Abstract
In this report, we used filtered noise bands to constrain listeners’ access to interaural level differences (ILDs) and interaural time differences (ITDs) in a sound source localization task. The samples of interest were listeners with single-sided deafness (SSD) who had been fit with a cochlear implant in the deafened ear (SSD-CI). The comparison samples included listeners with normal hearing and bimodal hearing, i.e. with a cochlear implant in 1 ear and low-frequency acoustic hearing in the other ear. The results indicated that (i) sound source localization was better in the SSD-CI condition than in the SSD condition, (ii) SSD-CI patients rely on ILD cues for sound source localization, (iii) SSD-CI patients show functional localization abilities within 1–3 months after device activation and (iv) SSD-CI patients show better sound source localization than bimodal CI patients but, on average, poorer localization than normal-hearing listeners. One SSD-CI patient showed a level of localization within normal limits. We provide an account for the relative localization abilities of the groups by reference to the differences in access to ILD cues.
Keywords: Cochlear implant, Interaural level differences, Single-sided deafness, Sound source localization
A small number of individuals with single-sided deafness (SSD) have received a cochlear implant (CI) in an effort to reduce tinnitus in the deafened ear and/or to improve health-related quality of life by improving speech understanding and sound source localization. Multiple reports suggest that CIs have been successful in achieving these goals [for a comprehensive review of findings see Vlastarakos et al., 2014].
One important component of the improvement in health-related quality of life is an improved ability to localize sound sources. Subjective reports of better sound source localization have been confirmed by laboratory studies showing that localization with a normal-hearing ear and a contralateral CI (SSD-CI) is superior to localization with a single, normal-hearing ear [Arndt et al., 2011a, b; Firszt et al., 2012; Hassepass et al., 2013; Jacob and Stelzig, 2011; Nawaz et al., 2014].
On the one hand, the improved sound source localization is unremarkable given that the baseline – localization with a single ear – is so poor. High levels of localization accuracy depend on the availability of binaural cues [Blauert, 1997], and SSD patients, by definition, do not receive binaural cues.
On the other hand, normal values of binaural cues do not exist for SSD patients fit with a CI. CIs, most generally, code temporal changes in amplitude (envelope information), and fine temporal information, the basis for ITDs, is not well transmitted [Wilson and Dorman, 2009]. Moreover, signal level information, the basis for ILDs, is severely compressed by CI signal processing. All commercial CI signal processing includes an automatic gain control at the front end of the signal path. In the MED-EL devices, for example, this produces a 2: 1 to 3.5: 1 compression for signal levels greater than 48 to 67 dB sound pressure level (SPL), depending on the processor’s sensitivity setting. Signal levels are further compressed when the acoustic signals are mapped by a logarithmic function into the electric dynamic range. In a recent paper [Dorman et al., 2014], we explored the effects of CI processing on ILDs for bilateral CI patients. The magnitude of the level compression is shown in the following example. Normally, the ILD at 3 kHz for a sound source at 45° azimuth is approximately 10 dB; at 15°, the ILD is approximately 3 dB. Following CI signal processing, at 45° the ILD is 1.6 dB, and at 15° it is 0.4 dB. Thus, SSD-CI patients, who have 1 normal-hearing ear and 1 CI ear, will experience a distorted representation of signal level as a function of signal azimuth. We would not expect a normal level of localization accuracy for SSD-CI patients if localization is based on access to either ITD or ILD information.
The cues used by listeners for sound source localization can be inferred from localization data when filtered noise bursts are used as signals. ILDs are largest for frequencies over 1,500 Hz, and ITDs are largest for frequencies under 1,500 Hz [Blauert, 1997]. Noise signals that are low-pass (LP)-filtered at 500 Hz contain strong ITD cues and minimal ILD cues. In contrast, noise signals high-pass (HP)-filtered at 1,500 Hz contain strong ILD cues and minimal ITD cues. In this report, using filtered noise bursts as stimuli, we (i) describe the accuracy of sound source localization by 4 SSD-CI patients, (ii) infer the acoustic cues used by the SSD-CI patients to localize sound sources, (iii) compare the localization ability of SSD-CI patients to the localization ability of normal hearing listeners and bimodal CI patients and (iv) provide a rationale for the sound source localization abilities of the various groups of listeners.
