ABSTRACT
Resident microbes promote many aspects of host development, although the mechanisms by which microbiota influence host tissues remain unclear. We showed previously that the microbiota is required for allocation of appropriate numbers of secretory cells in the zebrafish intestinal epithelium. Because Notch signaling is crucial for secretory fate determination, we conducted epistasis experiments to establish whether the microbiota modulates host Notch signaling. We also investigated whether innate immune signaling transduces microbiota cues via the Myd88 adaptor protein. We provide the first evidence that microbiota-induced, Myd88-dependent signaling inhibits host Notch signaling in the intestinal epithelium, thereby promoting secretory cell fate determination. These results connect microbiota activity via innate immune signaling to the Notch pathway, which also plays crucial roles in intestinal homeostasis throughout life and when impaired can result in chronic inflammation and cancer.
KEY WORDS: Microbiota, Notch, Myd88, Intestinal cell determination, Secretory cell, Zebrafish
Highlighted Article: Investigations in gnotobiotic zebrafish reveal that resident intestinal microbes determine intestinal secretory cell fate by modulating host Notch signaling, a highly conserved pathway involved in myriad host cell fate decisions.
INTRODUCTION
Host-associated microbiota play important roles in animal health and development (McFall-Ngai et al., 2013), yet the mechanisms by which they influence processes such as cell differentiation remain unknown. Understanding the relationship between microbiota and intrinsic developmental pathways is key for developing strategies to promote intestinal homeostasis in pathological situations. We showed previously that the microbiota is necessary to promote secretory fates in the larval zebrafish intestinal epithelium (Bates et al., 2006). Zebrafish larvae reared germ free (GF) have fewer secretory cells than their conventionally reared (CV) counterparts; this deficit is reversed by conventionalization (CVZ) of GF animals with zebrafish-associated microbes.
Intestinal secretory fate determination is regulated by Notch signaling across many species (Fre et al., 2011), including zebrafish (Crosnier et al., 2005) and mammals (Noah and Shroyer, 2013; Yuan et al., 2015). Intestinal stem cells differentiate into two primary cell types: absorptive enterocytes and secretory cells. Secretory cells are further distinguished into mucus-secreting goblet cells, hormone-secreting enteroendocrine cells (EECs), and, in mammals, antimicrobial-secreting Paneth cells and chemosensing, immunostimulatory tuft cells (Gerbe et al., 2016; Noah and Shroyer, 2013). The mechanisms that drive specific intestinal cell lineages are currently under investigation, but involve signaling between differentiating cells expressing Notch ligands, such as Delta, that activate Notch receptors on adjacent cells; signal-receiving cells become absorptive enterocytes, whereas signal-producing cells become secretory (Koch et al., 2013). In zebrafish, no molecular markers are known for intestinal stem or progenitor cells, but mature secretory cells are distinguished by multiple markers (Fig. 1). Zebrafish are well-suited to the study of vertebrate host-microbe interactions because it is easy to derive large numbers of GF individuals (Melancon et al., 2017), they are optically transparent allowing visualization of both host and microbial cells (Taormina et al., 2012), and they are genetically tractable.
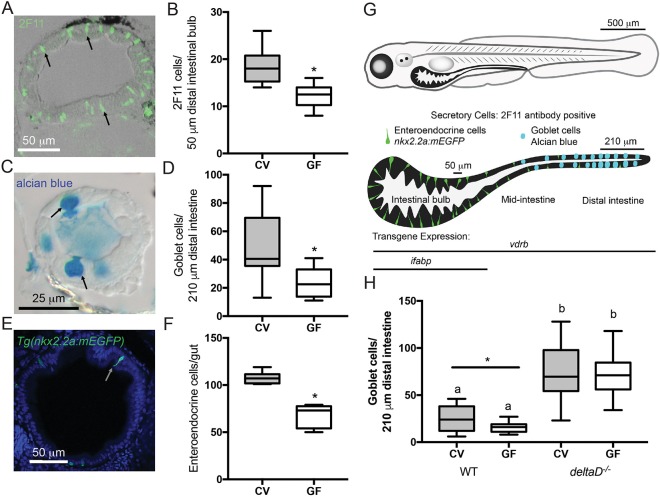
Fig. 1.
The microbiota promotes intestinal epithelium secretory cell fates through the Notch ligand DeltaD. (A) Representative image of cross-section stained with 2F11 antibody (e.g. black arrows). (B) Number of 2F11-positive secretory cells in CV and GF larvae; n=16. (C) Representative image of mucus-containing vacuole of goblet cells stained with Alcian Blue (black arrows). (D) Counts of Alcian Blue-positive goblet cells in CV and GF larvae; n=18 (CV), 14 (GF). (E) Representative cross-section of Tg(nkx2.2a:mEGFP) larva expressing GFP in (EECs (white arrow). (F) Number of GFP-positive EECs in GF and CV Tg(nkx2.2a:mEGFP) larvae; n=6 (CV), 9 (GF). (G) Schematic of larval zebrafish and larvae intestine. Enteroendocrine and goblet secretory cells are indicated in their normal intestinal locations. The transgene vdrb is expressed through the entire intestine whereas ifabp is expressed only in the bulb. Labeled bars by the isolated intestine schematic indicate the bulb and distal intestine regions scored for 2F11 and Alcian Blue, respectively. (H) Number of Alcian Blue-positive goblet cells in CV and GF WT and deltaD−/− larvae; n=15 (WT), 12 (CV deltaD−/−), 13 (GF deltaD−/−). *P<0.05, Student's t-test. Letters denote P<0.05, ANOVA followed by Tukey's post-hoc test. Each box represents the first to third quartiles, center bar the median, and whiskers the maximum and minimum of each dataset.
