ABSTRACT
Congenital laryngeal webs result from failure of vocal fold separation during development in utero. Infants present with life-threatening respiratory problems at birth, and extensive lifelong difficulties in breathing and voicing. The molecular mechanisms that instruct vocal fold formation are rarely studied. Here, we show, for the first time, that conditional inactivation of the gene encoding β-catenin in the primitive laryngopharyngeal epithelium leads to failure in separation of the vocal folds, which approximates the gross phenotype of laryngeal webbing. These defects can be traced to a series of morphogenesis defects, including delayed fusion of the epithelial lamina and formation of the laryngeal cecum, failed separation of the larynx and esophagus with reduced and disorganized cartilages and muscles. Parallel to these morphogenesis defects, inactivation of β-catenin disrupts stratification of epithelial cells and establishment of p63+ basal progenitors. These findings provide the first line of evidence that links β-catenin function to the cell proliferation and progenitor establishment during larynx and vocal fold development.
KEY WORDS: β-Catenin, Epithelial lamina, Laryngeal web, Vocal folds
Summary: The first demonstration that β-catenin signaling contributes to vocal fold morphogenesis by regulating proliferation and epithelial progenitor establishment, shedding light on the etiology of poorly understood congenital laryngeal malformations.
INTRODUCTION
Laryngeal webs are congenital malformations related to the incomplete recanalization of the laryngotracheal tube, when the vocal folds (VFs) fail to separate and obstruct the entrance of lower airway structures. These anomalies result from different degrees of failure of the epithelial lamina resorption during intrauterine development and they may present at the moment of birth with life-threating respiratory problems requiring immediate attention (Hartnick and Cotton, 2000; Wyatt et al., 2005). They can cause difficulties in breathing, swallowing and voicing across the lifespan. Webs range from thin and membranous to thicker, high-grade webs, with associated mesenchymal abnormalities, such as aberrant shape of the cricoid cartilage (Cohen, 1985; Hartnick and Cotton, 2000; Ahmad and Soliman, 2007). Thickness and size of webbing determines the severity of the breathing complications (Cohen, 1985; Ahmad and Soliman, 2007) (Fig. S1). Prevalence of laryngeal webbing at birth is 1 in 10,000 (Sacca et al., 2017). There is an association between anterior laryngeal webbing and velocardiofacial syndrome where laryngeal anomalies range from webs (Fokstuen et al., 1997) to complete atresia (Lipson et al., 1991) with a worldwide incidence estimated at 1 in 2000 to 1 in 4000 live births (Sacca et al., 2017). Despite the importance of a complete VF separation, little is known about how this is achieved during development.
Several studies have described laryngeal and VF embryonic development in humans (Sanudo and Domenech-Matteu, 1990; Zaw-Tun and Burdi, 1985; Hartnick and Cotton, 2000; Wyatt et al., 2005; Ahmad and Soliman, 2007) and rodent models (Lobcko et al., 1979; Henick, 1993). We have recently extended these findings and delineated five principal morphogenetic events that occur during murine VF development and provided gene expression pattern analysis that could serve as the foundation for studying mechanisms of normal and aberrant VF formation (Lungova et al., 2015). These developmental events include: (1) initiation of the larynx and VFs with apposition of the lateral walls of the primitive laryngopharynx (LPh) at E (embryonic day) 10.5; (2) establishment of the epithelial lamina (EL) with fusion of the lateral walls of the primitive LPh (E11.5); (3) EL recanalization and separation of VFs that is synchronized with (4) stratification of VF epithelium and development of laryngeal cartilages and muscles in the lamina propria (LP) (E13.5-18.5); and last, (5) maturation of VF epithelium and LP during postnatal stages (Fig. S2). Based on these steps, we hypothesize that precisely controlled EL formation together with proper specification of VF epithelial progenitors are prerequisites for the accurate final VF separation.
The β-catenin signaling pathway plays a crucial role during development and adult stem cell maintenance of many organs (Stevens et al., 2003; Dessimoz et al., 2005; Apte et al., 2007), including lungs and trachea (Mucenski et al., 2003; Goss et al., 2009; Harris-Johnson et al., 2009; Woo et al., 2011). It regulates diverse cellular processes such as cell proliferation, apoptosis or cell fate determination. In the ventral anterior foregut endoderm, β-catenin is required for the initiation of the respiratory lineage and its inactivation results in the absence of both trachea and lungs (Goss et al., 2009; Harris-Johnson et al., 2009). After lung specification, inactivation of β-catenin in the lung epithelium leads to aberrant branching and proximal-distal patterning (Mucenski et al., 2003; Shu et al., 2005). Finally, inactivation of β-catenin in the developing lung mesenchyme leads to decreased mesenchymal growth and disrupted endothelial differentiation (Yin et al., 2008; Li et al., 2008). In addition to its central role in Wnt signaling, β-catenin is a component of adherence junctions connecting classical cadherins through α-catenin to the actin cytoskeleton (Bienz, 2004; Lyashenko et al., 2011; Perez-Moreno et al., 2003). β-Catenin-mediated cell adhesion is particularly important for neuroepithelial and endoderm formation in embryoid bodies (Lyashenko et al., 2011), for directing coordinated cellular organization and movements within epithelia, and for transmitting information from the environment to the interior of cells (Perez-Moreno et al., 2003).
In the present study, we report, for the first time, the requirement of β-catenin function in VF and larynx morphogenesis. Conditional ablation of β-catenin in the primitive LPh epithelium during EL formation leads to failed VF separation that resembles human laryngeal webbing on a gross tissue level. The failure in VF separation is preceded by delayed EL fusion and disrupted establishment of VF basal epithelial progenitors. Delayed EL fusion may be caused by cell cycle arrest in the LPh epithelium and surrounding mesenchyme, consistent with the role of β-catenin in cell proliferation (Huang et al., 2007; Shutman et al., 1999; Rowlands et al., 2003). In more severe cases, inactivation of β-catenin affected the EL integrity. Mesenchymal cells filled the gap between the EL fragments and precluded VF separation. Besides reduced cell proliferation, loss of β-catenin also disrupted initial differentiation of VF basal progenitors into p63-expressing cells, consistent with its role in promoting progenitor identity (Goss et al., 2009; Harris-Johnson et al., 2009). Specification of basally positioned VF progenitors is necessary for their terminal conversion into functional basal cells giving rise to a suprabasal layer that disintegrates during VF separation. In summary, this study provides the first evidence linking β-catenin function to VF morphogenesis by regulating proliferation and cell fate determination of epithelial and mesenchymal cell lineages during the EL formation and recanalization, shedding light on the etiology of poorly understood congenital laryngeal malformations.
RESULTS
Inactivation of β-catenin in developing VFs
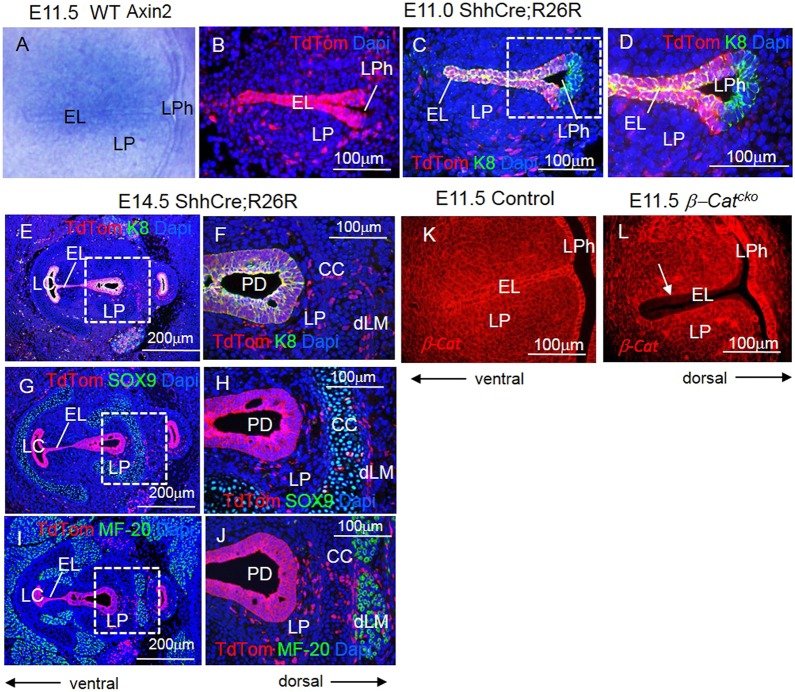
To determine whether β-catenin signaling is active in developing VFs, we examined the expression of Axin2, a reporter gene for WNT/ β-catenin activity. We found that during EL formation, Axin2 was expressed in the EL as well as in the surrounding LP (Fig. 1A). To investigate the requirements for β-catenin signaling in VF and larynx morphogenesis, we disrupted β-catenin function in the EL by conditional gene inactivation using ShhCre (Harris-Johnson et al., 2009; Harfe et al., 2004). By mating ShhCre mice to TdTomato reporter mice, we found that ShhCre was robust at the EL during EL formation (at E11.0), the site of prospective VFs. This is in contrast to the dorsal compartment of the primitive LPh, which gives rise to the esophagus (Fig. 1B-D). ShhCre was also robust in epithelial cells in more advanced stages of VF formation during EL recanalization at E14.5 (Fig. 1E,J). ShhCre lineaged cells were found at the EL, the laryngeal cecum (LC) and the pharyngoglottic duct (PD) (Fig. 1E-J). Moreover, the ShhCre lineage tracing analysis revealed that during EL formation ShhCre lineage signal extended into adjacent, cytokeratin (K) 8 negative mesenchyme (Fig. 1C-F). By RNA in situ hybridization, we found that Shh expression remained primarily in the epithelium (Lungova et al., 2015). These data suggest that the mesenchymal lineaged cells either arise from rare Shh expressing cells beyond the sensitivity of RNA in situ detection, or from epithelial cells that have undergone epithelial-mesenchymal transition (EMT) and delaminated into mesenchyme of the LP (Fig. 1G-J). These cells intermix with SOX9-expressing chondrocytes or surround MF-20-expressing myoblasts (Fig. 1G-J). By mating Shhcre mice to mice carrying a conditional knockout allele of β-catenin (Ctnnb1tm2Kem), we generated Shhcre/+; Ctnnb1tm2Kem/tm2Kem (hereafter referred to as β-Catcko, for conditional knockout) mutant embryos. In the epithelial cells of E11.5 embryos, we found that β-catenin was severely reduced in the EL, at the site of prospective VFs, whereas it remained present in the dorsal compartment of the primitive LPh when compared with control embryos (Fig. 1K,L). As Shhcre activity in the mesenchyme is rather minor compared with that in the epithelium, expression of β-catenin in mesenchymal cells remained strong (Fig. 1L). These data indicate that Shhcre is an effective tool for β-catenin inactivation in the epithelium of prospective VFs.
