Abstract
Non-surgical bleeding (NSB) in heart failure (HF) patients with continuous-flow left ventricular assist device (CF-LVAD) support is the most common clinical complication. The aim of this study was to investigate the association between oxidative stress and platelet glycoproteins GPIbα and GPVI shedding on the incidence of NSB in CF-LVAD patients. Fifty-one HF patients undergoing CF-LVAD implantation and 11 healthy volunteers were recruited. Fourteen patients developed NSB (bleeder group) during one month follow-up duration while others were considered non-bleeder group (n=37). Several biomarkers of oxidative stress were quantified at baseline and weekly intervals in all patients. Surface expression and plasma elements of platelet receptor glycoproteins GPIbα and GPVI were measured. Oxidative stress biomarkers and platelet GPIbα and GPVI receptor-shedding (decreased surface expression and higher plasma levels) were found to be pre-existing conditions in baseline samples of both groups of HF patients when compared to healthy volunteers. Significantly elevated oxidative stress biomarkers and platelet-glycoprotein-receptor-shedding were observed in post implant bleeder group temporarily when compared to non-bleeder group. Strong significant associations between biomarkers of oxidative stress and platelet-glycoprotein-receptor-shedding were observed, suggesting a possible role of oxidative stress in platelet integrin shedding leading to NSB in CF-LVAD patients. Receiver operating characteristic (ROC) analyses of GPIbα and GPVI indicated that the likelihood of NSB had a predictive power of bleeding complication in CF-LVAD patients. In conclusion, elevated oxidative stress may play a role in GPIbα and GPVI shedding in the event of NSB. Thus, oxidative stress and GPIbα and GPVI shedding may be used as potential biomarkers for bleeding risk stratification in those patients.
Keywords: Cardiac failure, Mechanical circulatory support device, non-surgical bleeding, oxidative stress, platelet glycoprotein shedding
Introduction
The use of a CF-LVAD either as a BTT (bridge to cardiac transplant) or DT (destination therapy) has significantly improved survival for the HF patients.1,2 These new generation of CF-LVADs have favorable characteristics; they are small, durable, and are associated with less adverse events than pulsatile LVADs. However, important co-morbidities associated with these devices do exist and non-surgical bleeding (NSB) is one of the major clinical complications contributes significantly to morbidity and mortality.3 Major bleeding rates among CF-LVAD patients ranging between 45% to 55% with gastrointestinal bleeding occurring in approximately 25%.1,4–7 This results in hospital readmissions and treatment, which increases the total cost of care considerably.8
Platelets are critical for the maintenance of the integrity of the vascular system and are the first line of defense against hemorrhage.9 GPIbα and GPVI are two key platelet receptors that bind vWF and collagen, respectively, which initiate hemostasis at sites of vascular injury.10 Metalloproteinase-mediated ectodomain shedding of platelet receptors has been recognized as a new mechanism for regulating platelet function.11 Platelet dysfunction is an important cause of major bleeding after cardiac surgery.12 Recent study reported that loss of the platelet surface receptors in HF patients, patients with mechanical circulatory support device and patients with extracorporeal membrane oxygenator support may contribute to ablated platelet adhesion/activation, and limit thrombus formation under high/pathologic shear conditions.13 Studies also revealed that non-physiological high shear stress may also contribute to the shedding of different important platelet receptor glycoproteins.14–16 Several biochemical pathways that induce shedding of GPIbα and/or GPVI have been identified.17–21 While these pathways might be dependent or independent of platelet activation, the loss of these functional receptors may result in defective platelets. Defects affecting normal surface expression and shedding of GPIbα and/or GPVI may result in mild or more severe bleeding complication.13
Oxidative stress, defined as an excess production of ROS relative to antioxidant defense, has been shown to play an important role in the pathophysiology of HF.22 Moreover, increased oxidative stress found to relate with the functional severity of HF, with the highest levels being noticed in patients in functional class III and IV.23 Our recent reports demonstrated that increased levels of oxidative stress biomarkers and mechanistic insight of platelet apoptosis are linked to bleeding complication in some HF patients with CF-LVAD support.24,25 Oxidative stress may also influence the alteration of platelet and leukocyte functionality in CF-LAVD patients with systemic inflammation, infection and sepsis.26 A previous study showed that higher levels of oxidative stress have a potential role in mediating abnormal bleeding and was associated with increased serum malondialdehyde and decreased SOD levels.27,28 The regulation of platelet function is finely tuned by a balance between the vasculature’s redox environment and the oxidative processes that occur in it.29 Prior study on murine and human model suggested that oxidative injury of platelets may attenuate their function by shedding key adhesion receptors on platelet surface.30 Thus the importance of redox-regulation of important platelet surface glycoprotein functionality cannot be underestimated. Unfavorable response to CF-LVAD implantation, leading to increased production of oxidative stress, in combination with non-physiological mechanical factors could induce platelet glycoprotein shedding. The role of platelet surface receptor glycoproteins and oxidative stress response of NSB associated with CF-LVADs has been elusive. The present study focused on the status of oxidative stress and their associations with platelet glycoproteins GPIbα and GPVI shedding during NSB in CF-LVAD patients.
Methods
Study population
Fifty one HF patients undergoing CF-LVAD implantation and 11 healthy volunteers were recruited in our study. The CF-LVAD therapies were for either as bridge to transplant or as destination therapy. Among the different CF-LVADs implanted; 21 patients received the HeartMate II (Thoratec Corp, Pleasanton, CA), 11 received the Jarvik 2000 (Jarvik Heart, New York, NY) and 19 had HeartWare HVAD (HeartWare Inc, Framingham, MA).
Patient selection criteria and ethical aspects
Patients between ages 18–70 years undergoing CF-LVAD implantation were enrolled in our study. All the subjects were enrolled after signing informed consent form in accordance with the Declaration of Helsinki. Patients with history of malignancy and preexisting inflammatory conditions were excluded from our study. Healthy volunteers without any history of any cardiovascular disease were included as reference. Blood samples and background information were collected from each patient according to our institutional Review Board (IRB) protocol.
Sample collection and processing
Baseline (pre-operative: Pre-OP) and post-operative (at 1, 2, 3 weeks and 1 month: POD-1W, 2W, 3W and 1M) blood samples from the HF patients were collected in EDTA/Citrate-anticoagulated vacutainer tubes. Reference blood samples from healthy volunteers were collected once. Samples were aliquoted, processed and used for further analysis according to the study protocol.