The mechanisms by which microbiota interact with Notch signaling and influence intestinal cell fate are unknown. Studies link Notch signaling to toll-like receptors (TLRs) that detect bacterial components and induce innate immune responses (Palaga et al., 2008; Shang et al., 2016; Zhang et al., 2012) via a conserved adaptor protein, Myd88 (Fre et al., 2011; Stein et al., 2007). We found that Myd88-dependent signaling is required for microbiota to promote zebrafish intestinal epithelial proliferation (Cheesman et al., 2011), suggesting that innate immunity can mediate cell fate responses to intestinal microbes.
Here, we tested the hypothesis that the microbiota promotes secretory fates through Myd88-dependent inhibition of Notch signaling, by examining epistatic relationships between the microbiota, Notch signaling and Myd88-dependent signaling in gnotobiotic zebrafish. We found that modulation of intestinal Notch signaling is downstream of microbial signals that promote secretory fates, and that the microbiota modulates Notch signaling via Myd88. We provide the first evidence that microbiota-induced Myd88-dependent signaling plays a role in Notch-mediated intestinal cell fate determination.
RESULTS AND DISCUSSION
Microbiota-induced Notch signaling promotes intestinal epithelial secretory cell fate determination
We previously showed a paucity of secretory cells in 8 days post-fertilization (dpf) GF zebrafish (Bates et al., 2006). Here, we provide evidence that 7 dpf GF larvae also have fewer secretory cells than their CV siblings, as revealed by 2F11 antibody staining (Fig. 1A,B; Crosnier et al., 2005; Zhang et al., 2014), goblet cell markers (Alcian Blue; Fig. 1C,D) and EEC transgene (nkx2.2a:mEGFP) expression (Fig. 1E,F; Pauls et al., 2007). We previously found no difference in the total number of intestinal epithelial cells in GF and CV larvae (Bates et al., 2006), suggesting that the paucity of secretory cells in GF intestines is due to reallocation of cells to the enterocyte fate. Fig. 1G shows a diagrammatic representation of secretory cell types along the length of the intestine (Wallace et al., 2005), and indicates the regions analyzed for Fig. 1A-F and the expression domains of transgenes used in subsequent experiments. Our finding that the GF intestinal epithelium contains fewer secretory cells mirrors reports from GF rats (Tomas et al., 2013; Uribe et al., 1994) and mice (Kandori et al., 1996). Additionally, in fruit flies the microbiota modulates intestinal secretory fate determination through Notch signaling, although in this case Notch signaling is decreased and enteroendocrine cells are more abundant in the GF state (Broderick et al., 2014).
Notch signaling is a key mechanism for cell fate determination throughout the animal kingdom. We combined gnotobiotic and genetic manipulations to test whether the microbiota influences secretory fate via Notch signaling. CV homozygous deltaD mutants have many more intestinal epithelial secretory cells than CV wild-type (WT) siblings (Fig. 1H), but not as many as observed in mind bombta52b zebrafish, which harbor a mutation that abrogates all Notch signaling, causing complete conversion of the intestinal epithelium to the secretory fate (Crosnier et al., 2005). The increased number of goblet cells in CV versus GF WT intestines is consistent with the hypothesis that the microbiota promotes secretory fates by inhibiting Notch signaling; alternatively, there could be a parallel, Notch-independent effect. We reasoned that if microbiota cues that promote goblet cells act in parallel with DeltaD, then we should observe fewer goblet cells in GF than in CV deltaD mutants. In contrast, we observed a similarly high number of goblet cells in CV and GF deltaD mutant intestines (Fig. 1H), consistent with the model that host perception of microbiota cues requires DeltaD to promote intestinal secretory fate.
Intestinal epithelial Notch signaling is required for microbial-dependent secretory cell fate
To determine whether Notch signaling is necessary in the intestinal epithelium to transduce microbial cues that promote secretory fates, we generated transgenic lines with intestine-specific expression of constructs that constitutively activate or repress Notch signaling. To establish whether intestinal epithelial Notch activation inhibits secretory fates, we used the Tg(UAS:nicd) line, which ectopically activates Notch signaling by expressing the Notch intracellular domain under control of the yeast upstream activating sequence (Cambier et al., 2014). To achieve intestine-specific expression, we used a transgenic line isolated in a Gal4-enhancer trap screen (Distel et al., 2009) that expresses the yeast Gal4 transcription factor throughout the larval intestinal epithelium (Fig. 2A), including in secretory cells (Fig. S1). We mapped the GAL4;Tg(UAS:mCherry) insertion to the vitamin D receptor (vdrb) gene, which in mammals is expressed in all intestinal epithelial cells including stem cells (Peregrina et al., 2015). Tg(vdrb:GAL4); Tg(UAS:ncid); Tg(UAS:mCherry) larvae had fewer goblet cells compared with sibling Tg(vdrb:GAL4); Tg(UAS:mCherry) larvae (Fig. 2B), consistent with our expectation that intestinal epithelial Notch activation is sufficient to inhibit secretory fates and promote absorptive fates.
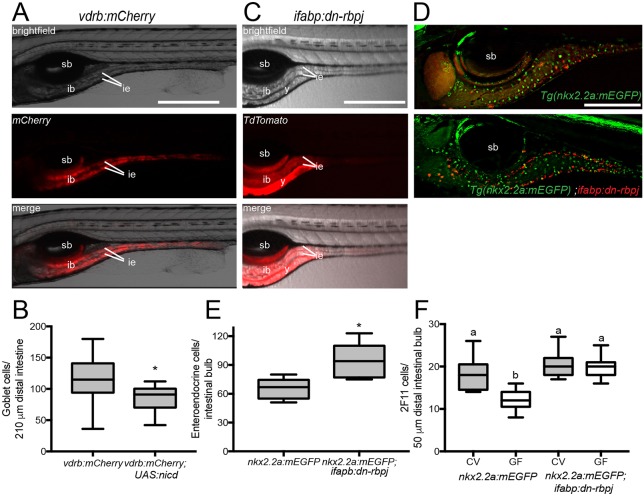
Fig. 2.