Fig. 1.
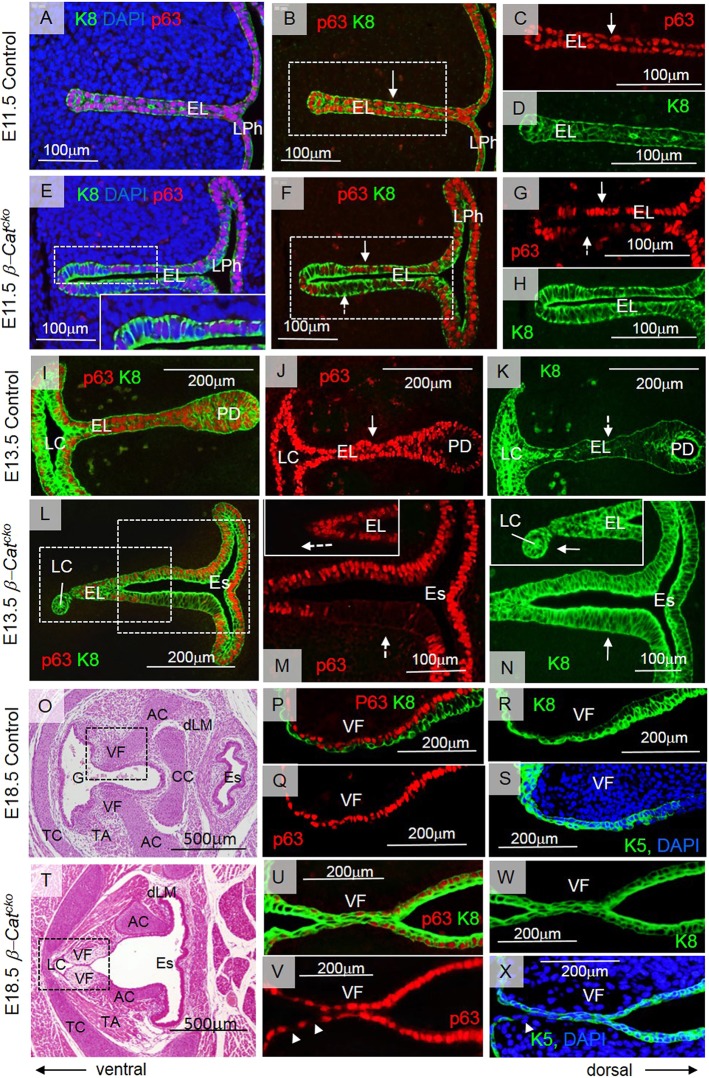
Inactivation of β-catenin in the primitive LPh leads to a failure in VF separation. (A) Axin2 expression, as determined by RNA in situ hybridization, in vibratome transverse sections at the level of developing VFs at E11.5. (B) Anti-TdTom (red) immunofluorescent staining in ShhCre; R26R embryos at E11.0 during EL formation. (C-F) Double immunofluorescent staining for anti-TdTom (red) and anti-cytokeratin K8 (green) in ShhCre; R26R embryos at E11.0 (C,D) and E14.5 (E,F). Boxed region in C is magnified in D. (G,H) Double immunofluorescent staining for anti-TdTom (red) and anti-SOX9 (green) in ShhCre; R26R embryos at E14.5. (I,J) Double immunofluorescent staining for anti-TdTom (red) and anti-MF-20 (green) in ShhCre; R26R embryos at E14.5. Boxed regions in E,G,I are magnified in F,H,J, respectively. (K,L) Immunofluorescent anti-β-catenin staining in transverse sections of the EL at E11.5, when the EL is established. Arrow in L indicates diminished β-catenin activity in the EL of the β-Catcko mutants. For each experiment, at least three different individuals were collected for analysis. For each individual, at least two transverse sections from cranial and caudal VF regions were characterized. The experiment was replicated twice. EL, epithelial lamina; LC, laryngeal cecum; LP, lamina propria; LPh, laryngopharynx; PD, pharyngoglottic duct; CC, cricoid cartilage; dLM, dorsal laryngeal muscles.
β-Catcko mutants exhibit incomplete recanalization of the laryngotracheal tube
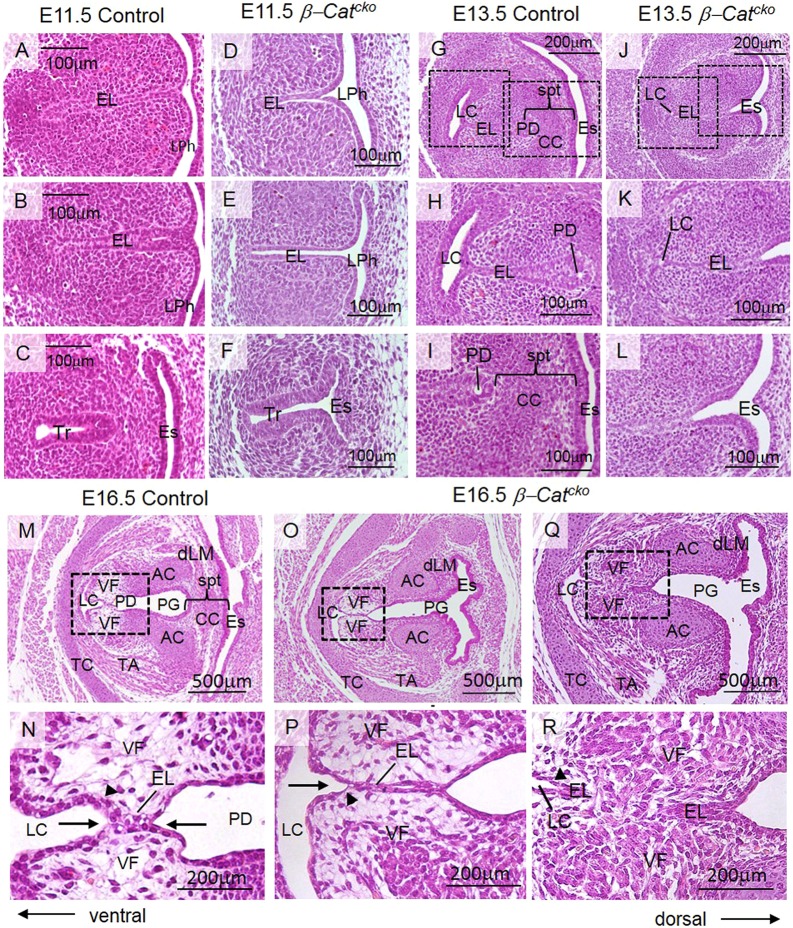
β-Catcko mutants die at birth with multiple defects, including agenesis of the lung (Harris-Johnson et al., 2009). We first analyzed the VF phenotype at E18.5, shortly before birth. β-Catcko mutants failed to completely disintegrate the EL, thereby resembling the phenotype described in laryngeal webbing at a gross tissue level. Unlike in control embryos (Fig. 2A,B), unseparated mutant VFs obstruct the entrance into the trachea in both the milder (Fig. 2D,E) and more severe (Fig. 2G,H) cases. Upon close examination, we found, that, in milder cases, mutant VFs, at the site of EL persistent fusion, are mostly lined with a single epithelial cell layer on each side. In contrast, there are two cell layers on each side in fully separated VFs in control embryos (Fig. 2B,E, insets). Our previous characterization of the wild-type (WT) VFs suggests that transition from one layer into two layers precedes successful VF separation (Lungova et al., 2015). In more severe cases, a large proportion of the EL is replaced by mesenchymal cells that have migrated into the space between VFs, suggesting that VFs have completely grown together (Fig. 2H). Ventral to the VFs is the LC (Fig. 2E,H), which in control embryos becomes a part of the glottis once VFs separate (Fig. 2B). The dorsal region opens into the posterior glottis (Fig. 2C). In the mutant, the dorsal extent of the glottis is not as clearly defined, owing to the absence of the septum (Fig. 2F,I). In more severe cases, unseparated VFs occluded more than 50% of the glottal region, and the posterior glottis was significantly narrowed, when compared with milder cases (Fig. 2F,I). These phenotypes in the severe cases are consistent with high-grade thick laryngeal webs in human (Wyatt et al., 2005; Ahmad and Soliman, 2007). We sought to further characterize the cause for the phenotypes in mice to understand the etiology and progression of laryngeal webbing.
Fig. 2.