Methods
Participants
Four patients with unilateral deafness served as listeners following approval by the IRB at Arizona State University. Demographic data for the listeners are shown in table 1. The patients ranged in age from 38 to 49 years. In the ear opposite the implant, all patients had pure-tone thresholds within the range of normal for frequencies from 125 Hz to 4 kHz. The patients had 1–6 years of severe-to-profound hearing loss before receiving a CI. The duration of implant experience ranged from 2 to 16 months.
Table 1.
Biographical data for the 4 SSD-CI patients
| S1 | S2 | S3 | S4 | |
|---|---|---|---|---|
| Age, years | 39 | 38 | 48 | 49 |
| Gender | Female | Female | Male | Male |
| Age at profound HL, years | 34 | 36 | 42 | 48 |
| Etiology | Ménière’s disease | Idiopathic | Idiopathic | Idiopathic |
| Time since activation, months | 2 | 9 | 3 | 16 |
| Implant processor | Advanced Bionics | MED-EL | MED-EL | MED-EL |
| Speech (CI only) | 77% sentences quiet | 96% sentences quiet | 76% sentences quiet | 60% sentences quiet |
The sentences are from the AzBio test set [Spahr et al., 2012]. HL = Hearing loss.
Patient S4, prior to testing in our laboratory at 16 months after the CI hookup, had been tested in another laboratory at 1 month after the CI hookup. We describe the data collected at 1 month in the discussion of the results.
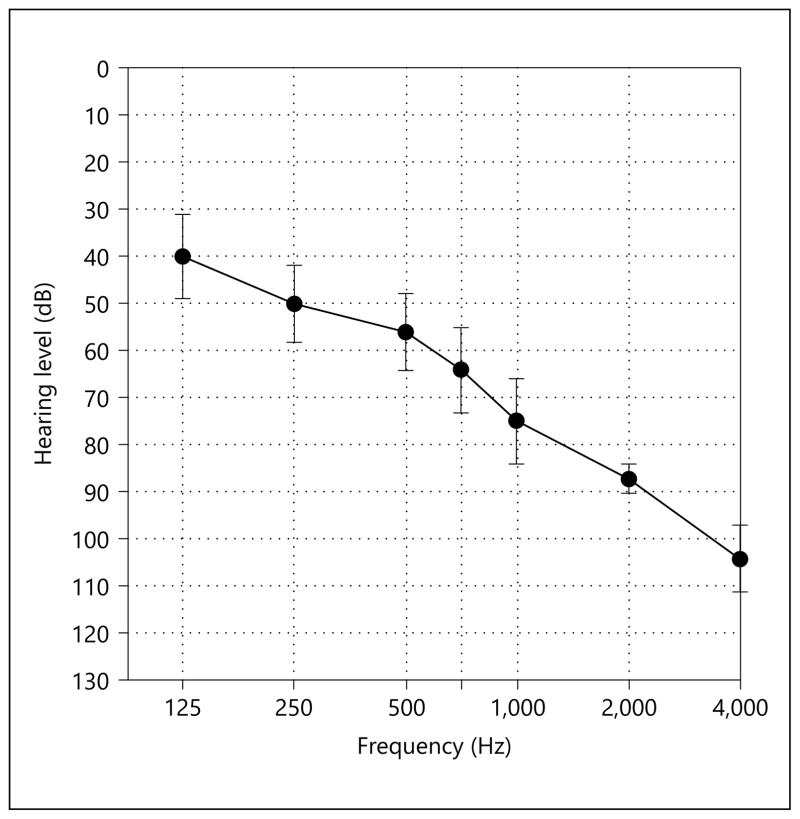
We also tested 45 young, normal-hearing listeners [Yost et al., 2013] and 9 bimodal CI patients (low-frequency acoustic hearing in the ear contralateral to the CI). The mean audiogram for the acoustically stimulated ear for the bimodal patients is shown in figure 1.
Fig. 1.
Mean thresholds for the acoustically stimulated ear for bimodal patients. Error bars indicate the standard error of the mean.