Modulating Notch signaling within the intestinal epithelium is sufficient to alter secretory cell numbers. (A) Expression of Tg(vdrb:GAL4); Tg(UAS:mCherry); (vdrb:mCherry) with brightfield, fluorescence and merged signals; vdrb:GAL4 is expressed throughout the intestinal epithelium. (B) Number of goblet cells in vdrb:mCherry and vdrb:mCherry; Tg(UAS:nicd) zebrafish. n=23 (vdrb:mCherry), 10 [vdrb:mCherry; Tg(UAS:nicd)]. (C) Expression of ifabp:dn-rbpj with brightfield, fluorescence and merged signals; note that ifabp drives expression primarily in posterior intestinal bulb and proximal intestine. (D) Representative images of Tg(nkx2.2a:mEGFP) and Tg(nkx2.2a:mEGFP) crossed with ifabp:dn-rbpj to determine the effect of modulating Notch on EECs (green) in regions in which Notch signaling is augmented or suppressed (red). (E) Number of EECs in ifabp:dn-rbpj larvae; n=5 [Tg(nkx2.2a:mEGFP)], 7 [Tg(nkx2.2a:mEGFP); ifabp:dn-rbpj]. (F) Quantification of 2F11-positive secretory cells in CV and GF Tg(nkx2.2a:mEGFP) and Tg(nkx2.2a:mEGFP); ifabp:dn-rbpj larvae; n=17 for each condition. ib, intestinal bulb; ie, intestinal epithelium; sb, swim bladder; y, yolk. *P<0.05, Student's t-test. Letters denote P<0.05, ANOVA followed by Tukey's post-hoc test. Each box represents the first to third quartiles, center bar the median, and whiskers the maximum and minimum of each dataset. Scale bars: 500 µm (A,C); 250 µm (D).
To confirm an intestine-specific role for Notch signaling in secretory fate specification, we generated a stable line that expresses the dn-rbpj transgene, encoding a dominant-negative form of the Notch pathway transcription factor Rbpj, to repress Notch (Guruharsha et al., 2012). We drove intestinal epithelial expression of dn-rbpj and a TdTomato reporter, using a 1.8 kb fragment of the intestinal fatty acid binding protein promoter (ifabp; fabp2) (Her et al., 2004). Using this same ifabp promoter element, we previously induced elevated epithelial cell proliferation upon ectopic expression of the Helicobacter pylori effector protein CagA (Neal et al., 2013), suggesting that the promoter's expression domain includes intestinal epithelial stem or progenitor cells. Expression of ifabp:TdTomato:p2a:dn-rbpj (ifabp:dn-rbpj) is restricted to proximal intestinal epithelium (Fig. 2C), where most secretory cells are EECs (Fig. 1G) (Wallace et al., 2005). To assess the effects of Notch signaling modulation on proximal intestinal secretory cell specification, we crossed the EEC marker Tg(nkx2.2a:mEGFP) into our transgenic line and quantified GFP-expressing cells in the region expressing TdTomato (Fig. 2D). Consistent with the ifabp promoter driving expression of Notch-modulating transgenes in progenitors of mature secretory cells, we observed more EECs in fish expressing ifabp:dn-rbpj (Fig. 2E). To exclude the possibility of tissue hyperplasia in ifabp:dn-rbpj intestines with elevated EECs, we estimated total epithelial cells by quantifying them in representative transverse sections in three regions [esophageal intestinal junction (eij), intestinal bulb (ib) and mid-intestine (mi)] and observed no differences between ifabp:dn-rbpj and WT larvae (Fig. S2).
We next addressed whether microbiota-derived cues promote secretory fates by activating intestinal epithelial Notch signaling. The low number of goblet cells in CV Tg(vdrb:GAL4); Tg(UAS:ncid); Tg(UAS:mCherry)-expressing larvae (Fig. 2B), similar to levels in GF WTs (Fig. 1D), is consistent with our model that the microbiota suppresses Notch signaling. However, the low number of goblet cells in both groups made it difficult to assess whether the microbiota affected the Tg(vdrb:GAL4); Tg(UAS:ncid); Tg(UAS:mCherry) goblet cell phenotype. In contrast, the high number of EECs in ifabp:dn-rbpj larvae (Fig. 2E) provided an informative phenotype for microbiota manipulations. We found that CV and GF dn-rbpj-expressing larvae had the same high number of 2F11-positive secretory cells (Fig. 2F), consistent with the microbiota functioning upstream of Rbpj in the Notch pathway within the intestinal epithelium.
The microbiota promotes secretory cell fates by signaling through Myd88
We next addressed how microbiota cues are perceived by the host Notch pathway. We showed previously that the innate immune signaling adaptor Myd88 is responsible for microbiota-dependent developmental processes (Bates et al., 2007; Cheesman et al., 2011). To learn whether Myd88 promotes secretory fates, we used myd88 mutants with a stop codon at amino acid 85, which results in a prematurely truncated protein containing only the N-terminal death domain (van der Vaart et al., 2013). This myd88 mutation resulted in fewer goblet cells in both heterozygotes and homozygotes (Fig. 3A), suggesting that it behaves in a dominant-negative manner. Because this mutant was severely immunocompromised and difficult to maintain, we also used a previously validated splice-blocking morpholino (MO) specific to the myd88 exon2/intron2 boundary (Bates et al., 2007; Cambier et al., 2014). As in myd88 mutants, MO-mediated reduction of myd88 expression resulted in fewer goblet cells compared with control siblings (Fig. 3B). One downstream effector of Myd88 is the pro-inflammatory cytokine tumor necrosis factor alpha (TNFα) (Akira and Takeda, 2004). We used previously validated splice-blocking MOs to knock down expression of tnfrsf1a (tumor necrosis factor receptor superfamily, member 1a), which encodes the TNF receptor (Bates et al., 2007), and observed fewer goblet cells in MO-injected animals compared with control siblings (Fig. 3C). These data support a requirement for Myd88-dependent signaling to promote intestinal secretory fates.