Assessment of oxidative stress
To investigate the extent of oxidative stress we measured several biomarkers of oxidative stress in platelets, leukocytes, erythrocytes and even in plasma samples of the study population according to previously published procedures.25, 31–33 In brief, generation of reactive oxygen species (ROS) in platelets, neutrophils, lymphocytes and monocytes were quantified by our standard laboratory procedures published earlier and the results were expressed as mean fluorescence intensity (MFI) in arbitrary unit for each cell types.33 As the status of oxidative stress can not be confirmed by measuring only ROS; it is important to look into the antioxidant status also, as the oxidative stress best explained by the balance between ROS and antioxidant enzyme. To evaluate the antioxidant status we further quantified potent antioxidant enzyme superoxide dismutase (SOD, kit: Cell Technology Inc. Mountain View, CA, USA) in erythrocytes and total antioxidant capacity (TAC, kit: Randox Laboratories, Antrim, UK) in plasma samples by using commercially available kits according to the manufacturer’s instructions. The data for SOD and TAC were expressed as units per milliliters (U/mL) and millimole per liters (mmol/L) respectively. We further estimated the concentration of oxidized low density lipoprotein (oxLDL) in plasma by ELISA using a commercially available kit (Mercodia Inc, Winston Salem, NC) following the manufacturer’s instruction to explore overall status of oxidative stress. Details of each experimental procedure were found in our previously published literatures.24,25,32,33
Assessment of platelet surface receptors GPIbα and GPVI shedding
To evaluate the shedding of two important platelet surface glycoprotein receptors GPIbα and GPVI; we looked in the surface expression and plasma elements of both receptors in all of our study samples. Reduction in the surface expression and elevation in the plasma levels indicated the shedding of those receptors. Flow cytometry and ELISA were used to estimate surface expression and plasma elements of these receptors according to the following procedures.
Assessment of platelet surface receptors GPIbα and GPVI by Flow cytometry
Evaluation of surface expression of platelet membrane glycoproteins GPIbα (CD42b, clone HIP1, from BioLegend, San Diego, CA) and GPVI (eBioscience, San Diego, CA) were performed with the use of specific antibodies and multi-color whole-blood flow cytometry, as described in our previous study [14]. In brief, 5 μl aliquot of EDTA anticoagulated whole blood samples with 25 μl of 10 mM HEPES buffer were incubated with 10 μl each of fluorescein isothiocyanate (FITC for CD42b)- and eFluor 660 (eFlu for GPVI)-conjugated monoclonal antibodies raised against human cells and isotype-matched negative controls (FITC and eFlu for mouse IgG1, κ). In each sample, another 10 μl phycoerythrin (PE)-conjugated anti-human CD41 antibody was added to identify platelet population. All the samples were incubated for 30 min in the dark at room temperature. Thereafter, the cells were fixed with 1% paraformaldehyde, kept in dark for 30 minutes at 40°C and analyzed in a flow cytometer.
Assessment of plasma levels of GPIbα and GPVI by ELISA
To determine the plasma levels of GPIbα (cat no: MBS701708) and GPVI (cat no: MBS915183), we used commercially available ELISA kits from MyBioSource Inc., San Diego, CA. These assays employ the quantitative sandwich enzyme immunoassay technique. Antibody specific for either GPIbα or GPVI has been pre-coated onto microplate. Standard or plasma (100 μL for both) was added to each well and they were incubated at 37°C for 2 hrs. The liquid of each well was removed, with 100 μL of biotin-antibody added to each well and the resultant samples were incubated at 37°C for 1 h. After washing three times, the plate was inverted and blotted against clean paper towels. HRP-avidin (100 μL) was added to each well and the sample was incubated at 37°C for 1 hr. Washing lasted for five times. TMB substrate (90 μL) was added to each well and the sample was incubated for 15–30 min at 37°C away from light. Stop solution (50 μL) was added and the plate was gently tapped to ensure thorough mixing. The absorbance was determined within 15 min, on a microplate reader set to 450 nm. The detection ranges of the kits were 0.156 – 10 μg/mL for GPIbα and 46.88 – 3000 pg/mL for GPVI.
Statistical analyses
All analyses were performed with GraphPad Prism, version 6.07 (GraphPad Software, Inc., La Jolla, California) and SAS software, version 9.4 (SAS Institute, Cary, North Carolina). The data are presented as mean ± SD (standard deviation) and/or median with inter quartile range (IQR). Statistical differences were determined by using Chi-square test, Student’s t-test, Mann–Whitney U test and One-Way ANOVA (Wilcoxon rank-sum test or Kruskal-Wallis test), as applicable. Univariate analysis was carried out using Spearman’s rank correlation test to find out the relation between two measurable parameters as continuous variables, and the result was expressed as rho value. Statistical significance was assigned at p<0.05. For spearman’s rank correlation analysis to identify any correlation between different biomarkers of oxidative stress and platelet receptor shedding, paired oxidative stress data for each biomarker from baseline to post operative day 30 in each patients plotted against the matched platelet receptor shedding data. Multivariate logistic regression models were created to evaluate impact of relevant baseline factors and change in levels of biomarkers (from baseline) on incidence of post operative bleeding. There were two multivariate logistic regression models created; one each using GPIbα and GPVI. The covariates in GPIb model included BMI and a variable derived from the equation of GPIbα change from baseline (described in the results). Similarly GPVI model included BMI and variable derived from the equation of GPVI change from baseline (described in the results). BSA was not included as a covariate as BMI and BSA use same factors in their calculations. Apart from biomarkers, the covariates were selected based on purposeful selection of variables i.e. any variables significantly different on univariate analysis. Any measured parameter was treated as a variable, either continuous (when computing univariately for correlation) or dichotomous (when examining association). We did not include variables known to interact (GPIbα and GPVI) in the same model to avoid interaction related bias. The ROC curves were generated for both models.
Results
NSB and demography
Among 51 HF patients, fourteen experienced at least one episode of NSB within one month after CF-LVAD support (bleeder group). NSB was defined according to INTERMACS classification. CF-LVAD patients having bleeding that requires a transfusion of 4 or more units of packed red blood cells and/or causes hemodynamic instability requiring inotropic infusion and/or surgical reintervention was considered bleeders. Comparative analyses of demographic and clinical characteristics of the patients in the bleeder group and those who did not experience NSB (non-bleeder group) before CF-LVAD implantation were summarized in Table I. All the patients were transfused with either RBCs or fresh frozen plasma or platelets. Anti-coagulation regimen at the time of bleeding was clinically optimized individually for each patient.
