Abstract
Objective
We aimed to compare perioperative morbidity and mortality and late survival amongst black, white, and Asian patients undergoing intact AAA repair.
Methods
We identified all patients undergoing intact, infrarenal AAA repair in the VQI from 2003–2017. We compared in-hospital outcomes by race using the Fisher Exact and Kruskal Wallis tests. Multivariable logistic and linear regression models of perioperative outcomes adjusted for differences in demographics, comorbidities, hospital volume, and procedure. We used Cox regression to evaluate late survival by race.
Results
In the cohort, 21,961 (94%) patients were white, 1,215 (5.2%) were black, and 318 (1.4%) were Asian. Black patients were more likely to be symptomatic (Black: 16%, White: 9.1%, Asian: 11%, P < .001) and to undergo EVAR (Black: 87%, White: 83%, Asian: 84%, P < .001). There were no differences in 30-day mortality after EVAR (Black: 1.1%, White: 1.1%, Asian: 0.8%, P = .80) or open repair (Black: 4.3%, White: 2.6%, Asian: 1.9%, P = .33). However, black patients were more likely to receive new postoperative dialysis (Black: 1.6%, White: 0.8%, Asian: 0.7%, P = .01) and to return to the operating room (Black: 4.3%, White: 2.9%, Asian: 0.9%, P < .01). Mean hospital length of stay was longer in black patients after EVAR (Black: 3.3 days, White: 2.6, Asian: 2.6, P < .001) and in Asian and black patients after open repair (Black: 10.5 days, White: 8.5, Asian: 13.0, P < .001). After multivariable adjustment, black patients were more likely than white patients to have postoperative dialysis (OR 2.2, 95% CI: 1.3–3.6, P < .01) and return to the operating room (OR 1.6, 95% CI: 1.2–2.2, P < .01). Five-year survival was highest for Asian patients (Black: 84%, White: 85%, Asian: 92%), even in the adjusted Cox model (Asian HR 0.6, 95% CI 0.4–0.97, P = .04).
Conclusions
Although perioperative mortality is comparable across races following AAA repair, black patients are more likely than white or Asian patients to develop new postoperative renal failure and return to the operating room, even after adjusting for differences in comorbidities, operative variables, and hospital volume. Additionally, while Asian patients have the highest rate of postoperative myocardial infarction, they also have the highest late survival. Further studies are warranted to elucidate the mechanism of these disparities.
Introduction
Racial disparities in surgical outcomes are well documented, including disparities following abdominal aortic aneurysm (AAA) repair.1–3 While the prevalence of AAA is lower in black compared to white patients, black patients have been repeatedly found to have worse outcomes following repair.4–6 Several early series using the Veterans Affairs National Surgical Quality Improvement Program data,7 the Nationwide Inpatient Sample,8 and Medicare2 all identified higher rates of perioperative mortality in black patients compared to white patients following open AAA repair on unadjusted analyses. However, these disparities were often mitigated at least in part by adjusting for patient demographics, including socioeconomic status, and comorbid conditions.
More recent studies have focused on the interplay between race, socioeconomic status, and hospital quality, and have found that black patients more often receive care in low-volume hospitals,8 and that hospitals that treat large proportions of black patients have higher mortality for all patients, including their non-black patients.2 Osborne et al. found that black patients had a higher mortality after AAA repair, and that 29% of the disparity was related to patient comorbidities, 26% to socioeconomic factors, and 25% was due to lower hospital quality.9 Additionally, they found that black patients less often underwent endovascular aneurysm repair, even after adjusting for procedure urgency and patient characteristics.10 Notably, however, these studies utilized data from Medicare beneficiaries undergoing intervention between 2001 and 2006, with only one-third to one-half of patients undergoing EVAR. In the modern era, greater than 80% of infrarenal AAA repairs are performed via an endovascular approach, and hospital-and surgeon-volume have less of an effect on perioperative outcomes.11 Furthermore, very few studies have evaluated outcomes in other minority ethnic groups, including Asian patients. While Asians make up a minority of patients undergoing AAA repair, small series have described shorter, more tortuous iliac arteries in Asian patients, and this may impact procedural complexity and therefore outcomes.12–14
We recently used the Society for Vascular Surgery Vascular Quality Initiative (VQI) registry to describe racial differences in presentation for an initial vascular procedure.15 We found that black patients more often presented with symptomatic or ruptured aneurysm, which may represent barriers to appropriate screening and referral to a specialist, and would certainly contribute to worse outcomes overall. However, the impact of race on outcomes following repair of intact aneurysms in the modern era is less clear. Furthermore, few studies have evaluated perioperative outcomes other than mortality. Therefore, the goal of this study is to expand upon our previous analysis by using the VQI to compare perioperative morbidity and mortality and late survival amongst black, white, and Asian patients undergoing intact AAA repair.
Methods
The Beth Israel Deaconess Medical Center Institutional Review Board approved this study and waived informed consent due to the use of de-identified data in the Vascular Quality Initiative.
