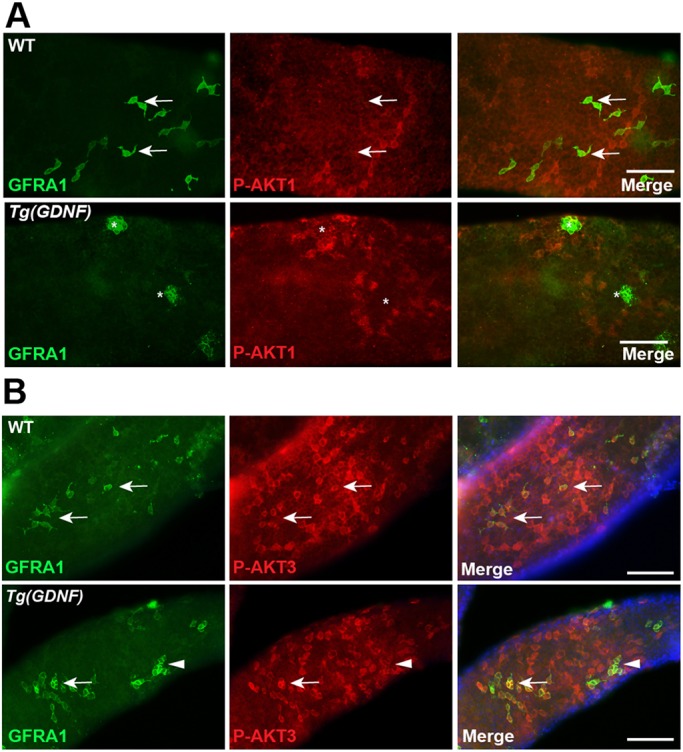
AKT1 and AKT3 are phosphorylated in different types of spermatogonia. (A) Whole-mount wild-type or Tg(Gdnf) tubules co-immunostained for phosphorylated AKT1 Ser473 (P-AKT1) and GFRA1. GFRA+ cells in wild-type testes (arrows) and large clusters of GFRA+ cells in Tg(GDNF) testes (asterisks) are P-AKT1 negative. (B) Whole-mount wild-type or Tg(Gdnf) tubules co-immunostained for phosphorylated AKT3 Ser472 (P-AKT3) and GFRA1. Arrows indicate GFRA1+ P-AKT3+ As and Apr spermatogonia. Arrowheads indicate GFRA1+ P-AKT3+ cluster in Tg(Gdnf) tubules. See also Figs S5 and S6. Scale bars: 100 µm.
Fig. 7.
Effect of germ cell-specific deletion of Akt1 on germ cell development. (A-C) No significant change in body weight (A), testis weight (B) or sperm count (C) in 2-month-old mice when Akt1 is deleted in germ cells (n=3). (D) Paraffin wax-embedded sections of testis from control and mutant testis stained with PSA and probed with PLZF antibody. (E) Average PLZF+ cells per tubule (200-250 tubules counted) in control and mutant testis (n=3). (F) Akt1 transcript is significantly (***P=0.002) downregulated in mutant testis (n=3) as determined by qRT-PCR. (G) Absence of P-AKT1 staining in the mutant germ cells. Arrows indicate GFRA1+ P-AKT1+ (double-positive) cells. (H) Detection of P-AKT3 in Akt1-deleted germ cells. Arrows indicate GFRA1+ P-AKT3+ (double-positive) cells. Scale bars: 100 µm.
Because there are three AKT isoforms in mammals, we asked whether there were differences in Akt gene expression levels in Tg(Gdnf) testes extracts. Unexpectedly, whereas Akt1 and Akt2 were downregulated in Tg(Gdnf) extracts compared with wild type, Akt3 was upregulated (Fig. 5A). Using an antibody specific for phosphorylated AKT3 (S472), we observed both cytoplasmic and nuclear P-AKT3 present in GFRA1+ cells in both wild-type and Tg(Gdnf) tubules, including induced As clusters (Fig. 6B). Furthermore, P-AKT3 was also co-expressed with LIN28A in stage VII-VIII tubules (Fig. S6A), and was not detected in differentiated KIT+ cells (Fig. S6). Staining was still detected in Akt1fl/lf; Tg(Ddx4-Cre) testes, confirming the specificity of the antibody for P-AKT3 (Fig. 7H). These results suggest that AKT3 phosphorylation is specific to self-renewing cells and progenitor cells, suggesting a potential role for AKT3 in undifferentiated spermatogonia.