Inactivation of β-catenin in the primitive LPh leads to laryngeal webs. (A-C) Hematoxylin and Eosin staining in transverse sections demonstrating the morphology of the VFs in control embryos at E18.5. Boxed regions in A are magnified in B and C. Boxed region in B is magnified in the inset. A solid black arrow indicates the two-layered VF epithelium in control embryos. (D-F) Hematoxylin and Eosin-stained transverse sections demonstrating morphology of the VFs in β-Catcko mutants at E18.5 in milder cases. Boxed regions in D are magnified in E and F. Boxed region in E is magnified in the inset. A dashed black arrow indicates aberrant stratification of VF epithelium in mutant VFs. (G-I) Hematoxylin and Eosin-stained transverse sections demonstrating morphology of the VFs in β-Catcko mutants at E18.5 in severe cases. Boxed regions in G are magnified in H and I. For each experimental group, at least three different individuals were collected for analysis. For each individual, at least two transverse sections from cranial and caudal VF regions were characterized. The experiment was replicated twice. AC, arytenoid cartilage; CC, cricoid cartilage; EL, epithelial lamina; Es, esophagus; dLM, dorsal laryngeal muscles; G, glottis; LC, laryngeal cecum; LL, lateral laminae; PG, posterior glottis; spt, septum; TA, thyroarytenoid muscle; TC, thyroid cartilage; VF, vocal fold.
The β-Catcko mutant laryngeal phenotype can be traced to an early defect of incomplete EL fusion
We found that aberrant VF development in β-Catcko mutants was already apparent at E11.5 during EL formation. In control embryos at E11.5, lateral walls of the primitive LPh obliterated the ventral lumen and formed the EL (Fig. 3A,B). More caudally, the trachea was already separated from the esophagus (Fig. 3C). In β-Catcko mutant embryos at E11.5, although the cranial part of the primitive LPh fused normally to establish the EL (Fig. 3D), the caudal region with the prospective VFs remained open (Fig. 3E). More caudally, the trachea was not separated from the esophagus (Fig. 3F). At the cranial level of the larynx and esophagus in the control, dorsal and ventral patterning was delineated by SOX2 expression dorsally and NKX2-1 expression ventrally (Fig. S3A). In the mutant, the SOX2 expression domain was extended ventrally at the expense of NKX2-1 (Fig. S3B). This is similar to failed patterning at the more caudal trachea and esophagus level shown previously (Minoo et al., 1999; Que et al., 2007; Domyan et al., 2011).
Fig. 3.
Inactivation of β-catenin in the primitive LPh leads to a delayed and aberrant EL formation and recanalization. (A-L) Hematoxylin and Eosin-stained transverse sections demonstrating morphology of the VFs in control embryos and β-Catcko mutants at E11.5 and at E13.5. Boxed regions in G,J are magnified in H,K and I,L, respectively. (M-R) Hematoxylin and Eosin-stained transverse sections demonstrating morphology of the VFs in control embryos (M,N), and in β-Catcko mutants at E16.5, in milder cases (O,P) and severe cases (Q,R). Boxed regions in the M,O,Q are magnified in N,P,R, respectively. Black solid arrows in N denote expansion of the LC and PD, respectively; an arrowhead indicates the two-layered VF epithelium. A black solid arrow in P indicates expansion of the LC. Arrowheads in P,R indicate a flat single layer of VF basal cells in milder (P) and severe (R) cases of laryngeal webbing. For each experimental group, at least three different individuals were collected for analysis. For each individual, at least two transverse sections from cranial and caudal VF regions were characterized. The experiment was replicated twice. AC, arytenoid cartilage; CC, cricoid cartilage; EL, epithelial lamina; Es, esophagus; dLM, dorsal laryngeal muscles; LC, laryngeal cecum; LPh, primitive laryngopharynx; PD, pharyngoglottic duct; PG, posterior glottis; spt, septum; TA, thyroarytenoid muscle; TC, thyroid cartilage; Tr, trachea; VF, vocal fold.
Two days later at E13.5, in control embryos, three morphogenetic events occurred. First, the LC appeared as a T-shaped lumen extending caudally along the ventral border of the EL, indicating the start of EL recanalization, whereas remaining epithelial cells at the EL were still fused (Fig. 3G,H). Second, a septum developed between the esophagus and VFs, and the cricoid cartilage formed in this space (Fig. 3G,I). Third, in the fused EL near the cricoid, a cavity could be detected, indicating the start of recanalization into the PD (Fig. 3G,I). In β-Catcko mutants at E13.5, part of the lateral walls finally fused to establish a shortened EL (Fig. 3J,K). However, all three subsequent morphogenetic events were disrupted. First, there was a delay in LC expansion (Fig. 3J,K). Second, no septum or cricoid cartilage separated the esophagus and future larynx with VFs (Fig. 3J,L). Third, because there was no septum delineating the dorsal region of the EL, there was no apparent PD (Fig. 3J,L).
At E16.5, in control embryos, with continuing dilation of the LC and PD (Fig. 3M,N), the EL gradually disintegrates to open the laryngotracheal tube by E18.5. Meanwhile, epithelial cells lining the developing VFs acquired a two-cell layer morphology (Fig. 3N), suggesting that these events may precede final VF separation at E18.5. In β-Catcko mutants at E16.5, the LC finally expands (Fig. 3O-R). However, in milder mutants, EL is lined by a single epithelial cell layer that fails to stratify, and a longer region of the EL remain fused compared with control (Fig. 3P). In more severe mutants, the ventral and dorsal EL are interrupted by mesenchymal cells that run through (Fig. 3Q,R). Staining for β-catenin indicates that the mesenchymal cells that disrupt the EL are a mixture of β-catenin+ and β-catenin− cells (Fig. S3E,F). As the overall β-catenin expression is low at this stage, the β-catenin− cells could still be wild type in genotype. This more severe gross phenotype resembles severe cases of human laryngeal webs.
To elucidate the origin of severe laryngeal webbing, we stained epithelial cells with cytokeratin (K) 8. In a subset of the more severe β-Catcko mutants, at E13.5, the ventral tip of the EL pinched off from the remaining cells of the EL, in contrast to controls (Fig. 4A-D), and the LC was formed in this small cluster of pinched off epithelial cells (Fig. 4D). At E16.5, in the control, the LC gradually expands and may drive the separation of the remainder of the EL as they are still connected (Fig. 4E,F). In β-Catcko mutants, however, the delayed LC expansion cannot drive the separation of the remainder of the EL, as the two epithelial regions are now separated by mesenchymal cells (Fig. 4G,H). Our findings demonstrate that the defects in EL formation preceded the failure of VF separation.
Fig. 4.
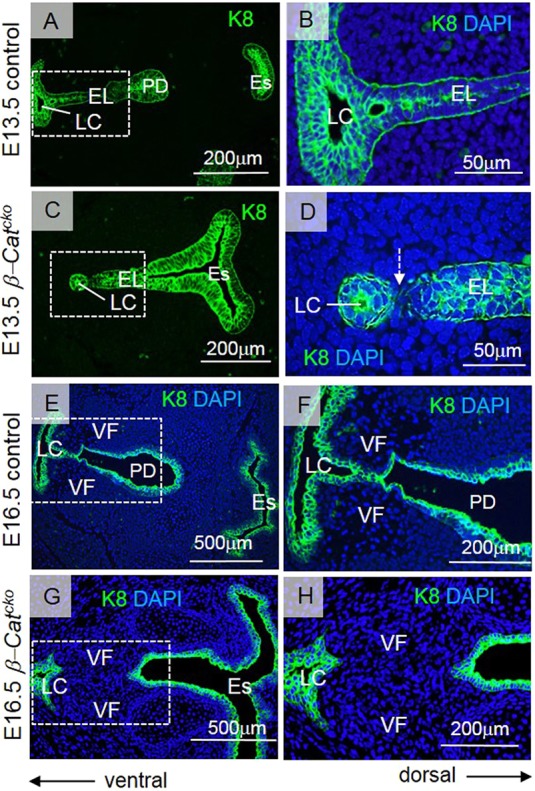
Inactivation of β-catenin affects the EL integrity. (A-H) Anti-K8 staining of cells at the EL in green at 13.5 (A-D) and E16.5 (E-H). (A,B) Anti-K8 staining in control embryos. (C,D) Anti-K8 staining in β-Catcko mutants. A white dashed arrow in D indicates the process of separation of the tip of the EL from the remaining cells at the EL. (E-H) Anti-K8 staining of cells at the LC, EL and PD in green at E16.5 in control embryos (E,F) and β-Catcko mutants (G,H). Boxed regions in A,C,E,G are magnified in B,D,F,H. For each experimental group, at least three different individuals were collected for analysis. For each individual, at least two transverse sections from cranial and caudal VF regions were characterized. The experiment was replicated twice. EL, epithelial lamina; Es, esophagus; LC, laryngeal cecum; PD, pharyngoglottic duct; VF, vocal fold.
Cellular mechanism underlying aberrant EL formation
To address whether β-catenin plays a role in cell survival and/or proliferation, we examined embryos at E11.5 using TUNEL assay for cell death and EdU incorporation in S-phase for cell proliferation. We found no increased cell death in the mutant EL or adjacent LP when compared with control mice (Fig. S4A,B). In contrast, there was a statistically significant decline of cell proliferation in the EL and LP, in the caudal, but not cranial, region of the developing larynx in β-Catcko mutants (EL caudal region, P=0.0001; LP caudal region, P=0.0320) (Fig. S4C-G). This reduction may contribute to the failed epithelial morphogenesis that follows, such as the expansion of the LC.