Fig. 3.
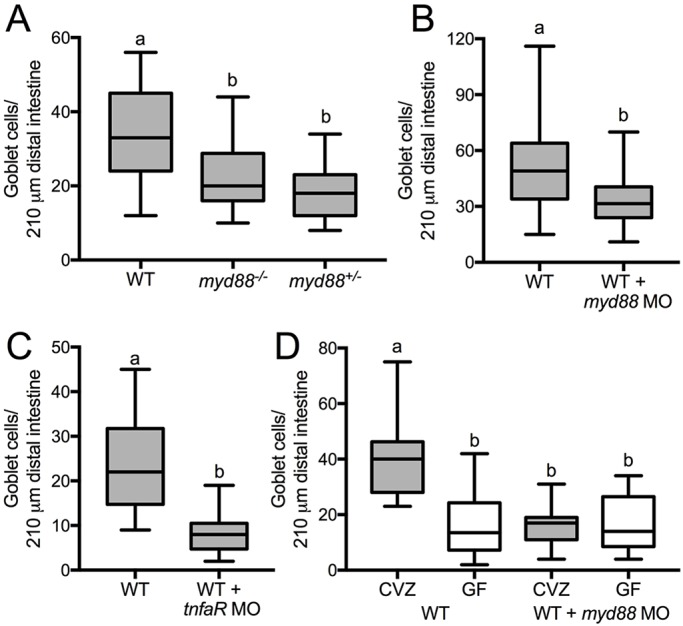
Myd88-dependent signaling is required for the microbiota to promote goblet cell fate. Alcian Blue-positive goblet cells were counted in multiple conditions. (A) Heterozygous and homozygous myd88 mutant larvae compared with WT siblings; n=17 (WT), 14 (myd88−/−), 15 (myd8+/−). (B) myd88 MO-injected WT larvae; n=19 (WT), 22 (WT+myd88 MO). (C) tnfrsf1a MO-injected WT larvae; n=14 (WT), 10 (WT+ tnfaR MO). (D) WT and myd88 MO-injected larvae siblings derived CVZ or GF; n=14 (CVZ WT), 12 (GF WT), 15 (CVZ WT+myd88 MO), 13 (GF WT+myd88 MO). Letters denote P<0.05, ANOVA followed by Tukey's post-hoc test. Each box represents the first to third quartiles, center bar the median, and whiskers the maximum and minimum of each dataset.
To test whether Myd88 acts downstream of the microbiota to promote secretory fates, we derived GF myd88 MO-injected and control mock-injected embryos and compared the secretory cell numbers in larvae reared GF or CVZ after the injections. We observed the expected reduction in secretory cells in GF versus CVZ larvae and found that secretory cell numbers in both CVZ and GF myd88 MO-injected larvae were comparable to GF controls, and were all significantly lower than the number of secretory cells in control CVZ larvae (Fig. 3D). The absence of an additive reduction in secretory cell number with the combined loss of myd88 and microbiota suggests that secretory cell-promoting signals from the microbiota are mediated through Myd88.
Myd88 requires intestinal epithelial Notch signaling to promote secretory cell fates
Our epistasis tests of the signaling pathways required for promoting intestinal epithelial secretory fates placed the microbiota upstream of both Myd88 and intestinal epithelial Notch signaling. To determine whether Myd88 acts directly in the Notch pathway, we knocked down Myd88 function in WTs and deltaD mutants. We found fewer goblet cells in myd88 MO-injected WT larvae compared with WT controls. Moreover, we saw an increase in goblet cells in both myd88 MO-injected and control deltaD mutants (Fig. 4A), demonstrating that Myd88 functions upstream of DeltaD to promote goblet cell fates.
Fig. 4.
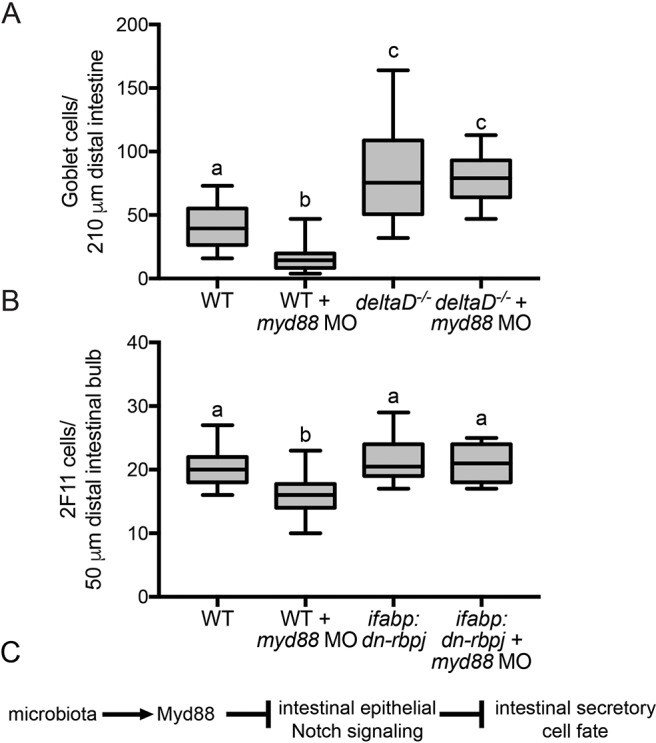
Myd88 requires Notch signaling to promote goblet cell fates. (A) Number of goblet cells in myd88 MO-injected WT and deltaD−/− larvae; n=22 (WT), 20 (WT+myd88 MO and deltaD−/−), 19 (deltaD−/−+myd88 MO). (B) Number of 2F11-positive secretory cells in myd88 MO-injected WT and ifabp:dn-rbpj larvae; n=18. (C) Schematic showing our model that the microbiota interacts with Myd88 to inhibit Notch signaling, which, in turn, inhibits intestinal secretory cell fate. Letters denote P<0.05, ANOVA followed by Tukey's post-hoc test. Each box represents the first to third quartiles, center bar the median, and whiskers the maximum and minimum of each dataset.