Clinical hematology
There were no significant differences in most of the common hematology and blood chemistry data (WBC, RBC, HCT, BUN, Creatinine, INR and PTT) between the non-bleeder and bleeder groups before and after CF-LVAD implantation throughout the study period as observed from our hospital database. At the baseline (Pre-OP) there was no significant difference in hemoglobin counts between non-bleeder and bleeders (11.8±1.9 vs 11.4±1.6, p>0.05). Hemoglobin content was reduced temporally in both the non-bleeder and bleeder groups with similar trends. After one month of CF-LVAD implantation, the hemoglobin counts were significantly reduced by 20% (11.8 vs 9.4, p<0.05) and 25% (11.4 vs. 8.5, p<0.05) in non-bleeder and bleeder groups respectively when compared to their corresponding baseline values. We did not notice significant difference in the platelet count between the bleeder and the non-bleeder groups at baseline (178.7 ± 46.7 vs 197.1 ± 85.4 ×103/mL; p=0.43) and at one week (191.1 ± 94.1 vs. 194.4 ± 73.9 ×103/mL; p=0.93) after CF-LVAD implantation. Platelet counts in both groups remained within the reference range (153 – 367×103/mL) up to two weeks after CF-LVAD support. At the 3rd week, we noticed significant reduction in the platelet counts in the bleeder group (137.0 ± 97.7 vs. 251.0 ± 107.2×103/mL; p=0.03) and at 4th week (93.2 ± 51.1 vs. 235.3 ± 99.7×103/mL; p=0.0008) it became lowest, below the reference values.
Change in ROS generation by platelets and leukocytes
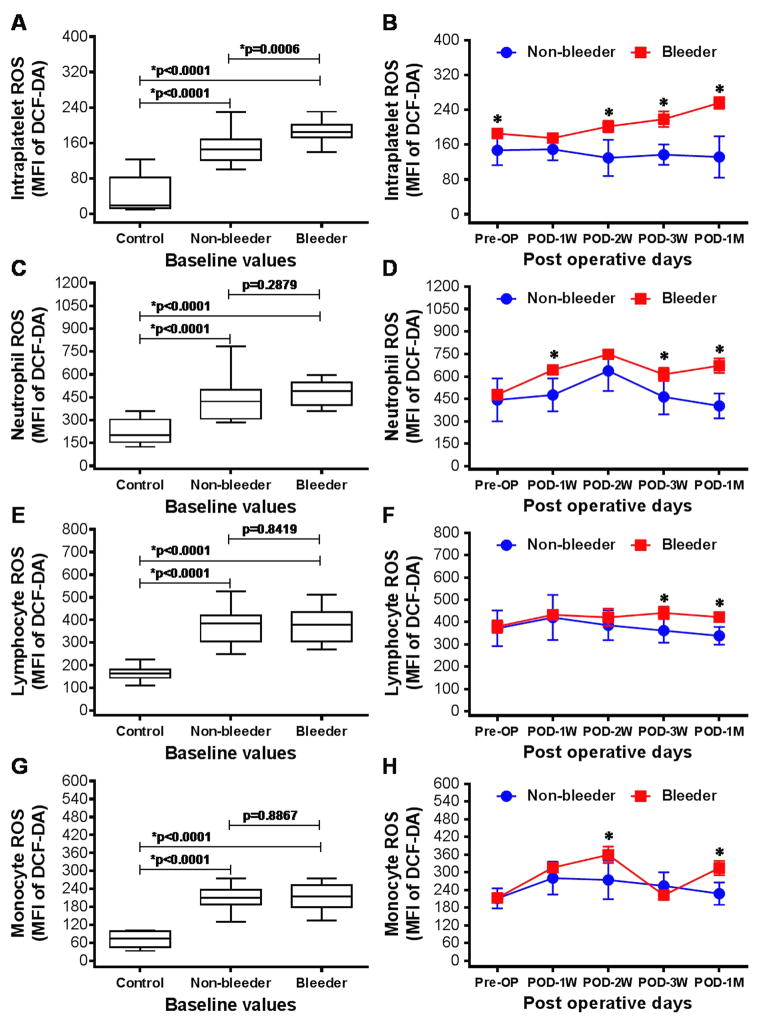
The flow cytometric analysis of the ROS generation from platelets, neutrophils, lymphocytes and monocytes were found to be significantly higher among both the non-bleeding and bleeding groups of HF patients at baseline compared to the healthy volunteers (Fig. 1). Moreover, ROS generation from platelets was significantly 26% elevated in the bleeder group compared to the non-bleeder group prior to CF-LVAD implantation. After CF-LVAD implantation, we noticed temporal changes in ROS generation from all cell types in both groups. Intraplatelet ROS generation was found to be temporarily increased up to the end of the study duration; while the levels of intraplatelet ROS decreased in the non-bleeder group. We noticed significant elevation of intraplatelet ROS in bleeder group compared to non-bleeder at post implant day 14, 21 and 30. The trends of ROS generation in neutrophils and lymphocytes were similar in both non-bleeder and bleeder groups. In all cases, ROS generation was found to be significantly higher in bleeder group compared to non-bleeder group at post-implant day 30 (POD-1M).
Figure 1.
Box-whisker plots and line arts to show the generation of ROS from platelets (A,B), neutrophils (C,D), lymphocytes (E,F) and monocytes (G,H) among healthy volunteers, non-bleeders and bleeders at baseline (A,C,E,G) and temporarily after CF-LVAD implantation (B,D,F,H). *p<0.05 is considered significant.
Change in SOD, TAC and oxLDL
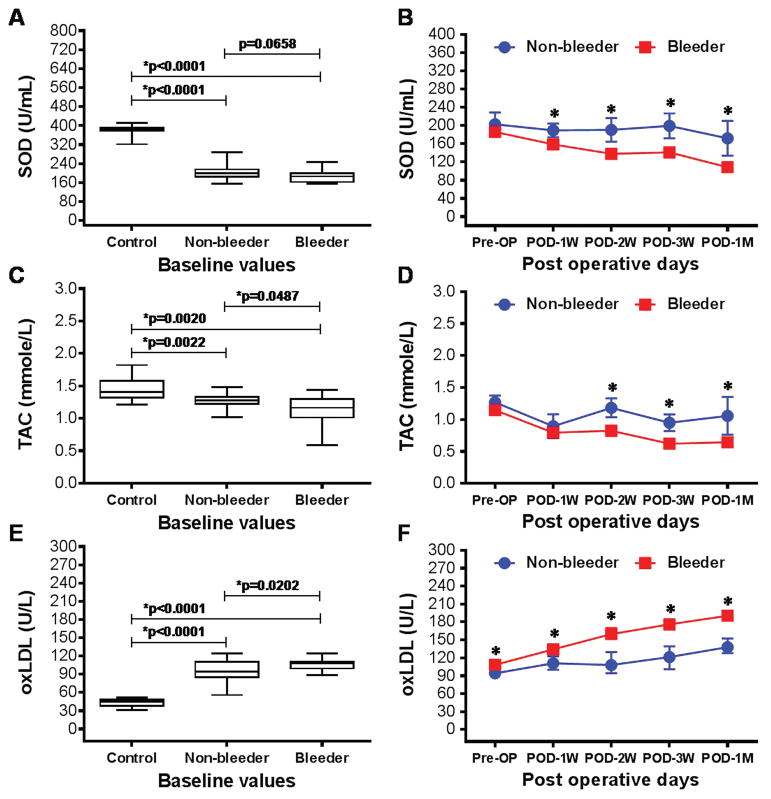
Significantly lower levels of antioxidant enzyme SOD in erythrocytes and TAC in plasma were detected among both the non-bleeding and bleeding groups of HF patients at baseline compared to the healthy volunteers (Fig. 2A,C). The baseline levels of SOD and TAC between the non-bleeder and bleeder groups were comparable to each other. After CF-LVAD implantation, temporal decrease in SOD and TAC levels were significantly prominent in bleeder group in comparison to non-bleeder group. The lowest levels of SOD and TAC were observed in the bleeder group at the end of the study (Fig. 2B,D). A higher level of plasma oxLDL, another important biomarker of oxidative stress, was also observed in both groups of HF patients compared to the healthy volunteers, which was also higher pre- and post-implant in the bleeder group and continued to increase temporally post-implant (Fig. 2E,F).