DISCUSSION
Cyclic GDNF expression is required for SSC homeostasis
SSCs reside in the basal compartment along the basement membrane and beneath Sertoli-cell tight junctions (Smith and Braun, 2012). Each Sertoli cell supports a characteristic number of germ cells (Russell and Peterson, 1984), partly through secretion of GDNF, which is necessary for SSC self-renewal in vivo and in vitro (Kanatsu-Shinohara et al., 2005, 2003a; Meng et al., 2000; Naughton et al., 2006). We show here that GDNF levels are highest during stages of SSC proliferation (X-III), and lowest during the stages in which spermatogonia are quiescent and eventually differentiate into A1 spermatogonia (IV-IX). Our findings are consistent with previous studies that also documented cyclical expression of GDNF during spermatogenesis in several species (Grasso et al., 2012; Johnston et al., 2011; Sato et al., 2011; Tokue et al., 2017) and its regulation by RA-induced signaling in Sertoli cells (Hasegawa et al., 2013). Expression of GDNF in stages VI-VIII in Tg(Ctsl-Gdnf) mice resulted in constitutive expression across all stages of the cycle, disrupting the balance between self-renewal and differentiation, and demonstrating the importance of cyclical GDNF expression in maintaining SSC homeostasis.
High expression of GDNF during proliferative stages is consistent with its role in SSC self-renewal, but it was unclear whether the cyclical nature of Sertoli cell expression was crucial for spermatogenesis. Previously, GDNF was overexpressed throughout all somatic and germ cells of transgenic testes using the EF-1α promoter (Meng et al., 2000). Large clusters of undifferentiated spermatogonia were observed within 3 weeks of age that disappeared by 10 weeks to give way to atrophic tubules devoid of sperm. However, autocrine germ cell overexpression of GDNF made it difficult to discern whether these phenotypes reflected the endogenous role of GDNF in the testis. In our experiments, we overexpressed GDNF specifically in Sertoli cells during the differentiation stages when endogenous GDNF is lowest – which in turn eliminated its cyclic expression. Similar to Meng et al., we observed large clusters of undifferentiated spermatogonia, suggesting that the reported phenotype was indeed because of Sertoli cell regulation by GDNF. Using a panel of markers unavailable in the previous work, we identified these clusters as groups of GFRA1+ As cells, a population considered to contain SSCs (Chakraborty et al., 2014; Hara et al., 2014; Nakagawa et al., 2010), and a population that had elevated stem cell colonization potential compared with wild type. Of note, these cells only rarely expressed LIN28A, furthering the notion that LIN28A is expressed in a subpopulation of As cells and in Apr and Aal cells (Chakraborty et al., 2014). Unlike in Meng et al., transgenic Ctsl-Gdnf mice have differentiated germ cells for the first few spermatogenic cycles, although spermatogenesis is halted by 6 months of age. This difference could be caused by differences in the levels of expression of GDNF in the two studies or, more likely, could reflect the consequences of ectopic expression of GDNF in germ cells in the previous work.
GDNF drives SSC self-renewal by inhibiting SSC differentiation and not by promoting proliferation
GDNF is required for germ cell self-renewal both in vitro and in vivo. However, it was unclear whether GDNF activation promotes proliferation of SSCs or blocks their differentiation. Here, we show that stage-specific ectopic expression of GDNF in the testis did not change the rate of proliferation of the GFRA1+ or GFRA1+ PLZF+ self-renewing population. Furthermore, high levels of GDNF resulted in increased numbers of GFRA1+ LIN28− As cells in transgenic testes, despite proliferating at similar rates to wild type. These results are consistent with a role for GDNF in maintaining SSC self-renewal by blocking differentiation into later spermatogonia cell types.
Treating Tg(Gdnf) mice with busulfan did not change the rate of spermatogonia cell loss during the cell death period. This was not surprising, as busulfan kills proliferating cells, present in similar numbers in both Tg(Gdnf) and wild type. However, we observed a significant lag in the ability of the Tg(Gdnf) stem cell compartment to self-renew during the regenerative period. This too is consistent with GDNF not promoting SSC proliferation, as we would expect a faster recovery than in wild type.