Population
We performed a retrospective cohort study using the Society for Vascular Surgery Vascular Quality Initiative (SVS-VQI), a national clinical registry established as a collaboration between regional quality groups to improve patient care through the prospective collection of clinical data. At the time of this study, the VQI included 17 regions and close to 400 participating hospitals. Within participating hospitals, complete capture of all included procedures is expected, with regular performance reviews to ensure compliance. More information about the VQI can be found at www.vascularqualityinitiative.org. We identified all patients undergoing open or endovascular repair of an infrarenal abdominal aortic aneurysm between 2003 and January 2017, and excluded those with rupture or those with missing race data or race other than white, black, or Asian (n = 818, 3.4%). Patients with missing race data or race other than white, black, or Asian were more often treated with open repair (20% vs. 17%, P = .04) and at low-volume hospitals (52% vs. 37%, P < .001), and had slightly lower rates of chronic obstructive pulmonary disease coronary artery disease, congestive heart failure, and chronic kidney disease (all P < .05). Hispanic ethnicity was coded separately, with Hispanic and non-Hispanic white patients grouped together and Hispanic and non-Hispanic black patients grouped together given the low percentage of Hispanic patients. Race and ethnicity were coded by the treating provider at each hospital who enters demographic data, which in many cases comes from how patients self-identify.
Variables
Demographics, comorbid conditions, operative details, and in-hospital postoperative outcomes were identified for all patients. We used the standard formula for BMI: BMI = weight (kg)/height (m2) and a single preoperative creatinine value to estimate the glomerular filtration rate (GFR) for each patient using the Modification of Diet in Renal Disease Study equation, which accounts for patient sex and race.16 Renal insufficiency was considered present with an estimated GFR < 30 mL/min/1.73m2 or current dialysis. We defined preoperative anemia as hemoglobin < 10 g/dL.
Hospital volume was calculated using de-identified center identification numbers from the VQI, and was calculated separately for each center’s open and endovascular volume in the prior year, with low-volume hospitals for the respective procedures defined as those performing < 18 open cases or < 30 endovascular cases, as per our prior work.11 Prior aneurysm repair included open or endovascular repair of any aortic aneurysm, and prior AAA repair was noted separately. The VQI defined aortic diameter as the maximum total aortic diameter within the diseased segment being treated. Symptomatic patients were those presenting with symptoms but without rupture, as defined by the VQI. Concomitant procedures during EVAR included hypogastric coiling (pre- or intraoperative), unplanned graft extension, femoral endarterectomy, iliofemoral bypass, thrombectomy, iliac angioplasty or stent placement, and renal angioplasty and/or stent placement. Concomitant procedures during open repair included thrombectomy, renal bypass, infrainguinal bypass, or other intraabdominal procedures.
Outcomes
Thirty-day and long-term mortality were captured using linkage between the VQI and the Social Security Death Index and therefore were considered complete. In-hospital postoperative complications were recorded per the VQI registry and included new stroke, myocardial infarction, pulmonary complications including pneumonia or reintubation, temporary or permanent renal replacement therapy, intestinal ischemia, any postoperative blood transfusion, and reoperation. In the VQI, myocardial infarction is defined as any troponin elevation, EKG changes, or clinical evidence of myocardial infarction. Pneumonia is defined as any treatment with antibiotics and a chart diagnosis of pneumonia using accepted individual institutional criteria, which may include clinical status, physical exam, chest X-ray findings, white blood cell count, and/or culture. Additionally, we recorded hospital and intensive care unit lengths of stay for each patient, including for the subgroup of patients with no complications.
Statistical Analysis
Categorical variables were presented as percentages. Continuous, non-normally distributed variables were presented as median (interquartile range [IQR]). Statistical tests compared three groups: white, black, and Asian patients. When appropriate, analyses of operative and outcome variables were stratified by repair type: EVAR or open repair. Differences between cohorts were assessed using the Fisher’s exact test for categorical variables. All continuous variables were non-normally distributed, so we used the Kruskal Wallis test for these comparisons. Logistic and Cox hazards regression modeling were utilized to assess the independent association between race and perioperative outcomes and late survival, respectively. White race was used as the reference group for all multivariable models. Purposeful selection was used to identify covariates for inclusion in the models, which allows for inclusion of select covariates identified on univariate analysis with P < .1 as well as clinically relevant factors shown to be predictive of adverse events in previous studies.17 Age, sex, relevant comorbidities, hospital volume, aortic diameter, symptoms, and repair type were thus accounted for in all multivariable models. All variables used in the multivariable models also had < 5% missing data. Kaplan-Meier survival estimates were stratified by race and compared using the Log Rank test, with all standard errors < 10%. All tests were conducted two-sided, and a P-value of less than 0.05 was considered significant. Statistical analysis was conducted using STATA version 14.2 (StataCorp LP, College Station, TX).
Results
Demographics
We identified 23,494 patients who underwent AAA repair, of which 21,961 were white, 1,215 black, and 318 Asian. In general, Asian patients were the oldest, least often female, and had the least comorbidities. Conversely, black patients were the youngest, most often female, and had the most comorbidities (Table I). Notably, black and Asian patients were more likely than white patients to have renal insufficiency, or estimated GFR less than 30 mg/mL/1.73m2 (Black: 6.9%, White: 3.6%, Asian: 6.9%, P < .001), and were more likely to be on dialysis preoperatively (Black: 3.6%, White: 0.8%, Asian: 3.1%, P < .001).
Table I.