Figure 2.
Box-whisker plots and line arts to show the production of SOD (A,B), TAC (C,D) and oxLDL (E,F) among healthy volunteers, non-bleeders and bleeders at baseline (A,C,E) and temporarily after CF-LVAD implantation (B,D,F). *p<0.05 is considered significant.
Change in shedding of platelet receptors glycoproteins: GPIbα and GPVI
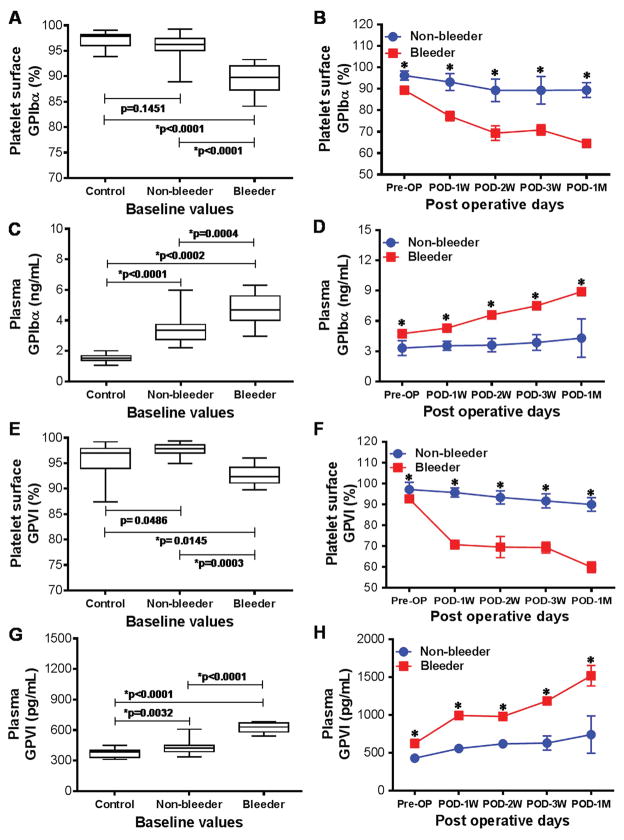
Figure 3 demonstrates data from flow cytometric and ELISA analysis to investigate surface expression and plasma levels of platelet GPIbα and GPVI receptors among healthy volunteers and HF patients before and after multiple time points of CF-LVAD implantation. At baseline, the surface expression of GPIbα and GPVI receptors were significantly lowered in the bleeder group when compared to the non-bleeder group and healthy volunteers; indicating pre-existing condition of decreased receptors in bleeder group prior to CF-LVAD implantation (Fig. 3A,E). While looking at post implant surface expression, we noticed a progressive decrease in GPIbα and GPVI receptors in bleeder groups and became lowest at one month after CF-LVAD support (Fig. 3B,F). In case of non-bleeder group, the temporal changes were not so prominent. The temporal change in GPIbα and GPVI receptors on platelet surface remained always significantly lowered in bleeder group in comparison to non-bleeder group. Contrary to reduced surface expression of platelet GPIbα and GPVI receptors, we noticed significantly elevated plasma levels of those two receptors among bleeder group compared to non-bleeder and healthy volunteers at baseline (Fig. 3C,G). Moreover, post-implant temporal changes in elevated plasma levels of GPIbα and GPVI were found to be significantly higher at all time points in bleeder group compared to non-bleeder (Fig. 3D,H). All indicating proteolytic shedding of GPIbα and GPVI receptors in circulating plasma especially in bleeder group.
Figure 3.
Box-whisker plots and line arts to show the glycoprotein receptor GPIbα (A–D) and GPVI (E–H) on platelet surface and in plasma among healthy volunteers, non-bleeders and bleeders at baseline (A,C,E,G) and temporarily after CF-LVAD implantation (B,D,F,H). *p<0.05 is considered significant.
Likelihood of bleeding prediction by GPIbα and GPVI receptor shedding
After assessing the GPIbα and GPVI shedding as a potential marker of the bleeding at different post-LVAD time points, we evaluated the marker by change in their value at these time points from baseline value. We hypothesized that scale of increase in the GPIbα and the GPVI shedding from the baseline level as well as the frequency of increase from baseline is proportional to the likelihood of bleeding. First, we calculated the difference of concentration from baseline at each available follow-up time points up to one month study period. Then we calculated the mean of the difference in those follow-up time point concentrations. We also assessed the frequency of increase in the concentrations from baseline. We then used following equation to calculate likelihood of bleeding for the GPIbα and the GPVI shedding.