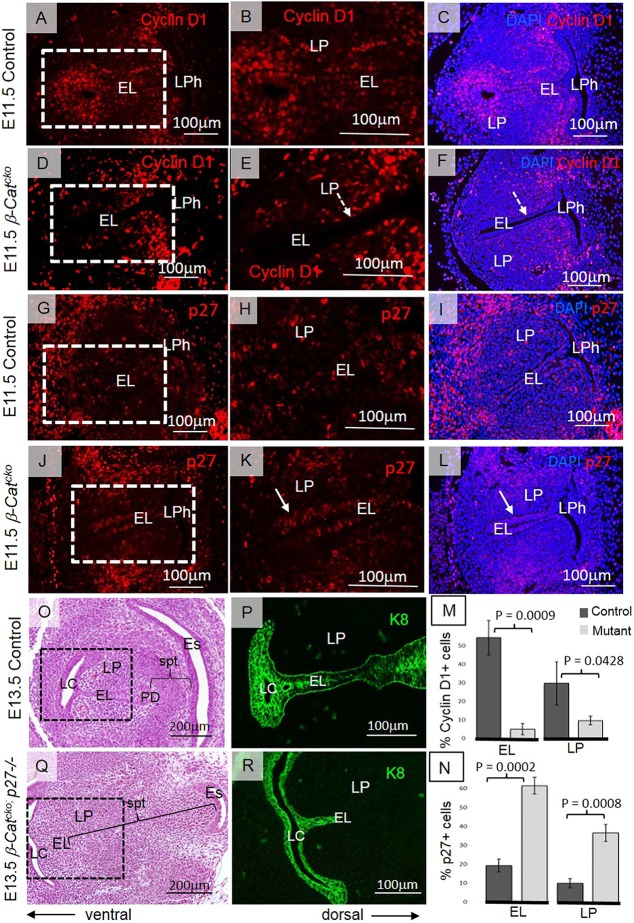
Previous studies have shown that β-catenin regulates cell proliferation via its interaction with cyclin D1 (Shutman et al., 1999; Rowlands et al., 2003; Atanasoski et al., 2001). Cyclin D1 also interacts with cell cycle inhibitors, such as p27Kip and p21Cip genes, that control the exit of cells from the cell cycle (Geng et al., 2001; Lee et al., 2006). Immunofluorescent staining revealed that cyclin D1 was strikingly absent in the mutant EL and was also reduced in surrounding LP when compared with control (Fig. 5A-F). In contrast, expression of p27Kip increased in both the EL and LP during EL formation (Fig. 5G–L). Low cyclin D1 levels and high p27Kip expression is consistent with the outcome that a majority of cells have withdrawn from the cell cycle and are arrested at a G1 checkpoint. Statistically significant differences in cyclin D1 and p27 expression levels were also confirmed by quantitative analysis using Student's t-test (EL cyclin D1, P=0.0009; LP cyclin D1, P=0.0428; EL p27, P=0.0002; LP p27, P=0.0008) (Fig. 5M,N).
Fig. 5.
Inactivation of β-catenin in the EL leads to downregulation of cyclin D1 and upregulation of p27Kip. (A-F) Red anti-cyclin D1 staining in control embryos (A-C) and in β-Catcko mutants (D-F) at E11.5. Boxed regions in A and D are magnified in B and E, respectively. White dashed arrows in E,F indicate cyclin D1-negative cells in the EL. (G-L) Red anti-p27Kip staining in control embryos (G-I) and β-Catcko mutants (J-L) at E11.5. White solid arrows in K,L indicate p27-positive cells at the EL. (M,N) Statistical analysis of quantitative distribution of cyclin D1- (M) and p27- (N) positive cells. Student's t-test was performed to compare cyclin D1 and p27 expression at the EL and LP in control embryos and in β-Catcko mutants. For each experimental group, at least three different individuals were collected for analysis. For each individual, at least two transverse sections from cranial and caudal VF regions were characterized. The experiment was replicated twice. (O,Q) Hematoxylin and Eosin-stained transverse sections demonstrating morphology of VFs in control (O) and double mutant (Q) embryos at E13.5. (P,R) Anti-K8 green staining in control embryos (P) and double mutants (R). Boxed regions in O,Q are magnified in P,R, respectively, showing the size of the EL. For a double-mutant embryo, only two individuals were collected for analysis due to the low frequency of double mutant production. For each individual, at least two transverse sections from cranial and caudal VF regions were characterized. The experiment was replicated twice. EL, epithelial lamina; Es, esophagus; LC, laryngeal cecum; LP, lamina propria; LPh, laryngopharynx; PD, pharyngoglottic duct; spt, septum.
To elucidate whether reduced cell proliferation is responsible for the mutant phenotypes, we stimulated proliferation by introducing p27Kip mutation into the β-catenin mutant (Shh-Cre/+; Ctmnb1F/F; p27−/−) and addressed whether it rescues EL fusion in the mutant. At E13.5, inactivation of p27Kip gene from the genotype of β-Catcko mutants rescued a septum between the larynx and esophagus, the EL ventral to this appears fused and is connected to the LC (Fig. 5O-R). However, the size of the EL remains shorter than in control embryos, as confirmed by K8 staining (Fig. 5P,R), possibly owing to the expansion of the mesenchymal cells between the esophagus and the larynx (Fig. 5O,Q). This ectopic expansion of the mesenchyme may be due to global knockout of p27Kip, which led to increased proliferation in the mesenchyme independently of its interaction with β-catenin in the epithelium. These results demonstrate that inactivation of p27Kip led to a partial rescue of the β-catenin mutant morphogenesis defect, suggesting that epithelial cell proliferation defects contribute to, but are not the sole reason for, the morphogenesis phenotypes in the mutant.
β-Catenin inactivation causes defects in differentiation of mesenchymal cells in the LP
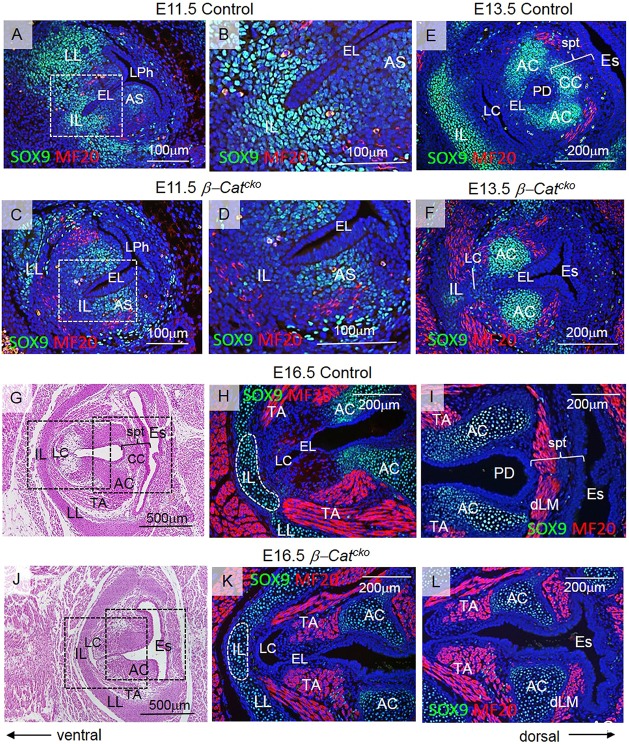
Abnormalities of mesenchyme-derived structures, such as cartilage and muscle, are common features in high-grade laryngeal webs (Hartnick and Cotton, 2000; Ahmad and Soliman, 2007). Accompanying epithelium defects, we also found that β-Catcko mutants exhibited defects of the cartilaginous and muscular structures in the mesenchyme (Fig. 2D-I). The middle region of the thyroid cartilage and the shape of arytenoids were also altered (Fig. 2A,D,G), affecting the attachment of the thyroarytenoid (TA) muscle (Fig. 2B,E,H). To more precisely define the mesenchymal defects revealed by histological observation, we outlined cell types with anti-SOX9 antibody for laryngeal cartilage and with anti-myosin heavy chain MF-20 antibody for developing myoblasts. In control embryos at E11.5, there were intensely stained SOX9-expressing cells subjacent to the ventral tip, and these will give rise to the middle region of the thyroid cartilage (intermediate lamina, IL) (Fig. 6A,B). Intensely stained cells lateral to the larynx will give rise to lateral laminae (LL) of the thyroid cartilage (Fig. 6A). Lightly stained cells subjacent to the dorsal EL (at the site of the arytenoid swelling) will give rise to the arytenoids (Fig. 6A). In β-Catcko mutants at E11.5, SOX9-expressing cells were detected principally at the sites of the arytenoid swellings and developing lateral lamina, whereas SOX9 expression around the ventral tip of the EL was much reduced (Fig. 6C,D). In β-Catcko mutants at E13.5, the cluster of SOX9-expressing cells ventrally from the EL was much smaller when compared with controls, resulting in an underdeveloped intermediate lamina (Fig. 6E,F). Although arytenoids formed, the cricoid did not develop, likely owing to absence of a septum (Fig. 6F).
Fig. 6.
