Abstract
Background
Survivors of childhood cancer are at risk for non-surgical premature menopause (NSPM). Risk factors for NSPM and impact of NSPM on reproduction remain poorly defined.
Methods
Menopausal status of 2930 survivors diagnosed 1970–1986, (median age 6, range 0–20); older than 18 at study (median age 35, range 18–58) was compared to 1399 siblings. NSPM was defined as menses cessation ≥six months 5 years after diagnosis prior to age 40 not due to pregnancy, surgery or medications. Among survivors, multivariable logistic regression identified risk factors for NSPM. Pregnancy and live birth rates were compared between survivors with and without NSPM.
Results
110 survivors developed NSPM (median age 32, range 16–40), prevalence at age 40 9.1% (95% confidence interval [CI] 4.9%–17.2%); odds ratio (OR) 10.5 (95% CI 4.2–26.3) compared to siblings. Independent risk factors included exposure to procarbazine ≥ 4,000mg/m2 OR 8.96 (95% CI 5.02–16.00), any dose of ovarian radiation (OvRT) (OvRT<500 cGy, OR 2.73 (95% CI 1.33–5.61); OvRT ≥500 cGy, OR 8.02 (95% CI 2.81–22.85); referent RT=0), and receipt of a stem cell transplant OR 6.35 (95% CI 1.19–33.93). Compared to survivors without NSPM, those who developed NSPM were less likely to ever be pregnant, rate ratio (RR) 0.41 (95% CI 0.22–0.68) or have a live birth RR 0.35, (95% CI 0.16–0.66) between ages 31–40.
Conclusions
Survivors of childhood cancer are at risk for NSPM associated with lower rates of live birth in their thirties. Those at risk should consider fertility preservation if they anticipate delaying childbearing.
Keywords: Premature Menopause, Reproductive Outcomes, Survivorship, Childhood Cancer, Late Effects
INTRODUCTION
Contemporary, combined modality therapy has resulted in five-year survival rates exceeding 80% among children and adolescents diagnosed with cancer.1 It is estimated that 500,000 individuals will be survivors of childhood cancer by the year 2020.2 With increasing numbers of children surviving into adulthood,3 the long-term complications of exposure to chemotherapy, radiation therapy (RT) and surgery have become apparent, including impairment of gonadal function and fertility.4, 5
Females are born with a finite supply of follicles that naturally decline with age through atresia, apoptosis and maturation during menstrual cycles, culminating in menopause at a median age of 52.5 years in the general population.6, 7 Cancer-directed therapies can accelerate this decline resulting in menopause earlier than would otherwise be expected.8–13 Previous Childhood Cancer Survivor Study (CCSS) investigations have demonstrated acute ovarian failure (menopause occurring within five years from diagnosis) in 6.3% of female survivors and a cumulative incidence of non-surgical premature menopause ((NSPM), menopause occurring before age 40 but after 5 years from diagnosis, not related to surgical intervention) in 8%.8, 9
Treatment-related risk factors including higher doses of alkylating agents (e.g. cyclophosphamide, busulfan, procarbazine and ifosfamide), and increasing doses of RT to the ovaries (OvRT), have been implicated in premature menopause.8–16 One study has also identified unilateral oophorectomy as a risk factor.12 Host factors, such as attained age, have also been associated with increasing risk for diminished ovarian reserve.17–19 We undertook this study to provide more precise estimates of the prevalence of and risk factors for NSPM in the CCSS population utilizing an additional 7 years of longitudinal follow-up. As little is known about the implications of premature menopause on reproductive outcomes in childhood cancer survivors, we also assessed pregnancy and live birth rates among those survivors who ultimately developed NSPM.
SUBJECTS AND METHODS
Childhood Cancer Survivor Study
Detailed descriptions of the design, cohort characteristics and baseline data collection of CCSS have previously been published.20–22 In brief, the CCSS is a 26 center retrospective cohort study with longitudinal follow-up of 14,364 long term survivors of childhood cancer in North America diagnosed before the age of 21 and between January 1st 1970 and December 31st, 1986. Participants completed a comprehensive baseline and follow-up questionnaires that included information about demographics and chronic health conditions. Treatment information was abstracted from medical records at the individual institutions at study entry. These data included all treatments in the first five years from diagnosis for the primary cancer, and, if relevant, treatment for relapse and preparatory regimens for stem cell transplantation (SCT). Exposure to chemotherapy was collected either quantitatively (22 agents) or qualitatively (20 additional agents). A cyclophosphamide equivalent dose (CED) was calculated where relevent.23 Additional information about cancer treatment included surgeries performed from the time of diagnosis, and region- and organ-specific radiation dosimetry. Dosimetry methods have previously been described.24 Specifically, the RT record for each patient was abstracted for date of RT, prescription dose(s), and specific treatment parameters of each radiation field including, energy, weighting, configuration, field size, blocking, and anatomic borders by the CCSS study team. Ovary doses for individual patients were determined by reconstructing their RT fields on age-specific computational phantoms and calculating the average absorbed dose separately to the right and left ovaries. The minimum ovary doses used for the analyses were the lesser of the two average doses (either the right or left). A cohort of 3899 siblings, randomly selected, also completed questionnaires for comparison. Institutional Review Board approval was obtained at the coordinating institution and at each individual participating site. Participants provided informed consent.