Demographics and preoperative comorbidities of patients undergoing AAA repair by race
| % or median (IQR) | Black N = 1,215 |
White N = 21,961 |
Asian N = 318 |
P-value |
|---|---|---|---|---|
| Age, years | 70 (64–78) | 73 (67–79) | 75 (68–81) | < .001 |
| Female | 363 (30%) | 4,229 (19%) | 44 (14%) | < .001 |
| Body Mass Index, kg/m2 | 26.6 (23.2–30.8) | 27.4 (24.3–31.0) | 24.4 (22.0–26.6) | < .001 |
| Smoker, ever | 1,033 (85%) | 19,239 (88%) | 203 (64%) | < .001 |
| Smoker, current | 497 (41%) | 7,361 (34%) | 53 (17%) | < .001 |
| COPD | 322 (27%) | 7,360 (34%) | 58 (18%) | < .001 |
| Hypertension | 1,110 (91%) | 18,180 (83%) | 253 (80%) | < .001 |
| Coronary artery disease | 309 (25%) | 6,346 (29%) | 69 (22%) | < .01 |
| Congestive heart failure | 175 (14%) | 2,461 (11%) | 24 (7.6%) | < .001 |
| Renal Insufficiency | 84 (6.9%) | 789 (3.6%) | 22 (6.9%) | < .001 |
| Dialysis Dependence | 44 (3.6%) | 170 (0.8%) | 10 (3.1%) | < .001 |
| Diabetes | 332 (27%) | 4,239 (19%) | 85 (27%) | < .001 |
| Anemia | 117 (9.6%) | 901 (4.1%) | 19 (6.0%) | < .001 |
| Family History of Aneurysm | 24 (5.0%) | 1,321 (11%) | 5 (4.4%) | < .001 |
| Prior AAA Repair, any | 29 (2.4%) | 298 (1.4%) | 6 (1.9%) | .01 |
| Prior Open AAA Repair | 17 (1.4%) | 178 (0.8%) | 2 (0.6%) | .09 |
| Prior Endovascular Repair | 12 (1.0%) | 125 (0.6%) | 4 (1.3%) | .04 |
Operative Details
Black patients were more likely to undergo EVAR as opposed to open repair (Black: 87% EVAR, White: 83%, Asian: 84%, P < .01), although there was little clinical difference in these rates. Black patients were also the most likely to be symptomatic (Black: 16%, White: 9.1%, Asian: 11%, P < .001) (Table II). Asian patients were most likely to undergo repair at a low-volume hospital (Black: 40%, White: 37%, Asian: 50%, P < .001). There was no clinically significant difference in aortic diameter. However, we identified statistically significant differences in rates of concomitant iliac artery aneurysms by race, with the highest among black patients (Black: 40%, White: 25%, Asian: 31%, P < .001). Among those with iliac artery aneurysms, the mean diameter was greatest among Asian patients (Black: 31 mm, White: 30 mm, Asian: 44 mm, P < .01).
Table II.
Presentation and type of repair by race
| % or median (IQR) | Black N = 1,215 |
White N = 21,961 |
Asian N = 318 |
P-value |
|---|---|---|---|---|
| Repair Type | < .01 | |||
| EVAR | 1,052 (87%) | 18,152 (83%) | 266 (84%) | |
| Open Repair | 163 (13%) | 3,809 (17%) | 52 (168%) | |
| Urgent Repair | 195 (16%) | 2,000 (9.1%) | 35 (11.0%) | < .001 |
| Low Volume Hospital | 481 (40%) | 8,039 (37%) | 159 (50%) | < .001 |
| Aneurysm diameter, mm | 55 (50–61) | 55(51–60) | 55 (51–62) | .049 |
| Iliac Aneurysm, any | 482 (40%) | 5,581 (25%) | 99 (31%) | < .001 |
| Maximum Diameter, mm | 31 (26–45) | 30 (23–40) | 44 (29.5–49) | < .01 |
| Iliac Aneurysm, bilateral | 299 (25%) | 2,733 (12%) | 46 (14%) | < .001 |
IQR = interquartile range; EVAR = endovascular aneurysm repair
Operative variables among the 19,470 patients (83%) undergoing EVAR are outlined in Table IIIa. Black and Asian patients had longer operative times (Black: 124 minutes, White: 115 minutes, Asian: 124 minutes, P < .001), but the clinical difference was not substantial. Black patients had statistically more blood loss and were more likely to be transfused. Asian patients were most likely to have any endoleak at the end of the case (Black: 26%, White: 30%, Asian: 36%, P < .01), including higher rates of type II endoleak (Black: 21%, White: 25%, Asian: 28%, P = .02). However, there was no difference in rates of type I or III endoleak. There was no difference in the amount of contrast used by race. Not surprisingly given the disparities in rates of iliac aneurysms, black and Asian patients more often underwent hypogastric coverage with or without coiling (Black: 20%, White: 9.3%, Asian: 22%, P < .001). Hypogastric coiling was most common amongst Asian patients and, interestingly, least common among black patients (Black: 14%, White: 16%, Asian: 19%, P < .001), although the rates were clinically similar across all three groups. There was no difference in rates of renal artery angioplasty or stenting but iliac artery stenting was highest among black patients (Black: 12%, White: 6.8%, Asian: 5.7%, P < .001).
Table IIIa.