Where: (A) = mean difference in concentration from baseline; (B) = frequency of increased concentrations from baseline; (C) = total frequency of follow-up concentrations
Multivariate regression and ROC analysis for Likelihood of bleeding prediction model
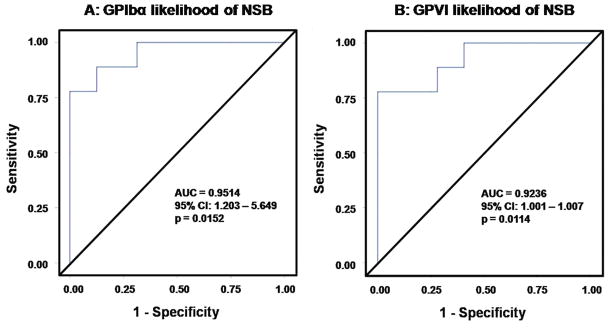
In a multivariate logistic regression model, we applied the likelihood bleeding data points to evaluate its relation to the bleeding. The ROC curve for the GPIbα and the GPVI likelihood of bleeding unit is shown in Figure 4. The estimated AUCs were 0.9514 (95% CI: 1.203 – 5.649; p=0.0152) for GPIbα and 0.9236 (95% CI: 1.001 – 1.007; p=0.0114) for GPVI. According to these statistics, if we randomly select a patient with NSB and a patient without NSB, the probability of bleeding (while controlling BMI) being greater for the bleeding patient than that of non-bleeding patient are 0.9514 (GPIbα) and 0.9236 (GPVI). To test whether the AUCs of GPIbα and GPVI were significantly different from 0.5, wald Chi-square statistic of 5.89 and 6.40 were obtained (using the estimated standard errors of 0.395 and 0.001), which yield p values of 0.015 and 0.011 respectively, significant at a Type I error rate of 5%. Thus the discriminating power of the biomarker of the predicted probability for bleeding by the GPIbα and the GPVI likelihood of bleeding units are significantly greater than that of chance alone. Thus, the measured GPIbα and GPVI shedding as a potential biomarker have a predictive power for NSB.
Figure 4.
Receiver operating characteristic (ROC) curve for measurement of the predictive power of GPIbα (A) and the GPVI (B) likelihood of bleeding in CF-LVAD patients. AUC (area under the curve) was calculated using a method designed for ROC analysis.
Correlation between biomarkers of oxidative stress and platelet receptor shedding
To investigate relationship between biomarkers of oxidative stress and shedding of platelet GPIbα and GPVI receptors, we conducted Spearman’s rank correlation test in all CF-LVAD patients (Table 2). Our data clearly indicated that surface expression of GPIbα and GPVI receptors correlated negatively with of generation of ROS and levels of oxLDL; and correlated positively with concentration of SOD and TAC. As expected, opposite strong correlations were evident in case of plasma elements of GPIbα and GPVI receptors and oxidative stress markers. Thus, the shedding of these two important platelet receptors correlated with the elevation of oxidative stress among HF patients with CF-LVAD support. In multivariate regression analysis, oxidative stress biomarkers were significantly associated with platelet receptor glycoproteins shedding after controlling possible covariates (p<0.01).
Table 2.
Spearman correlation between biomarkers of oxidative stress and platelet glycoproteins in CF-LVAD patients
| Biomarkers of oxidative stress (Baseline to one month) | Platelet glycoproteins (baseline to one month) | |||
|---|---|---|---|---|
| GPIbα Surface | GPIbα Plasma | GPVI Surface | GPVI Plasma | |
| Platelet ROS | ||||
| Spearman’s ρ | −0.24 | 0.35 | −0.30 | 0.28 |
| 95% confidence intervals (CI) | −0.41 to −0.06 | 0.18 to 0.50 | −0.48 to −0.09 | 0.11 to 0.44 |
| p-value | 0.01* | <0.0001* | 0.004* | 0.002* |
|
| ||||
| Neutrophil ROS | ||||
| Spearman’s ρ | −0.54 | 0.32 | −0.20 | 0.44 |
| 95% confidence intervals (CI) | −0.67 to −0.37 | 0.10 to 0.50 | −0.47 to 0.10 | 0.24 to 0.60 |
| p-value | < 0.0001* | 0.004* | 0.17 | < 0.0001* |
|
| ||||
| Lymphocyte ROS | ||||
| Spearman’s ρ | −0.32 | 0.33 | −0.09 | 0.26 |
| 95% confidence intervals (CI) | −0.50 to −0.12 | 0.11 to 0.51 | −0.37 to 0.22 | 0.05 to 0.46 |
| p-value | 0.002* | 0.003* | 0.57 | 0.02* |
|
| ||||
| Monocyte ROS | ||||
| Spearman’s ρ | −0.38 | 0.39 | −0.23 | 0.36 |
| 95% confidence intervals (CI) | −0.54 to −0.18 | 0.19 to 0.56 | −0.49 to 0.07 | 0.15 to 0.53 |
| p-value | 0.0002* | 0.0002* | 0.12 | 0.001* |
|
| ||||
| SOD | ||||
| Spearman’s ρ | 0.54 | −0.51 | 0.59 | −0.57 |
| 95% confidence intervals (CI) | 0.40 to 0.66 | −0.64 to −0.36 | 0.42 to 0.72 | −0.69 to −0.43 |
| p-value | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* |
|
| ||||
| TAC | ||||
| Spearman’s ρ | 0.53 | −0.53 | 0.64 | −0.59 |
| 95% confidence intervals (CI) | 0.39 to 0.65 | −0.65 to −0.39 | 0.49 to 0.75 | −0.70 to −0.46 |
| p-value | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* |
|
| ||||
| oxLDL | ||||
| Spearman’s ρ | −0.57 | 0.60 | −0.63 | 0.60 |
| 95% confidence intervals (CI) | −0.68 to −0.44 | 0.47 to 0.71 | −0.75 to −0.48 | 0.48 to 0.71 |
| p-value | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* |
Note: Result was expressed as ρ (rho) value.
statistically significant in spearman’s rank correlation test.
Discussion
The major bleeding complication in CF-LVAD patients may result in significant morbidity, and its incidence has been estimated to range between 18–40%.3,34–36 The etiology of bleeding diathesis can be multi-factorial in CF-LVAD patients. The development of acquired von Willebrand syndrome (AvWS) is one of the most discussed factor in which patients experienced with mechanical destruction and proteolysis of high-molecular-weight multimers (HMWM) of von Willebrand factor (vWF), induced by CF-LVADs generated non-physiological high shear stress.37–40 Although the CF-LVAD patients with AvWS may result in reduced vWF associated platelet activity and aggregation, not all of them experience major bleeding.40–42 The key function of platelets is to prevent bleeding; subsequently, it stands to reason that abnormal platelet function may be a contributing factor to NSB events.25 We recently reported mechanistic insight of platelet apoptosis is linked to elevated oxidative stress in CF LVAD patients and were associated with bleeding complications as well as there is an important role of oxidative stress and platelet mitochondrial damage due to inflammation/infection during CF-LVAD support.24–26 In our present study we hypothesized that persistent oxidative stress and reduced surface expression or shedding of tow important platelet-receptor-glycoproteins GPIbα and GPVI may give rise to NSB complication among CF-LVAD patients.