Previous studies have suggested that GDNF promotes SSC proliferation. Addition of GDNF to GC cultures induces long-term proliferation in vitro (Lee et al., 2007) and deletion of Gfra1 in vivo decreases phosphorylated H3 detection in SSCs (Hasegawa et al., 2013). GC cultures are inherently heterogeneous and may not reflect GDNF action in vivo. It is possible that, in wild-type animals, GDNF normally promotes proliferation. If so, abolishment of GDNF signaling by deletion of Gfra1 would be predicted to decrease SSC proliferation at those stages, as previously observed (Hasegawa et al., 2013). GDNF levels are normally at their lowest during the stages in which Aal spermatogonia differentiate into A1 spermatogonia. It is possible that, in wild-type animals, the reduction in GDNF signaling during these stages is required for RA-induced differentiation, and that in Tg(Gdnf) mice, GFRA1+ LIN28− cells accumulate because they are resistant to RA-induction. This model is consistent with previous data showing that GFRA1+ cells are not able to respond to RA as efficiently as NGN3+ cells, and that ectopic expression of RARγ in GFRA1+ cells confers the ability to directly differentiate into KIT+ cells in response to RA (Ikami et al., 2015). It is also consistent with the suggestion that MEK/ERK signaling downstream of GFRA1/RET antagonizes RA signaling and inhibits differentiation (Hasegawa et al., 2013).
AKT signaling
The signaling pathway(s) downstream of GFRA1/RET in SSCs has yet to be fully elucidated. In vitro, addition of GDNF to GC cultures rapidly induces phosphorylation of AKT, whereas inhibition of MAPK signaling has little effect on culture dynamics (Lee et al., 2007). In contrast, in vivo studies have implicated MEK/ERK signaling in SSC self-renewal (Hasegawa et al., 2013). Reduction of MEK/ERK signaling in germ cells results in downregulation of Gdnf/Ret mRNA and upregulation of differentiation markers, suggesting that MEK/ERK promotes SSC self-renewal by blocking differentiation.
The role of PI3K/AKT and mTORC1 activation in SSC self-renewal and differentiation in vivo has been previously studied. PI3K/AKT signaling can induce mTORC1 activation through inhibition of TSC protein inhibitors, and a recent report shows that Tsc2, an inhibitor of mTORC1 activation downstream of P-AKT, is required specifically in SSCs to prevent mTORC1 activation (Hobbs et al., 2015). Furthermore, blocking AKT phosphorylation using a PI3K inhibitor prevents GC culture self-renewal (Lee et al., 2007), and reduction in PI3K/AKT signaling suppresses differentiation (Hasegawa et al., 2013). Consistent with this, deletion of Pdk1, which is required for activation of AKT, causes a block in differentiation (Goertz et al., 2011).
Given the numerous studies suggesting a role for PI3K/AKT signaling in SSC and progenitor cell dynamics, we examined whether there was a correlation between PI3K/AKT and mTORC1 activation in the testis of Tg(Gdnf) mice. Our observation that phosphorylation of RPS6 does not occur in most GFRA1+ or PLZF+ undifferentiated spermatogonia, but is present in differentiated KIT+ cells, is consistent with previously reported studies (Hobbs et al., 2015). However, another marker of mTORC1 activation, phosphorylated 4EBP, is detected in LIN28A+ progenitors (Hobbs et al., 2015), and previous studies have suggested that PLZF is required for inhibition of mTORC1 activation in SSCs (Hobbs et al., 2010), suggesting that mTORC1 is active in progenitor cells.
Previous studies have implicated a requirement for Akt1 in spermatogenesis (Chen et al., 2001). Although males were fertile, a whole-body knockout of Akt1 resulted in reduced sperm counts and increased germ cell apoptosis. Our failure to detect P-AKT1 in undifferentiated spermatogonia, and the absence of a spermatogenic phenotype of Akt1fl/fl; Ddx4-Cre mice, strongly suggest that AKT1 does not couple with GFRA1/RET in undifferentiated spermatogonia, suggesting that mutation of Akt1 outside of the germline may be responsible for the previously observed phenotype.
