Abstract
Purpose
Cell-based therapies to replace corneal endothelium depend on culture methods to optimize human corneal endothelial cell (HCEC) function and minimize endothelial-mesenchymal transition (EnMT). Here we explore contribution of low-mitogenic media on stabilization of phenotypes in vitro that mimic those of HCECs in vivo.
Methods
HCECs were isolated from cadaveric donor corneas and expanded in vitro, comparing continuous presence of exogenous growth factors (“proliferative media”) to media without those factors (“stabilizing media”). Identity based on canonical morphology and expression of surface marker CD56, and function based on formation of tight junction barriers measured by trans-endothelial electrical resistance assays (TEER) were assessed.
Results
Primary HCECs cultured in proliferative media underwent EnMT after three to four passages, becoming increasingly fibroblastic. Stabilizing the cells before each passage by switching them to a media low in mitogenic growth factors and serum preserved canonical morphology and yielded a higher number of cells. HCECs cultured in stabilizing media increased both expression of the identity marker CD56 and also tight junction monolayer integrity compared to cells cultured without stabilization.
Conclusions
HCECs isolated from donor corneas and expanded in vitro with a low-mitogenic media stabilizing step before each passage demonstrate more canonical structural and functional features and defer EnMT, increasing the number of passages and total canonical cell yield. This approach may facilitate development of HCEC-based cell therapies.
Keywords: human corneal endothelial cells, endothelial-mesenchymal transition, corneal cell therapy, in vitro production
The corneal endothelium is a thin cellular monolayer (∼5 μm) coating the inner side of the cornea. It is responsible for maintaining corneal transparency by pumping water out of the stromal layer.1 With ageing, the density of human corneal endothelial cells (HCECs) gradually decreases, with a loss of almost 50% from newborn to elderly.2–4 Arrested in phase G1 of the cell cycle, HCECs do not regenerate in vivo,5 and further cell loss by accidental or surgery-induced trauma,6–8 or genetic dystrophies9–12 leads to loss of endothelial function. As a consequence, the corneal stroma and epithelium swell with edema and lose transparency causing corneal blindness, a major cause of vision loss worldwide.13–15 Current treatments are based on tissue replacement,16–19 with either full corneal transplant (penetrating keratoplasty [PK]), or the selective replacement of only the damaged endothelial layer (Descemet stripping endothelial keratoplasty [DSEK]; Descemet membrane endothelial keratoplasty [DMEK]; or Descemet membrane endothelial transfer [DMET]).20–23 These surgeries present risks related to anesthesia, infection and inflammation, and immune rejection.7,24–26 Moreover, access to donor corneas is increasingly difficult due to higher demand related to population ageing, and a global shortage of tissue. Thus, a cell therapy based on the use of in vitro expanded HCECs from cadaveric donor corneas has been proposed as a solution.27
In vitro expansion of HCECs using a two-step peel-and-digest culture method28,29 faces the challenge of endothelial-to-mesenchymal transition (EnMT). This transformation of canonical, hexagonal-shaped HCECs toward a fibroblastic fate becomes evident after only a few passages,30–35 and leads to the disruption of the cellular monolayer, loss of tight junctions and cell–cell contact inhibition, as well as changes in the extracellular matrix composition, cell morphology, and function.
Here we test different culture strategies, using media additives and a novel two-step proliferation-stabilization culture technique, to decrease fibroblastic transformation and increase the number of functional cells that can be obtained from single donor corneas, and characterize cell identity and function after shipping and freezing steps.
Materials and Methods
Donor Tissue
Human cadaveric corneas from male and female donors were obtained from Eversight (Ann Arbor, MI, USA), San Diego Eye Bank (San Diego, CA, USA), and Lions VisionGift (Portland, OR, USA). Tissue was preserved in Optisol-GS (Bausch & Lomb, Rochester, NY, USA). Donor confidentiality was preserved at all steps according to the tenets of the Declaration of Helsinki. A total of 36 corneas from 27 donors were used for this study. The age of donors ranged from 10 to 70 years, and 74% of the donors were younger than 40 years old. The specular endothelial cell counts were greater than 2500 cells/mm2 in 89% of the corneas (see Supplementary Table S1). Corneas from donors undergoing chemotherapy at the time of death and with history of diabetes or sepsis were excluded. The time from death to tissue preservation in Optisol-GS (Bausch & Lomb) was less than 24 hours, and primary cultures of HCECs were initiated within 14 days of preservation. For every experiment, we used at least three independent biological replicates.
HCEC Culture
Primary HCECs were cultured following previously described methods.27 In brief, corneas were washed three times in M199 media (Gibco, Rockville, MD, USA) with 50 μg/mL gentamicin (Gibco). Corneal endothelium, attached to the Descemet's membrane, was peeled off, and stripped into smaller pieces that were incubated overnight at 37°C in a humidified 5% CO2 chamber in proliferative growth media, composed of Opti-MEM-I (Gibco) supplemented with 8% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 5 ng/mL human recombinant EGF (PeproTech, Rocky Hill, NJ, USA), 20 ng/mL human recombinant NGF (PeproTech), 100 μg/mL bovine pituitary extract (Biomedical Technologies, Stoughton, MA, USA), 0.5 mM L-ascorbic acid 2-phosphate (Sigma-Aldrich Corp., St. Louis, MO, USA), 200 mg/L calcium chloride (Invitrogen, Carlsbad, CA, USA), 0.08% chondroitin sulfate (Sigma-Aldrich Corp.), 50 μg/mL gentamicin, and 1× antibiotic/antimycotic solution (Invitrogen). The next day, tissue was washed in Hank's Balanced Salt Solution (Gibco) and incubated in 0.02% EDTA (Sigma-Aldrich Corp.) for 1 hour at 37°C. Tissue was passed 15 to 20 times through a glass pipette to release the cells. Single cells and remaining pieces of Descemet's membrane from one single cornea were plated in one well of a 12-well tissue culture plate precoated with FNC Coating Mix (Athena Environmental Sciences, Inc., Baltimore, MD, USA); this was considered passage 0. All cultures were grown at 37°C in a 5% CO2, humidified atmosphere. Media was replaced every other day. Clinical grade reagents were used whenever available.
Media Optimization, Media Additives, Survival, and Proliferation Assays
HCECs were cultured as indicated above, and then at passages two to four, specific drugs were tested when added to the growth media. L-ascorbic acid 2-phosphate (AA-2P) (Sigma-Aldrich Corp.), versus L-ascorbic acid (AA) at a concentration of 20 μg/mL were tested on HCECs plated at 40,000/well in triplicate and cultured for 2 days to examine survival and function by trans-endothelial electrical resistance assay (TEER; see below). Y27632 ROCK inhibitor (Tocris Bioscience, Minneapolis, MN, USA), SB154352 TGF-β inhibitor (Cayman Chemicals, Ann Arbor, MI, USA), and human recombinant Rspondin-1 (StemRD, Burlingame, CA, USA) were tested on HCECs plated at 5000 cells/well for 12 hours before adding drugs at increasing concentrations for 72 hours. Cells treated with dimethyl sulfoxide (DMSO) or H2O only were used as controls. To measure survival, a 5 mg/mL stock solution of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma-Aldrich Corp.) in water was prepared, and added to the culture wells with media in a 1:100 dilution. After 15 minutes at 37°C in 5% CO2, cells were imaged, and cell counts were compared to control wells. Morphology was assessed by dividing the longest axis by the shortest axis of the cell (length/width ratio), using a grid to image unbiased locations within the culture wells.