Finally, we addressed whether Myd88 requires intestine-specific Notch signaling to promote secretory fates. We compared the effect of Myd88 on secretory cells by injecting myd88 MO into WT and ifabp:dn-rbpj fish. ifabp:dn-rbpj larvae with inhibited intestinal epithelial Notch signaling showed no response to MO-mediated myd88 inhibition (Fig. 4B). Additionally, we confirmed that the change in secretory cell number did not alter total intestinal cells in myd88 MO-injected fish (Fig. S2).
Microbiota modulation of intestinal epithelial Notch signaling regulates proportions of absorptive and secretory cells
Our results support a model in which the microbiota promotes intestinal secretory fates through Myd88-mediated inhibition of Notch signaling in the intestinal epithelium (Fig. 4C). Myd88 is also required for microbiota activation of Wnt signaling and promoting intestinal epithelial cell proliferation in the zebrafish larval intestine (Cheesman et al., 2011). A subtle distinction between these two phenomena is that Tnfr is required for secretory fate determination and dispensable for normal epithelial cell renewal (Cheesman et al., 2011), but plays a role in pathological, inflammation-associated intestinal epithelial hyperplasia (Rolig et al., 2017). Because Notch and Wnt signaling are intimately coordinated in the balance of intestinal epithelial cell proliferation and differentiation (Sancho et al., 2015), it will be interesting to dissect the microbiota cues that stimulate these different pathways.
Host-microbe interactions involving developmental signaling pathways likely evolved because they provide an advantage to the host and its resident microbiota. In the vertebrate intestine, secretory cells are crucial for shaping the environment to foster controlled microbial growth and confer protection against microbial insults. Goblet cells secrete mucus that creates a barrier between luminal contents and epithelial cells, and also provides microbiota with habitats and nutrients (Desai et al., 2016; Johansson and Hansson, 2016; Kashyap et al., 2013). EECs impact the intestinal environment through secretion of over 30 peptide hormones (Vincent et al., 2011) that aid digestion and absorption (Tolhurst et al., 2012), glucose metabolism (Pais et al., 2016), and motility (Sikander et al., 2009). Motility in turn can profoundly impact the microbiota (Rolig et al., 2017; Wiles et al., 2016).
Secretory cells shape the resident microbes' intestinal environment and emerging evidence suggests they also are sensors for microbial products. For example, goblet cells respond to both host factors, such as acetylcholine, and microbial factors, through host innate signaling receptors, including NLRP6 (Birchenough et al., 2015; Wlodarska et al., 2014) and Myd88 (Knoop et al., 2017, 2015; Miller et al., 2014). Similarly, EECs are luminal sensors of microbiota (Bogunovic et al., 2007) and short chain fatty acids (Gribble and Reimann, 2016; Reigstad et al., 2015), which may prime the host immune system during intestinal dysbiosis (Worthington, 2015). The Myd88-dependent sensing of microbiota cues we describe here might be mediated by secretory cells, which could modulate Notch signaling in the tissue to maintain an appropriate census of secretory cells for the organ's microbial environment. Our results suggest that development of at least two secretory cell types, goblet cells and EECs, are subject to similar genetic and environmental controls. It would be interesting to learn whether there are additional regional and cell type-specific processes involved that affect development of these cells in distinct ways along the length of the gut.
Not only is Notch signaling required for cell fate determination in the developing intestine, but it also plays crucial roles in tissue homeostasis throughout life (Sancho et al., 2015). For example, mice with intestinal epithelial-specific deletion of Rbpj exhibit impaired barrier function and develop microbiota-dependent spontaneous colitis (Obata et al., 2012). On the other end of the spectrum, mice overexpressing intestinal epithelial claudin 1 exhibit increased Notch signaling, loss of goblet cells and protective mucus, and increased sensitivity to dextran sulfate sodium-induced colitis (Pope et al., 2014). The finding that resident microbiota can modulate host intestinal epithelial Notch signaling offers the potential for new therapeutic approaches to target this crucial pathway to promote intestinal health throughout life.
MATERIALS AND METHODS
Animal husbandry, gnotobiology and genetics
Experiments were conducted according to protocols approved by the University of Oregon Institutional Animal Care and Use Committee and followed standard zebrafish protocols (Westerfield, 2007). GF embryos were generated by surface sterilization (Bates et al., 2007, 2006), and then reared without feeding in sterile embryo media in tissue culture flasks. Experiments were conducted at 7 dpf, rather than at 8 dpf as in Bates et al. (2006), to reduce possible nutrient deprivation secondary effects. Sterility was verified by plating the media to count colony forming units (CFUs) and by visual inspection through a compound microscope. CV fish were naturally spawned sibling embryos not subjected to surface sterilization and instead kept in UO Zebrafish Facility system water but were otherwise reared under the same conditions as GF counterparts. We verified that media chemistry (pH, conductivity, ammonia, nitrates, nitrites, alkalinity) was the same for GF, CV and CVZ conditions.