Interestingly, Akt3 is upregulated in Tg(Gdnf) testes and we consistently observed both cytoplasmic and nuclear P-AKT3 in GFRA1+ cells and in LIN28A+ progenitor cells. Because GFRA1+ cells do not have activated mTORC1, this suggests that P-AKT3 is not sufficient to induce mTORC1 activation in SSCs. However, P-AKT3 signaling may play a role in LIN28A+ progenitor cells. Consistent with this possibility, mice carrying a single allele of Akt1, and lacking both alleles of Akt2 and Akt3 (Akt+/−; Akt2−/−; Akt3−/−), have reduced testis size (Dummler et al., 2006). Given the absence of a phenotype in Akt1fl/fl; Ddx4-Cre mice, no reported phenotype in Akt2−/− mice (Cho et al., 2001; Garofalo et al., 2003) and the presence of P-AKT3 in GFRA1+ and LIN28A+ cells, we suggest that AKT3 plays the major role in SSCs and its progenitors, and it is possible that AKT is coupled to mTORC1 activation (Hobbs et al., 2015). Studies show that the three AKT isoforms can perform distinct functions despite having similar structures (Altomare and Testa, 2005) and that AKT3 can function in the nucleus to promote cell cycle progression and cell survival among other functions (Martelli et al., 2012). Notably, AKT3 (and not AKT1 or AKT2) protects cells from irradiation and chemically induced DNA damage in vitro (Turner et al., 2015), which is an important consideration in any stem cell niche. Future studies will be focused on distinguishing the role of AKT3 in SSC self-renewal and/or progenitor cells.
A model for GDNF signaling in SSCs
GDNF is expressed cyclically in Sertoli cells and is at its highest during the stages when SSCs self-renew where it specifically acts on the GFRA1+ LIN28− As pool (X-IV, Fig. 6C). We propose that instead of controlling proliferation of SSCs during these stages, GDNF acts to promote self-renewal by inhibiting LIN28− As cells from differentiating into LIN28+ As, Apr and Aal spermatogonia. In Tg(Gdnf) mice, GDNF is expressed throughout all stages of the spermatogenic cycle, blocking cells from differentiating into LIN28+ cell types. LIN28− As cells continue to proliferate at their normal rate and accumulate because of their reduced ability to differentiate into transit amplifying Aundiff spermatogonia. Notably, LIN28− As SSCs remain close together in Tg(Gdnf) clusters, and can be found close to the interstitial region as previously reported for SSCs (Chiarini-Garcia and Russell, 2002; Yoshida et al., 2007), suggesting that they may also have an inability to migrate away from the stem cell niche in order to continue the cycle of spermatogenesis. Further studies will be required to understand what limits GDNF expression to specific stages during spermatogenesis and what transcriptional effectors lie downstream of GDNF-GFRA1-AKT3 signaling in SSCs.
If GDNF is not responsible for regulating SSC proliferation, then what is? The busulfan challenge experiment suggests that SSCs regulate their own density. During the recovery period (days 9-15), the rate of proliferation of PLZF+ spermatogonia was statistically less than in control animals (Fig. 4C). At day 9, when spermatogonia numbers were at their lowest in both groups of animals, they were still significantly higher in Tg(Gdnf) mice than in controls. One explanation for this is that SSCs communicate with their neighbors to regulate their density through secretion of an unknown chalone: the greater the number of SSCs, the higher the concentration of chalone and the lower the proliferative rate. Alternatively, SSCs could be sensing and degrading a mitogen coming from the somatic environment. A greater number of SSCs would dampen the mitogenic signal and decrease the proliferative rate. Further studies are needed to distinguish between these competing hypotheses.
MATERIALS AND METHODS
Animals
All mice were maintained in a barrier facility at the Jackson Laboratory, and experimental procedures were approved by the Jackson Lab Institutional Animal Care and Use Committee (ACUC). The Gdnf cDNA gene was cloned in frame with Egfp from the Pem212 clone provided by Dr Wilkinson (UCSD, CA, USA), where the hMT2A intron 1 was inserted into the Egfp-coding region at nucleotides 898 (Fig. S1B) (Rao et al., 2002). The Pem promoter was replaced by the Cathepsin L promoter KpnI-SalI fragment from a luciferase reporter construct gifted from Dr Wright (Johns Hopkins School of Medicine, Baltimore, MD, USA) (Charron et al., 2003). Transgenic mice were generated in the B6.C3H.F1 mouse strain by pronuclear injection. Founders were genotyped using the primer sets Cath-2831 (seq 5′-CACATGCAAAGATGCTTCAACTCCAA-3′) and Gdnf-97R (seq 5′-CGGGCGCTTCGAGAAGCCTCTTAC-3′). One of six characterized transgenic lines was used for further analysis.