Premature Menopause
CCSS subjects included in the current study were older than 18 years of age at the time they completed either of the follow-up questionnaires, which included items providing sufficient information to define the menstrual and reproductive outcomes required for this analysis. Information related to menstrual status and reproductive outcomes was not part of the comprehensive baseline questionnaire. Figure 1 details the exclusions from the 9242 female participants in the CCSS cohort, resulting in 2930 subjects eligible for this analysis. The cohort did not include survivors with a second malignancy prior to menopause or primary tumor in the region of the hypothalamic-pituitary gland. Subjects self-reported age at menarche, current menstrual status and age at last menstrual period. Individuals no longer menstruating were asked for the cause of menstrual cessation. Subjects also reported whether or not they had ever been pregnant, the age at which they were pregnant in 5 year age ranges and the outcome of each pregnancy. Of the 2930 participants in this analysis, 2570 reported their menstrual history on the 2000 Follow-up questionnaire, and 2162 reported it on the 2007 Follow-up questionnaire. 1802 subjects completed both questionnaires.
Figure 1.
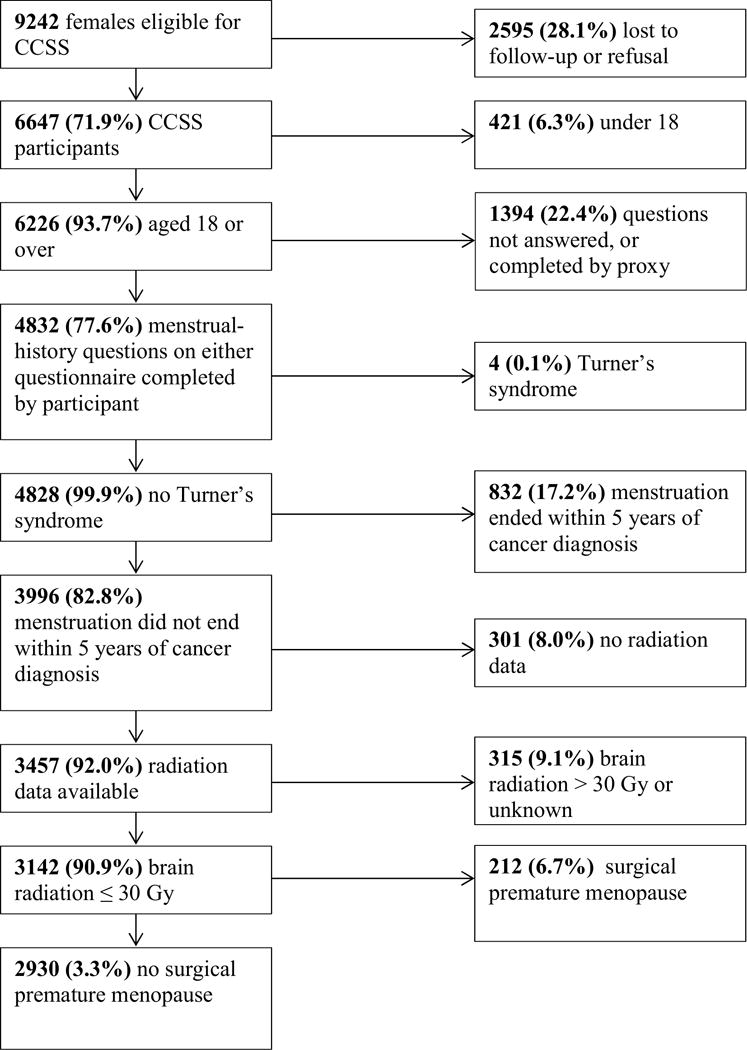
Consort diagram for study population.
NSPM was defined as sustained menses cessation occurring for six months or greater beginning five years after cancer diagnosis but prior to age 40 not due to pregnancy, surgery or medications. The sibling control group was comprised of 1399 females older than the age of 18 who had achieved spontaneous menarche.