Platelets are unique in their structural assembly, though they are anucleate but have distinct mitochondria and play major role in hemostasis and thrombosis.43 Plasma membrane of platelets composed of phospholipid bilayer and is the site of expression of various surface receptors including GPIbα, GPVI and lipid rafts which helps in signalling and intracellular trafficking related to cellular homeostasis.43 Platelets are well known as key mediators of hemostasis and their activity and fate have been reported to be controlled oxidative stress during cell activation.44–48 These conditions can dramatically affect platelet physiology. Other study demonstrated that redox control of cellular homeostasis is a key determinant of platelet destiny.49 However, the precise mechanisms involved in redox modification of platelet surface receptor dysfunction are not clear. It is noted that the clinical role of oxidative stress in platelet function and thrombosis/bleeding is unclear and complex so far. Nonetheless, redox regulation of the platelet is crucial to the modulation of its function. Prior studies showed that redox sites on the surface of the platelet most likely impacts platelet function, as it does in other biological processes. These include integrin-mediated adhesion, virus entry into the cell, and importantly receptor shedding.50 In our study, we noticed significantly higher ROS generation not only from platelets but also from neutrophils, lymphocytes and monocytes in the CF-LVAD patients who developed NSB when compared to patients without bleeding complication. We also noticed concomitant decrease in SOD, TAC and elevated oxLDL, suggesting that the total antioxidant capacity might not be strong enough to minimize the deleterious effect of ROS during NSB. These observations explain the severity of oxidative stress in CF-LVAD patients, especially in bleeder group. The increased formation and release of ROS, as well as the modified content of endogenous or exogenous antioxidants may play a role not only in platelet destiny and thrombus formation, but also in the development of other diseases including bleeding complications.
The balance between hemostasis and thrombosis relies on a finely tuned adhesive response of blood platelets. Inadequate adhesion leads to bleeding, whereas excessive or inappropriate adhesion leads to thrombosis.51 We noticed temporarily reduced surface expression and elevated plasma levels of two important platelet glycoproteins (GPIbα and GPVI) in bleeder group when compared to non-bleeder group, - indicating possibilities of proteolytic shedding during the CF-LVAD support. Thus, due to the proteolytic shedding of those glycoprotein receptors from platelet surface may produce abnormal platelets which may facilitate the process of NSB event in CF-LVAD patients. In univariate analysis, strong correlations between the biomarkers of oxidative stress and platelet glycoprotein receptors shedding in the HF patients were observed in our study. In multivariate regression analysis, oxidative stress significantly associated with platelet glycoprotein receptors shedding after controlling for BMI. Thus, our study may suggest that oxidative stress may partially play a role in accelerating receptor shedding leading to platelet dysfunction in CF-LVAD patients and subsequent NSB. We further verified the predictability of the likelihood of NSB using the platelet shedding values and performed ROC analysis. Based on this statistical analysis, the likelihood of NSB would be a good biomarker as a diagnostic test for bleeding complication in CF-LVAD patients. Thus, the implications, therefore, are that the regulation of platelets by oxidative stress is an important biomedical issue. A thorough understanding of these mechanisms and how they interact with other platelet signaling events is of the utmost importance for the development of novel therapeutic targets so that we can protect against inappropriate thrombus formation or bleeding complications in CF-LVAD patients. Platelet glycoprotein shedding could be due to other factors such as long-term exposure to the high shear stress flow environment as well as the artificial blood contacting materials found in CF-LVADs. Recently, it was shown that the non-physiological high shear stress (NPHSS) environment that occurs with CF-LVADs may be responsible for platelet dysfunction as evidenced by GPIbα, GPVI and GPIIbIIIa receptor shedding.14–16 Understanding of the role of oxidative stress and NPHSS from CF-LVAD as contributory factor in platelet glycoprotein shedding may enable development of effective medical management strategy.
Study Limitations
We acknowledge that there are some limitations in this prospective observational study. The sample size was relatively small. Not all CF-LVAD patients were enrolled for this study. Medication might have some impact on oxidative stress status. Recent changes in medications should be addressed in further studies to rule out drug–drug interactions or a new side effect. A larger cohort of CF-LVAD patients with bleeding complication is needed to confirm our initial findings.
Conclusions
Elevated oxidative stress may play a role in GPIbα and GPVI shedding in the event of NSB. Thus, oxidative stress and GPIbα and GPVI shedding may be used as potential biomarkers for bleeding risk stratification in those patients.
Table 1.
Demographic and baseline clinical characteristics of patients prior to CF-LVAD implantation
| Characteristics | Pre-implant HF patients (n = 51) | ||
|---|---|---|---|
| Non-bleeder group(n = 37) | Bleeder group(n =14) | p-value | |
| Demography | |||
| Age in years | |||
| Mean ± SD | 59.2 ± 10.7 | 59.5 ± 12.6 | 0.92 |
| Median (IQR) | 60.0 (31.0 – 76.0) | 63.5 (25.0 – 70.0) | 0.59 |
| Sex, n (% male) | 36 (97.3 %) | 11 (78.6 %) | 0.10 |
| Age in years | |||
| Caucasian white, n (%) | 28 (75.7 %) | 6 (42.9 %) | 0.06 |
| Black, n (%) | 9 (24.3 %) | 8 (57.1 %) | |
| Height in meter | |||
| Mean ± SD | 1.8 ± 0.1 | 1.7 ± 0.1 | 0.25 |
| Median (IQR) | 1.8 (1.6 – 1.9) | 1.7 (1.6 – 1.9) | 0.17 |
| Weight in kilograms | |||
| Mean ± SD | 99.0 ± 19.4 | 81.3 ± 17.4 | 0.01* |
| Median (IQR) | 99.8 (60.0 – 127.9) | 80.3 (53.0 – 112.0) | 0.01* |
| Body mass index (BMI) (kg/m2) | |||
| Mean ± SD | 30.7 ± 5.0 | 26.6 ± 4.9 | 0.02* |
| Median (IQR) | 31.2 (20.8 – 39.0) | 26.1 (19.5 – 34.3) | 0.02* |
| Body surface area (BSA) (m2) | |||
| Mean ± SD | 2.2 ± 0.3 | 2.0 ± 0.3 | 0.02* |
| Median (IQR) | 2.3 (1.6 – 2.5) | 2.0 (1.6 – 2.4) | 0.03* |
| History of smoking, n (%) | 10 (27.0 %) | 3 (21.4 %) | 0.96 |
| History of substance abuse | |||
| Ethyl alcohol abuse, n (%) | 7 (18.9 %) | 2 (14.3 %) | 0.70 |
| Drug abuse, n (%) | 9 (24.3 %) | 3 (21.4 %) | 0.83 |
|
| |||
| Vital signs | |||
| Systolic blood pressure (mmHg) | |||
| Mean ± SD | 103.1 ± 10.7 | 107.7 ± 11.9 | 0.28 |
| Median (IQR) | 105.0 (79.0 – 129.0) | 109.0 (86.0 – 131.0) | 0.13 |
| Diastolic blood pressure (mmHg) | |||
| Mean ± SD | 62.1 ± 8.6 | 67.6 ± 13.5 | 0.17 |
| Median (IQR) | 63.0 (46.0 – 83.0) | 64.0 (60.0 – 105.0) | 0.43 |
|
| |||
| Etiology of heart disease | |||
| Ischemic cardiomyopathy, n (%) | 21 (56.8 %) | 10 (71.4 %) | 0.53 |
| Non-ischemic cardiomyopathy, n (%) | 16 (43.2 %) | 4 (28.6 %) | 0.53 |
|
| |||
| Echocardiographic parameters | |||
| Left ventricular end diastolic diameter (mm) | |||
| Mean ± SD | 66.1 ± 8.6 | 67.4 ±11.7 | 0.76 |
| Median (IQR) | 63.0 (53.0 – 88.0) | 67.5 (52.0 – 88.0) | 0.70 |
| Left ventricular ejection fraction (%) | |||
| Mean ± SD | 13.7 ± 3.3 | 15.0 ± 5.6 | 0.37 |
| Median (IQR) | 15.0 (10.0 – 20.0) | 12.5 (10.0 – 25.0) | 0.66 |
Demographic and clinical parameters of non-bleeder versus bleeder groups of HF patients were statistically compared by Mann-Whitney ‘U’ test (for median values with IQR), χ2-test (for results presented as percentages) and Student’s t-test (for results presented as mean ± SD) as applicable,
p<0.05 were considered significant.