Antibodies and whole-mount immunostaining
Antibodies used were: mouse PLZF (Santa Cruz Biotech); rat GFRA1 (R&D Systems); rat LIN28A (a gift from Dr Eric G. Moss, UMDNJ, NJ, USA); rabbit SOHLH1 (Abcam); rabbit phospho-S6 (Ser240/244; #2215) and rabbit phospho-AKT1 (S473; D7F10) (Cell Signaling Technology); rabbit phospho-Akt3 (S472, #AP3468a) (Abgent); and mouse KIT (#CBL1360, Millipore) all at 1:200. Seminiferous tubules were dispersed in PBS by removing the testis tunica, rinsed with PBS and fixed in 4% paraformaldehyde (Electron Microscopy Sciences) for 4-6 h at 4°C. Tubules were permeabilized with 0.25% NP-40 in 0.05% Tween in PBS (PBST) for 25 min at room temperature before blocking in 5% normal goat serum and then incubated with primary antibodies overnight at room temperature or 4°C. The next day, tubules were washed three times with PBST and incubated with secondary antibodies conjugated to Alexa Fluors (Molecular Probes) for 1 h at room temperature. After washing in PBST, the tubules were mounted in VectaShield with DAPI (Vector Laboratories) and imaged using a Nikon Eclipse E600 epifluorescence microscope equipped with an EXi Aqua Camera from Q-Imaging.
Histology and immunohistochemistry
Testes were fixed in Bouin's solution overnight at 4°C, rinsed in water and dehydrated for paraffin wax embedding. For histological analysis, 5 µm sections were cut and stained with Periodic acid–Schiff. For immunohistochemistry, sections were dewaxed in xylene and rehydrated through a series of graded alcohols. Slides were boiled in 10 mM sodium citrate (pH 6.0) for 10 min for antigen retrieval. The sections were blocked in 5% normal goat serum for 1 h at room temperature. Antibodies were diluted in 3% normal goat serum in PBST and incubated overnight at 4°C. After washing in PBS, sections were incubated with conjugated secondary antibodies and mounted in Vectashield medium with DAPI.
Stage dissection
Whole-mount tubules were rinsed in ice-cold PBS and pulled apart carefully using fine forceps, then viewed under a transilluminating dissection microscope. The greater the level of spermatid chromatin condensation, the greater the amount of light absorbed, resulting in the differential appearance of tubule segments based on the stage of spermatogenesis. Weak spots represent stages X-I, strong spots stages II-VI and dark zones stages VII-VIII (Fig. S1A) (Nakagawa et al., 2010).
RNA extraction, RT-PCR and qRT-PCR
Total RNA was extracted from 6-week-old or older mouse testes using a Total RNA extraction kit (Ambion, Austin, TX). For the transgene transcript, the Access RT-PCR kit from Promega was used and the β-actin RT-PCR product was a loading control. Real-time quantitative PCR was performed using the Applied Biosystems 7500 Real-Time PCR system using SYBR green. Primer sets for q-RT-PCR are available upon request. Equal amounts of total RNA from wild-type and transgenic testes were used in each reaction and each sample run in triplicate. β-actin was used for normalization of the transcripts.
Flow cytometry
Single-cell suspensions from 6-week-old testis were prepared using a two-step enzymatic digestion procedure, filtered with a 80 µm nylon membrane followed by a 25 µm filter (Brinster and Avarbock, 1994). DNase I was added to the cell suspension to prevent clumping. Cells were suspended in 200 ml ice-cold PBS containing 2% BSA and 1 mM EDTA, and incubated with anti-THY-1.2 antibody conjugated to PE, anti-GFRA1 antibody conjugated to Alex 647 and anti-KIT antibody conjugated to APC at 4°C for 45 min. Cells were washed once in PBS, re-suspended at a concentration of 1-2×106 cells/ml and analyzed on a BD FACSVantage cell sorter. Cells not receiving primary antibody were used to gate the positive staining cell population. Propidium iodide was used to gate out dead cells.
Transplant experiments
Donor cells were obtained from 6-week-old mice crossed to B6.129S7-Gt(ROSA)26Sor/J. C57BL/6J recipient mice were treated with busulfan (40 mg/kg) at 3 weeks of age. Donor cells from wild-type or transgenic mice were injected 5-6 weeks after busulfan treatment. Single-cell suspensions from donor testes were made by the two-step digestion procedure described above. Cells for transplantation were re-suspended in DMEM media containing 0.5 mM pyruvate, 6 mM L-glutamine, 100 units of penicillin, 100 mg/ml of streptomycin and 2% fetal calf serum. A concentration of 108 cells/ml was injected into efferent ducts of recipient mice. Tubules were analyzed at 6 weeks for colony number and colony length. The tunica of the testes was removed and the seminiferous tubules dispersed. Tubules were fixed in 2% PFA for 2 h at 4°C and incubated overnight with X-Gal.