Statistical Analysis
Since data sufficient for defining onset of NSPM was included in the Follow-up questionnaires but not the baseline questionnaire, prevalence, rather than incidence, of NSPM was analyzed. That is, the relevant data from the Follow-up questionnaires supports cross-sectional, but not time-to-event, analyses. If menses had ceased, we used the reported ages at last menstrual period to determine the prevalence of NSPM among subjects with a Follow-up questionnaire after that time point at five-year age points from 20 to 40. Generalized estimating equations (GEE) logistic models were used to estimate the associations of risk factors with prevalence, while adjusting for attained age. Predicted prevalence as a function of age was estimated and plotted from these models. A multivariable model was constructed by sequentially adding and removing candidate risk factors including diagnosis, age at diagnosis, exposure to alkylating agents individually and as a group, ovarian radiation, unilateral oophorectomy, smoking status and body mass index (BMI). The quasi-likelihood information criterion (QIC) was used to determine goodness-of-fit; we ultimately reported the best-fitting parsimonious final model.25
Rates of pregnancy and live birth were modelled using Poisson models, utilizing the number of events and number of person-years prior to age of menopause or 40th birthday, and within age categories adjusted for age. Comparisons of rates between women who ultimately experienced NSPM and those who did not should be viewed as a retrospective summary of their fertility.
RESULTS
Non-surgical Premature Menopause
Demographic and treatment characteristics of the 2930 survivors eligible for this analysis are provided in Table 1. Median age at primary cancer diagnosis was 6 years (range 0 – 20), self-reported median age at menarche was 12 years (range 7 – 23), and median age at the time of the current study was 35 years (range 18 – 58). Survivors were compared to 1399 siblings with a median age at menarche 13 years (range 7 – 23), median age at study 38 years (range 19 – 63). A total of 110 survivors developed NSPM at a median age of 32 years (range 16–40), 46 were less than age 30 at the time of menopause, 35 were between 31–35 years and 29 greater than 35. The prevalence of NSPM was 9.1% among survivors at age 40 compared to a prevalence of 0.9% among siblings, (odds ratio [OR] 10.5, 95% confidence interval [CI] 4.2 – 26.3). Frequency according to diagnosis, treatment and demographics is provided in Table 2. Frequency of NSPM by the combination of diagnosis and exposure is provided in Supplementary Table 1.
Table 1.
Characteristics of survivor cohort.
| Total in Cohort N=2930 | ||
|---|---|---|
| N (%) | ||
| Age at diagnosis | 0–9 | 1875 (64.0) |
| 10–14 | 601 (20.5) | |
| 15–20 | 485 (16.6) | |
| Age at study | 21–25 | 302 (10.3) |
| 26–30 | 692 (23.6) | |
| 31–35 | 658 (22.5) | |
| 36–40 | 601 (20.5) | |
| >40 | 655 (22.4) | |
| Diagnosis | Leukemia | 1149 (39.2) |
| Hodgkin Lymphoma | 348 (11.9) | |
| Kidney Tumors | 344(11.7) | |
| Bone Tumors | 311 (10.6) | |
| Neuroblastoma | 254 (8.7) | |
| Soft Tissue Sarcomas | 224 (7.6) | |
| CNS Tumors | 157 (5.4) | |
| Non Hodgkin Lymphoma | 143 (4.9) | |
| Treatment exposure | Alkylating Agent Only | 552 (18.8) |
| Ovarian RT Only | 792 (27.0) | |
| Alkylating Agent and Ovarian RT | 804 27.4) | |
| Unilateral Oophorectomy | 62 (2.1) | |
| Stem Cell Transplant | 17 (0.5) | |
| Smoking History | Yes | 786 (26.8) |
| No | 1741 (59.4) | |
| Unknown | 403 (13.8) | |
| Body Mass Index | Above 30 | 555 (18.9) |
| between 25 and 29.9 | 542 (18.5) | |
| Under 24.9 | 1117 (38.1) | |
| Unknown | 313 (10.7) | |
Table 2.