Test Signals
Three 200-ms noise band signals were created and shaped with 20-ms rise-decay times. The wideband (WB) signal was band-pass filtered between 125 and 6,000 Hz. The LP signal was filtered between 125 and 500 Hz. The HP signal was filtered between 1,500 and 6,000 Hz. In all cases, the filter roll-offs were 48-dB/octave. The broadband overall signal level was 65 dBA.
Test Environment
The stimuli were presented from 11 of 13 loudspeakers arrayed within an arc of 180° on the frontal plane. The speakers (Boston Acoustics 110×) were 15° apart. The speakers at the extreme ends of the loudspeaker array were not used for signal delivery in order to minimize edge effects [Rakerd and Hartmann, 1986]. However, the speakers were listed as response options for the listeners. The 3.04 m × 3.35 m room was lined with 4-inch acoustic foam (noise reduction coefficient = 0.9) on all 6 surfaces along with special sound treatment on the floor and ceiling. The broadband reverberation time (RT60) was 90 ms. The subjects sat in a chair at a distance of 1.67 m from the loudspeakers. The loudspeakers were located at the height of the listeners’ pinna.
Test Conditions
Presentation of the 3 noise stimuli was controlled by Matlab. Each stimulus was presented 4 times from each loudspeaker. The presentation level was 65 dBA with a 2-dB rove-in level. Level roving was used to reduce any cues that might be provided by the acoustic characteristics of the loudspeakers. Subjects were instructed to look at the midline (center loudspeaker) until a stimulus was presented. They entered the number of the loudspeaker (1–13) on a keypad.
Results
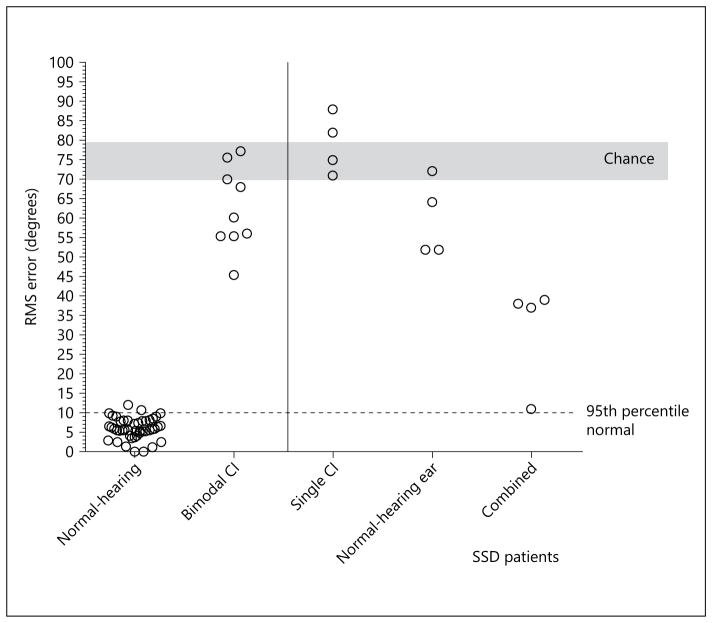
Localization accuracy was calculated in terms of root mean square (RMS) error using the D statistic of Rakerd and Hartman [1986]. Chance performance, calculated using a Monte Carlo method, was 73.5° (SD = 3.2). Localization accuracy, using the WB noise signal, for all listeners is shown in figure 2. The RMS error for the normal-hearing group was 6.0° (SD = 2.7) and for the bimodal CI group, 63° (SD = 11).
Fig. 2.
Sound source localization (RMS error) in response to a WB noise stimulus as a function of the test group. Each circle indicates the performance of a single subject. Chance performance (±1 SD) is shaded in grey. The horizontal dotted line indicates the 95th percentile of the localization scores for the normal-hearing listeners. The solid vertical line is drawn to visually segregate the data of the SSD patients from that of the other groups.
For the SSD-CI patients 1–4, the RMS error scores for the normal-hearing ear alone were 64, 52, 72 and 52°; for the CI alone, 88, 75, 82 and 71°. The error scores in the combined condition were 39, 38, 37 and 11°.