Inactivation of β-catenin at the EL leads to defective differentiation of mesenchymal cells. (A-D) Green anti-SOX9 and red anti-myosin MF-20 staining in transverse sections of control embryos (A,B) and β-Catcko mutants (C,D) at E11.5. Boxed regions in A,C are magnified in B,D, respectively. (E,F) Green anti-SOX9 and red anti-myosin MF-20 staining in transverse sections of control embryos (E) and β-Catcko mutants (F) at E13.5. (G,J) Hematoxylin and Eosin transverse sections demonstrating morphology of the VFs at E16.5 in control embryos (G) and β-Catcko mutants (J). Boxed regions are magnified in H,K (H, control; K, β-Catcko mutants) and I,L (I, control embryos; L, β-Catcko mutants), respectively. Green anti-SOX9 staining shows differentiation of cells into cartilage; red anti-MF-20 staining shows differentiation of mesenchymal cells into muscle. For each experimental group, at least three different individuals were collected for analysis. For each individual, at least two transverse sections from cranial and caudal VF regions were characterized. The experiment was replicated twice. AC, arytenoid cartilage; AS, arytenoid swelling; CC, cricoid cartilage; EL, epithelial lamina; Es, esophagus; dLM, dorsal laryngeal muscles; IL, intermediate lamina; LC, laryngeal cecum; LL, lateral lamina; LPh, laryngopharynx; PD, pharyngoglottic duct; spt, septum; TA, thyroarytenoid muscle.
Concurrent with differentiation of laryngeal cartilages, other mesenchymal cells initiated differentiation into laryngeal muscles. At E11.5 and E13.5, in β-Catcko mutants, accompanying a decrease in SOX9 expression, there were slightly more MF20-expressing mesenchymal cells, especially in the ventral laryngeal region when compared with controls (Fig. 6A-F). At E16.5 in the control, MF20-expressing cells have given rise to three principal groups of laryngeal muscles. Ventrally there is the thyroarytenoid muscle that is a paired muscle attached to the intermediate lamina (Fig. 6G,H). Dorsally there are the laryngeal muscles, which consist of the interarytenoid muscle connecting the posterior surfaces of arytenoid cartilages, and posterior cricoarytenoid muscles that connect arytenoid cartilages with the cricoid (Fig. 6G,I). The thyroarytenoid muscle functions in phonation and acts as a sphincter that tightens and narrows the laryngeal inlet during swallowing. The muscles are brought closer together in parallel to close the glottis by the contraction of interarytenoid muscles. On the other hand, the posterior cricoarytenoid muscle opens VFs for breathing. Both muscles, interarytenoid and posterior cricoarytenoid, are located in the septum (here referred to for simplicity as dorsal laryngeal muscles) (Fig. 6G,I). β-Catenin ablation in developing VF epithelium disrupts the attachment and function of all these muscles. In β-Catcko mutants, the thyroarytenoid muscle inserts into the lateral laminae instead of the intermediate lamina of the thyroid, and its mass seems to be reduced (Fig. 6J,K). This defect may lead to insufficient glottal closure, which significantly affects swallowing and voicing. Concurrently, the dorsal laryngeal muscles are disconnected due to the absence of the septum and cricoid cartilage (Fig. 6J,L); they cannot function together to approximate the VFs or abduct them. These data suggest that epithelial cells interact with the surrounding mesenchyme starting from early stages of VF morphogenesis during EL formation. Primarily, loss of β-catenin in the epithelium leads to profound disorganization of the adjacent mesenchymal cells.
Inactivation of β-catenin affects remodeling of VF basement membrane
Next, we characterized changes in shape and cellular organization of VF, including remodeling of their basement membrane during EL formation. Using images from transmission electron microscopy and expression analysis of laminin α 5 (Lam 5), we confirmed that in control embryos, during EL fusion at E11.5, prospective basal cells had a well-defined basement membrane (Fig. S5A-J). Cells that lost contact with the lumen, e.g. cells at the ventral tip of the EL, also lost apical characteristics such as filopodia (Fig. S5). Cells at the EL tip formed basal protrusions that pointed towards the surrounding mesenchyme (Fig. S5C,E). In β-Catcko mutants, cells at the ventral tip of the EL retained the original elongated cell shape with apical filopodia and tight junctions (Fig. S5G-I, arrowheads). Their Lam 5 (Fig. S5F) positive basement membrane was smooth and without protrusions (Fig. S5H,J). During EL recanalization at E16.5, compared with the continuous Lam 5-positive basement membrane in control embryos (Fig. S5K), there is fragmentation of Lam 5 expression between the interrupted EL segments (Fig. S5L,M), suggesting a breakdown of basement membrane. Despite these changes, E-cadherin expression remains robust in the remainder of the EL in the mutant (Fig. S5N,O), suggesting that β-catenin loss does not have a direct effect on adherence junctions.
β-Catenin inactivation in the EL disrupts establishment of VF epithelial basal progenitors
To understand whether β-catenin inactivation affects specification of prospective VF basal progenitors and their subsequent differentiation, we assayed for the expression of p63 and cytokeratins K8 and K5. p63 is a nuclear marker of basal cells, and at E11.5 it was expressed in all epithelial cells along with a simple epithelial cell marker K8 (Fig. 7A-D).p63+ K8+ cells were cuboidal and perfectly organized in two single-cell layers that were fused along the midline (Fig. 7A-D). In the β-Catcko mutant, in epithelial layers that have not yet fused, K8 was expressed in all cells whereas p63 was detected in a mosaic fashion (Fig. 7E-H). Interestingly, even though p63+ cells are cuboidal as in the control, p63− cells were slightly taller (Fig. 7E). Negative staining for Alcian Blue shows that p63-negative cells in β-Catcko mutants did not adopt a different fate (Fig. S3C,D). At E13.5 in control embryos, p63+ cells increased in number along the EL midline (Fig. 7I,J). K8 expression in these cells became weaker than in cells lining the PD or LC (Fig. 7K). These p63+ cells with downregulated K8 expression represent basal cell progenitors for the stratified squamous VF epithelium. In β-Catcko mutants, the prospective LC cells in the ventral tip of the EL remained p63− K8+, whereas cells at the EL expressed p63 and K8 (Fig. 7L-N). More dorsally, there is a large domain of p63− cells expressing K8, which are columnar (Fig. 7M,N). At E18.5, after EL separation in the wild type and control, expression of cytokeratin 5 (K5), a stratified epithelial marker, begins to be detected (Lungova et al., 2015). The epithelium is composed of two cell layers, the basal p63+, K5+ and K8-low cell layer and an apical p63-low, K5+ and K8+ cell layer (Fig. 7O-S). This expression of cytokeratins is unique for VFs within the foregut (Lungova et al., 2015). In comparison, in β-Catcko mutant mice, fused VFs were lined with a single layer of p63+ and K8+ epithelial cells, most of which, but not all, also expressed K5 (Fig. 7T-X). A few basal cells were p63− and K8+, suggesting that these cells either did not initiate p63 expression or did not maintain it (Fig. 7V,W). In the dorsal compartment near the PD region, the previously observed columnar cells remain, and were p63−, K5− and K8+ (data not shown). In summary, these data suggest that inactivation β-catenin disrupts the normal formation of p63+, K5+ and K8− basal progenitor cells.
Fig. 7.
Inactivation of β-catenin at the EL leads to defective specification of VF basal progenitors. (A-H) Red anti-p63 and green anti-K8 staining in control embryos (A-D) and mutants (E-H) at E11.5. Boxed region in E is magnified in the inset. Boxed regions in B and F are magnified in C,D and G,H, respectively. White solid arrows in B,C,F,G indicate p63+ cells; white dashed arrows in F,G indicate p63− cells. (I-K) Red anti-p63 and green anti-K8 staining in control embryos at E13.5. A solid arrow in J indicates p63+ cells and a dashed white arrow in K indicates K8 downregulation in the EL. (L-N) Red anti-p63 and green anti-K8 staining in mutants at E13.5. Boxed regions in L are magnified in M and N, with dorsal and ventral regions magnified in the insets. Dashed arrows indicate p63− cells (M), solid arrows indicate K8+ expression in mutants (N). (O,T) Hematoxylin and Eosin staining of fully separated VFs in control embryos (O) and fused VFs in mutants in milder cases (T). Boxed region in O is magnified in P-S. (P,Q) Red anti-p63 staining, (P,R) green anti-K8 staining and (S) green anti-K5 staining. Boxed region in T is magnified in U-X. (U-W) Anti-p63 (red) and anti-K8 (green) staining. (X) Green anti-K5 staining. Arrowheads in V and X indicate p63− and K5− cells, respectively. For each experimental group, at least three different individuals were collected for analysis. For each individual, at least two transverse sections from cranial and caudal VF regions were characterized. The experiment was replicated twice. AC, arytenoids; CC, cricoid; EL, epithelial lamina; Es, esophagus; dLM, dorsal laryngeal muscles; G, glottis; LC, laryngeal cecum; LPh, laryngopharynx; PD, pharyngoglottic duct; TC, thyroid cartilage; TA, thyroarytenoid muscle; VF, vocal fold.
DISCUSSION
The larynx is a vitally important organ, sitting at the crossroads between the gastrointestinal and respiratory tracts, thereby orchestrating swallowing, breathing, coughing and, in humans, vocalization. Loss of a functioning airway is life-threatening, as is loss of airway protection from ingested food and drink. Impaired voice production holds significant implications for individual health and wellness, social and occupational function, and societal productivity. Molecular mechanisms that cause congenital laryngeal webbing have not been elucidated and there is also little histological data that further characterize tissue from patients. In humans with laryngeal webbing, fibrous tissue covers the larynx (Cohen, 1985; Hartnick and Cotton, 2000; Wyatt et al., 2005; Ahmad and Soliman, 2007). This gross phenotype has been modeled for the first time in the β-Catcko mutant. The described phenotype in β-Catcko mutants ranges from milder to severe laryngeal webs that occlude more than 50% of the glottis; this range on a gross tissue level is similar with clinical observations (Cohen, 1985; Ahmad and Soliman, 2007). In this mouse model, we traced the morphological defects to surprisingly early events of VF morphogenesis, when epithelial cells fuse to establish the EL and initiate their differentiation into VF basal epithelial progenitors. Our finding illustrates that a thorough understanding of the origin of defects, as demonstrated through animal models, can provide important insights into the etiology of human congenital laryngeal malformations.