Frequency of non surgical premature menopause by diagnosis, treatment and demographics.
| Non-surgical premature menopause | |||||
|---|---|---|---|---|---|
| Yes | No | ||||
| N | % | N | % | ||
| Age at diagnosis | 0–9 | 38 | 2.0 | 1837 | 98.0 |
| 10–14 | 29 | 5.1 | 542 | 94.9 | |
| 15–20 | 43 | 8.9 | 442 | 91.1 | |
| Diagnosis | Leukemia | 34 | 3.0 | 1115 | 97.0 |
| Hodgkin Lymphoma | 56 | 16.1 | 292 | 83.9 | |
| Kidney Tumors | 7 | 2.0 | 337 | 98.0 | |
| Bone Tumors | 2 | 0.6 | 309 | 99.4 | |
| Neuroblastoma | 3 | 1.2 | 251 | 98.8 | |
| Soft Tissue Sarcomas | 4 | 1.8 | 220 | 98.2 | |
| CNS Tumors | 1 | 0.6 | 156 | 99.4 | |
| Non Hodgkin Lymphoma | 3 | 2.1 | 140 | 97.9 | |
| Treatment exposure | Any Alkylating Agents | 78 | 5.7 | 1283 | 94.3 |
| Any Procarbazine | 47 | 23.4 | 154 | 76.6 | |
| No procarbazine | 63 | 2.3 | 2666 | 97.7 | |
| Procarbazine > 0 – 4000 mg/m2 | 4 | 13.8 | 25 | 86.2 | |
| Procarbazine ≥ 4000 mg/m2 | 31 | 24.2 | 97 | 75.8 | |
| No OvRT | 16 | 1.2 | 1290 | 98.8 | |
| Minimum OvRT dose > 0–500 cGy |
78 | 5.2 | 1418 | 94.8 | |
| Minimum OvRT dose >500 cGy | 15 | 13.0 | 100 | 87.0 | |
| Alkylating Agent Only | 12 | 2.2 | 540 | 97.8 | |
| OvRT Only | 27 | 3.4 | 765 | 96.6 | |
| Alkylating Agent and OvRT | 66 | 8.2 | 738 | 91.8 | |
| Procarbazine and OvRT | 41 | 23.8 | 131 | 76.2 | |
| Unilateral Oophorectomy | 5 | 8.1 | 57 | 91.9 | |
| Stem Cell Transplant | 3 | 17.6 | 14 | 82.4 | |
| Smoking History | Yes | 29 | 3.7 | 757 | 96.3 |
| No | 61 | 3.5 | 1680 | 96.5 | |
| Unknown | |||||
| Body mass inidex | Above 30 | 21 | 3.8 | 534 | 96.2 |
| between 25 and 29.9 | 26 | 4.8 | 516 | 95.2 | |
| Under 24.9 | 40 | 3.6 | 1077 | 96.4 | |
| Unknown | |||||
OvRT=Ovarian Radiation
Univariate analysis, adjusted for age point, revealed the following to be significant for risk of NSPM: age greater than 15 at the time of diagnosis, exposure to a dose of procarbazine ≥ 4,000mg/m2, CED of ≥ 6,000mg/m2 (with procarbazine included in the CED), any radiation to the ovaries, receipt of a stem cell transplant and a diagnosis of Hodgkin Lymphoma (HL). The following variables were not found to be significant: exposure to any dose of cyclophosphamide, unilateral oophorectomy, smoking status or BMI. When patients who had received procarbazine were removed from the analysis, CED at any dose was no longer significant (Supplementary Table 2).
In the final multivariable analyses, significant variables included exposure to a dose of procarbazine ≥ 4,000mg/m2 [OR 8.96 (95% CI 5.02–16.00), p<.0001], any dose of radiation to the ovaries [OR 2.73 (95% CI 1.33–5.61), p = 0.0062) for an OvRT dose < 500 cGy and 8.02 (95% CI 2.81–22.85), P < 0.0001) for OvRT ≥ 500 cGy]. and receipt of a stem cell transplant [OR 6.35 (95% CI 1.19–33.93), p=0.0307]. (Table 3)
Table 3.
Results of multivariable model for risk for non-surgical premature menopause.
| Parameter | OR | 95% CI | p | ||
|---|---|---|---|---|---|
| Min ovary RT dose | 0 | 1.00 | |||
| >0 – 500 cGy | 2.73 | 1.33 | 5.61 | 0.0062 | |
| >500 cGy | 8.02 | 2.81 | 22.85 | <.0001 | |
| Procarbazine | 0 mg/m2 | 1.00 | |||
| <4000 mg/m2 | 3.07 | 0.76 | 12.43 | 0.1154 | |
| >=4000 mg/m2 | 8.96 | 5.02 | 16.00 | <.0001 | |
| Stem Cell Transplant | No | 1.00 | |||
| Yes | 6.35 | 1.19 | 33.93 | 0.0307 | |
For survivors who received procarbazine ≥ 4, 000 mg/m2 the prevalence of NSPM at age 40 was 39.7% (95% CI 21.2–74.5), compared with 4.2 (95% 2.8–6.2) among those who did not receive any procarbazine (p < 0.0001; Figure 2). Radiation exposure to the ovaries of > 500 cGy resulted in a prevalence of NSPM at age 40 of 24.1% (95% CI 9.5 – 49.0) compared to a prevalence of 3.0% in those who did not receive RT to the ovaries (p < 0.0001; Figure 3).