Regeneration of SSCs after busulfan treatment
A single dose of 10 mg/kg body weight of busulfan was injected intraperitoneally in wild-type C57BL/6 adult mice. Three mice were analyzed at each time point, and their testes processed for whole-mount immunostaining. EdU labeled PLZF+ cells were counted over equal length of seminiferous tubules for each mouse. The data are presented as the number of cells ±s.e.m. per 1000 Sertoli cells.
Supplementary Material
Acknowledgements
We are grateful to Dr William Wright for the Ctsl promoter and to Dr Dayana Krawchuk for her help in writing the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: M.S., R.E.B.; Methodology: M.S., R.E.B.; Formal analysis: M.S., R.E.B.; Investigation: M.S.; Resources: R.E.B.; Writing - original draft: M.S., R.E.B.; Writing - review & editing: M.S., R.E.B.; Supervision: R.E.B.; Project administration: R.E.B.; Funding acquisition: R.E.B.
Funding
This work was supported by a grant from National Institute of Child Health and Human Development/National Institutes of Health (HD042454UW, to R.E.B.) and from the National Cancer Institute (CA34196 to The Jackson Laboratory) in support of The Jackson Laboratory's shared services. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.151555.supplemental
References
- Altomare D. A. and Testa J. R. (2005). Perturbations of the AKT signaling pathway in human cancer. Oncogene 24, 7455-7464. 10.1038/sj.onc.1209085 [DOI] [PubMed] [Google Scholar]
- Ballow D., Meistrich M. L., Matzuk M. and Rajkovic A. (2006). Sohlh1 is essential for spermatogonial differentiation. Dev. Biol. 294, 161-167. 10.1016/j.ydbio.2006.02.027 [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P., Jiang G., Hyman R., Lee K.-F., O'Gorman S. and Hunter T. (2000). Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3′-kinase is essential for male fertility. Nat. Genet. 24, 157-162. 10.1038/72814 [DOI] [PubMed] [Google Scholar]
- Brinster R. L. and Avarbock M. R. (1994). Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. USA 91, 11303-11307. 10.1073/pnas.91.24.11303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas F. W., Kirsh A. L., Sharma M., McLean D. J., Morris J. L., Griswold M. D., de Rooij D. G. and Braun R. E. (2004). Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 36, 647-652. 10.1038/ng1366 [DOI] [PubMed] [Google Scholar]
- Castilho R. M., Squarize C. H., Chodosh L. A., Williams B. O. and Gutkind J. S. (2009). mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell 5, 279-289. 10.1016/j.stem.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P., Buaas F. W., Sharma M., Snyder E., de Rooij D. G. and Braun R. E. (2014). LIN28A marks the spermatogonial progenitor population and regulates its cyclic expansion. Stem Cells 32, 860-873. 10.1002/stem.1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron M., Folmer J. S. and Wright W. W. (2003). A 3-kilobase region derived from the rat cathepsin L gene directs in vivo expression of a reporter gene in sertoli cells in a manner comparable to that of the endogenous gene. Biol. Reprod. 68, 1641-1648. 10.1095/biolreprod.102.011619 [DOI] [PubMed] [Google Scholar]
- Chen W. S., Xu P. Z., Gottlob K., Chen M. L., Sokol K., Shiyanova T., Roninson I., Weng W., Suzuki R., Tobe K. et al. (2001). Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15, 2203-2208. 10.1101/gad.913901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.-Y., Willis W. D. and Eddy E. M. (2016). Targeting the Gdnf Gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development. Proc. Natl. Acad. Sci. USA 113, 1829-1834. 10.1073/pnas.1517994113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarini-Garcia H. and Russell L. D. (2002). Characterization of mouse spermatogonia by transmission electron microscopy. Reproduction 123, 567-577. 10.1530/rep.0.1230567 [DOI] [PubMed] [Google Scholar]
- Cho H., Mu J., Kim J. K., Thorvaldsen J. L., Chu Q., Crenshaw E. B. III, Kaestner K. H., Bartolomei M. S., Shulman G. I. and Birnbaum M. J. (2001). Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292, 1728-1731. 10.1126/science.292.5522.1728 [DOI] [PubMed] [Google Scholar]