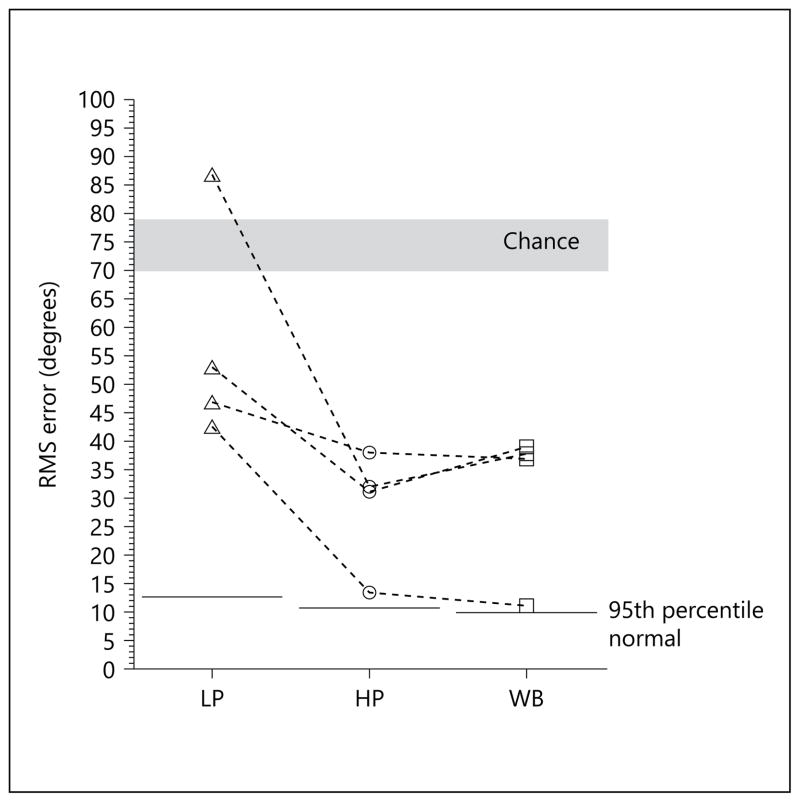
Localization accuracy for the SSD-CI patients in response to the LP, HP and WB noise stimuli is shown in figure 3. RMS error was greatest in response to the LP noise (53, 47, 87 and 43° of RMS error for S1–S4) and was much smaller, and very similar, in response to the HP noise (31, 38, 32 and 13°) and the WB noise (39, 37, 38 and 11°).
Fig. 3.
Sound source localization (RMS error) for SSD-CI patients as a function of stimulus characteristics. Data for the 95th percentile of the localization scores for normal-hearing listeners was taken from Yost et al. [2013].
Discussion
In the introduction, we noted that multiple papers, commonly with relatively small samples, have reported improved sound source localization when SSD patients were fit with a CI. Our findings, shown in figure 2, add to the studies with this outcome. What unique information we provide to the literature is in the cue-specific localization data for the SSD-CI population. Specifically, the data shown in figure 3 suggest that SSD-CI patients use ILD information for sound source localization in the WB noise condition. This follows from (i) the smaller RMS error scores with HP noise as compared to LP noise and (ii) the high degree of similarity of RMS error scores in the WB and HP noise conditions. The performance of patients S3 and S4 is especially informative as performance in the LP noise condition was much poorer than in the HP noise condition and, critically, performance in the WB and HP conditions was nearly identical.
SSD-CI Patients and ITD Cues
Although the performance in the LP noise condition was poorer than in the HP or WB noise conditions, inspection of figure 2 indicates that the performance for 3 of the 4 SSD-CI patients was better than chance in the LP noise condition. Does this mean that some SSD-CI patients have access to ITD cues? For SSD-CI patients, the normal-hearing ear has an excellent representation of this information, but the CI ear has, at best, a poor representation. Perhaps having a good representation from the normal-hearing ear allows whatever information there is from the CI ear to be minimally useful. On the other hand, the level of performance for the 3 patients with the best performance in the LP noise condition (43–53° of error) was in the same range as the level of performance of 1 patient using a single ear (52°) [see Grantham et al., 2008, for a report of better-than-chance localization by patients with a single implant]. Moreover, the level of performance was similar to that for some of the ‘better’ bimodal patients (45 and 55°) for whom binaural ITD information is very unlikely.