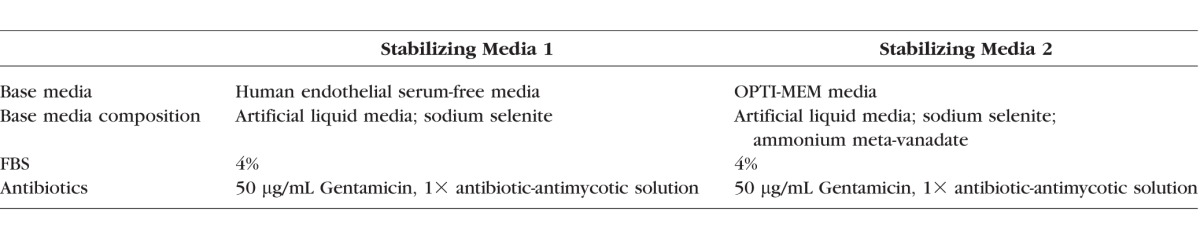
Low Mitogen Stabilization Media
HCEC cultures were established as indicated above, and passage 0 cells were grown in proliferative growth media. At confluence, cells were passaged after incubation with 0.05% Trypsin (Gibco) for 5 minutes at 37°C in 5% CO2. Cells were counted and equal numbers of cells were seeded into three wells of a 12-well tissue culture plate precoated with FNC. Well 1 was considered a control. Cells were grown in proliferative culture media until confluence. Well 1 was then passaged, and samples were taken for flow cytometry and TEER; wells 2 and 3 were rinsed with Dulbecco's phosphate-buffered saline (DPBS) and proliferative growth media was replaced by two different types of stabilizing media (Table): stabilizing media 1, composed of human endothelial serum free media (Gibco), 4% FBS (Hyclone), 50 μg/mL gentamicin (Gibco), and 1× antibiotic/antimycotic solution (Invitrogen); and stabilizing media 2, composed of Opti-MEM-I (Gibco), 4% FBS (Hyclone), 50 μg/mL gentamicin (Gibco), and 1× antibiotic/antimycotic solution (Invitrogen). Cells were stabilized and media changed every other day for 7 days. Then, cells were passaged and samples were taken for flow cytometry and TEER. Cells were then cultured for up to four passages. Cell viability was assessed by trypan blue exclusion assay (Sigma-Aldrich Corp.). Images of the cultures were taken on an Axio Observer A1 phase-contrast microscope (Carl Zeiss Microscopy, GmbH, Germany).
Table.
Stabilizing Media Components
Flow Cytometry
HCECs cultured in proliferative growth media and in stabilizing growth media 1 and 2 were trypsinized, dissociated into single cells, and counted using a hemocytometer. Two-hundred thousand cells/condition were used, with 100,000 cells re-suspended in 100 μL DPBS (Gibco) supplemented with 2% FBS as negative controls, and 100,000 cells re-suspended in 100 μL DPBS (Gibco) supplemented with 2% FBS for staining. Cells were stained in the dark at room temperature for 30 minutes with mouse anti-human CD56-APC antibody (BD Biosciences Pharmingen, San Jose, CA, USA). Flow cytometric data were acquired using a BD Facs Canto flow cytometer (BD Biosciences Pharmingen) with the FACSDiva software. For each run, an unstained HCEC control sample, and single color positive control (anti-mouse beads, stained with CD56-APC: AbC Anti-Mouse Bead Kit; Invitrogen) were used to calibrate the instrument. Computer compensation was applied during data acquisition. Data were stored in a FCS file format, and further analysis was performed using FlowJo V X.0.7. Briefly, the HCEC population was isolated by gating on the FSC-A versus SSC-A dot plot (population 1). Doublets were excluded by performing two consecutive additional gatings (FSC-A versus FSC-W, “population 2” and SSC-A versus SSC-W, “population 3”). To quantify the expression of each marker, population 3 of each sample was used to create a fluorescence histogram. An overlay of the stained sample histogram, the unstained control sample histogram, and the positive control beads was created for each sample. CD56-negative gate included the negative control, and contained less than 1% of the positive cells. CD56-high gate contained at least 99% of the positive control. CD56-low gate corresponds to the region in between. At least three independent experiments were conducted for each condition.27
Trans-Endothelial Electrical Resistance (TEER)
Twenty thousand cells/well in passage 4 were seeded per triplicate transwell (6.5 mm diameter, 0.4 μM pore [Costar, Corning, NY, USA]), coated with FNC. Each triplicate was first grown in proliferative media for 1 week, then media was changed to either stabilizing 1, stabilizing 2, or proliferative media. Fibroblastic HCECs were used as a negative control. TEER was measured with an EVOM2 epithelial volt-ohmmeter (World Precision Instruments, Sarasota, FL, USA) for 30 days or until readings reached a steady state, whichever happened first. TEER measurements were normalized to the value of the control wells (growth media without cells). The reported values represent the average reading of at least three independent experiments.
Cryopreservation and Shipping of HCECs
Cells from three different corneas at passage 0 or 1 cultured in proliferative media were harvested at confluence and their quality was assessed using CD56 expression measurements by flow cytometry as described above. Fibroblastic HCECs were used as a control. Cells were then frozen down in 80% FBS (Hyclone) in DMSO (Sigma-Aldrich Corp.) at −80°C for 5 days, then stored in liquid nitrogen for 3 more days. Cryovials were thawed at 37°C, their content diluted up to 1 mL with DPBS (Gibco), and spun down at 930g. The supernatant was removed and the cells were plated on FNC-coated 12-well plates (Gibco). Cells were plated in triplicate wells, and cultured in proliferative media until 70% confluence. Then, media in each well of the triplicate was replaced either by proliferative, stabilizing 1 or stabilizing 2 media, and the cells cultured until reaching confluence. Expression of CD56 was assessed by flow cytometry as described above.
Stability of HCECs During Transport
Canonical HCECs cultured in proliferative media up to passage 3 were harvested and their viability and numbers were assessed. One-hundred thousand cells were separated for CD56 expression testing by flow cytometry, as described above. Remaining cells were placed in 1 mL syringes (BD Biosciences Pharmingen), at a concentration of 800,000 cells/500 μL BSS Plus (Alcon, Fort Worth, TX, USA). Syringes were placed in a temperature-controlled container at 2 to 8°C, and shipped back to the laboratory via FedEx overnight. Upon receipt of the shipment, container was opened at 72 and 96 hours after harvesting and the quality and survival of the cells were assessed by trypan blue exclusion, flow cytometry, and TEER. Syringes with fibroblastic HCECs were shipped together with canonical HCECs, and used as controls for CD56 expression assays.
Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM) unless noted otherwise. Statistical analysis was performed using unpaired, two-tailed Student's t-test; a χ2 test was performed to analyze the data distribution of Figure 1B. P < 0.05 was considered statistically significant.
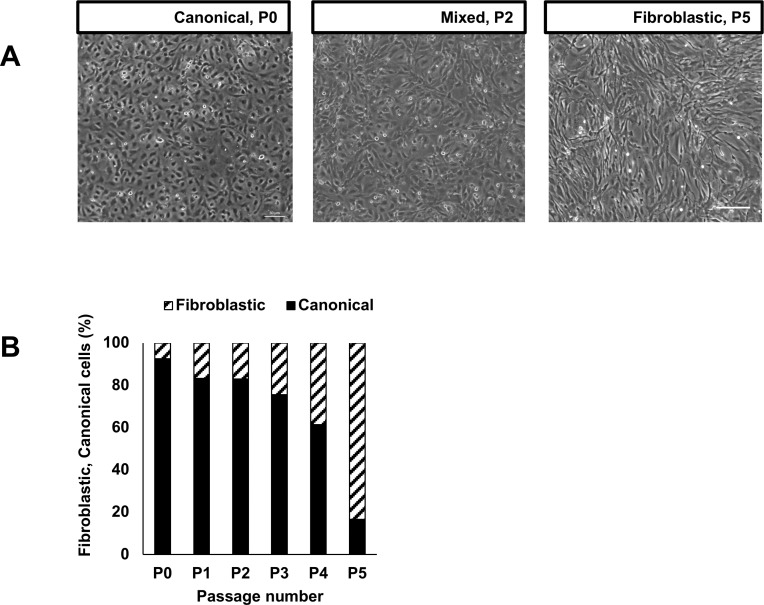
Figure 1.