- Costoya J. A., Hobbs R. M., Barna M., Cattoretti G., Manova K., Sukhwani M., Orwig K. E., Wolgemuth D. J. and Pandolfi P. P. (2004). Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 36, 653-659. 10.1038/ng1367 [DOI] [PubMed] [Google Scholar]
- de Rooij D. G. and Russell L. D. (2000). All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 21, 776-798. [PubMed] [Google Scholar]
- Dummler B., Tschopp O., Hynx D., Yang Z.-Z., Dirnhofer S. and Hemmings B. A. (2006). Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol. Cell. Biol. 26, 8042-8051. 10.1128/MCB.00722-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo R. S., Orena S. J., Rafidi K., Torchia A. J., Stock J. L., Hildebrandt A. L., Coskran T., Black S. C., Brees D. J., Wicks J. R. et al. (2003). Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. J. Clin. Invest. 112, 197-208. 10.1172/JCI16885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goertz M. J., Wu Z., Gallardo T. D., Hamra F. K. and Castrillon D. H. (2011). Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J. Clin. Invest. 121, 3456-3466. 10.1172/JCI57984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso M., Fuso A., Dovere L., de Rooij D. G., Stefanini M., Boitani C. and Vicini E. (2012). Distribution of GFRA1-expressing spermatogonia in adult mouse testis. Reproduction 143, 325-332. 10.1530/REP-11-0385 [DOI] [PubMed] [Google Scholar]
- Hara K., Nakagawa T., Enomoto H., Suzuki M., Yamamoto M., Simons B. D. and Yoshida S. (2014). Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell 14, 658-672. 10.1016/j.stem.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Namekawa S. H. and Saga Y. (2013). MEK/ERK signaling directly and indirectly contributes to the cyclical self-renewal of spermatogonial stem cells. Stem Cells 31, 2517-2527. 10.1002/stem.1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs R. M., Seandel M., Falciatori I., Rafii S. and Pandolfi P. P. (2010). Plzf regulates germline progenitor self-renewal by opposing mTORC1. Cell 142, 468-479. 10.1016/j.cell.2010.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs R. M., La H. M., Makela J.-A., Kobayashi T., Noda T. and Pandolfi P. P. (2015). Distinct germline progenitor subsets defined through Tsc2-mTORC1 signaling. EMBO Rep. 16, 467-480. 10.15252/embr.201439379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth C. A., Arnold S., Kent T., Mitchell D., Isoherranen N. and Griswold M. D. (2015). Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production. Biol. Reprod. 92, 37 10.1095/biolreprod.114.126326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikami K., Tokue M., Sugimoto R., Noda C., Kobayashi S., Hara K. and Yoshida S. (2015). Hierarchical differentiation competence in response to retinoic acid ensures stem cell maintenance during mouse spermatogenesis. Development 142, 1582-1592. 10.1242/dev.118695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D. S., Olivas E., DiCandeloro P. and Wright W. W. (2011). Stage-specific changes in GDNF expression by rat Sertoli cells: a possible regulator of the replication and differentiation of stem spermatogonia. Biol. Reprod. 85, 763-769. 10.1095/biolreprod.110.087676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Ogonuki N., Inoue K., Miki H., Ogura A., Toyokuni S. and Shinohara T. (2003a). Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 69, 612-616. 10.1095/biolreprod.103.017012 [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Toyokuni S., Morimoto T., Matsui S., Honjo T. and Shinohara T. (2003b). Functional assessment of self-renewal activity of male germline stem cells following cytotoxic damage and serial transplantation. Biol. Reprod. 68, 1801-1807. 10.1095/biolreprod.102.012575 [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Miki H., Inoue K., Ogonuki N., Toyokuni S., Ogura A. and Shinohara T. (2005). Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol. Reprod. 72, 985-991. 10.1095/biolreprod.104.036400 [DOI] [PubMed] [Google Scholar]
- Kotaja N., Kimmins S., Brancorsini S., Hentsch D., Vonesch J.-L., Davidson I., Parvinen M. and Sassone-Corsi P. (2004). Preparation, isolation and characterization of stage-specific spermatogenic cells for cellular and molecular analysis. Nat. Methods 1, 249-254. 10.1038/nmeth1204-249 [DOI] [PubMed] [Google Scholar]