Operative variables by race amongst patients undergoing EVAR
| % or median (IQR) | Black N = 1,052 |
White N = 18,152 |
Asian (N = 266 |
P-value |
|---|---|---|---|---|
| General Anesthesia | 974 (93%) | 16,603 (91%) | 232 (87%) | .03 |
| Procedure Time, minutes | 124 (91–175) | 115 (87–157) | 124 (92–179) | < .001 |
| Estimated Blood Loss, mL | 100 (50–225) | 100 (50–200) | 120 (50–250) | < .001 |
| Intraoperative Transfusion, any | 116 (11%) | 1,054 (5.8%) | 25 (9.4%) | < .001 |
| Hypogastric Coverage/Coiling | 121 (26%) | 916 (11%) | 34 (28%) | < .001 |
| Any Endoleak, end of case | 211 (26%) | 4,294 (30%) | 81 (36%) | < .01 |
| Type I Endoleak | 38 (5.0%) | 725 (5.5%) | 18 (8.6%) | .13 |
| Type II Endoleak | 164 (21%) | 3,445 (25%) | 62 (28%) | .02 |
| Type III Endoleak | 6 (0.8%) | 96 (0.7%) | 0 (0%) | .59 |
| Type IV Endoleak | 4 (0.5%) | 107 (0.8%) | 1 (0.5%) | .76 |
| Contrast Used | 85 (55–125) | 85 (60–120) | 85 (55–130) | .74 |
| Hypogastric Coverage | 94 (20%) | 797 (9.3%) | 27 (22%) | < .001 |
| Concomitant Procedure, any | 167 (35%) | 2,446 (29%) | 43 (35%) | < .01 |
| Hypogastric Coiling | 68 (14%) | 505 (16%) | 18 (19%) | < .001 |
| Unplanned Graft Extension | 39 (8.2%) | 725 (8.5%) | 15 (12%) | .30 |
| Femoral Endarterectomy | 19 (4.0%) | 366 (4.3%) | 2 (1.6%) | .41 |
| Iliofemoral Bypass | 2 (0.4%) | 43 (0.5%) | 1 (0.8%) | .63 |
| Thrombectomy | 6 (1.3%) | 86 (1.0%) | 1 (0.8%) | .81 |
| Iliac Artery Angioplasty | 40 (8.4%) | 753 (8.8%) | 5 (4.1%) | .18 |
| Iliac Artery Stenting | 59 (12%) | 578 (6.8%) | 7 (5.7%) | < .001 |
| Renal Artery Stent | 13 (2.7%) | 328 (3.8%) | 2 (1.6%) | .29 |
IQR = interquartile range
Operative characteristics among open repair patients are shown in Table IIIb. Procedure time was longest in black patients (Black: 282 minutes, White: 210, Asian: 209, P < .001). White and Asian patients were most likely to have an epidural placed in addition to general anesthesia (Black: 32%, White: 51%, Asian: 52%, P < .001). White patients were more likely to have a tube graft as opposed to a bifurcated graft (Black: 21%, White: 41%, Asian: 27%, P < .001). Among patients with bifurcated grafts, Asian patients were the least likely to have an anastomosis to the femoral artery (Black: 21%, White: 25%, Asian: 5.4%, P < .01). As in EVAR, black patients had the highest blood loss and were most likely to be transfused. Notably, black patients were the most likely to have been an early (< 30 days) conversion from EVAR (Black: 3.1%, White: 1.0%, Asian: 1.9%, P = .045). There was no difference in rates of concomitant procedures by race, except for concurrent infrainguinal bypass (Black: 5.5%, White: 2.3%, Asian: 1.9%, P = .04)
Table IIIb.
Operative variables by race amongst patients undergoing open surgical repair
| % or median (IQR) | Black N = 163 |
White N = 3,809 |
Asian N = 52 |
P-value |
|---|---|---|---|---|
| Procedure Time, minutes | 282 (218–355) | 210 (164–280) | 209 (178–247) | < .001 |
| Epidural Used with GETA | 52 (32%) | 1,946 (51%) | 27 (52%) | < .001 |
| Retroperitoneal Incision | 23 (14%) | 651 (17%) | 7 (14%) | .57 |
| Tube Graft | 35 (21%) | 1,555 (41%) | 14 (27%) | < .001 |
| Bifurcated Graft | 128 (79%) | 2,239 (59%) | 37 (73%) | < .001 |
| Femoral Anastomosis | 27 (21%) | 565 (25%) | 2 (5.4%) | < .01 |
| Estimated Blood Loss, mL | 1,700 (900–2,926) | 1,100 (700–1,950) | 1,175 (850–1,975) | < .001 |
| Intraoperative Transfusion, any | 84 (52%) | 1,095 (29%) | 18 (35%) | < .001 |
| Heparin Used | 160 (99%) | 3,754 (99%) | 50 (98%) | .63 |
| Conversion from EVAR, any | 12 (7.4%) | 126 (3.3%) | 2 (3.9%) | .03 |
| Early (< 30 day) Conversion | 5 (3.1%) | 39 (1.0%) | 1 (1.9%) | .045 |
| Late Conversion | 7 (4.3%) | 87 (2.3%) | 1 (1.9%) | .24 |
| IMA Ligated/Occluded | 132 (81%) | 3,311 (87%) | 44 (85%) | .08 |
| Hypogastric Ligated/Occluded | 32 (20%) | 406 (11%) | 6 (12%) | < .01 |
| Unilateral | 24 (15%) | 275 (7.3%) | 4 (7.8%) | < .01 |
| Bilateral | 8 (4.9%) | 131 (3.5%) | 2 (3.9%) | .51 |
| Concomitant Procedures, any | 28 (17%) | 649 (17%) | 9 (17%) | .99 |
| Thrombectomy | 9 (5.5%) | 218 (5.7%) | 4 (7.7%) | .72 |
| Renal Bypass | 2 (1.2%) | 75 (2.0%) | 1 (1.9%) | .83 |
| Infrainguinal Bypass | 9 (5.5%) | 88 (2.3%) | 1 (1.9%) | .04 |
| Other Abdominal Procedure | 14 (8.6%) | 337 (8.9%) | 5 (9.6%) | .94 |
IQR = interquartile range; GETA = general endotracheal anesthesia; EVAR = endovascular aneurysm repair; IMA = inferior mesenteric artery
Outcomes
Thirty-day mortality was similar across races following EVAR (Black: 1.1%, White: 1.1%, Asian: 0.8%, P = 1.0) and open repair (Black: 4.3%, White: 2.6%, Asian: 1.9%, P = .33) (Table IV). Asian patients more likely had a postoperative myocardial infarction (Black: 1.3%, White: 1.5%, Asian: 4.7%, P < .001) or pneumonia (Black: 1.1%, White: 0.8%, Asian: 2.2%, P = .01). Black patients more often started new dialysis (Black: 1.6%, White: 0.8%, Asian: 0.7%, P = .01), were transfused (Black: 17%, White: 9.9%, Asian: 13%, P < .001), and returned to the operating room (Black: 4.3%, White: 2.9%, Asian: 0.9%, P < .01). Additionally, black patients had the longest intensive care unit (Open: Black: 3 [Interquartile Range IQR 2–6] days, White: 2 [1–4], Asian: 2 [2–5], P < .001) and hospital lengths of stay (EVAR: Black: 2 [1–3] days, White: 1 [1–2], Asian: 1 [1–2], P < .001; Open: Black: 7 [6–12] days, White: 7 [5–9], Asian: 7 [6–10], P < .001), and were more likely discharged to a skilled nursing facility (Black: 12%, White: 8.6%, Asian: 5.1%, P < .001).