(A) HCECs undergo EnMT in early passages (P) when maintained in mitogenic media. The morphology of the cells changes from canonical with regular polygonal patterning to fibroblastic and irregular with increasing number of passages. (B) At confluence, the proportion of fibroblastic cells within a culture increased significantly and canonical cells decreased significantly with the number of passages (N = 5 biological replicates; n = 100 cells per well counted per condition; χ2 test P < 0.0001).
Results
Effects of Media Additives on Survival, Proliferation, and Morphology of HCECs
In vitro HCEC culture following previously published methods yields monolayers of canonical HCECs at low passage numbers, comparable to the in vivo morphology of these cells, but fibroblastic phenotypes by passage 5 due to a well-described phenomenon known as EnMT (Fig. 1).27,30 We tested a number of media additives that have been previously described to have a positive effect on HCEC proliferation, survival, and morphology. First, we analyzed the efficiency of ascorbic acid (AA), an intracellular antioxidant that is an essential component of the standard growth media.30 AA reduces the deleterious effect of reactive oxygen species that are accumulated within HCECs as a normal consequence of light transmission.36,37 However, AA is very unstable and prone to be oxidized in aqueous environment (Alvarez-Delfin K, et al. IOVS 2013;54:ARVO E-Abstract 1648).38 We therefore tested the effect of substituting AA with a more stable form, AA-2P, in the growth media. After 2 days in culture, cells in AA-2P demonstrated higher cell counts per well than cells in the control media (Fig. 2A). The ability of HCECs to form a functional barrier measured by TEER showed no difference between AA and AA-2P (Fig. 2B). Thus, AA-2P was substituted instead of AA in HCEC culture media for all subsequent experiments.
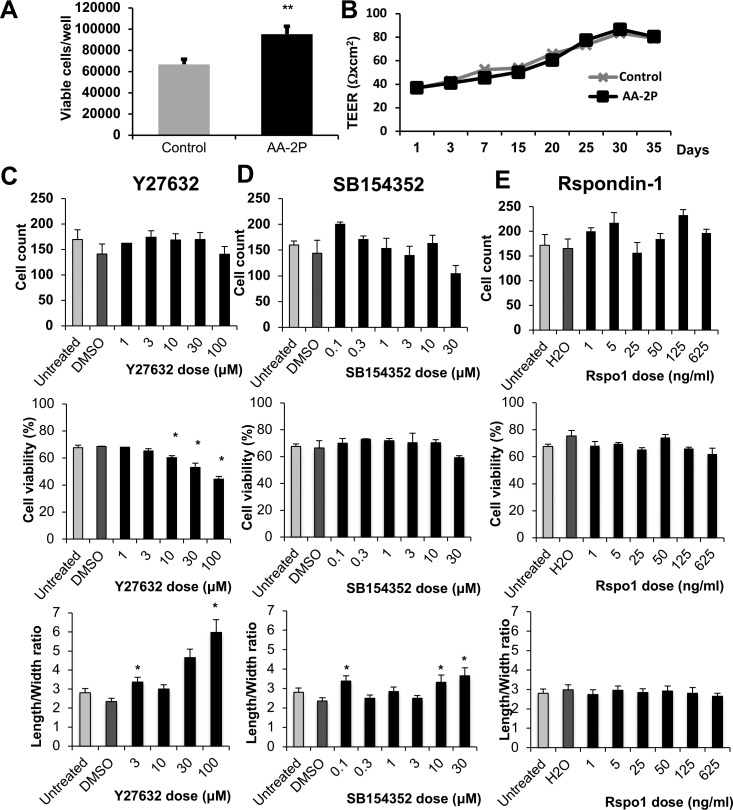
Figure 2.
(A) HCECs cultured in media with 0.5 mM AA-2P showed a 30% increase in cell number compared to cells in control media containing AA (N = 5; mean ± SEM; P = 0.006). (B) Cell function, measured by TEER, was not affected by the addition of AA-2P to culture media, compared to the control media containing ascorbic acid. (C–E) Dose titration of Y27632, SB154352, and Rspondin-1 was performed on HCECs, examining cell yield, viability, and fibroblastic EnMT morphology defined by increasing length-to-width ratio. Increasing concentrations of Y27632 decreased viability and promoted fibroblastic transformation; SB154352 increased fibroblastic transformation without affecting viability or proliferation; and Rspondin-1 increased cell yield demonstrated higher proliferation rates at specific concentrations as marked but did not affect cell viability or morphology (*P < 0.05). Each experiment was repeated at least three times.
Next, we asked whether further modifying the culture media composition might enhance HCECs' proliferative capacity and help retain their canonical morphology. Three different drugs, Y27632 (Rho kinase inhibitor), SB154352 (TGF-β inhibitor), and Rspondin-1 (Wnt pathway activator) whose effects on corneal endothelial cells were previously described39–45 were examined, and the proliferation, viability, and morphology of treated cells were assessed. Cells were plated in triplicate in 96-well plates coated with FNC, and treated for 72 hours with increasing concentrations of each drug as labeled, stained with MTT, and imaged. Cell count, viability, and morphology were determined. We found that Y27632 did not affect cell proliferation. Higher doses of Y27632 had a negative effect on cell viability and, contrary to what has previously been reported,39,40,44,46–49 appeared significantly more elongated than their controls, suggesting drug-induced EnMT (Figs. 2C, 3). SB154352 treatment did not have any effect on cell proliferation or survival; similarly, to Y27632, at high doses, an increased length/width ratio compared to control suggested EnMT induced by the drug (Figs. 2D, 3). Finally, Rspondin-1 treatment increased the number of cells at the doses of 5 ng/mL (as has been previously described42), and 125 ng/mL. No significant effect on proliferation was detected in the intermediate concentrations, and no significant changes in cell survival or morphology were observed with Rspondin-1 (Figs. 2E, 3). Thus, Rspondin-1 may be a suitable candidate for further HCEC culture optimization, but none of these three drugs were carried forward into our subsequent experiments.
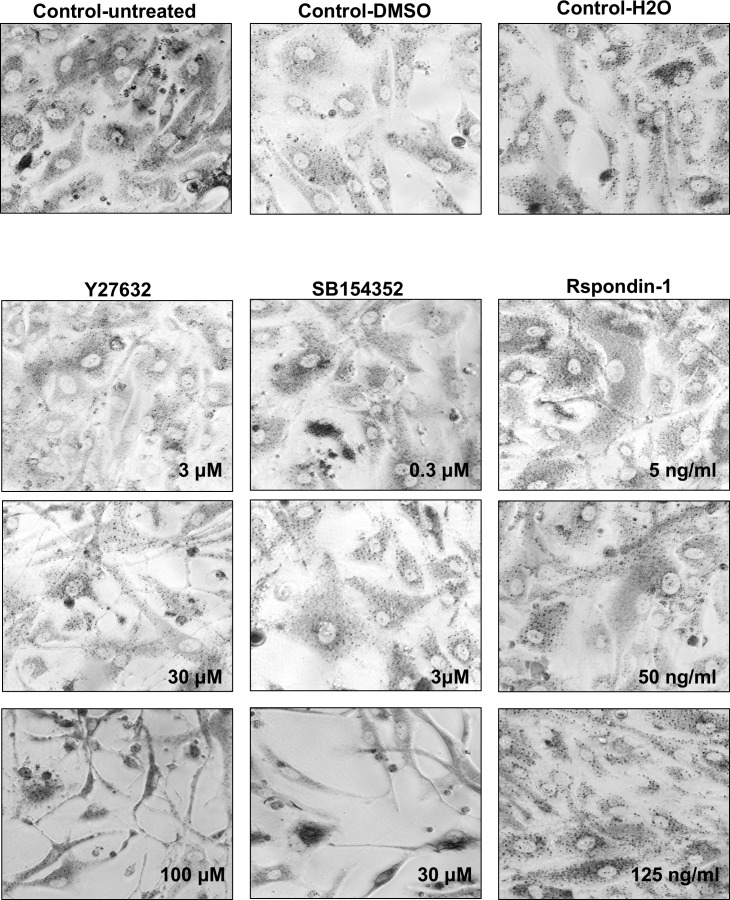
Figure 3.