Generation of the ifabp:TdTomato:p2a:dn-rbpj (ifabp:dn-rbpj) transgenic line was accomplished using splicing by overlap extension (SOE) PCR as described further in the supplementary Materials and Methods. Briefly, a construct in which a 1.8 kb region of the ifabp promoter sequence (Table S1) drove the expression of TdTomato and dn-rbpj was generated using primers presented in Table S2.
myd88 and tnfrsf1a MO-injected animals were generated as described previously (Bates et al., 2007) and detailed further in the supplementary Materials and Methods. Briefly, splice-blocking MOs (Gene Tools) were injected into embryos at the one-cell stage. Morpholino sequences are presented in Table S3 and splice blocking was verified by RT-PCR using the primers in Table S4. For GF derivation of myd88 MO-injected animals, embryos were generated by in vitro fertilization in antibiotic-containing embryo media (Pham et al., 2008). Embryos were injected at the one-cell stage, derived GF, and then a portion of the population immediately conventionalized by immersion in UO Zebrafish Facility system water containing zebrafish-associated bacteria (Stephens et al., 2016). AB/Tü was the WT reference for all experiments.
deltaDtr233 mutants were maintained as homozygotes (Holley et al., 2000; Jiang et al., 1996) and out-crossed with WT every other generation. Homozygous deltaDtr233 mutant embryos were identified by somite defects (Holley et al., 2000). myd88hu3568 mutants were maintained as homozygotes and identified by a PCR reaction showing a single base pair mutation at bp 390 from a T to an A (van der Vaart et al., 2013). The vdrb:GAL4;UAS:mCherry line was isolated in a Gal4-enhancer trap screen (Distel et al., 2009) and was maintained as a homozygous line. The vdrb:GAL4 insertion site was identified using a method similar to that developed to identify bacterial transposon insertion sites (Langridge et al., 2009), as described in the supplementary Materials and Methods using primers presented in Table S5.
Histological analysis
Details of histological analysis are provided in the supplementary Materials and Methods. Briefly, to quantify goblet cells, 7 dpf larvae were fixed in 4% paraformaldehyde in PBS overnight at 4°C, stained with 0.04% Alcian Blue (Sigma-Aldrich), embedded in paraffin, cut in 7 µm transverse sections and mounted on glass slides. We estimated total epithelial cells by staining sections with DAPI and counting nuclei in three different regions [esophageal intestinal junction (eij), intestinal bulb (ib), and mid-intestine (mi)].
Statistics
All secretory cell counting experiments were performed a minimum of two times with at least n=10. Absolute numbers of secretory cells varied between experiments, possibly owing in part to fluctuations in the composition of the CV microbiota; however, the trends in relative abundance of secretory cells between treatment groups were consistent across experiments. When only two groups were compared, significance was determined using Student's t-test assuming unequal variances. When more than two groups were compared, significance was determined using ANOVA and Tukey's pairwise comparisons, family error rate=0.05. All statistics were performed using the JMP9 software package.
Supplementary Material
Acknowledgements
Thanks to Erika Mittge, Rose Sockol, and the UO Zebrafish Facility staff for technical support.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.V.T., J.M.B., J.S.E., K.G.; Methodology: J.V.T., M.L.A., J.G., J.M.B., W.Z.S., E.M.; Validation: J.V.T.; Formal analysis: J.V.T., M.K.H.; Investigation: J.V.T.; Resources: M.V., A.H.M., M.D., J.S.E., K.G.; Data curation: J.S.E., K.G.; Writing - original draft: J.V.T., M.K.H.; Writing - review & editing: J.V.T., M.K.H., J.G., W.Z.S., E.M., J.S.E., K.G.; Visualization: J.V.T., M.K.H.; Supervision: J.S.E., K.G.; Project administration: J.V.T., M.K.H.; Funding acquisition: J.V.T., J.S.E., K.G.
Funding
Research reported in this publication was supported by an American Cancer Society postdoctoral fellowship (120188-PF-11-272-01-MPC to J.V.T.), and the National Institutes of Health (32DK089716 to J.V.T.; P01HD22486 and P50GM098911 to J.S.E. and K.G.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.155317.supplemental
References
- Akira S. and Takeda K. (2004). Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499-511. 10.1038/nri1391 [DOI] [PubMed] [Google Scholar]
- Bates J. M., Mittge E., Kuhlman J., Baden K. N., Cheesman S. E. and Guillemin K. (2006). Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev. Biol. 297, 374-386. 10.1016/j.ydbio.2006.05.006 [DOI] [PubMed] [Google Scholar]
- Bates J. M., Akerlund J., Mittge E. and Guillemin K. (2007). Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2, 371-382. 10.1016/j.chom.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchenough G. M. H., Johansson M. E. V., Gustafsson J. K., Bergström J. H. and Hansson G. C. (2015). New developments in goblet cell mucus secretion and function. Mucosal. Immunol. 8, 712-719. 10.1038/mi.2015.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M., Davé S. H., Tilstra J. S., Chang D. T. W., Harpaz N., Xiong H., Mayer L. F. and Plevy S. E. (2007). Enteroendocrine cells express functional Toll-like receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1770-G1783. 10.1152/ajpgi.00249.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick N. A., Buchon N. and Lemaitre B. (2014). Microbiota-induced changes in drosophila melanogaster host gene expression and gut morphology. MBio 5, e01117-14 10.1128/mBio.01117-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier C. J., Takaki K. K., Larson R. P., Hernandez R. E., Tobin D. M., Urdahl K. B., Cosma C. L. and Ramakrishnan L. (2014). Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 505, 218-222. 10.1038/nature12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesman S. E., Neal J. T., Mittge E., Seredick B. and Guillemin K. (2011). Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc. Natl. Acad. Sci. USA 108 Suppl 1, 4570-4577. 10.1073/pnas.1000072107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C., Vargesson N., Gschmeissner S., Ariza-McNaughton L., Morrison A. and Lewis J. (2005). Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 132, 1093-1104. 10.1242/dev.01644 [DOI] [PubMed] [Google Scholar]