Acknowledgments
Source of Funding: The described research was sponsored by the National Institutes of Health (Grant 1R01HL124170-01A1).
Footnotes
Conflicts of Interests: The authors have no conflicts of interest to report.
References
- 1.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Pagani FD, et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;44:584–603. doi: 10.1016/j.jtcvs.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey L, Holley CT, John R. Gastrointestinal bleed after left ventricular assist device implantation: incidence, management, and prevention. Ann Cardiothorac Surg. 2014;3:475–479. doi: 10.3978/j.issn.2225-319X.2014.08.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stulak JM, Lee D, Haft JW, et al. Gastrointestinal bleeding and subsequent risk of thromboembolic events during support with a left ventricular assist device. J Heart Lung Transplant. 2014;33:60–64. doi: 10.1016/j.healun.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Shrode C, Draper K, Huang R, et al. Significantly higher rates of gastrointestinal bleeding and thromboembolic events with left ventricular assist devices. Clin Gastroenterol Hepatol. 2014;12:1461–1467. doi: 10.1016/j.cgh.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 6.John R, Kamdar F, Eckman P, et al. Lessons learned from experience with over 100 consecutive HeartMate II left ventricular assist devices. Ann Thorac Surg. 2011;92:1593–9159. doi: 10.1016/j.athoracsur.2011.06.081. [DOI] [PubMed] [Google Scholar]
- 7.Demirozu ZT, Radovancevic R, Gregoric ID, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with HeartMate II left ventricular assist device. J Heart Lung Transplant. 2011;30:849–853. doi: 10.1016/j.healun.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Petrovic M, Nathan S, Radovancevic R, et al. Adverse Events in Continuous Flow LVAD Recipients: Gastrointestinal Bleeding is Still Notable? The VAD Journal. 2016 doi: 10.13023/VAD.2016.22. [DOI]
- 9.Payrastre B, Missy K, Trumel C, Bodin S, Plantavid M, Chap H. The integrin alpha IIb/beta 3 in human platelet signal transduction. Biochem Pharmacol. 2000;60:1069–1074. doi: 10.1016/s0006-2952(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 10.Kauskot A, Hoylaerts MF. Platelet receptors. Handb Exp Pharmacol. 2012;210:23–57. doi: 10.1007/978-3-642-29423-5_2. [DOI] [PubMed] [Google Scholar]
- 11.Qiao JL, Shen Y, Gardiner EE, Andrews RK. Proteolysis of platelet receptors in humans and other species. Biol Chem. 2010;391:893–900. doi: 10.1515/BC.2010.081. [DOI] [PubMed] [Google Scholar]
- 12.Forestier F, Coiffic A, Mouton C, Ekouevi D, Chêne G, Janvier G. Platelet function point-of-care tests in post-bypass cardiac surgery: are they relevant? Br J Anaesth. 2002;89:715–721. [PubMed] [Google Scholar]
- 13.Lukito P, Wong A, Jing J. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J Thromb Haemost. 2016;14:2253–2260. doi: 10.1111/jth.13497. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Mondal NK, Ding J, Gao J, Griffith BP, Wu ZJ. Shear-induced platelet receptor shedding by non-physiological high shear stress with short exposure time: glycoprotein Ibα and glycoprotein VI. Thromb Res. 2015;135:692–698. doi: 10.1016/j.thromres.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Mondal NK, Ding J, Wu ZJ. Activated expression and shedding of platelet glycoprotein IIb/IIIa under nonphysiological shear stress. Mol Cell Biochem. 2015;409:93–101. doi: 10.1007/s11010-015-2515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Wu ZJ. Paradoxical Effect of Non-Physiological Shear Stress on Platelets and von Willebrand factor. Artif Organs. 2016;40:459–468. doi: 10.1111/aor.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardiner EE, Arthur JF, Kahn ML, Berndt MC, Andrews RK. Regulation of platelet membrane levels of glycoprotein VI by a platelet-derived metalloproteinase. Blood. 2004;104:3611–3617. doi: 10.1182/blood-2004-04-1549. [DOI] [PubMed] [Google Scholar]
- 18.Gardiner EE, Arthur JF, Berndt MC, Andrews RK. Role of calmodulin in platelet receptor function. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:283–287. doi: 10.2174/156801605774322283. [DOI] [PubMed] [Google Scholar]
- 19.Aktas B, Pozgajova M, Bergmeier W, et al. Aspirin induces platelet receptor shedding via ADAM17 (TACE) J Biol Chem. 2005;280:39716–3922. doi: 10.1074/jbc.M507762200. [DOI] [PubMed] [Google Scholar]
- 20.Bergmeier W, Piffath CL, Cheng G, et al. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates GPIb alpha shedding from platelets in vitro and in vivo. Circ Res. 2004;95:677–683. doi: 10.1161/01.RES.0000143899.73453.11. [DOI] [PubMed] [Google Scholar]
- 21.Bergmeier W, Rabie T, Strehl A, et al. GPVI down-regulation in murine platelets through metalloproteinase-dependent shedding. Thromb Haemost. 2004;91:951–958. doi: 10.1160/TH03-12-0795. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 23.Keith M, Geranmayegan A, Sole MJ, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 24.Mondal NK, Sorensen EN, Hiivala NJ. Intraplatelet reactive oxygen species, mitochondrial damage and platelet apoptosis augment non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist device. Platelets. 2015;26:536–544. doi: 10.3109/09537104.2014.948840. [DOI] [PubMed] [Google Scholar]