Representative images of cells treated for 72 hours with Y27632, SB154352, and Rspondin-1, after incubation with MTT. Cells treated with DMSO, H2O, and untreated cells are shown as controls.
Effects of Low-Mitogen Media on HCEC Culture
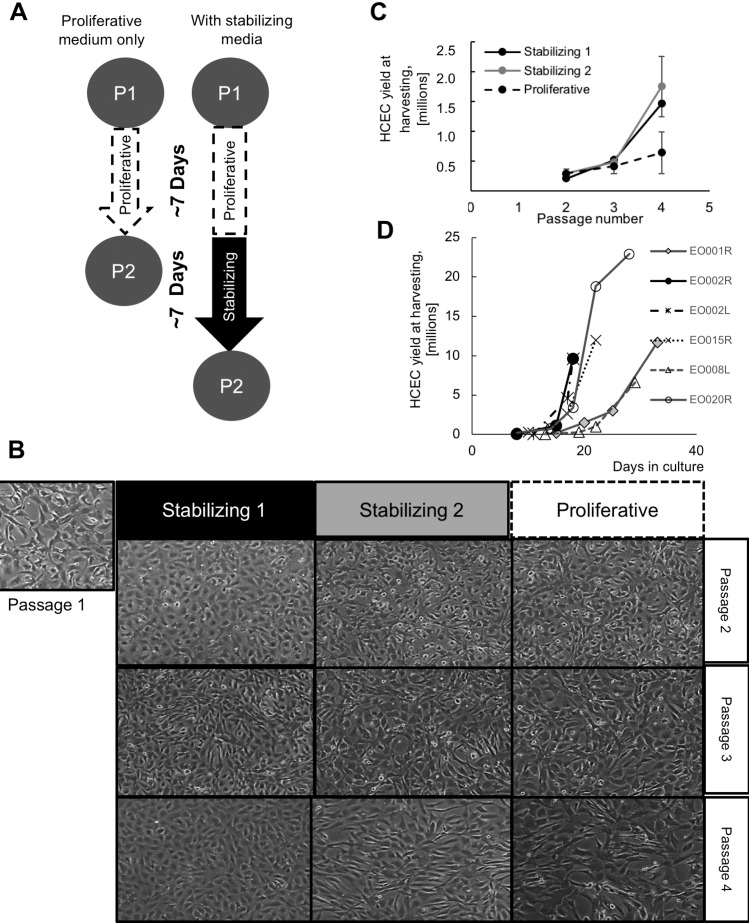
We hypothesized that withdrawing mitogenic growth factors from the growth media might improve cell morphology and delay EnMT, similar to a dual media strategy previously described.50 We used two different types of stabilizing media: the stabilizing media 1, based in endothelial serum-free media, and stabilizing media 2, based in Opti-MEM media. Human endothelial serum free media is a liquid artificial media containing sodium selenite. Opti-MEM media is a liquid artificial media containing sodium selenite and ammonium meta-vanadate. Both media were supplemented with antibiotics and a low percentage of FBS (4%); exogenous growth factors were not added. We then compared morphology, proliferative ability, viability, identity (by CD56 expression), and function (by TEER) of HCECs grown only in proliferative growth media versus parallel cultures exposed to either stabilizing media before each passage (Fig. 4A). Cells grown with stabilizing steps up to passage 4 presented a better morphology throughout the cultures than cells grown in proliferative media only. At passage 4, cells in stabilizing media 1 presented better morphology than cells in stabilizing media 2 (Fig. 4B). Moreover, stabilizing HCECs increased culture time, and cell counts at each passage were higher (Fig. 4C). When repeated in a Good Manufacturing Practice setting, total cell yields per cornea crested 10 million in 18 to 33 days (Fig. 4D, right). Thus extending culture time with additional days in media low in mitogenic factors or serum enhanced canonical morphology and cell yield.
Figure 4.
(A) HCECs cultured in proliferative growth media for 1 week and passaged were compared to HCECs cultured in proliferative media for 1 week, followed by 1 week of stabilization before passaging. (B) Primary HCECs stabilized in stabilizing media 1 or stabilizing media 2 showed more canonical morphology than cells cultured in proliferative media only, with stabilizing media 1 showing better morphology than stabilizing media 2. (C) Culture in stabilizing low mitogen media before each passage increased cell yield by passage 4. (D) Replicated in a GMP setting, HCECs cultured with a stabilization step yielded 10 million or more cells in 5 to 10 times more cells than those cultured in 18 to 33 days.
Stabilization of HCEC Cultures and Expression of Canonical Surface Markers
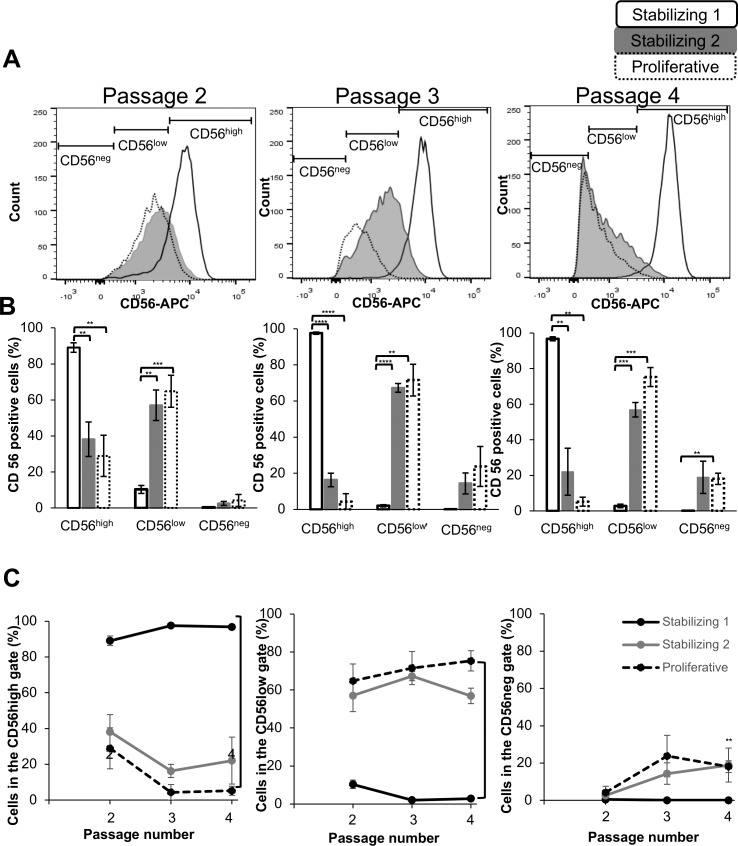
We next asked whether stabilizing HCECs in low mitogenic media would affect the expression of CD56, a surface marker highly expressed in canonical HCECs.27 At each passage, a subset of HCECs was isolated, stained, and analyzed by flow cytometry. At passage 2, there was a 3-fold increase in CD56 expression in stabilization media 1 compared to cells grown in stabilization media 2 or proliferative media only (Fig. 5A). This difference in CD56 expression between these conditions was even greater (>20-fold) at passages 3 and 4 (Figs. 5B, 5C), reflecting the loss of CD56 expression by HCECs maintained in proliferative media and undergoing EnMT with fibroblastic morphology. Thus, stabilizing the cells in low-mitogenic media promoted canonical cell identity, with significantly higher levels of CD56 in stabilized cells.
Figure 5.