Figure 2.

Prevalence and 95% confidence intervals of non-surgical premature menopause among survivors by procarbazine dose.
Figure 3.
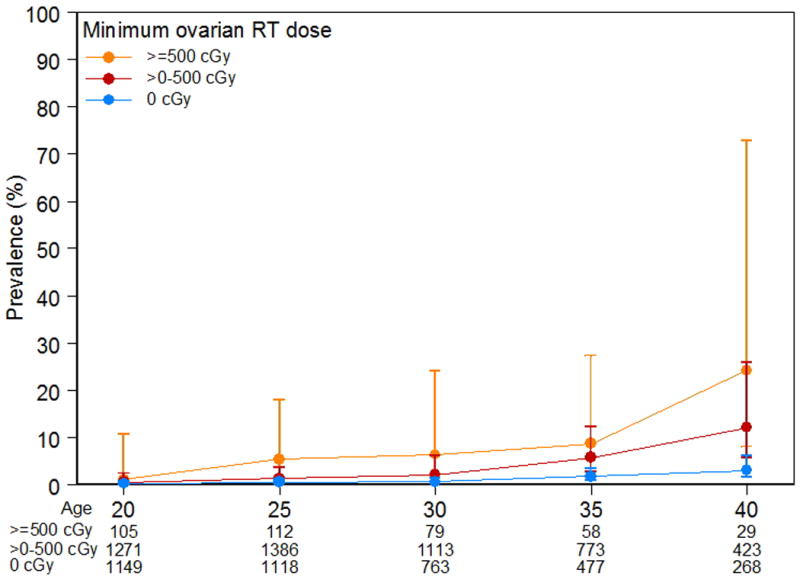
Prevalence and 95% confidence intervals of non-surgical premature menopause among survivors by ovarian radiation dose.
Pregnancy and Live Birth
Pregnancy and live birth rates per patient years by attained age in survivors who ultimately did and did not develop NSPM are presented in Table 4. A total of 103 pregnancies and 66 live births were reported among survivors who ultimately developed NSPM. Thirteen pregnancies (12.6%) occurred within five years of the onset of menopause, and 51 (49.5%) occurred within ten years of the onset of menopause. The rate ratio (RR) for pregnancy among those who developed NSPM was 0.88 (95% CI = 0.72 to 1.06; p = 0.2) compared to those who did not develop NSPM. The RR of ever having a live birth was 0.80 (95% CI = 0.62 to 1.01, p = 0.07) among those who developed NSPM, compared to those without NSPM. While subsequent NSPM did not reduce the risk for pregnancy or live birth among survivors between the ages of 21–30, the RRs for ever being pregnant or ever having a live birth among survivors between the ages of 31–40 were 0.41 (95% CI 0.22 to 0.68; p = 0.0014) and 0.35 (95% CI 0.16–0.66; p = 0.003), respectively for those who developed NSPM compared to those who did not (Table 5).
Table 4.
Pregnancy and live birth rates per patient years by attained age.
| Non-surgical premature menopause | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | |||||||||
| Person-years | # pregnancies | Pregnancy rate per 1000PY | # Live births | Live birth rate per 1000PY | Person-years | # pregnancies | Pregnancy rate per 1000PY | # live births | Live birth rate per 1000PY | |
| Ages 21 to 25 | 452 | 46 | 101.9 | 29 | 64.2 | 12179 | 1172 | 96.2 | 770 | 63.2 |
| Ages 26 to 30 | 364 | 44 | 120.9 | 29 | 79.7 | 9223 | 1223 | 132.6 | 899 | 97.5 |
| Ages 31 to 35 | 242 | 10 | 41.4 | 6 | 24.8 | 5955 | 642 | 107.8 | 485 | 81.4 |
| Ages 36 to 40 | 60 | 3 | 50.3 | 2 | 33.5 | 2833 | 194 | 68.5 | 117 | 41.3 |
| Ages 21 to 30 | 816 | 90 | 110.3 | 58 | 71.1 | 21402 | 2395 | 111.9 | 1669 | 78.0 |
| Ages 31 to 40 | 301 | 13 | 43.2 | 8 | 26.6 | 8788 | 836 | 95.1 | 602 | 68.5 |
| Ages 21 to 40 | 1117 | 103 | 92.2 | 66 | 59.1 | 30190 | 3231 | 107.0 | 2271 | 75.2 |
Table 5.