Genetic evidence from β-Catcko mutants establishes β-catenin as a key player involved in early larynx and VF morphogenesis. This is consistent with the dual role of β-catenin in regulating expression of genes involved in cell proliferation, likely mediated by cyclin D1, and, simultaneously, genes that are involved in specification of VF basal epithelial progenitors. Moreover, as a part of cell adherent junctions, β-catenin can also affect cell adhesion and EL integrity. Inactivation of β-catenin in the epithelium and/or in a small number of cells in the mesenchyme also disrupts normal patterning of laryngeal cartilages and muscles.
Our data stipulate a combination of mechanical and genetic controls that drive EL recanalization and VF separation (Fig. S2). This mechanism is unique to the VFs and has not been described in any other tissues. Mechanical forces are generated within the epithelium, as the cavities, the LC and PD, expand and exert pressure on the epithelial walls, and in the mesenchyme by initial contraction of the dorsal laryngeal muscles that pull on the VFs (Fig. S2). Recent research has shown that the epithelial wall adapts to enable the expansion of the lumen (Andrew and Ewald, 2010; Hoijman et al., 2015). Besides cell division, which increases the number of cells in the epithelial layer, mitotically active epithelial cells remodel their shape and contract apicobasally, as shown during Drosophila tracheal or zebrafish inner ear morphogenesis (Nelson et al., 2012; Hoijman et al., 2015). Both mechanisms contribute to lumen expansion and simultaneously determine its shape (Hoijman et al., 2015). Therefore, cell cycle arrest in β-Catcko mutants may delay the LC expansion and thus affect EL recanalization (Fig. 8A,B). Our results also indicate that the cells at the ventral tip of the EL may guide the LC expansion. Prior to the LC expansion, these cells send finger-like projections into the surrounding mesenchyme. These projections of the basement membrane can have endocytic or exocytic properties (Hexige et al., 2015) or can work as mechanosensors (Murphy and Courtneidge, 2011). However, because the LC appears in this region 2 days later, we hypothesize that these basal cell progenitors may coordinate extracellular matrix degradation with the basal cell membrane extension, as the LC expands. Absence of these protrusions in the mutant EL can be associated with the delay in LC extension in β-Catcko mutants. In the more severe cases of failed EL separation, when the EL loses its integrity and mesenchymal cells fill the space in between, they block the EL separation, despite the pressure exerted from LC expansion (Fig. 8C,D).
Fig. 8.
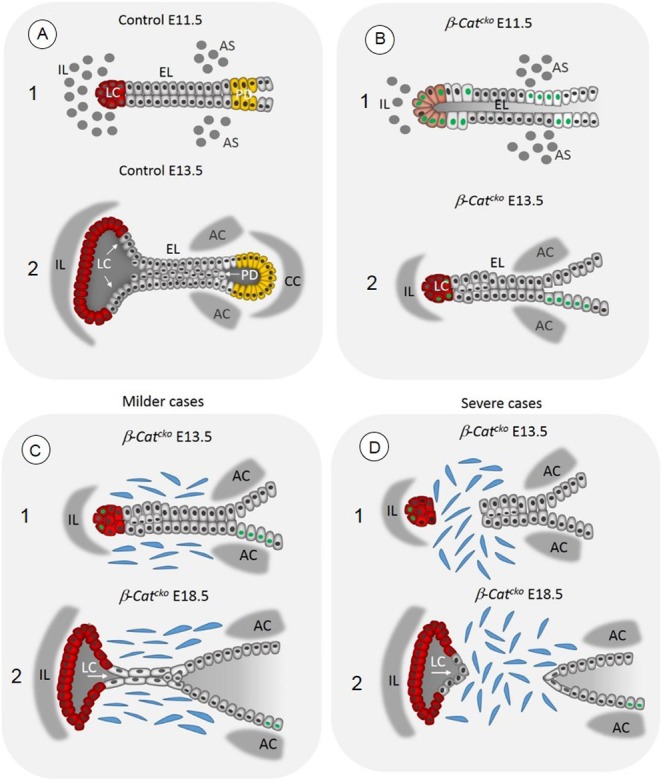
Schematic illustration demonstrating the EL fusion and recanalization in control embryos and β-Catcko mutants, in milder and more severe cases of laryngeal webbing. (A1) Formation of the EL in control embryos at E11.5. (A2) Formation of LC (red cells) and PD (yellow cells) in control embryos at E13.5. Epithelial cells in the EL express p63 (gray nuclei), proliferate and accumulate along the EL midline. Meanwhile mesenchymal cells differentiate into laryngeal cartilages. (B1) Inactivation of β-catenin in β-Catcko mutants leads to the failure in the EL fusion at E11.5 and disrupted initial differentiation of VF basal progenitors. Some VF epithelial cells remain columnar and fail to upregulate p63 (green nuclei). (B2) The delay in the EL fusion leads to the delay in LC expansion (in red) and the absence of the PD and the septum with the cricoid cartilage. (C) Failure in specification of basally positioned of VF progenitors versus a suprabasal cell layer may prevent EL recanalization in milder web cases. (D) In more severe cases of laryngeal webs, inactivation of β-catenin in the epithelium affects the EL integrity. After delayed EL fusion, cells at the tip of the EL can pinch off from the remaining EL, enabling mesenchymal cells to migrate into the space in between. It is also possible that some epithelial cells undergo aberrant epithelial-mesenchymal transition and contribute to laryngeal web formation. AS, arytenoid swelling; AC, arytenoid cartilage; CC, cricoid cartilage; EL, epithelial lamina; IL, intermediate lamina; LC, laryngeal cecum; PD, pharyngoglottic duct.
The β-catenin mutant also exhibits a number of mesenchymal defects, including cartilage and muscle malformation. While ShhCre activity is detected in a few of the mesenchymal cells in addition to robust signal in the epithelium, the mesenchymal cells do not make major contribution to either the cartilage or muscle. This suggests that the cartilage and muscle defects are not due to direct loss of β-catenin in these cells. Secondary causes could include earlier cellular defects. For example, failed formation of the laryngeal septum would lead to the lack of cricoid cartilage. The mesenchymal defects could also be due to altered signals from the epithelium following inactivation of β-catenin in the epithelium. Prior research has shown that changes in signals such as SHH and BMP would affect cartilage or muscle formation (Murtaugh et al., 1999; Zeng et al., 2002). The defects in mesenchyme could also feedback to regulate epithelial formation. For example, dorsal laryngeal muscles connect arytenoid cartilages and arytenoids with the cricoid cartilage, and together they contribute mechanical force to EL recanalization. In the β-Catcko mutant, the altered attachment of the dorsal laryngeal muscles, misshapen thyroid and arytenoid cartilages, and absence of cricoid may significantly reduce the force necessary to pull VFs apart.
In the β-catenin mutant, failure in VF separation leading to anterior laryngeal webbing is preceded by delayed EL fusion and aberrant specification of VF basal epithelial progenitors (Fig. 8). Our data obtained from β-Catcko mutants supported previous observations that EL fusion coincides with intensive cell proliferation (Lobsko et al., 1979; Müller et al., 1985; Lungova et al., 2015). In β-Catcko mutants, low cyclin D1 levels and high p27Kip expression in epithelial and mesenchymal cells in the LP show that during early stages of VF morphogenesis, the majority of cells withdraw from the cell cycle and arrest at a G1 checkpoint. The lateral walls remain closely juxtaposed and fail to fuse at the midline at the appropriate time. Eventually, the ventral part of the EL fuses (Fig. 8B). In the control, during EL formation, the juxtaposed epithelium is a single layer where all cells are p63+ K8+. Two days later, they start to convert into two layers: a basal p63+ K8− VF progenitor layer, and a suprabasal p63−K8+ cell layer. The basal layer represents a reservoir of stem cells for epithelial regeneration and tissue repair (Yu et al., 2005; Mou et al., 2016). In the mutant, large patches of the epithelium are p63− K8+, suggesting that these cells may have failed to establish their basal cell state, and became K8 suprabasal only. For the p63+ cells that are present, they failed to transition into the bilayer structure with P63+ K8− mature progenitors in the basal layer. Future studies may focus on the role of β-catenin in regulation of VF basal cell differentiation during development and/or postnatally during VF epithelial regeneration.
Advances in the understanding of morphogenetic processes during VF embryonic development will promote greater insight into the pathophysiology of vocal fold congenital disorders. We have shown, for the first time, that loss of β-catenin during early stages of VF morphogenesis is associated with congenital laryngeal malformations resembling laryngeal webbing. Characterization of the regulatory mechanisms controlling the establishment and expansion of VF progenitors will help in devising therapeutic strategies for targeting these disorders and optimizing surgical techniques for their corrections. The suggested role of β-catenin in differentiation of the VF basal cell population also opens a new avenues for researching the function of β-catenin in postnatal VF epithelial regeneration and tissue repair.
MATERIALS AND METHODS
Generation of β-catenin mutant mice and embryo isolation
Mice carrying a conditional loss-of-function allele of β-catenin (Ctnnb1tm2Kem) were mated to mice carrying a ShhCre allele to generate Shhcre/+; Ctnnb1tm2Kem/tm2Kem (β-Catcko, for conditional knockout) (Harris-Johnson et al., 2009; Harfe et al., 2004; Brault et al., 2001). Offspring were genotyped using the following primer pairs: for Cre, 5′-TGATGAGGTTCGCAAGAACC-3′ and 5′-CCATGAGTGAACGAACCTGG-3′, product size 420 bp; for Ctnnb1tm2Kem, 5′-AAGGTAGAGTGATGAAAGTTGTT-3′ and 5′-CACCATGTCCTCTGTCTATTC-3′, product sizes 324 bp from theCtnnb1tm2Kem allele and 221 bp from the wild-type allele. Timed pregnant females were dissected, and mutant and control animals studied at the indicated embryonic stages, following the regulations of protocols approved by the University of Wisconsin Animal Care and Use Committee. Shhcre/+; Ctnnb1tm2Kme/− embryos were used as controls.