Table IV.
Unadjusted 30-day postoperative mortality and complications by race
| % or median (range) | Black N = 1,215 |
White N = 21,961 |
Asian N = 318 |
P-value |
|---|---|---|---|---|
| Mortality | ||||
| 30-Day | 18 (1.5%) | 298 (1.4%) | 3 (0.9%) | .80 |
| EVAR | 11 (1.1%) | 200 (1.1%) | 2 (0.8%) | 1.0 |
| Elective only | 6 (0.7%) | 143 (0.9%) | 1 (0.4%) | .80 |
| Open | 7 (4.3%) | 98 (2.6%) | 1 (1.9%) | .33 |
| Elective only | 5 (3.9%) | 80 (2.4%) | 1 (2.1%) | .43 |
|
| ||||
| Complications | ||||
| Stroke | 7 (0.6%) | 52 (0.3%) | 1 (0.3%) | .09 |
| Cardiac Complications | 66 (5.5%) | 1,229 (5.6%) | 30 (9.4%) | .02 |
| Myocardial Infarction | 16 (1.3%) | 323 (1.5%) | 15 (4.7%) | < .001 |
| Dysrhythmia | 45 (3.7%) | 845 (3.9%) | 15 (4.7%) | .65 |
| Congestive Heart Failure | 12 (1.0%) | 292 (1.3%) | 7 (2.2%) | .22 |
| Respiratory Complications | 39 (3.2%) | 656 (3.0%) | 11 (3.5%) | .19 |
| Pneumonia | 13 (1.1%) | 174 (0.8%) | 7 (2.2%) | .02 |
| Reintubation | 26 (2.1%) | 482 (2.2%) | 4 (1.3%) | .59 |
| Colitis | 9 (0.7%) | 199 (0.9%) | 5 (1.6%) | .36 |
| Postoperative Dialysis | 19 (1.6%) | 170 (0.8%) | 2 (0.7%) | .01 |
| Permanent Dialysis | 9 (0.8%) | 74 (0.3%) | 1 (0.3%) | .08 |
| Any Transfusion | 202 (17%) | 2,184 (9.9%) | 42 (13%) | < .001 |
| Return to Operating Room | 52 (4.3%) | 632 (2.9%) | 3 (0.9%) | < .01 |
| ICU Length of Stay, Open only | 3 (2–6) | 2 (1–4) | 3 (2–5) | < .001 |
| Hospital Length of Stay | 2 (1–5) | 2 (1–4) | 2(1–4) | < .001 |
| EVAR only | 2 (1–3) | 1 (1–2) | 1 (1–2) | < .001 |
| Open only | 7 (6–12) | 7(5–9) | 7(6–10) | < .001 |
| Discharge to SNF | 143 (12%) | 1,876 (8.6%) | 16 (5.1%) | < .001 |
EVAR = endovascular aneurysm repair; ICU = intensive care unit; SNF = skilled nursing facility
After adjustment for age, sex, comorbidities (including smoking, coronary artery disease, renal insufficiency, and diabetes), hospital volume, repair type, symptom status, and aortic diameter, there was still no difference in 30-day mortality by race (Table V). However, after adjustment, Asian patients had higher rates of myocardial infarction compared to white patients (Odds Ratio (OR) 4.0 [95% Confidence Interval (CI) 2.3 – 7.0), P < .001). Black patients were significantly more likely than white patients to be started on new dialysis (2.3 [1.7 – 3.1], P < .001), even after adjusting for contrast volume and intraoperative renal artery intervention, and were also more likely to return to the operating room (1.6 [1.2 – 2.2], P < .01). Length of stay after adjustment was 0.6 days longer in black patients than white patients, although this was not significant (P = .07). However, black patients were significantly more likely to be discharged to a skilled nursing facility (OR 1.7 [1.4 – 2.0], P < .001), whereas Asian patients were significantly less likely to be (OR 0.6 [0.3 – 0.9], P = .03).
Table V.