(A) HCECs cultured with a stabilizing low mitogen media step expressed higher levels of CD56 expression at passages 2 to 4 (as marked) than HCECs cultured in proliferative media only. (B) A significantly higher number of cells expressed high CD56 levels when cultured in stabilizing media 1 (P values: * < 0.05; ** < 0.01; *** < 0.001; **** < 0.0001). (C) Cells cultured in stabilizing media 1 expressed significantly higher levels of CD56 over multiple passages compared to stabilizing media 2 and proliferative media (P ≤ 0.01). The number of cells cultured in stabilizing media 1 that express low levels of CD56 was significantly lower over multiple passages compared to stabilizing media 2 and proliferative media (P ≤ 0.01).
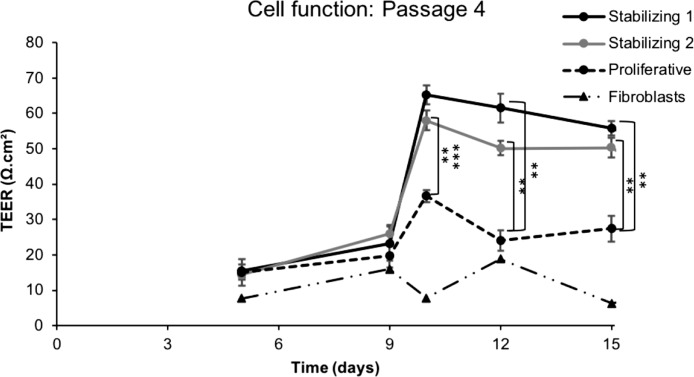
Effect of Low-Mitogenic Stabilization Media on Monolayer Barrier Function
HCECs in passage 4 with or without stabilization in low-mitogenic media were collected and seeded in transwells, and barrier formation was followed by measuring TEER associated with tight junction monolayer formation.27,51 Cells in passage 4 were seeded and cultured in proliferative media for 7 days, and then the media was replaced by either stabilizing media 1 or 2. Fibroblasts cultured in transwells in proliferative media were used as control. Three days 7after media replacement, a significant increase in trans-endothelial electrical resistance was detected in cells cultured in stabilizing media 1 and 2, compared to cells cultured in proliferative media. Stabilized cells maintained significantly higher resistance throughout the whole experiment (Fig. 6), suggesting that stabilization in low-mitogenic media had a beneficial effect on the ability of HCECs to form functional barriers. Taken together, these data demonstrate that a two-step method of alternating proliferation and stabilization media when culturing HCECs improves the yield and function of these cells.
Figure 6.
The barrier function of HCECs cultured with stabilizing steps in stabilizing media 1 or 2 was significantly higher compared to cells cultured in proliferative media only. Fibroblastic cells are shown as a negative control (**P < 0.01).
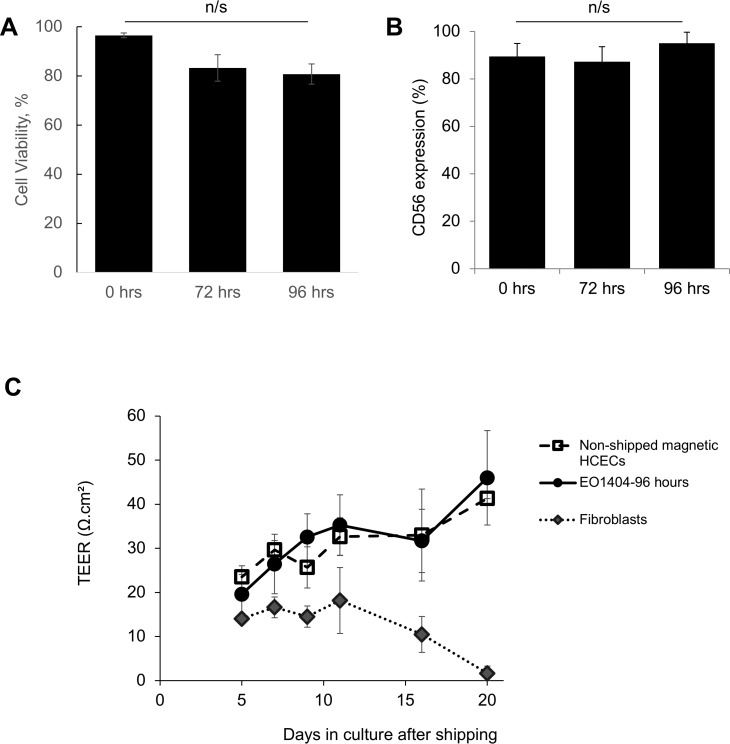
Stability of HCECs After Shipping at 4°C
One of the major challenges in the cell therapy field is shipping the cell product, while preserving viability and function. We wished to test stability of HCECs from a Good Manufacturing Practice (cGMP) setting following the method described above, but our access to GMP-grade HCECs was limited to cells grown in the media above with added biocompatible, magnetic superparamagnetic nanoparticles to produce magnetic HCECs.51 These magnetic HCECs have been previously tested in a preclinical model of corneal endothelial dysfunction and were able to restore corneal transparency when used in combination with an external magnetic eye patch (Xia X, et al. IOVS 2017;58:ARVO E-Abstract 1474; Kunzevitzky NJ, et al. IOVS 2014;55:ARVO E-Abstract 2040). We also established in prior work that adding the superparamagnetic nanoparticles does not affect viability nor function of cultured HCECs for up to 30 days51; however, shipping stability had not been investigated. We tested the viability of magnetic HCECs, their expression of identity and functional marker CD56, and their ability to form monolayers when shipped at 4°C in ready-to-use syringes. Immediately after suspension in BSS Plus, syringes were shipped overnight via FedEx back to the laboratory in temperature controlled containers. Upon arrival, the containers were kept at room temperature, opened after 72 or 96 hours, and the contents of the syringes retrieved in a biosafety cabinet and placed in culture in proliferative media for 7 days. HCEC viability remained high (81%) after shipping (Fig. 7A), and expression of canonical marker CD56 did not change significantly (Fig. 7B). Shipped cells formed monolayers with TEER measures indistinguishable from those of sister cultures that were not shipped (Fig. 7C). Thus, these data suggest that cultured HCECs can be shipped at 4°C and are stable for at least 96 hours.
Figure 7.
(A) There was no significant difference in survival between cells shipped at 4°C for up to 96 hours and the same cells prior to shipping (N = 4). (B) Canonical HCECs cultured up to P3, harvested and shipped, retained high levels of CD56 expression not statistically different from baseline up to 96 hours after shipping (N = 4). (C) HCECs shipped for 96 hours showed similar tight junction monolayer function measured by TEER to sister cultures that had not been shipped (0 hours), but higher barrier function than fibroblastic HCECs (N = 6).
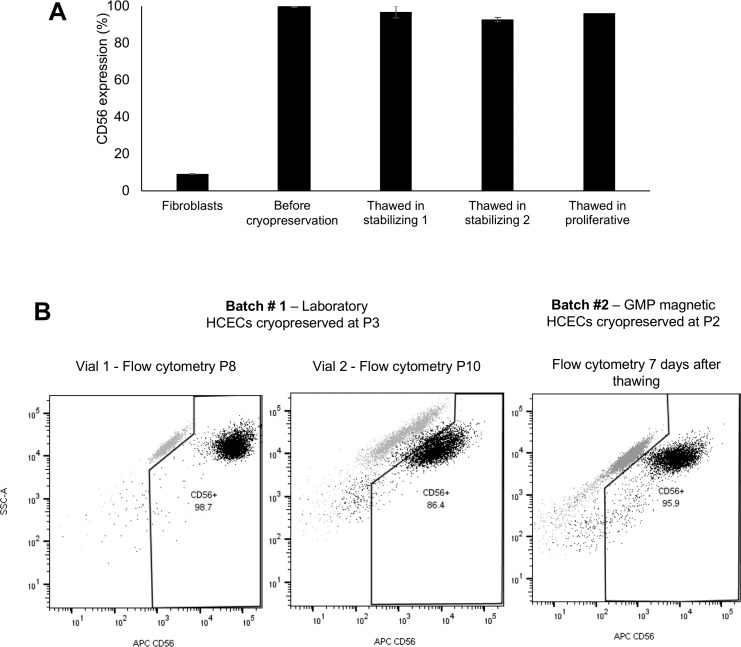
Effect of Cryopreservation on HCEC Viability and Function
We tested the viability and function of cryopreserved HCECs. Cells in passage 0 or 1 were expanded to confluence in proliferative media, collected, and cryopreserved by conventional methods. The expression of CD56 was tested in a subset of cells at harvesting, prior to cryopreservation. The cells were canonical in morphology, and thus they expressed high levels (>95%) of CD56, while fibroblastic HCECs (used as negative controls) had a significantly lower expression of the marker (9%). We next thawed the HCECs, cultured them until confluence with either proliferative or stabilization media, and re-assessed expression of CD56. We found that the media used to thaw the cells had no effect on the expression of CD56 (Fig. 8A). We repeated the experiments with cells at higher passage number (up to P8) and obtained similar results (Fig. 8B). Taken together, these data demonstrate that HCECs can be cryopreserved, opening a door to intercontinental cell therapy solutions.