Age-specific rate ratios (RR) for pregnancies and live births in survivors with non-surgical premature menopause compared to survivors without non-surgical premature menopause.
| Age | RR | 95% CI | p-value | |
|---|---|---|---|---|
| Pregnancy | All ages (21–40) | 0.88 | 0.72–1.06 | 0.20 |
| Ages 21–30 | 1.00 | 0.81–1.23 | 0.97 | |
| Ages 31–40 | 0.49 | 0.27–0.80 | 0.009 | |
| Live Birth | All ages (21–40) | 0.80 | 0.62–1.01 | 0.07 |
| Ages 21–30 | 0.92 | 0.70–1.19 | 0.55 | |
| Ages 31–40 | 0.42 | 0.19–0.79 | 0.015 |
CI= Confidence Interval
DISCUSSION
Adequately counseling female survivors about their reproductive capacity relies on identifying risk factors for NSPM as well as estimating the window of fertility for those with risk factors. The large size and ongoing longitudinal follow-up of aging adult survivors within the CCSS provides a unique opportunity to obtain precise estimates of NSPM after gonadotoxic exposures and to explore the impact of NSPM on pregnancy and live birth rates. We have identified that pregnancy and live birth rates before the age of 30 are not different between patients who ultimately develop NSPM and those who do not.
In the current study, we identified that 9% of female survivors developed NSPM by age 40, in a population with a median age of 34 years, providing the most stable estimates of NSPM to date, and similar to those in our previous report.9 Furthermore, compared to siblings, the risk among these aging survivors was similar to the original estimates (RR 10.5 vs 13.2). This suggests that the risk of NSPM among survivors is not disproportionate to that in the general population as they continue to age. Moreover, this provides clinicians with greater certainty in providing estimates of risk for NSPM as they counsel patients.
In addition to the CCSS, the Five Center Study, the Ontario Cancer Registry, the Euro 2K study, and the St. Jude Lifetime study have all evaluated premature menopause in large cohorts of childhood cancer survivors.10–13 The Euro2K study identified a cumulative incidence of NSPM of 2.1% at age 40 among 706 female survivors. This difference is likely explained, at least in part, to differences in treatment exposures as the Euro2K study was weighted towards survivors of kidney tumors and neuroblastoma (50% of participants vs 21% CCSS), while the CCSS had 39% survivors of leukemia vs. no leukemia survivors in the Euro K study.12 The St. Jude Lifetime study, a cohort more similar in distribution to the CCSS, assessed premature menopause via self-report and clinical measurements, reported a prevalence of 10.9% among its cohort of 921 participants.13
Across these studies, exposure to OvRT has consistently emerged as a risk factor implicated in the development of premature menopause in the childhood cancer population.8–13 In the current study, we again demonstrate the adverse effect of exposure to any OvRT on the risk of NSPM, with risk of NSPM increasing with increasing dose.
Exposure to alkylating agents has also consistently been recognized as a risk factor for diminished ovarian reserve.26, 27 However, honing in on the toxicity of specific agents, as well as the impact of dose and age at exposure (and the interplay among these factors) has proven to be more difficult. In this study, exposure to doses of procarbazine ≥ 4,000mg/m2 was an independent risk factor for NSPM. The deleterious effect of procarbazine on gonadal function has been well described. While both procarbazine and cyclophosphamide were associated with NSPM in the Euro K study, the magnitude of effect was much greater for procarbazine.12 In cohort studies of Hodgkin Lymphoma survivors, cumulative procarbazine dose was strongly associated with risk of premature menopause.28
The impact of other alkylating agents on risk for NSPM has been more variable across different studies. The St. Jude Cohort study found a CED of ≥8,000mg/m2 to be a significant risk factor for development of NSPM, although in a multivariable model that categorized exposure to alkylating agents, ORT, both or neither, exposure to alkylating agents was not significant.13 Of note, in this study, a CED of ≥ 6,000mg/m2 was significant in the univariate analysis but not when patients exposed to procarbazine were removed from the CED calculation, suggesting that the gonadal toxicity of procarbazine may be underestimated in this formula. Additional studies are necessary to further assess the utility the CED in the setting of gonadal toxicity.
Given the reliance on total body radiation and/or high-dose alkylating agents as components of conditioning regimens, patients who undergo stem cell transplant are at high risk for gonadal toxicity.29–32 Although the numbers of patients who had undergone a stem cell transplant were small in this study, this group of patients were identified as being independently at risk for the development of NSPM.