Multivariable regression models for 30-day mortality and complications a
| Black vs. White | Asian vs. White | |||
|---|---|---|---|---|
| Effect Estimate (95% CI) | P-Value | Effect Estimate (95% CI) | P-Value | |
| Mortality | OR 1.0 (0.6 – 1.7) | .96 | OR 0.6 (0.2 – 2.0) | .45 |
| Myocardial Infarction | OR 1.0 (0.6 – 1.8) | .86 | 4.0 (2.3 – 7.0) | < .001 |
| New Dialysis b | OR 2.3 (1.7 – 3.1) | < .001 | 0.7 (0.3 – 1.7) | .43 |
| Reoperation | OR 1.6 (1.2 – 2.2) | < .01 | OR 0.4 (0.1 – 1.1) | .08 |
| Length of Stay | 0.6 days (−0.04 – 1.2) | .07 | −0.1 days (−1.3 – 1.1) | .87 |
| Discharge to SNF | OR 1.7 (1.4 – 2.0) | < .001 | OR 0.6 (0.3 – 0.9) | .03 |
CI = confidence interval; OR = odds ratio; SNF = skilled nursing facility
Covariates in all models included: age, sex, smoking status, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, preoperative dialysis, diabetes, low-volume hospital, repair type (EVAR vs. open), urgency of repair, and aortic diameter.
Excludes all patients on preoperative dialysis. Adjusts for preoperative estimated glomerular filtration rate as well as operative renal revascularization.
Unadjusted late survival was higher in Asian patients compared to white and black patients (Figure 1), with 5-year survivals of 92%, compared to 85% in white patients and 84% in black patients. After adjusting for differences in age, sex, comorbidities, and type and urgency of repair, Asian patients still had better late survival (Hazard Ratio (HR) 0.6 [0.4 – 0.97], P = .04) (Table VI).
Figure 1.
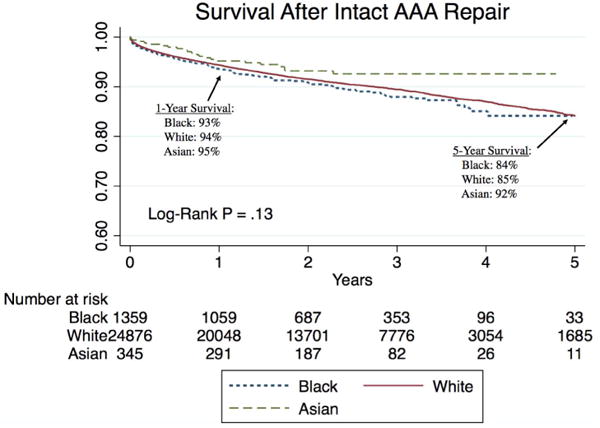
Long-term survival by race after intact abdominal aortic aneurysm repair suggests that Asian patients have slightly higher unadjusted survival than white and black patients, although this is not statistically significant. All standard errors are < 10%.
Table VI.
Cox regression for long-term mortality, with white race as a reference group.
| Hazard Ratio | 95% Confidence Interval | P-Value | |
|---|---|---|---|
| Black Race | 0.9 | 0.7 – 1.1 | .33 |
| Asian Race | 0.6 | 0.4 – 0.97 | .04 |
Other covariates include age, sex, smoking status, chronic obstructive pulmonary disease, coronary artery disease, congestive heart failure, preoperative dialysis, diabetes, statin use, low-volume hospital, repair type, symptom status, and aortic diameter.
Discussion
Following repair of intact abdominal aortic aneurysms, we found no differences in rates of perioperative mortality by race, but black patients had higher rates of complications including transfusion, renal failure resulting in dialysis, and reoperation in the index hospitalization. Conversely, Asian patients had similar rates of perioperative morbidity and lower mortality compared to white patients, although they did have higher rates of postoperative myocardial infarction. Identifying and understanding the etiology of these disparities will allow us to improve the vascular care provided to these patients in the future.
We found no difference in perioperative mortality amongst black, white, and Asian patients, in contrast to much of the literature, in which black patients have been shown to have higher perioperative mortality than white patients. The rates following EVAR were the same across races, but when we compared the unadjusted mortality rates following open repair, there was a trend towards higher mortality in black patients (4.3% vs. 2.6%), although the sample size of nonwhite patients was likely too small to detect a difference. However, it is important to note that prior work identifying mortality disparities evaluated patients either exclusively or primarily undergoing open AAA repair.2, 7–9, 18 For instance, Collins et al. evaluated patients in the Veterans Affairs Hospital System and found a 7.2% mortality in black versus 3.2% in white patients following open repair; however, after adjusting for demographics and comorbidities, race was no longer associated with mortality.7 Trivedi et al.8 used the Nationwide Inpatient Sample and Lucas et al.2 used Medicare beneficiary data to compare outcomes following open AAA repair, and both series found higher mortality in black patients. Notably, all three of these studies used patient data from early in the endovascular era, with none evaluating patients after the year 2001. More recently, Osborne et al. used Medicare data from 2001–2006 and again found higher mortality in black patients, at least some of which was explained by higher rates of open repair compared to EVAR amongst black patients.9, 10 Again, less than one-half of patients in that series underwent endovascular repair (even less so amongst black patients), so this is poorly representative of contemporary practice where the majority of cases are EVAR. However, when the stakes are higher and these high-risk patients undergo open AAA repair, such as in the recent study from Hughes et al. evaluating nonagenarians, the racial disparity broadens, with black patients over the age of 90 experiencing an 8-fold higher perioperative mortality compared to white patients.19 However, in our current series, 83% of patients underwent EVAR, and, in contrast to prior studies,10 black patients more often underwent EVAR than white patients (87% vs. 83%), which is likely reflective of changing practice patterns and increasing availability of EVAR. This increasing use of EVAR could explain the narrowing gap in outcomes across racial groups.