Figure 8.
(A) Cryopreservation of canonical HCECs in early passages did not alter CD56 expression compared to the same cells prior to a single freeze-thaw cycle. There was no significant difference between cryopreserved cells cultured with low-mitogen stabilizing media prior to final harvest, versus cells cultured without that step. CD56 expression in fibroblastic HCECs is shown as a negative control. (B) Flow cytometry of HCECs cryopreserved at higher passages, thawed, and expanded up to passage 10, maintained high expression of CD56.
Discussion
Corneal endothelial cells, essential for corneal transparency, do not regenerate in vivo,2,52–54 and HCEC loss with injury or disease leads to corneal endothelial dysfunction, a major cause of blindness worldwide.13–15 To date, the primary treatment strategy consists of corneal transplant surgery, which carries inconveniences of cost, technical challenge, potential side effects, and shortage of donor tissue. Alternative strategies are currently being developed, including corneal scaffold engineering,55–59 and injectable cell therapies.39,60–62 Injectable cell therapies would not only dramatically increase the number of patients treated per donor cornea, but could also transform a complicated and highly specialized surgery into a simple injection, thus increasing worldwide access to treatment.
To achieve this aim, it is essential to produce functional HCECs in vitro. In vivo, at weeks 5 to 6 of human gestation, HCECs are arrested in phase G1 of the cell cycle, and HCEC loss from that point on is not significantly replaced through endogenous replenishment.52–54 The reasons for regenerative failure are not completely elucidated, but may include the presence of antimitogenic factors such as TGF-β in the aqueous humor of the anterior chamber54,63 or cell–cell or cell–Descemet's membrane contact inhibition. In vitro, however, this mitotic block can be overcome, and HCECs proliferate in response to mitogenic signals in culture.30,64
A number of different techniques to isolate and media to culture HCECs have been developed but diverse parameters, from donor age and health status to the use of chemotherapy or other toxic substances, have an influence on the success of HCEC culture.27,30,50,65,66 Even in the best conditions, HCEC cultures are fragile, and can be expanded only up to four to five passages before changes in cell morphology and function are observed. This EnMT is characterized by fibroblastic transformation and loss of tight-junction formation and barrier function of HCECs.33,35,43,67 In vivo, EnMT is often triggered by excessive loss of HCECs, with remaining HCECs unable to compensate. Indeed, with extensive endothelial injury, inflammatory mechanisms leading to the activation of PI-3 kinase and fibroblast growth factor 2 (FGF2) stimulate the proliferation of the nonproliferative HCECs, but also trigger fibroblastic transformation with loss of barrier function.32,67–69
After multiple passages, a similar change in morphology and function is observed in vitro. Research is ongoing for novel HCEC culture media additives that would increase cell proliferation while maintaining cell function and morphology. A recent study,43 linking EnMT to TGF-β signaling activation, demonstrated an effect of TGF-β blocking drug SB431542 on delaying EnMT. However, SB431542 failed to exhibit the expected effect when added to our cell culture media, and the drug induced a fibroblastic morphology at high concentrations. The Rspondin-1 molecule previously described as enhancer of cell proliferation in HCECs42,45 stimulated cell proliferation without any negative effects, and is a potential candidate for future culture media improvements or perhaps to explore for in vivo use.
Similarly, the Rho-kinase (ROCK) inhibitor has recently been shown to increase HCEC proliferation, while maintaining cell morphology.39,40,44,46 The proliferative effect has also been observed in a recent study that uses the dual media approach culture strategy to maintain cell morphology.70 When added to our media, low doses of ROCK inhibitor had no significant effect on cell proliferation or morphology; at higher doses, the drug negatively impacted cell survival, proliferation, and morphology. Differences between donor tissues including age or genetic factors may account for variation in results. Pairing a ROCK inhibitor with another strategy to preserve cell morphology and function, such as a dual media approach, may be a good way to potentiate positive effects of both on HCEC growth.
A recently developed dual media strategy50 relies on the potential of HCECs to revert from fibroblastic phenotypes back to canonical morphology and function. This two-step culture strategy uses two types of media: the first step media is a proliferative media enriched in mitogenic growth factors and serum, promotes HCEC proliferation in vitro, but perhaps at cost of canonical morphology in later passages. Once confluence is achieved, the proliferative media is replaced by a stabilizing media lower in serum and mitogenic factors and the cells regain their canonical morphology. Previous studies have risen concerns of such dual media approaches in terms of cell proliferation, yield and viability. In our data, however, cell viability was similar between cells in low-mitogenic stabilizing media and cells grown exclusively in proliferative media. In addition, the final number of cells after four passages was higher when using the dual media approach, compared to the proliferative media approach.
In addition, our work demonstrated that stabilizing HCECs in low-mitogenic media also showed positive impact on cell identity (measured with canonical marker CD56)27 and function (measured by TEER). Omitting the stabilizing step significantly lowered CD56 expression, with increased number of CD56 negative cells throughout the passages, consistent with observed EnMT. As previously demonstrated,27 CD56 expression was a strong correlate for tight junction formation as measured by TEER. Our experimental design used two types of stabilizing media with different solutions. Stabilizing media 1 contained Human Endothelial Serum-Free basic media, and stabilizing media 2 contained OPTI-MEM. While HCECs stabilized in either media regained canonical morphology and CD56 expression, cultures in stabilizing media 1 yielded more HCECs with high CD56 expression over multiple passages, although their ability to form barriers did not differ significantly compared to cultures in stabilizing media 2. Thus, human endothelial serum-free media is the preferred basic component of stabilizing media for future applications.
These novel findings suggest that using the dual media approach not only improves the morphology, but also preserves the canonical identity and function of in vitro cultured HCECs for multiple passages. While the underlying mechanisms of these effects remain to be investigated, the use of a low-mitogenic dual media approach may represent an important step toward high-scale production of a reliable HCEC therapy product, creating a path toward cell transport for human studies.
Supplementary Material
Acknowledgments
The authors thank Jonathan Van Dyke, Gerhard Bauer, and Brian Fury (University of California Davis) and Katherine Drews-Elger (Universidad CES, Colombia) for technical assistance with flow cytometry, and Lions VisionGift, Eversight, and the families of the tissue donors.
Supported by grants from the National Eye Institute (P30-EY026877 and P30-EY022125), the California Institute of Regenerative Medicine (DISC1-08848), and Research to Prevent Blindness, Inc.