Age at diagnosis as a risk factor for NSPM has been seen in some but not all studies. The Five Center Study, Ontario Cancer Registry Study, and Euro2K study identified increased risk in survivors diagnosed in the post pubertal period compared to those diagnosed in the pre-pubertal period.10–12 In the current analysis, age greater than 15 was significant in a univariate analysis but was not an independent risk factor. This is likely a result of the confounding influence of procarbazine being used primarily as treatment for Hodgkin Lymphoma, a disease more common in adolescence. Age at diagnosis was not a significant risk factor for premature menopause in the St. Jude Lifetime Cohort Study, which, as noted above, is demographically similar to the CCSS cohort.13
There are limited data examining the rates of pregnancy and live births between those who ultimately develop NSPM and those who do not. We determined that for those who ultimately develop NSPM, rates of pregnancy and live birth are substantially reduced prior to NSPM between the ages of 31 and 40. However, pregnancy and live birth rates did not differ for those aged 21–30 years, based on ultimate menopausal status, even for NSPM occurring before age 30. Although we do not have information on whether pregnancies were achieved spontaneously or with assisted reproduction, it is worth noting that almost half of pregnancies in the total NSPM cohort occurred within 10 years of the onset of menopause and 12.6% within five years suggesting conception is possible in the peri-menopausal period.
A range of fertility preserving strategies exist that can improve the chance of having a biologic child despite being exposed to gonadotoxic therapies as part of cancer directed therapy. Two recent publications have demonstrated that, even in the setting of decreased ovarian reserve and/or clinical infertility, pregnancy and live births with or without assisted reproduction are possible.33, 34 These data reinforce the need for providers to educate survivors of childhood cancer regarding their risks for infertility and the fertility preservation options that are available to them.
In interpreting our findings, some limitations should be considered. NSPM is self-reported and may therefore be subject to both over- and under- reporting. Cases of NSPM may be masked by women who are taking oral contraceptives or other hormone medication that result in persistent menstruation. The study cohort was treated in the 1970s and 1980s and therefore was exposed to treatment combinations and doses that may no longer be employed. Data on radiation exposure was calculated according to body region dosimetry which does not differentiate between flank RT (i.e. to one ovary) vs. whole abdomen RT (i.e. involving both ovaries). Therefore, we are limited in being able to comment on the effect of radiation to the ovary when only one ovary is in the field. Our data on pregnancy and live birth does not take into account an individual’s desire to become pregnant and may have led to an overestimate of impaired fertility. Furthermore, as noted above, we are not able to comment on whether pregnancies were achieved spontaneously or via assisted reproduction.
In summary, our data demonstrate that treatment with procarbazine, ovarian RT and SCT are significant risk factors for NSPM. We have also determined that the odds ratio for pregnancy and live birth over the age of 30 is decreased for those patients who ultimately develop NSPM. Clinicians should incorporate this information as they counsel female childhood cancer patients and their families at the time of cancer diagnosis and in the years following completion of cancer directed treatment.
Supplementary Material
Acknowledgments
Funding Sources: National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator). Support to St. Jude Children’s Research Hospital: Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
Footnotes
Conflict of Interest: None.
Author Contributions:
Jennifer Levine, Conceptulization, Methodology, Investigation, Writing, Project Administration
John Whitton, Methodology, Formal Analysis, Investigation, Writing
Jill Ginsberg, Investigation, Writing
Daniel Green, Investigation, Writing
Wendy Leisenring, Methodology, Formal Analysis, Investigation, Writing
Marilyn Stovall, Investigation, Writing
Leslie Robison, Investigation, Writing, Supervision, Funding
Gregory Armstrong, Investigation, Writing, Supervision, Funding
Charles Sklar, Conceptualization, Methodology, Investigation, Writing, Supervision
Oral Presentation: American Society of Clinical Oncology Annual Meeting, Chicago, IL, 6/3/2016
Contributor Information
Jennifer M. Levine, Weill Cornell Medical College, NY, NY.
John A. Whitton, Fred Hutchinson Cancer Research Center, Seattle, WA.
Jill P. Ginsberg, Children’s Hospital of Philadelphia, Philadelphia, PA.
Daniel M. Green, St. Jude Children’s Research Hospital. Memphis, TN.
Wendy M. Leisenring, Fred Hutchinson Cancer Research Center, Seattle, WA.
Marilyn Stovall, University of Texas, MD Anderson, Houston, TX.