An important contributing factor to racial disparities is unequal access to high-quality centers. Black patients have repeatedly been shown to receive care in lower-volume centers with lower-volume surgeons.2, 9, 20, 21 In this series, however, rates of low-volume centers were similar between black and white patients, and higher only in Asian patients. While we adjusted for hospital volume in this analysis, it is important to note that, although the VQI is nationally representative and includes both large, academic medical centers and smaller community hospitals, it is still composed of centers interested in quality improvement in vascular surgery. It is likely that there are procedures performed at even lower-volume centers that are not included in the VQI, which could also explain the low rate of inclusion of nonwhite patients overall. Thus, we may not be fully capturing the extent of the disparities in this analysis.
Our analysis also suggests that black patients may undergo more complex procedures. While we only included patients with infrarenal proximal aneurysm extent in this analysis, black patients more often had iliac artery aneurysms and hypogastric interventions, suggesting more distal extent of disease. Furthermore, the longer operative time and higher blood loss seen may also suggest more difficult procedures. However, we found that black patients were actually more likely to undergo EVAR than white patients, contrary to what has previously been reported.10 Despite these findings, black patients still experienced comparable perioperative mortality.”
Our study is somewhat unique in that we compared not only black and white patients but also Asian patients, who have been infrequently studied following AAA repair given the low prevalence of disease within this cohort.22, 23 Interestingly, despite being older and more often undergoing open repair than the black or white patients, Asian patients still had comparable perioperative mortality. Similarly small series of Asian patients noted disparities in iliac artery morphology, with Asian patients having shorter common iliac arteries,12, 13 narrower external iliac diameters, and more tortuous iliac systems.14 Although we could not identify these parameters with the VQI registry, we did find higher rates of iliac artery aneurysms among Asian and black patients compared to white patients, with subsequently higher rates of concomitant iliac artery procedures. The implication of this is not well understood as, at least in the early postoperative period, Asian patients did not have higher rates of reintervention. Although we are unable to identify late reinterventions in this series, Asian patients had the highest late survival. Despite being older, Asian patients were generally healthier prior to their operation – most likely the basis for their higher rates of late survival. Further study of late outcomes by race, especially reintervention, may provide more explanation.
Finally, while several studies compare perioperative mortality by race, few have evaluated additional perioperative morbidity. Although overall rates of morbidity were similar by race, there were some notable discrepancies. Black patients had the highest rates of preoperative renal insufficiency and dialysis but, even after accounting for this, had higher rates of perioperative renal failure resulting in new dialysis. Black patients were more likely to undergo EVAR and therefore receive a contrast load and, among patients undergoing EVAR, black patients received more contrast. Notably, although this difference was statistically significant, it may not have been clinically significant as the median contrast load varied by only 5mL across races. Perhaps other intraoperative events contributed to higher rates of postoperative acute kidney injury, including the higher blood loss, higher transfusion rates, and longer operative times in black patients. Racial disparities in postoperative renal insufficiency are well described in other specialties, but, to our knowledge, no other studies have identified disparities in renal outcomes following AAA repair.24–26
Interestingly, black patients were also slightly more likely to return to the operating room in the index hospitalization, with two-fold higher rates of return to the operating room after both open repair and EVAR. Unfortunately, we do not have data regarding the indications for reintervention, but this could be related to either the higher perioperative blood loss and transfusion rate, or could potentially be graft-related, especially with the higher rates of concomitant iliac artery aneurysms in black patients. Further research into the explanation of this may be warranted.
These data must be interpreted in the context of the study design. Clinical registries often have incomplete data and limited variable definitions and, in particular, the VQI contains only limited long-term follow-up information. Therefore, although we can account for late mortality given linkage to the Social Security Death Index, we cannot compare late rates of graft-related complications or reinterventions by race. There are also very low numbers of patients with nonwhite race in the VQI, although this is unsurprising given the distribution of aneurysmal disease by race, with far fewer aortic aneurysms in black and Asian patients.4, 6, 22, 23 Additionally, several potentially confounding variables cannot be accounted for due to limitations in the dataset, including preoperative ejection fraction and other markers of severity of comorbidities. Also, to fully understand racial disparities, social factors must be considered, including socioeconomic status and education, and these variables are difficult to quantify or even capture in any registry. We attempted to account for hospital volume, but are unable to account for socioeconomic status as zip code and other identifiers are removed from the VQI dataset. Furthermore, there are high amounts of missing data regarding the insurance variable in the VQI, so this is also unable to be accounted for in the analyses. Further study of racial differences following AAA repair are warranted to mitigate these disparities as best as possible.
Conclusion
Although black patients are more likely to present with symptomatic AAA, we found no difference in perioperative mortality following EVAR or open repair of intact infrarenal AAA by race, even after adjusting for demographics, comorbidities, and hospital volume. However, black patients more often develop postoperative renal complications and return to the operating room in the early postoperative period. Asian patients have the highest rate of postoperative myocardial infarction but the best late survival. Further research is warranted to understand the mechanism of these disparities to provide excellent quality care to patients of all backgrounds.