- 25.Mondal NK, Li T, Chen Z, et al. Mechanistic insight of platelet apoptosis leading to non-surgical bleeding among heart failure patients supported by continuous-flow left ventricular assist devices. Mol Cell Biochem. 2017 Mar 25; doi: 10.1007/s11010-017-3021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondal NK, Sorensen EN, Pham SM. Systemic Inflammatory Response Syndrome in End-Stage Heart Failure Patients Following Continuous-Flow Left Ventricular Assist Device Implantation: Differences in Plasma Redox Status and Leukocyte Activation. Artif Organs. 2016;40:434–443. doi: 10.1111/aor.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deveer R, Deveer M, Engin-Üstün Y, et al. Role of Oxidative Stress on Vaginal Bleeding during the First Trimester of Pregnant Women. Int J Fertil Steril. 2014;7:271–274. [PMC free article] [PubMed] [Google Scholar]
- 28.Ozkaya O, Sezik M, Kaya H. Serum malondialdehyde, erythrocyte glutathione peroxidase, and erythrocyte superoxide dismutase levels in women with early spontaneous abortions accompanied by vaginal bleeding. Med Sci Monit. 2008;14:CR47–51. [PubMed] [Google Scholar]
- 29.Eble JA, de Rezende FF. Redox-relevant aspects of the extracellular matrix and its cellular contacts via integrins. Antioxid Redox Signal. 2014;20:1977–1993. doi: 10.1089/ars.2013.5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brill A, Chauhan AK, Canault M, Walsh MT, Bergmeier W, Wagner DD. Oxidative stress activates ADAM17/TACE and induces its target receptor shedding in platelets in a p38-dependent fashion. Cardiovasc Res. 2009;84:137–144. doi: 10.1093/cvr/cvp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2, 7-dichlorofluorescein. J Leukoc Biol. 1990;47:440–448. [PubMed] [Google Scholar]
- 32.Mondal NK, Mukherjee B, Das D. Micronucleus formation, DNA damage and repair in premenopausal women chronically exposed to high level of indoor air pollution from biomass fuel use in rural India. Mutat Res. 2010;697:47–54. doi: 10.1016/j.mrgentox.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Mondal NK, Sorensen E, Hiivala N, Feller E, Griffith B, Wu ZJ. Oxidative stress, DNA damage and repair in heart failure patients after implantation of continuous flow left ventricular assist devices. Int J Med Sci. 2013;10:883–893. doi: 10.7150/ijms.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suarez J1, Yang D. Gastrointestinal Symptoms from Left-Ventricular Assist Device External Compression of the Gastric Lumen. ACG Case Rep J. 2016;3:e180. doi: 10.14309/crj.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassell B, Kushnir VM. Gastrointestinal bleeding following left ventricular assist device (LVAD) implantation: Taking the pulse of the problem. Dig Dis Sci. 2015;60:3507–3509. doi: 10.1007/s10620-015-3810-x. [DOI] [PubMed] [Google Scholar]
- 36.Guha A, Eshelbrenner CL, Richards DM, Monsour HP. Gastrointestinal bleeding after continuous-flow left ventricular device implantation: Review of pathophysiology and management. Houston Methodist Debakey Cardiovasc J. 2015;11:24–27. doi: 10.14797/mdcj-11-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldmann C, Zayat R, Goetzenich A. Perioperative onset of acquired von Willebrand syndrome: Comparison between HVAD, HeartMate II and on-pump coronary bypass surgery. PLoS One. 2017;12:e0171029. doi: 10.1371/journal.pone.0171029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldauf C, Schneppenheim R, Stacklies W, et al. Shear-induced unfolding activates von Willebrand factor A2 domain for proteolysis. J Thromb Haemost. 2009;7:2096–2105. doi: 10.1111/j.1538-7836.2009.03640.x. [DOI] [PubMed] [Google Scholar]
- 39.Slaughter MS. Hematologic effects of continuous flow left ventricular assist devices. J Cardiovasc Transl Res. 2010;3:618–624. doi: 10.1007/s12265-010-9222-6. [DOI] [PubMed] [Google Scholar]
- 40.Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Chen D, Milano C, Thomas W, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–1269. doi: 10.1016/j.athoracsur.2010.04.099. [DOI] [PubMed] [Google Scholar]
- 42.Meyer AL, Malehsa D, Bara C, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail. 2010;3:675–681. doi: 10.1161/CIRCHEARTFAILURE.109.877597. [DOI] [PubMed] [Google Scholar]
- 43.Ghoshal K, Bhattacharyya M. Overview of platelet physiology: its hemostatic and nonhemostatic role in disease pathogenesis. Scientific World Journal. 2014;2014:781857. doi: 10.1155/2014/781857. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexandru N, Popov D, Georgescu A. Intraplatelet oxidative/nitrative stress: inductors, consequences, and control. Trends Cardiovasc Med. 2010;20:232–238. doi: 10.1016/j.tcm.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Essex DW. Redox control of platelet function. Antioxid Redox Signal. 2009;11:1191–1225. doi: 10.1089/ars.2008.2322. [DOI] [PubMed] [Google Scholar]
- 46.Krötz F, Sohn H-Y, Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler Thromb Vasc Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 47.Freedman JE. Oxidative Stress and Platelets. Arterioscler Thromb Vasc Biol. 2008;28:s11–s16. doi: 10.1161/ATVBAHA.107.159178. [DOI] [PubMed] [Google Scholar]
- 48.Essex DW. The role of thiols and disulfides in platelet function. Antioxid Redox Signal. 2004;6:736–746. doi: 10.1089/1523086041361622. [DOI] [PubMed] [Google Scholar]
- 49.Pietraforte D, Vona R, Marchesi A. Redox control of platelet functions in physiology and pathophysiology. Antioxid Redox Signal. 2014;21(1):177–193. doi: 10.1089/ars.2013.5532. [DOI] [PubMed] [Google Scholar]
- 50.Murphy DD, Reddy EC, Moran N, O’Neill S. Regulation of platelet activity in a changing redox environment. Antioxid Redox Signal. 2014;20:2074–2089. doi: 10.1089/ars.2013.5698. [DOI] [PubMed] [Google Scholar]
- 51.Bergmeier Wolfgang, Richard O. Hynes Extracellular Matrix Proteins in Hemostasis and Thrombosis. Cold Spring Harb Perspect Biol. 2012;4:a005132. doi: 10.1101/cshperspect.a005132. [DOI] [PMC free article] [PubMed] [Google Scholar]