- Kubota H., Avarbock M. R. and Brinster R. L. (2003). Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc. Natl. Acad. Sci. USA 100, 6487-6492. 10.1073/pnas.0631767100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H., Avarbock M. R. and Brinster R. L. (2004). Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA 101, 16489-16494. 10.1073/pnas.0407063101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kanatsu-Shinohara M., Inoue K., Ogonuki N., Miki H., Toyokuni S., Kimura T., Nakano T., Ogura A. and Shinohara T. (2007). Akt mediates self-renewal division of mouse spermatogonial stem cells. Development 134, 1853-1859. 10.1242/dev.003004 [DOI] [PubMed] [Google Scholar]
- Martelli A. M., Tabellini G., Bressanin D., Ognibene A., Goto K., Cocco L. and Evangelisti C. (2012). The emerging multiple roles of nuclear Akt. Biochim. Biophys. Acta 1823, 2168-2178. 10.1016/j.bbamcr.2012.08.017 [DOI] [PubMed] [Google Scholar]
- Meng X., Lindahl M., Hyvonen M. E., Parvinen M., de Rooij D. G., Hess M. W., Raatikainen-Ahokas A., Sainio K., Rauvala H., Lakso M. et al. (2000). Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287, 1489-1493. 10.1126/science.287.5457.1489 [DOI] [PubMed] [Google Scholar]
- Meng X., de Rooij D. G., Westerdahl K., Saarma M. and Sariola H. (2001). Promotion of seminomatous tumors by targeted overexpression of glial cell line-derived neurotrophic factor in mouse testis. Cancer Res. 61, 3267-3271. [PubMed] [Google Scholar]
- Nakagawa T., Sharma M., Nabeshima Y., Braun R. E. and Yoshida S. (2010). Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328, 62-67. 10.1126/science.1182868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton C. K., Jain S., Strickland A. M., Gupta A. and Milbrandt J. (2006). Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol. Reprod. 74, 314-321. 10.1095/biolreprod.105.047365 [DOI] [PubMed] [Google Scholar]
- Rao M. K., Wayne C. M. and Wilkinson M. F. (2002). Pem homeobox gene regulatory sequences that direct androgen-dependent developmentally regulated gene expression in different subregions of the epididymis. J. Biol. Chem. 277, 48771-48778. 10.1074/jbc.M209417200 [DOI] [PubMed] [Google Scholar]
- Russell L. D. and Peterson R. N. (1984). Determination of the elongate spermatid-Sertoli cell ratio in various mammals. J. Reprod. Fertil. 70, 635-641. 10.1530/jrf.0.0700635 [DOI] [PubMed] [Google Scholar]
- Russell L. D., Ettlin R. A., SinhaHikim A. P. and Clegg E. D. (1990). Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press. [Google Scholar]
- Sato T., Aiyama Y., Ishii-Inagaki M., Hara K., Tsunekawa N., Harikae K., Uemura-Kamata M., Shinomura M., Zhu X. B., Maeda S. et al. (2011). Cyclical and patch-like GDNF distribution along the basal surface of Sertoli cells in mouse and hamster testes. PLoS ONE 6, e28367 10.1371/journal.pone.0028367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. E. and Braun R. E. (2012). Germ cell migration across Sertoli cell tight junctions. Science 338, 798-802. 10.1126/science.1219969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Sada A., Yoshida S. and Saga Y. (2009). The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev. Biol. 336, 222-231. 10.1016/j.ydbio.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Tegelenbosch R. A. J. and de Rooij D. G. (1993). A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res. 290, 193-200. 10.1016/0027-5107(93)90159-D [DOI] [PubMed] [Google Scholar]
- Tokue M., Ikami K., Mizuno S., Takagi C., Miyagi A., Takada R., Noda C., Kitadate Y., Hara K., Mizuguchi H. et al. (2017). SHISA6 confers resistance to differentiation-promoting Wnt/beta-catenin signaling in mouse spermatogenic stem cells. Stem Cell Rep. 8, 561-575. 10.1016/j.stemcr.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner K. M., Sun Y., Ji P., Granberg K. J., Bernard B., Hu L., Cogdell D. E., Zhou X., Yli-Harja O., Nykter M. et al. (2015). Genomically amplified Akt3 activates DNA repair pathway and promotes glioma progression. Proc. Natl. Acad. Sci. USA 112, 3421-3426. 10.1073/pnas.1414573112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Sukeno M. and Nabeshima Y. (2007). A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 317, 1722-1726. 10.1126/science.1144885 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.