Disclosure: A. Bartakova, None; O. Kuzmenko, None; K. Alvarez-Delfin, None; N.J. Kunzevitzky, Emmecell (E), P; J.L. Goldberg, Emmecell (S), P
References
- 1. DelMonte DW, Kim T. . Anatomy and physiology of the cornea. J Cartaract Refract Surg. 2011; 37: 588– 598. [DOI] [PubMed] [Google Scholar]
- 2. Bahn CF, Glassman RM, MacCallum DK,et al. Postnatal development of corneal endothelium. Invest Ophthalmol Vis Sci. 1986; 27: 44– 51. [PubMed] [Google Scholar]
- 3. Bourne WM, Nelson LR, Hodge DO. . Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997; 38: 779– 782. [PubMed] [Google Scholar]
- 4. Gipson IK. . Age-related changes and diseases of the ocular surface and cornea. Invest Ophthalmol Vis Sci. 2013; 54: ORSF48– ORSF53. [DOI] [PubMed] [Google Scholar]
- 5. Joyce NC, Meklir B, Joyce SJ, Zieske JD. . Cell cycle protein expression and proliferative status in human corneal cells. Invest Ophthalmol Vis Sci. 1996; 37: 645– 655. [PubMed] [Google Scholar]
- 6. Bourne WM, Brubaker RF. . Use of air to decrease endothelial cell loss during intraocular lens implantation. Arch Ophthalmol. 1979; 97: 1473– 1475. [DOI] [PubMed] [Google Scholar]
- 7. Patel SV, Hodge DO, Bourne WM. . Corneal endothelium and postoperative outcomes 15 years after penetrating keratoplasty. Am J Ophthalmol. 2005; 139: 311– 319. [DOI] [PubMed] [Google Scholar]
- 8. Garcia-Pous M, Udaondo P, Garcia-Delpech S, Salom D, Díaz-Llopis M. . Acute endothelial failure after cosmetic iris implants (NewIris®). Clin Ophthalmol. 2011; 5: 721– 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Afshari NA, Pittard, AB, Siddiqui A, Klintworth GK. . Clinical study of Fuchs corneal endothelial dystrophy leading to penetrating keratoplasty: a 30-year experience. Arch Ophthalmol. 2006; 124: 777– 780. [DOI] [PubMed] [Google Scholar]
- 10. Schmedt T, Silva MM, Ziaei A, Jurkunas U. . Molecular bases of corneal endothelial dystrophies. Exp Eye Res. 2012; 95: 24– 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vincent AL. . Corneal dystrophies and genetics in the International Committee for Classification of Corneal Dystrophies era: a review. Clin Exp Ophthalmol. 2013; 42: 4– 12. [DOI] [PubMed] [Google Scholar]
- 12. McLaren JW, Bachman LA, Kane KM, Patel SV. . Objective assessment of the corneal endothelium in Fuchs' endothelial dystrophy. Invest Ophthalmol Vis Sci. 2014; 55: 1184– 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stevens GA, White RA, Flaxman SR,et al. Global prevalence of vision impairment and blindness: magnitude and temporal trends, 1990-2010. Ophthalmology. 2013; 120: 2377– 2384. [DOI] [PubMed] [Google Scholar]
- 14. Bourne RA, Stevens GA, White RA,et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. The Lancet Global Health. 2013; 1: e339– e349. [DOI] [PubMed] [Google Scholar]
- 15. Pascolini D, Mariotti SP. . Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012; 96: 614– 618. [DOI] [PubMed] [Google Scholar]
- 16. Price MO, Gorovoy M, Benetz BA,et al. Descemet's stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor Study. Ophthalmology. 2010; 117: 438– 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anshu A, Price MO, Tan DTH, Price FW Jr.. Endothelial keratoplasty: a revolution in evolution. Surv Ophthalmol. 2012; 57: 236– 252. [DOI] [PubMed] [Google Scholar]
- 18. Feng MT, Price MO, Miller JM, Price FW Jr.. Air reinjection and endothelial cell density in Descemet membrane endothelial keratoplasty: five-year follow-up. J Cartaract Refract Surg. 2014; 40: 1116– 1121. [DOI] [PubMed] [Google Scholar]
- 19. Chaurasia S, Price MO, McKee Y, Price FWJ. . Descemet membrane endothelial keratoplasty combined with epithelial debridement and mitomycin-C application for Fuchs dystrophy with preoperative subepithelial fibrosis or anterior basement membrane dystrophy. Cornea. 2014; 33: 335– 339. [DOI] [PubMed] [Google Scholar]
- 20. Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. . Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998; 105: 1855– 1865. [DOI] [PubMed] [Google Scholar]
- 21. Melles G, Wijdh R, Nieuwendaal CP. . A technique to excise the Descemet membrane from a recipient cornea (descemetorhexis). Cornea. 2004; 23: 286– 288. [DOI] [PubMed] [Google Scholar]
- 22. Melles GRJ, Ong TS, Ververs B, van der Wees J. . Descemet membrane endothelial keratoplasty (DMEK). Cornea. 2006; 25: 987– 990. [DOI] [PubMed] [Google Scholar]
- 23. Dirisamer M, Yeh RY, van Dijk K, Ham L, Dapena I, Melles GRJ. . Recipient endothelium may relate to corneal clearance in Descemet membrane endothelial transfer. Am J Ophthalmol. 2012; 154: 290– 296.e1. [DOI] [PubMed] [Google Scholar]
- 24. Burkhardt B. . 2012 Eye Banking Statistical Report. Eye Bank Association of America. April 2013: 1– 115.
- 25. Wu EI, Ritterband DC, Yu G, Shields RA, Seedor JA. . Graft rejection. Am J Ophthalmol. 2012; 153: 949– 957.e1. [DOI] [PubMed] [Google Scholar]
- 26. Anshu A, Price MO, Price FW. . Risk of corneal transplant rejection significantly reduced with Descemet's membrane endothelial keratoplasty. OPHTHA. 2012; 119: 536– 540. [DOI] [PubMed] [Google Scholar]
- 27. Bartakova A, Alvarez-Delfin K, Weisman AD,et al. Novel identity and functional markers for human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2016; 57: 2749– 2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu C, Joyce NC. . Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci. 2004; 45: 1743– 1751. [DOI] [PubMed] [Google Scholar]
- 29. Chen KH, Azar D, Joyce NC. . Transplantation of adult human corneal endothelium ex vivo: a morphologic study. Cornea. 2001; 20: 731– 737. [DOI] [PubMed] [Google Scholar]
- 30. Joyce NC, Zhu CC. . Human corneal endothelial cell proliferation: potential for use in regenerative medicine. Cornea. 2004; 23 8 suppl: S8– S19. [DOI] [PubMed] [Google Scholar]
- 31. Lee JG, Kay EP. . Cross-talk among Rho GTPases acting downstream of PI 3-kinase induces mesenchymal transformation of corneal endothelial cells mediated by FGF-2. Invest Ophthalmol Vis Sci. 2006; 47: 2358. [DOI] [PubMed] [Google Scholar]
- 32. Lee HT. . FGF-2 induced by interleukin-1 through the action of phosphatidylinositol 3-kinase mediates endothelial mesenchymal transformation in corneal endothelial cells. J Biol Chem. 2004; 279: 32325– 32332. [DOI] [PubMed] [Google Scholar]
- 33. Lee JG, Kay EP. . FGF-2-mediated signal transduction during endothelial mesenchymal transformation in corneal endothelial cells. Exp Eye Res 2006; 83: 1309– 1316. [DOI] [PubMed] [Google Scholar]
- 34. Karamichos D, Hutcheon AEK, Zieske JD. . Reversal of fibrosis by TGF-β3 in a 3D in vitro model. Exp Eye Res. 2014; 124: 31– 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakano Y, Oyamada M, Dai P, Nakagami T, Kinoshita S, Takamatsu T. . Connexin43 knockdown accelerates wound healing but inhibits mesenchymal transition after corneal endothelial injury in vivo. Invest Ophthalmol Vis Sci. 2008; 49: 93– 104. [DOI] [PubMed] [Google Scholar]
- 36. Joyce NC, Zhu CC, Harris DL. . Relationship among oxidative stress, DNA damage, and proliferative capacity in human corneal endothelium. Invest Ophthalmol Vis Sci. 2009; 50: 2116– 2122. [DOI] [PubMed] [Google Scholar]