Acknowledgments
SD, TOD, KS, and PS supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant 5T32HL007734-22
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jha AK, Fisher ES, Li Z, Orav EJ, Epstein AM. Racial trends in the use of major procedures among the elderly. N Engl J Med. 2005;353(7):683–91. doi: 10.1056/NEJMsa050672. [DOI] [PubMed] [Google Scholar]
- 2.Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. Race and surgical mortality in the United States. Ann Surg. 2006;243(2):281–6. doi: 10.1097/01.sla.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravi P, Sood A, Schmid M, Abdollah F, Sammon JD, Sun M, et al. Racial/Ethnic Disparities in Perioperative Outcomes of Major Procedures: Results From the National Surgical Quality Improvement Program. Ann Surg. 2015;262(6):955–64. doi: 10.1097/SLA.0000000000001078. [DOI] [PubMed] [Google Scholar]
- 4.Costa M, Robbs JV. Abdominal aneurysms in a black population: clinicopathological study. Br J Surg. 1986;73(7):554–8. doi: 10.1002/bjs.1800730713. [DOI] [PubMed] [Google Scholar]
- 5.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126(6):441–9. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Johnson G, Jr, Avery A, McDougal EG, Burnham SJ, Keagy BA. Aneurysms of the abdominal aorta. Incidence in blacks and whites in North Carolina. Arch Surg. 1985;120(10):1138–40. doi: 10.1001/archsurg.1985.01390340036006. [DOI] [PubMed] [Google Scholar]
- 7.Collins TC, Johnson M, Daley J, Henderson WG, Khuri SF, Gordon HS. Preoperative risk factors for 30-day mortality after elective surgery for vascular disease in Department of Veterans Affairs hospitals: is race important? J Vasc Surg. 2001;34(4):634–40. doi: 10.1067/mva.2001.117329. [DOI] [PubMed] [Google Scholar]
- 8.Trivedi AN, Sequist TD, Ayanian JZ. Impact of hospital volume on racial disparities in cardiovascular procedure mortality. J Am Coll Cardiol. 2006;47(2):417–24. doi: 10.1016/j.jacc.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 9.Osborne NH, Upchurch GR, Jr, Mathur AK, Dimick JB. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. J Vasc Surg. 2009;50(4):709–13. doi: 10.1016/j.jvs.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Osborne NH, Mathur AK, Upchurch GR, Jr, Dimick JB. Understanding the racial disparity in the receipt of endovascular abdominal aortic aneurysm repair. Arch Surg. 2010;145(11):1105–8. doi: 10.1001/archsurg.2010.213. [DOI] [PubMed] [Google Scholar]
- 11.Zettervall SL, Schermerhorn ML, Soden PA, McCallum JC, Shean KE, Deery SE, et al. The effect of surgeon and hospital volume on mortality after open and endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2016 doi: 10.1016/j.jvs.2016.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banzic I, Lu Q, Zhang L, Stepak H, Davidovic L, Oszkinis G, et al. Morphological Differences in the Aorto-iliac Segment in AAA Patients of Caucasian and Asian Origin. Eur J Vasc Endovasc Surg. 2016;51(6):783–9. doi: 10.1016/j.ejvs.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Cheng SW, Ting AC, Ho P, Poon JT. Aortic aneurysm morphology in Asians: features affecting stent-graft application and design. J Endovasc Ther. 2004;11(6):605–12. doi: 10.1583/04-1268R.1. [DOI] [PubMed] [Google Scholar]
- 14.Masuda EM, Caps MT, Singh N, Yorita K, Schneider PA, Sato DT, et al. Effect of ethnicity on access and device complications during endovascular aneurysm repair. J Vasc Surg. 2004;40(1):24–9. doi: 10.1016/j.jvs.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 15.Soden PA, Zettervall SL, Deery SE, Hughes K, Stoner MC, Goodney PP, et al. Disparities in Patient Selection and Presentation for Initial Vascular Procedure Between Black and White Patients. J Vasc Surg. 2017 In Revision. [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 19.Hughes K, Abdulrahman H, Prendergast T, Rose DA, Ongu’ti S, Tran D, et al. Abdominal aortic aneurysm repair in nonagenarians. Ann Vasc Surg. 2015;29(2):183–8. doi: 10.1016/j.avsg.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 20.Epstein AJ, Gray BH, Schlesinger M. Racial and ethnic differences in the use of high-volume hospitals and surgeons. Arch Surg. 2010;145(2):179–86. doi: 10.1001/archsurg.2009.268. [DOI] [PubMed] [Google Scholar]
- 21.Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, et al. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296(16):1973–80. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- 22.Salem MK, Rayt HS, Hussey G, Rafelt S, Nelson CP, Sayers RD, et al. Should Asian men be included in abdominal aortic aneurysm screening programmes? Eur J Vasc Endovasc Surg. 2009;38(6):748–9. doi: 10.1016/j.ejvs.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Hobbs SD, Sam RC, Bhatti A, Rehman A, Wilmink AB, Adam DJ, et al. The low incidence of surgery for non-cardiac vascular disease in UK Asians may be explained by a low prevalence of disease. Eur J Vasc Endovasc Surg. 2006;32(5):494–9. doi: 10.1016/j.ejvs.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Grams ME, Matsushita K, Sang Y, Estrella MM, Foster MC, Tin A, et al. Explaining the racial difference in AKI incidence. J Am Soc Nephrol. 2014;25(8):1834–41. doi: 10.1681/ASN.2013080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, et al. Acute Kidney Injury After Major Surgery: A Retrospective Analysis of Veterans Health Administration Data. Am J Kidney Dis. 2016;67(6):872–80. doi: 10.1053/j.ajkd.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thakar CV, Liangos O, Yared JP, Nelson D, Piedmonte MR, Hariachar S, et al. ARF after open-heart surgery: Influence of gender and race. Am J Kidney Dis. 2003;41(4):742–51. doi: 10.1016/s0272-6386(03)00021-0. [DOI] [PubMed] [Google Scholar]