Accounting for Between-Group Differences in Localization
One of our principal interests in this report was the performance of the SSD-CI patients relative to other CI patients. Our interest stems from the different alterations to the information available to the listeners and the different normalization routines necessary to overcome the distortions to the cues for sound source localization. Here we provide a rationale for the performance of the patient groups based on the access to, and the symmetry of, ILD information.
Normal-hearing listeners have access to both ILD and ITD information, and either source of information is sufficient for a high level of localization accuracy. For example, Yost et al. [2013] reported 7° of error for an LP noise, 7° of error for an HP noise and 6° of error for a WB noise. Bimodal patients had the poorest mean performance of the 2 CI groups. Bimodal patients have relatively good access to fine temporal structure from the ear with low-frequency acoustic hearing and have access to signal level information from the ear fit with a CI. Neither timing nor level information is well represented at both ears. The very poor sound source localization for these patients [Potts et al., 2009; Tyler et al., 2002] follows from this circumstance.
Consider, finally, the SSD-CI patients. When tested with a single CI or a single hearing ear, the localization accuracy was in the range of the bimodal patients. This is reasonable as there were no consistent binaural cues for the patients in either group. When tested with the combination of a normal-hearing ear and a CI, signal level information could be processed binaurally, and localization accuracy increased.
As noted in the Method section, patient S4 had been tested at another laboratory at 1 month after the CI hookup. Using stimuli derived from head-related transfer functions and presented over headphones, a localization score of 13° RMS error was obtained using a pink noise stimulus. This score is in stark contrast to the scores of patients S1, S2 and S3 who had 2, 3 and 9 months of experience and whose error scores were 39, 37 and 38° in response to the WB noise. When tested in our laboratory at 16 months after the CI hookup, patient S4 achieved an RMS error score of 11° in response to the WB noise. This score is surprisingly close to the score he achieved at 1 month after the hookup and is at the 95th percentile mark for localization scores for normal-hearing listeners. Thus, one of the computational problems confronting the SSDCI patients, a large asymmetry in signal level at the 2 ears, can be solved, and solved very soon after initial activation. This suggests that experience is not the critical difference underlying the different levels of performance for the patients in our sample. It is also not the case that high levels of speech understanding performance are needed with the CI alone. In fact, S4 demonstrated the lowest speech recognition scores with the CI alone, yet the most acute localization scores of the 4 subjects in this sample. One possibility is that for S4 the electrodes in the implanted ear are nearer the ‘correct’ tonotopic place than for the other listeners, thus allowing a more normal relationship of ILDs by frequency and azimuth. Our data do not speak to this issue.
Finally, it is clear from the data of S1–S3 that SSD-CI patients, like bilateral CI patients, do not need to show a completely normal level of sound source localization in order to enjoy the benefits of cochlear implantation. Indeed, one of the most valuable aspects of implantation for SSD-CI patients, according to patient report, is the ability to localize sound sources.
Conclusion
We have replicated previous findings that SSD-CI patients achieve better scores on tests of sound source localization than SSD patients. We add to the literature by demonstrating that SSD-CI patients use ILD cues for sound source localization and that functional localization abilities appear within 1–3 months after device activation. The performance of 1 of our 4 patients indicates that the large asymmetry in the range of signal levels at the 2 ears for SSD-CI patients can be normalized to produce localization performance within the normal range.
Acknowledgments
The research reported here was supported by grants from the NIDCD to M.F.D. and R.H.G. (R01-DC010821) and from the AFOSR to W.A.Y. (FA9550–12–1-0312). Dr. Josh Stohl of the MED-EL Corporation provided the data from S4 when tested at 1 month after the CI hookup.