- 37. Joyce NC, Harris DL, Zhu CC. . Age-related gene response of human corneal endothelium to oxidative stress and DNA damage. Invest Ophthalmol Vis Sci. 2011; 52: 1641– 1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shima N, Kimoto M, Yamaguchi M, Yamagami S. . Increased proliferation and replicative lifespan of isolated human corneal endothelial cells with L-ascorbic acid 2-phosphate. Invest Ophthalmol Vis Sci. 2011; 52: 8711– 8717. [DOI] [PubMed] [Google Scholar]
- 39. Okumura N, Koizumi N, Ueno M,et al. Enhancement of corneal endothelium wound healing by Rho-associated kinase (ROCK) inhibitor eye drops. Br J Ophthalmol. 2011; 95: 1006– 1009. [DOI] [PubMed] [Google Scholar]
- 40. Okumura N, Koizumi N, Ueno M,et al. The new therapeutic concept of using a Rho kinase inhibitor for the treatment of corneal endothelial dysfunction. Cornea. 2011; 30: S54– S59. [DOI] [PubMed] [Google Scholar]
- 41. de Lau W, Barker N, Low TY,et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011; 476: 293– 297. [DOI] [PubMed] [Google Scholar]
- 42. Hirata-Tominaga K, Nakamura T, Okumura N,et al. Corneal endothelial cell fate is maintained by LGR5 through the regulation of hedgehog and Wnt pathway. Stem Cells. 2013; 31: 1396– 1407. [DOI] [PubMed] [Google Scholar]
- 43. Okumura N, Kay EP, Nakahara M, Hamuro J, Kinoshita S, Koizumi N. . Inhibition of TGF-β signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. In: Connon CJ, . ed PLoS One. 2013; 8: e58000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Okumura N, Koizumi N, Ueno M,et al. PIIS0002944012003227. Am J Pathol. 2012; 181: 268– 277. [DOI] [PubMed] [Google Scholar]
- 45. Okumura N, Nakamura T, Kay EP, Nakahara M, Kinoshita S, Koizumi N. . R-spondin1 regulates cell proliferation of corneal endothelial cells via the Wnt3a/beta-catenin pathway. Invest Ophthalmol Vis Sci. 2014; 55: 6861– 6869. [DOI] [PubMed] [Google Scholar]
- 46. Okumura N, Koizumi N, Kay EP,et al. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2013; 54: 2493– 2502. [DOI] [PubMed] [Google Scholar]
- 47. Okumura N, Kinoshita S, Koizumi N. . Application of Rho kinase inhibitors for the treatment of corneal endothelial diseases. J Ophthalmol. 2017;2017:2646904. [DOI] [PMC free article] [PubMed]
- 48. Koizumi N, Okumura N, Kinoshita S. . Human corneal endothelium regeneration: effect of ROCK inhibitor. Invest Ophthalmol Vis Sci. 2013; 54: 5594– 5595. [DOI] [PubMed] [Google Scholar]
- 49. Okumura N, Nakano S, Kay EP,et al. Involvement of cyclin D and p27 in cell proliferation mediated by ROCK inhibitors Y-27632 and Y-39983 during corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2014; 55: 318– 329. [DOI] [PubMed] [Google Scholar]
- 50. Peh GSL, Chng Z, Ang H-P,et al. Propagation of human corneal endothelial cells: a novel dual media approach. Cell Transplant. 2015; 24: 287– 304. [DOI] [PubMed] [Google Scholar]
- 51. Moysidis SN, Alvarez-Delfin K, Peschansky VJ,et al. Magnetic field-guided cell delivery with nanoparticle-loaded human corneal endothelial cells. Nanomedicine. 2015; 11: 499– 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Joyce NC, Meklir B, Joyce SJ, Zieske JD. . Cell cycle protein expression and proliferative status in human corneal cells. Invest Ophthalmol Vis Sci. 1996; 37: 645– 655. [PubMed] [Google Scholar]
- 53. Joyce NC, Navon SE, Roy S, Zieske JD. . Expression of cell cycle-associated proteins in human and rabbit corneal endothelium in situ. Invest Ophthalmol Vis Sci. 1996; 37: 1566– 1575. [PubMed] [Google Scholar]
- 54. Joyce NC, Harris DL, Mello DM. . Mechanisms of mitotic inhibition in corneal endothelium: contact inhibition and TGF-beta2. Invest Ophthalmol Vis Sci. 2002; 43: 2152– 2159. [PubMed] [Google Scholar]
- 55. Griffith M. . Functional human corneal equivalents constructed from cell lines. Science. 1999; 286: 2169– 2172. [DOI] [PubMed] [Google Scholar]
- 56. Takezawa T, Ozaki K, Nitani A, Takabayashi C, Shimo-Oka T. . Collagen vitrigel: a novel scaffold that can facilitate a three-dimensional culture for reconstructing organoids. Cell Transplant. 2004; 13: 463– 473. [DOI] [PubMed] [Google Scholar]
- 57. Koizumi N, Sakamoto Y, Okumura N,et al. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci. 2007; 48: 4519– 4526. [DOI] [PubMed] [Google Scholar]
- 58. Fagerholm P, Lagali NS, Merrett K,et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Science Trans Med. 2010; 2: 46ra61. [DOI] [PubMed] [Google Scholar]
- 59. Karamichos D, Hutcheon AEK, Zieske JD. . Transforming growth factor-β3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J Tissue Eng Regen Med. 2011; 5: e228– e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Koizumi N, Sakamoto Y, Okumura N,et al. Cultivated corneal endothelial transplantation in a primate: possible future clinical application in corneal endothelial regenerative medicine. Cornea. 2008; 27 suppl 1: S48– S55. [DOI] [PubMed] [Google Scholar]
- 61. Patel SV, Bachman LA, Hann CR, Bahler CK, Fautsch MP. . Human corneal endothelial cell transplantation in a human ex vivo model. Invest Ophthalmol Vis Sci. 2009; 50: 2123– 2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Peh GSL, Beuerman RW, Colman A, Tan DT, Mehta JS. . Human corneal endothelial cell expansion for corneal endothelium transplantation: an overview. Transplantation. 2011; 91: 811– 819. [DOI] [PubMed] [Google Scholar]
- 63. Joyce NC. . Proliferative capacity of corneal endothelial cells. Exp Eye Res. 2012; 95: 16– 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mimura T, Joyce NC. . Replication competence and senescence in central and peripheral human corneal endothelium. Invest Ophthalmol Vis Sci. 2006; 47: 1387– 1396. [DOI] [PubMed] [Google Scholar]
- 65. Baum JL, Niedra R, Davis C, Yue BY. . Mass culture of human corneal endothelial cells. Arch Ophthalmol. 1979; 97: 1136– 1140. [DOI] [PubMed] [Google Scholar]
- 66. Peh GSL, Toh K-P, Wu F-Y, Tan DT, Mehta JS. . Cultivation of human corneal endothelial cells isolated from paired donor corneas. Mohan RR, . ed PLoS One. 2011; 6: e28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee JG, Kay EP. . FGF-2-induced wound healing in corneal endothelial cells requires Cdc42 activation and Rho inactivation through the phosphatidylinositol 3-kinase pathway. Invest Ophthalmol Vis Sci. 2006; 47: 1376– 1386. [DOI] [PubMed] [Google Scholar]
- 68. Lee JG, Ko MK, Kay EP. . Endothelial mesenchymal transformation mediated by IL-1β FGF-2 in corneal endothelial cells. Exp Eye Res. 2012; 95: 35– 39. [DOI] [PubMed] [Google Scholar]
- 69. Lee JG, Kay EP. . NF-κB is the transcription factor for FGF-2 that causes endothelial mesenchymal transformation in cornea. Invest Ophthalmol Vis Sci. 2012; 53: 1530– 1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Peh GSL, Adnan K, George BL,et al. The effects of Rho-associated kinase inhibitor Y-27632 on primary human corneal endothelial cells propagated using a dual media approach. Sci Rep. 2015; 5: 9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.