Leslie L. Robison, St. Jude Children’s Research Hospital, Memphis, TN.
Gregory T. Armstrong, St. Jude Children’s Research Hospital, Memphis, TN.
Charles A. Sklar, Memorial Sloan Kettering Cancer Center, NY, NY.
References
- 1.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 2.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laverdiere C, Liu Q, Yasui Y, et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:1131–1140. doi: 10.1093/jnci/djp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow EJ, Stratton KL, Leisenring WM, et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2016;17:567–576. doi: 10.1016/S1470-2045(16)00086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston RJ, Wallace WH. Normal ovarian function and assessment of ovarian reserve in the survivor of childhood cancer. Pediatr Blood Cancer. 2009;53:296–302. doi: 10.1002/pbc.22012. [DOI] [PubMed] [Google Scholar]
- 7.Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178:70–83. doi: 10.1093/aje/kws421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91:1723–1728. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- 9.Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:890–896. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 10.Byrne J, Fears TR, Gail MH, et al. Early menopause in long-term survivors of cancer during adolescence. Am J Obstet Gynecol. 1992;166:788–793. doi: 10.1016/0002-9378(92)91335-8. [DOI] [PubMed] [Google Scholar]
- 11.Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964–1988 in Ontario, Canada. Am J Epidemiol. 1999;150:245–254. doi: 10.1093/oxfordjournals.aje.a009995. [DOI] [PubMed] [Google Scholar]
- 12.Thomas-Teinturier C, El Fayech C, Oberlin O, et al. Age at menopause and its influencing factors in a cohort of survivors of childhood cancer: earlier but rarely premature. Hum Reprod. 2013;28:488–495. doi: 10.1093/humrep/des391. [DOI] [PubMed] [Google Scholar]
- 13.Chemaitilly W, Li Z, Krasin MJ, et al. Premature Ovarian Insufficiency in Childhood Cancer Survivors: A Report from the St. Jude Lifetime Cohort. J Clin Endocrinol Metab. 2017 doi: 10.1210/jc.2016-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2009;27:2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green DM, Sklar CA, Boice JD, Jr, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2374–2381. doi: 10.1200/JCO.2008.21.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738–744. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 18.Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6:209–218. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 19.Behringer K, Breuer K, Reineke T, et al. Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2005;23:7555–7564. doi: 10.1200/JCO.2005.08.138. [DOI] [PubMed] [Google Scholar]
- 20.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 21.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 25.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 26.Thomas-Teinturier C, Allodji RS, Svetlova E, et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum Reprod. 2015;30:1437–1446. doi: 10.1093/humrep/dev060. [DOI] [PubMed] [Google Scholar]
- 27.Gracia CR, Sammel MD, Freeman E, et al. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97:134–140 e131. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Bruin ML, Huisbrink J, Hauptmann M, et al. Treatment-related risk factors for premature menopause following Hodgkin lymphoma. Blood. 2008;111:101–108. doi: 10.1182/blood-2007-05-090225. [DOI] [PubMed] [Google Scholar]
- 29.Teinturier C, Hartmann O, Valteau-Couanet D, Benhamou E, Bougneres PF. Ovarian function after autologous bone marrow transplantation in childhood: high-dose busulfan is a major cause of ovarian failure. Bone Marrow Transplant. 1998;22:989–994. doi: 10.1038/sj.bmt.1701483. [DOI] [PubMed] [Google Scholar]
- 30.Sanders JE, Hawley J, Levy W, et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87:3045–3052. [PubMed] [Google Scholar]
- 31.Grigg AP, McLachlan R, Zaja J, Szer J. Reproductive status in long-term bone marrow transplant survivors receiving busulfan-cyclophosphamide (120 mg/kg) Bone Marrow Transplant. 2000;26:1089–1095. doi: 10.1038/sj.bmt.1702695. [DOI] [PubMed] [Google Scholar]
- 32.Borgmann-Staudt A, Rendtorff R, Reinmuth S, et al. Fertility after allogeneic haematopoietic stem cell transplantation in childhood and adolescence. Bone Marrow Transplant. 2012;47:271–276. doi: 10.1038/bmt.2011.78. [DOI] [PubMed] [Google Scholar]
- 33.Barton SE, Najita JS, Ginsburg ES, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013;14:873–881. doi: 10.1016/S1470-2045(13)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dillon KE, Sammel MD, Ginsberg JP, Lechtenberg L, Prewitt M, Gracia CR. Pregnancy after cancer: results from a prospective cohort study of cancer survivors. Pediatr Blood Cancer. 2013;60:2001–2006. doi: 10.1002/pbc.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