References
- Arndt S, Aschendorff A, Laszig R, Beck R, Schild C, Kroeger S, Ihorst G, Wesarg T. Comparison of pseudo-binaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011a;32:39–47. doi: 10.1097/MAO.0b013e3181fcf271. [DOI] [PubMed] [Google Scholar]
- Arndt S, Laszig R, Aschendorff A, Schild C, Beck R, Kroeger S, Ihorst G, Kirchem P, Wesarg T. The University of Freiburg Asymmetric Hearing Loss Study. Audiol Neurotol. 2011b;16(suppl 1):3–25. [Google Scholar]
- Blauert J. Spatial hearing: The Psychophysics of Human Sound Localization. Cambridge: MIT Press; 1997. [Google Scholar]
- Dorman M, Loiselle L, Yost W, Stohl J, Spahr A, Brown C, Cook S. Interaural level differences and sound source localization for bilateral cochlear implant patients. Ear Hear. 2014;35:633–640. doi: 10.1097/AUD.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firszt J, Holden L, Reeder R, Waltzman S, Arndt S. Auditory abilities after cochlear implantation in adults with unilateral deafness: a pilot study. Otol Neurotol. 2012;33:1339–1346. doi: 10.1097/MAO.0b013e318268d52d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham W, Ricketts T, Ashmead D, Labadie R, Haynes D. Localization by postlingually deafened adults fitted with a single cochlear implant. Laryngoscope. 2008;118:145–151. doi: 10.1097/MLG.0b013e31815661f9. [DOI] [PubMed] [Google Scholar]
- Hassepass F, Aschendorff A, Wesarg T, Kroger S, Laszig R, Beck RL, Schild C, Arndt S. Unilateral deafness in children: audiologic and subjective assessment of hearing ability after cochlear implantation. Otol Neurotol. 2013;34:53–60. doi: 10.1097/MAO.0b013e31827850f0. [DOI] [PubMed] [Google Scholar]
- Jacob R, Stelzig Y. The Koblenz experience in treating single-sided deafness with cochlear implants. Audiol Neurotol. 2011;16(suppl 1):6–8. [Google Scholar]
- Nawaz S, McNeill C, Greenberg S. Improving sound localization after cochlear implantation and auditory training for the management of single-sided deafness. Otol Neurotol. 2014;35:271–276. doi: 10.1097/MAO.0000000000000257. [DOI] [PubMed] [Google Scholar]
- Potts L, Skinner M, Litovsky R, Strube M, Kuk F. Recognition and localization of speech by adult cochlear implant recipients wearing a digital hearing aid in the non-implanted ear (bimodal hearing) J Am Acad Audiol. 2009;20:353–373. doi: 10.3766/jaaa.20.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakerd B, Hartmann WM. Localization of sound in rooms. III. Onset and duration effects. J Acoust Soc Am. 1986;80:1695–1706. doi: 10.1121/1.394282. [DOI] [PubMed] [Google Scholar]
- Spahr A, Dorman M, Litvak L, van Wie S, Gifford R, Loiselle L, Oakes T, Cook S. Development and validation of the AzBio sentence lists. Ear Hear. 2012;33:112–117. doi: 10.1097/AUD.0b013e31822c2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler R, Parkinson A, Wilson B, Witt S, Preece J, Noble W. Patients utilizing a hearing aid and a cochlear implant: speech perception and localization. Ear Hear. 2002;23:98–105. doi: 10.1097/00003446-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Vlastarakos P, Nazos K, Tavoulari E-F, Nikolopoulos T. Cochlear implantation for singlesided deafness: the outcomes. An evidence-based approach. Eur Arch Otorhinolaryngol. 2014;271:2119–2126. doi: 10.1007/s00405-013-2746-z. [DOI] [PubMed] [Google Scholar]
- Wilson B, Dorman M. The design of cochlear implants. In: Niparko J, editor. Cochlear Implants, Principals and Practices. Lippincott, Philadelphia: 2009. pp. 95–136. [Google Scholar]
- Yost W, Loiselle L, Dorman M, Brown C, Burns J. Sound source localization of filtered noises by listeners with normal hearing: a statistical analysis. J Acoust Soc Am. 2013;133:2876–2882. doi: 10.1121/1.4799803. [DOI] [PMC free article] [PubMed] [Google Scholar]