- Desai M. S., Seekatz A. M., Koropatkin N. M., Kamada N., Hickey C. A., Wolter M., Pudlo N. A., Kitamoto S., Terrapon N., Muller A. et al. (2016). A dietary fiber-deprived Gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339-1353.e21. 10.1016/j.cell.2016.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel M., Wullimann M. F. and Koster R. W. (2009). Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc. Natl. Acad. Sci. USA 106, 13365-13370. 10.1073/pnas.0903060106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S., Bardin A., Robine S. and Louvard D. (2011). Notch signaling in intestinal homeostasis across species: the cases of Drosophila, Zebrafish and the mouse. Exp. Cell Res. 317, 2740-2747. 10.1016/j.yexcr.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Gerbe F., Sidot E., Smyth D. J., Ohmoto M., Matsumoto I., Dardalhon V., Cesses P., Garnier L., Pouzolles M., Brulin B. et al. (2016). Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226-230. 10.1038/nature16527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble F. M. and Reimann F. (2016). Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 78, 277-299. 10.1146/annurev-physiol-021115-105439 [DOI] [PubMed] [Google Scholar]
- Guruharsha K. G., Kankel M. W. and Artavanis-Tsakonas S. (2012). The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 13, 654-666. 10.1038/nrg3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Her G. M., Yeh Y.-H. and Wu J.-L. (2004). Functional conserved elements mediate intestinal-type fatty acid binding protein (I-FABP) expression in the gut epithelia of zebrafish larvae. Dev. Dyn. 230, 734-742. 10.1002/dvdy.20081 [DOI] [PubMed] [Google Scholar]
- Holley S. A., Geisler R. and Nusslein-Volhard C. (2000). Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity. Genes Dev. 14, 1678-1690. [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. J., Brand M., Heisenberg C. P., Beuchle D., Furutani-Seiki M., Kelsh R. N., Warga R. M., Granato M., Haffter P., Hammerschmidt M. et al. (1996). Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development 123, 205-216. [DOI] [PubMed] [Google Scholar]
- Johansson M. E. V. and Hansson G. C. (2016). Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 16, 639-649. 10.1038/nri.2016.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandori H., Hirayama K., Takeda M. and Doi K. (1996). Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp. Anim. 45, 155-160. 10.1538/expanim.45.155 [DOI] [PubMed] [Google Scholar]
- Kashyap P. C., Marcobal A., Ursell L. K., Smits S. A., Sonnenburg E. D., Costello E. K., Higginbottom S. K., Domino S. E., Holmes S. P., Relman D. A. et al. (2013). Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc. Natl. Acad. Sci. USA 110, 17059-17064. 10.1073/pnas.1306070110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop K. A., McDonald K. G., McCrate S., McDole J. R. and Newberry R. D. (2015). Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 8, 198-210. 10.1038/mi.2014.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop K. A., Gustafsson J. K., McDonald K. G., Kulkarni D. H., Kassel R. and Newberry R. D. (2017). Antibiotics promote the sampling of luminal antigens and bacteria via colonic goblet cell associated antigen passages. Gut Microbes 8, 1-12. 10.1080/19490976.2017.1299846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch U., Lehal R. and Radtke F. (2013). Stem cells living with a Notch. Development 140, 689-704. 10.1242/dev.080614 [DOI] [PubMed] [Google Scholar]
- Langridge G. C., Phan M.-D., Turner D. J., Perkins T. T., Parts L., Haase J., Charles I., Maskell D. J., Peters S. E., Dougan G. et al. (2009). Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res. 19, 2308-2316. 10.1101/gr.097097.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall-Ngai M., Hadfield M. G., Bosch T. C. G., Carey H. V., Domazet-Lošo T., Douglas A. E., Dubilier N., Eberl G., Fukami T., Gilbert S. F. et al. (2013). Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 110, 3229-3236. 10.1073/pnas.1218525110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon E., Gomez De La Torre Canny S., Sichel S., Kelly M., Wiles T. J., Rawls J. F., Eisen J. S. and Guillemin K. (2017). Best practices for germ-free derivation and gnotobiotic zebrafish husbandry. Methods Cell Biol. 138, 61-100. 10.1016/bs.mcb.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Knoop K. A. and Newberry R. D. (2014). Mind the GAPs: insights into intestinal epithelial barrier maintenance and luminal antigen delivery. Mucosal Immunol. 7, 452-454. 10.1038/mi.2014.4 [DOI] [PubMed] [Google Scholar]
- Neal J. T., Peterson T. S., Kent M. L. and Guillemin K. (2013). H. pylori virulence factor CagA increases intestinal cell proliferation by Wnt pathway activation in a transgenic zebrafish model. Dis. Model. Mech. 6, 802-810. 10.1242/dmm.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noah T. K. and Shroyer N. F. (2013). Notch in the intestine: regulation of homeostasis and pathogenesis. Annu. Rev. Physiol. 75, 263-288. 10.1146/annurev-physiol-030212-183741 [DOI] [PubMed] [Google Scholar]
- Obata Y., Takahashi D., Ebisawa M., Kakiguchi K., Yonemura S., Jinnohara T., Kanaya T., Fujimura Y., Ohmae M., Hase K. et al. (2012). Epithelial cell-intrinsic Notch signaling plays an essential role in the maintenance of gut immune homeostasis. J. Immunol. 188, 2427-2436. 10.4049/jimmunol.1101128 [DOI] [PubMed] [Google Scholar]
- Pais R., Gribble F. M. and Reimann F. (2016). Stimulation of incretin secreting cells. Ther. Adv. Endocrinol. Metab. 7, 24-42. 10.1177/2042018815618177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaga T., Buranaruk C., Rengpipat S., Fauq A. H., Golde T. E., Kaufmann S. H. E. and Osborne B. A. (2008). Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur. J. Immunol. 38, 174-183. 10.1002/eji.200636999 [DOI] [PubMed] [Google Scholar]