Phenotype analyses of β-Catcko mutants
To assay for Cre activity through TdTom expression, the R26R reporter line was introduced into the background of the ShhCre line (Harris-Johnson et al., 2009; Muzumdar et al., 2007). TdTom activity was detected using standard immunofluorescence staining protocol on paraffin wax-embedded transverse sections using anti-red fluorescent protein at E11.0 and E14.5. Whole-mount in situ hybridization with digoxigenin-labeled probes for Axin2 was performed on transverse 120 µm vibratome sections at the level of developing larynx and vocal folds at embryonic stage E11.5, as described by Neubuser et al. (1997).
Generation of β-Catcko; p27−/− double mutants and embryo isolation
Shhcre/+; Ctnnb1tm2Kem/+; Cdkln1btm1Mlf/+ males were crossed to Ctnnb1tm2Kem/tm2Kem; Cdkln1btm1Mlf/+ females to generate Shhcre/+; Ctnnb1tm2Kem/tm2Kem; Cdkln1btm1Mlf/tm1Mlf double mutants (here referred to as β-Catcko; p27−/− double mutants). Offspring were genotyped using the following primer pairs for Cre (as mentioned above): Ctnnb1tm2Kem (as mentioned above); and Cdkln1btm1Mlf, 5′-GATGGACGCCAGACAAGC and 5′-CTCCTGCCATTCGTATCTGC for the wild-type allele (product size 190 bp), and 5′-CTTGGGTGGAGAGGCTATTC and 5′-AGGTGAGATGACAGGAGATC for the mutant allele (product size 280 bp). Timed-mated pregnant females were dissected, and mutant and control animals studied at the indicated embryonic stages.
Tissue collection for immunofluorescent staining
Pregnant females were sacrificed at E11.5, E13.5, E16.5 and E18.5 (for β-Catcko mutants), at E11 and E14.5 (for ShhCre lineage tracing experiment), and at E13.5 (for double β-Catcko p27−/− mutants) following regulations of protocols approved by the University of Wisconsin Animal Care and Use Committee. For each experimental group, at least three different mouse embryos were collected for analysis. We included nine wild-type embryos for characterization of Axin2 expression and lineage-tracing experiments (E11 and E14.5); 18 control mouse embryos and 18 β-Cat cko mutants were used for characterization of the β-Catcko mutant phenotype. In double-mutant mice, only two embryos were collected for analysis due to the low frequency of the double mutants (1:16) (total number of mouse embryos=47). Mouse neck regions were dissected and immediately fixed in 4% paraformaldehyde in phosphate-buffered saline at 4°C overnight, dehydrated in a gradient series of ethanol, treated with xylene and embedded in paraffin wax. Paraffin wax blocks were cut into serial sections (5 µm), dewaxed and rehydrated, heated to boiling in 10 mM citrate buffer (pH 6) for antigen retrieval and treated with 0.5% triton in PBS for cell membrane permeabilization. Sections were then stained using a standard immunofluorescent protocol (Lungova et al., 2015). For each individual at stage E11.5 and E13.5, two transverse sections (one cranial and one caudal section) of developing VFs were characterized, accounting for a very small size of the larynx during early stages of VF formation. In more advanced stages of VF development, four transverse sections (two cranial and two caudal) were characterized. Each experiment was replicated twice in the laboratory. Primary antibodies used are listed in Table S1. They were applied overnight at 4°C. Secondary antibodies used are listed in the Table S1, they were applied for 1 h and 30 min at room temperature. Slides were mounted using Vectashield with DAPI (Vector Laboratories). Images were taken with a Nikon Eclipse E600 with a camera Olympus DP71, images were adjusted for brightness using the installed DP 71 software. Figure panels were created using Powerpoint 2010. Alcian Blue staining was performed using the Alcian Blue 1% 2.5 pH stain kit (Newcomer Supply, number 9102A), according the manufacturer's protocol.
TUNEL assay
TdT-mediated dUTP-biotin nick end labeling (TUNEL, Chemicon-Millipore) was employed to detect apoptosis. After rehydration, sectioned samples were treated with proteinase K at 20 μg/ml at room temperature for 15 min (EMD Millipore). After equilibration buffer, the reaction mixture was prepared as per manufacturer instructions and applied at 37°C for 50 min. After an anti-digoxigenin-peroxidase reaction for 30 min at room temperature, positive cells were visualized using the chromogen substrate diaminobenzidine (DAB kit, Vector Laboratories) and slides were counterstained with Hematoxylin.
Cell proliferation assay, cell quantification and statistical analysis
Pregnant females received an intraperitoneal injection of 100 µg EdU (Sigma-Aldrich) per gram of bodyweight 1 h before sacrifice. Embryos were fixed and processed to produce paraffin wax-embedded sections. EdU detection was performed according to the manufacturer's protocol (Invitrogen). Immunofluorescently labeled cells in the EL and LP were manually counted using ImageJ. For each experiment at E11.5, three separate mutant-control littermate pairs were analyzed. For each mutant and control sample, two sections were quantified – cranial and caudal sections (three control embryos, n=6 sections and three mutant embryos, n=6 sections). The percentages of EdU+ nuclei were compared using General Linear Model procedure with Repeated Measures Analysis of Variance. Results are reported as mean±s.d., and were considered statistically significant at P≤0.05.
Quantification of cyclin D1 and p27 expression and statistical analysis
Similar to quantification of EdU-positive cells, cyclin D1 and p27 immunofluorescently labeled cells were manually counted using ImageJ. For each experiment at E11.5, three separate mutant-control littermate pairs were analyzed. For each mutant and control sample, one section was quantified at the caudal region of the developing VFs (three control embryos, n=3 sections and three mutant embryos, n=3 sections). The percentage of cyclin D1+ or p27+ nuclei were compared using the Student's t-test. Results are reported as mean±s.d., and were considered statistically significant at P≤0.05.
Supplementary Material
Acknowledgements
We gratefully acknowledge Drew Ronnenberg and Sierra Raglin for their expert assistance with the tissue sample preparation for this study. We also gratefully acknowledge Glen Leverson for providing the statistical analysis.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: V.L., X.S., S.L.T.; Methodology: V.L., J.M.V., X.S., S.L.T.; Formal analysis: V.L., J.M.V., X.S., S.L.T.; Writing - original draft: V.L., X.S., S.L.T.; Writing - review & editing: V.L., J.M.V., X.S., S.L.T.; Supervision: X.S., S.L.T.; Project administration: S.L.T.; Funding acquisition: S.L.T.