- Pauls S., Zecchin E., Tiso N., Bortolussi M. and Argenton F. (2007). Function and regulation of zebrafish nkx2.2a during development of pancreatic islet and ducts. Dev. Biol. 304, 875-890. 10.1016/j.ydbio.2007.01.024 [DOI] [PubMed] [Google Scholar]
- Peregrina K., Houston M., Daroqui C., Dhima E., Sellers R. S. and Augenlicht L. H. (2015). Vitamin D is a determinant of mouse intestinal Lgr5 stem cell functions. Carcinogenesis 36, 25-31. 10.1093/carcin/bgu221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham L. N., Kanther M., Semova I. and Rawls J. F. (2008). Methods for generating and colonizing gnotobiotic zebrafish. Nat. Protoc. 3, 1862-1875. 10.1038/nprot.2008.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J. L., Bhat A. A., Sharma A., Ahmad R., Krishnan M., Washington M. K., Beauchamp R. D., Singh A. B. and Dhawan P. (2014). Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut 63, 622-634. 10.1136/gutjnl-2012-304241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigstad C. S., Salmonson C. E., Rainey J. F. III, Szurszewski J. H., Linden D. R., Sonnenburg J. L., Farrugia G. and Kashyap P. C. (2015). Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395-1403. 10.1096/fj.14-259598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolig A. S., Mittge E. K., Ganz J., Troll J. V., Melancon E., Wiles T. J., Alligood K., Stephens W. Z., Eisen J. S. and Guillemin K. (2017). The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol. 15, e2000689 10.1371/journal.pbio.2000689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho R., Cremona C. A. and Behrens A. (2015). Stem cell and progenitor fate in the mammalian intestine: Notch and lateral inhibition in homeostasis and disease. EMBO Rep. 16, 571-581. 10.15252/embr.201540188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Smith S. and Hu X. (2016). Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell 7, 159-174. 10.1007/s13238-016-0250-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikander A., Rana S. V. and Prasad K. K. (2009). Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin. Chim. Acta 403, 47-55. 10.1016/j.cca.2009.01.028 [DOI] [PubMed] [Google Scholar]
- Stein C., Caccamo M., Laird G. and Leptin M. (2007). Conservation and divergence of gene families encoding components of innate immune response systems in zebrafish. Genome Biol. 8, R251 10.1186/gb-2007-8-11-r251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens W. Z., Burns A. R., Stagaman K., Wong S., Rawls J. F., Guillemin K. and Bohannan B. J. M. (2016). The composition of the zebrafish intestinal microbial community varies across development. ISME J. 10, 644-654. 10.1038/ismej.2015.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taormina M. J., Jemielita M., Stephens W. Z., Burns A. R., Troll J. V., Parthasarathy R. and Guillemin K. (2012). Investigating bacterial-animal symbioses with light sheet microscopy. Biol. Bull. 223, 7-20. 10.1086/BBLv223n1p7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G., Reimann F. and Gribble F. M. (2012). Intestinal sensing of nutrients. Handb. Exp. Pharmacol. 209, 309-335. 10.1007/978-3-642-24716-3_14 [DOI] [PubMed] [Google Scholar]
- Tomas J., Wrzosek L., Bouznad N., Bouet S., Mayeur C., Noordine M.-L., Honvo-Houeto E., Langella P., Thomas M. and Cherbuy C. (2013). Primocolonization is associated with colonic epithelial maturation during conventionalization. FASEB J. 27, 645-655. 10.1096/fj.12-216861 [DOI] [PubMed] [Google Scholar]
- Uribe A., Alam M., Johansson O., Midtvedt T. and Theodorsson E. (1994). Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology 107, 1259-1269. 10.1016/0016-5085(94)90526-6 [DOI] [PubMed] [Google Scholar]
- van der Vaart M., van Soest J. J., Spaink H. P. and Meijer A. H. (2013). Functional analysis of a zebrafish myd88 mutant identifies key transcriptional components of the innate immune system. Dis. Model. Mech. 6, 841-854. 10.1242/dmm.010843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent K. M., Sharp J. W. and Raybould H. E. (2011). Intestinal glucose-induced calcium-calmodulin kinase signaling in the gut-brain axis in awake rats. Neurogastroenterol. Motil. 23, e282-e293. 10.1111/j.1365-2982.2011.01673.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace K. N., Akhter S., Smith E. M., Lorent K. and Pack M. (2005). Intestinal growth and differentiation in zebrafish. Mech. Dev. 122, 157-173. 10.1016/j.mod.2004.10.009 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2007). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th edn. Eugene: Univ. of Oregon Press. [Google Scholar]
- Wiles T. J., Jemielita M., Baker R. P., Schlomann B. H., Logan S. L., Ganz J., Melancon E., Eisen J. S., Guillemin K. and Parthasarathy R. (2016). Host gut motility promotes competitive exclusion within a model intestinal microbiota. PLoS Biol. 14, e1002517 10.1371/journal.pbio.1002517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarska M., Thaiss C. A., Nowarski R., Henao-Mejia J., Zhang J.-P., Brown E. M., Frankel G., Levy M., Katz M. N., Philbrick W. M. et al. (2014). NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045-1059. 10.1016/j.cell.2014.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington J. J. (2015). The intestinal immunoendocrine axis: novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem. Soc. Trans. 43, 727-733. 10.1042/BST20150090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Wu H., Xu H., Xiong H., Chu Q., Yu S., Wu G. S. and Wu K. (2015). Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett. 369, 20-27. 10.1016/j.canlet.2015.07.048 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wang C., Liu Z., Liu X., Han C., Cao X. and Li N. (2012). Notch signal suppresses Toll-like receptor-triggered inflammatory responses in macrophages by inhibiting extracellular signal-regulated kinase 1/2-mediated nuclear factor kappaB activation. J. Biol. Chem. 287, 6208-6217. 10.1074/jbc.M111.310375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Golubkov V. S., Han W., Correa R. G., Zhou Y., Lee S., Strongin A. Y. and Dong P. D. S. (2014). Identification of Annexin A4 as a hepatopancreas factor involved in liver cell survival. Dev. Biol. 395, 96-110. 10.1016/j.ydbio.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.