Funding
This work was supported by National Institutes of Health grants (NIDCD R01 DC004336 and R01 DC012773). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.157677.supplemental
References
- Ahmad S. M. and Soliman A. M. S. (2007). Congenital anomalies of the larynx. Otolaryngol. Clin. N. Am. 40, 177-191. 10.1016/j.otc.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Andrew D. J. and Ewald A. J. (2010). Morphogenesis of epithelial tubes: insights into tube formation, elongation, and elaboration. Dev. Biol. 341, 34-55. 10.1016/j.ydbio.2009.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte U., Zeng G., Thompson M. D., Muller P., Micsenyi A., Cieply B., Kaestner K. H. and Monga S. P. S. (2007). β-Catenin is critical for early postnatal liver growth. Am. J. Physiol. Gastrointest. Liver Physiol 292, G1578-G1585. 10.1152/ajpgi.00359.2006 [DOI] [PubMed] [Google Scholar]
- Atanasoski S., Shumas S., Dickson C., Scherer S. S. and Sutera U. (2001). Differential cyclin D1 requirements of proliferating schwann cells during development and after injury. Mol. Cell. Neurosci. 18, 581-592. 10.1006/mcne.2001.1055 [DOI] [PubMed] [Google Scholar]
- Bienz M. (2004). β-Catenin: a pivot between cell adhesion and Wnt signalling. Curr. Biol. 15, 64-67. 10.1016/j.cub.2004.12.058 [DOI] [PubMed] [Google Scholar]
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., Sommer L., Boussadia O. and Kemler R. (2001). Inactivation of the -catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253-1264. [DOI] [PubMed] [Google Scholar]
- Cohen S. R. (1985). Congenital glottis webs in children. A retrospective review of 51 patients. Ann. Otol. Rhinol. Laryngol. Suppl. 121, 2-16. 10.1177/00034894850940S601 [DOI] [PubMed] [Google Scholar]
- Dessimoz J., Bonnard C., Huelsken J. and Grapin-Botton A. (2005). Pancreas-specific deletion of β-catenin reveals Wnt-dependent and Wnt-independent functions during development. Curr. Biol. 15, 1677-1683. 10.1016/j.cub.2005.08.037 [DOI] [PubMed] [Google Scholar]
- Domyan E. T., Ferretti E., Throckmorton K., Mishina Y., Nicolis S. K. and Sun X. (2011). Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development 138, 971-981. 10.1242/dev.053694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokstuen S., Bottani A., Medeiros P. F., Antonarakis S. E., Stoll C. and Schinzel A. (1997). Laryngeal atresia type III with 22q11.2 microdeletion: report of three patients. Am. J. Med. Genet. 70, 130-133. [DOI] [PubMed] [Google Scholar]
- Geng Y., Yu Q., Sicinska E., Das M., Bronson R. T. and Sicinski P. (2001). Deletion of the p27Kip1 gene restores normal development in cyclin D1-deficient mice. Proc. Natl. Acad. Sci. USA 98, 194-199. 10.1073/pnas.98.1.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss A. M., Tian Y., Tsukiyama T., Cohen E. D., Zhou D., Lu M. M., Yamaguchi T. P. and Morrisey E. E. (2009). Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell 17, 290-298. 10.1016/j.devcel.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe B. D., Scherz P. J., Nissim S., Tian H., McMahon A. P. and Tabin C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517-528. 10.1016/j.cell.2004.07.024 [DOI] [PubMed] [Google Scholar]
- Harris-Johnson K. S., Domyan E. T., Vezina C. M. and Sun X. (2009). β-Catenin promotes respiratory progenitor identity in mouse foregut. Proc. Natl. Acad. Sci. USA 106, 16287-16292. 10.1073/pnas.0902274106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnick C. J. and Cotton R. T. (2000). Congenital laryngeal anomalies laryngeal atresia, stenosis, webs, and clefts. Otolaryngol. Clin. N. Am. 33, 1293-1308. 10.1016/S0030-6665(05)70282-6 [DOI] [PubMed] [Google Scholar]
- Henick D. H. (1993). Three dimensional analysis of murine laryngeal development. Ann. Otol. Rhinol. Larynol. Suppl. 156, 3-24. 10.1177/00034894931020S301 [DOI] [PubMed] [Google Scholar]
- Hexige S., Ardito-Abraham C. M., Wu Y., Wei Y., Fang Y., Han X., Li J., Zhou P., Yi Q., Maitra A. et al. (2015). Identification of novel vascular projections with cellular trafficking abilities on the microvasculature of pancreatic ductal adenocarcinoma. J. Pathol. 236, 142-154. 10.1002/path.4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoijman E., Rubbini D., Colombelli J. and Alsina B. (2015). Mitotic cell rounding and epithelial thinning regulate lumen growth and shape. Nat. Commun. 6, 7355 10.1038/ncomms8355 [DOI] [PubMed] [Google Scholar]
- Huang W.-S., Wang J.-P., Wang T., Fang J.-Y., Lan P. and Ma J.-P. (2007). ShRNA-mediated gene silencing of beta-catenin inhibits growth of human colon cancer cells. World J. Gastroenterol. 13, 6581-6587. 10.3748/wjg.v13.i48.6581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-S., Liu F. and Segil N. (2006). A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development 133, 2817-2826. 10.1242/dev.02453 [DOI] [PubMed] [Google Scholar]
- Li Y., Gordon J., Manley N. R., Litingtung Y. and Chiang C. (2008). Bmp4 is required for tracheal formation: A novel mouse model for tracheal agenesis. Dev. Biol. 322, 145-155. 10.1016/j.ydbio.2008.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson A. H., Yuille D., Angel M., Thompson P. G., Vandervoord J. G. and Beckenham E. J. (1991). Velocardiofacial (Shprintzen) syndrome: an important syndrome for the dysmorphologist to recognise. J. Med. Genet. 28, 596-604. 10.1136/jmg.28.9.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobcko P. I., Petrova R. M. and Chaika E. N. (1979). Functional anatomy of physiologic atresia in human and mammal embryogenesis. Anat. Anz. 145, 338-352. [PubMed] [Google Scholar]
- Lungova V., Verheyden J. M., Herriges J., Sun X. and Thibeault S. L. (2015). Ontogeny of the mouse vocal fold epithelium. Dev. Biol. 399, 263-282. 10.1016/j.ydbio.2014.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyashenko N., Winter M., Migliorini D., Biechele T., Moon R. T. and Hartmann C. (2011). Differential requirement for the dual functions of β-Catenin in embryonic stem cell self-renewal and germ layer formation. Nat. Cell Biol. 13, 753-761. 10.1038/ncb2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P., Su G., Drum H., Bringas P. and Kimura S. (1999). Defects in tracheoesophageal and lung morphogenesis in Nkx2-1(−/−) mouse embryos. Dev. Biol. 209, 60-71. 10.1006/dbio.1999.9234 [DOI] [PubMed] [Google Scholar]
- Mou H., Vinarsky V., Tata P. R., Brazauskas K., Choi S. H., Crooke A. K., Zhang B., Solomon G. M., Turner B., Bihler H. et al. (2016). Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell 19, 217-231. 10.1016/j.stem.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski M. L., Wert S. E., Nation J. M., Loudy D. E., Huelsken J., Birchmeier W., Morrisey E. E. and Whitsett J. A. (2003). β-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J. Biol. Chem. 278, 40231-40238. 10.1074/jbc.M305892200 [DOI] [PubMed] [Google Scholar]
- Müller F., O'rahilly R. and Tucker J. A. (1985). The human larynx at the end of the embryonic period proper. 2 The laryngeal cavity and the innervation of its lining. Ann. Otol. Rhinol. Laryngol. 94, 600-617. 10.1177/000348948509400617 [DOI] [PubMed] [Google Scholar]
- Murphy D. A. and Courtneidge S. A. (2011). The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nature 12, 413-426. 10.1038/nrm3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh L. C., Chyung J. H. and Lassar A. B. (1999). Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev. 13, 225-237. 10.1101/gad.13.2.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M. D., Tasic B., Miyamichi K., Li L. and Luo L. (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593-605. 10.1002/dvg.20335 [DOI] [PubMed] [Google Scholar]
- Nelson K. S., Khan Z., Molnár I., Mihály J., Kaschube M. and Beitel G. J. (2012). Drosophila Src regulates anisotropic apical surface growth to control epithelial tube size. Nat. Cell Biol. 14, 518-525. 10.1038/ncb2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubuser A., Peters H., Balling R. and Martin G. R. (1997). Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell 90, 247-255. 10.1016/S0092-8674(00)80333-5 [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M., Jamora C. and Fuchs E. (2003). Sticky business: orchestrating cellular signals at adherens junctions. Cell 112, 535-548. 10.1016/S0092-8674(03)00108-9 [DOI] [PubMed] [Google Scholar]
- Que J., Okubo T., Goldenring J. R., Nam K.-T., Kurotani R., Morrisey E. E., Taranova O., Pevny L. H. and Hogan B. L. M. (2007). Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134, 2521-2531. 10.1242/dev.003855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands T. M., Pechenkina I. V., Hatsell S. J., Pestell R. G. and Cowin P. (2003). Dissecting the roles of β-catenin and cyclin D1 during mammary development and neoplasia. Proc. Natl. Acad. Sci. USA 100, 11400-11405. 10.1073/pnas.1534601100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacca R., Zur K. B., Crowley B. T., Zackai E. H., Valverde K. D. and McDonald-McGinn M. D. (2017). Association of airway abnormalities with 22q11.2 deletion syndrome. Int. J. Pediatr. Otorhinolaryngol. 6, 11-14. 10.1016/j.ijporl.2017.02.012 [DOI] [PubMed] [Google Scholar]
- Sanudo J. R. and Domenech-Matteu J. M. (1990). The laryngeal primordium and epithelial lamina. A new interpretation. J. Anat. 171, 207-222. [PMC free article] [PubMed] [Google Scholar]
- Shu W., Guttentag S., Wang Z., Andl T., Ballard P., Lu M. M., Piccolo S., Birchmeier W., Whitsett J. A., Millar S. E. et al. (2005). Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev. Biol. 283, 226-239. 10.1016/j.ydbio.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Shutman M., Zhurinsky J., Simcha I., Albanese C., D'Amico M., Pestell R. and Ben-Ze'ev A. (1999). The cyclin D1 gene is a target of the b-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. 96, 5522-5527. 10.1073/pnas.96.10.5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. B., Davies A. L., Battista S., Lewisc J. H. and Fekete D. M. (2003). Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev. Biol. 261, 149-164. 10.1016/S0012-1606(03)00297-5 [DOI] [PubMed] [Google Scholar]
- Woo J., Miletich I., Kim B. M., Sharpe P. T. and Shivdasani R. A. (2011). Barx1-mediated inhibition of Wnt signaling in the mouse thoracic foregut controls tracheo-esophageal septation and epithelial differentiation. PLoS ONE 6, e22493 10.1371/journal.pone.0022493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt M. E., Ernest B. and Hartley J. (2005). Laryngotracheal reconstruction in congenital laryngeal webs and atresias. Otolaryngol. Head Neck Surg. 132, 232-238. 10.1016/j.otohns.2004.09.032 [DOI] [PubMed] [Google Scholar]
- Yin Y., White A. C., Huha S.-H., Hilton M. J., Kanazawa H., Longa F. and Ornitz D. M. (2008). An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev. Biol. 319, 426-436. 10.1016/j.ydbio.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W. Y., Slack J. M. and Tosh D. (2005). Conversion of columnar to stratified squamous epithelium in the developing mouse oesophagus. Dev. Biol. 284, 157-170. 10.1016/j.ydbio.2005.04.042 [DOI] [PubMed] [Google Scholar]
- Zaw-Tun H. A. and Burdi A. R. (1985). Reexamination of the origin and early development of the human larynx. Acta Anat. 122, 163-184. 10.1159/000145998 [DOI] [PubMed] [Google Scholar]
- Zeng L., Kempf H. L., Murtaugh L. C., Sato E. M. and Lassar A. B. (2002). Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev. 16, 1990-2005. http://www.genesdev.org/cgi/doi/10.1101/ gad.1008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.