Abstract
Background
Holistic approaches are sought to improve lifestyle behaviors and health of cancer survivors long term.
Objective
To explore whether a home-based vegetable gardening intervention is feasible, and improves diet and other health-related outcomes among older cancer survivors.
Design
Feasibility trial in which cancer survivors were randomized to receive a yearlong gardening intervention immediately or to a wait-list control arm. Home visits at baseline and 1-year assessed physical performance, anthropometric indices, behavioral and psychosocial outcomes, and biomarkers.
Participants/setting
Forty-six older (age 60+) survivors of locoregionally-staged cancers across Alabama from 2014–2016, of whom 42 completed 1-year follow-up.
Intervention
Cooperative Extension Master Gardeners delivered guidance to establish three seasonal vegetable gardens at survivors’ homes. Plants, seeds and gardening supplies were provided.
Outcomes
Primary outcomes were feasibility targets of 80% accrual and retention, and an absence of serious adverse events; other outcomes were secondary and explored potential benefits.
Statistical Analyses
Baseline to follow-up changes were assessed within- and between-arms using paired-t, McNemars and chi-square tests.
Results
This trial proved to be safe and demonstrated 91.3% retention; 70% of intervention participants rated their experience as “excellent,” and 85% would “do it again.” Data suggest significantly increased reassurance of worth (+0.49 v. −0.45) and attenuated increases in waist circumference (+2.30 cm v. +7.96 cm) in the gardening versus control arms (p-values=0.02). Vegetable and fruit consumption increased by ~1 serving/day within the gardening arm from baseline to follow-up [1.34(1.2) to 2.25(1.9); p=.02)] compared to controls [1.22(1.1) to 1.12(0.7), p=0.77]; between-arm p-value=0.06.
Conclusions
The home vegetable gardening intervention among older cancer survivors was feasible and suggested improvements in vegetable and fruit consumption and reassurance of worth; data also suggest attenuated increases in waist circumference. Continued study of vegetable gardening intervention is warranted to improve health, health behaviors, and well-being of older cancer survivors.
Keywords: Gardening, Diet, Cancer Survivors, Physical Function
INTRODUCTION/BACKGROUND
Currently, there are 15.5 million cancer survivors in the United States; 70% are age 60 or older.1 These older survivors often report complex health needs, of which functional decline is a major concern that threatens ability to live independently, reduces quality-of-life, and increases the burden borne by survivors, their families, and the health care system as a whole.1
Research shows that individuals who garden, and especially those who grow their own vegetables and fruits (V&F), are more physically active,2, 3 and tend to have healthier diets,3, 4 body weight status,5 and better mental health and acuity.4, 6 In a study by Brown et al. of 66 nursing home residents, a 5-week gardening intervention significantly improved ability to perform three activities of daily living (i.e., transferring, eating and toileting) and also enhanced reassurance of worth (defined as a feeling of adding value or deserving a place in society).7 These results may apply to cancer survivors who are at greater risk for many health conditions that are influenced by health behaviors (e.g., second cancers, cardiovascular disease and diabetes),1 as well as functional decline. Moreover, data indicate that few cancer survivors eat at least five servings of V&F daily or obtain sufficient physical activity (PA); therefore, the activities of gardening and access to fresh produce may have particular value in this population.8
While there have been some interventions aimed at gleaning (harvesting),9 Blair et al.3 reported the first and only study of a gardening intervention among cancer survivors. In this one-arm, quasi-experimental study, 12 cancer survivors (equally comprised of breast, prostate, and childhood cancer survivors) received supplies needed to grow a spring, summer, and fall vegetable garden. Home gardens, as opposed to community-based gardens, were used given the relatively low population density of cancer survivors and well-known barriers to travel.10 Given that many cancer survivors lacked the knowledge and skills to garden, volunteer Cooperative Extension Master Gardeners (MG) provided needed guidance. Results of this study found that all adult survivors who completed the 1-year study (6-of-8) achieved improvements in at least 2-of-3 of the following goals: (1) increase of ≥ 1 V&F serving/day; (2) increase of ≥ 30 minutes of moderate PA/day; and (3) improvement in 3-of-4 physical performance measures (30-second chair stand, hand grip strength, timed up-and-go, and the 6-minute walk test).
We adapted the intervention used by Blair et al.3 and re-evaluated it in a larger 2-arm National Cancer Institute (NCI)-sponsored pilot, feasibility trial among 46 older cancer survivors across Alabama, hypothesizing that the study would achieve the following benchmarks: 1) enrollment of at least 80% of the accrual target; 2) retention of at least 80% of participants; 3) an absence of serious adverse events attributable to the intervention; and 4) results that, if not statistically significant, showed satisfaction and some evidence of favorable effects on health behaviors and other health-related outcomes.
MATERIALS AND METHODS
A detailed description of the study protocol was published previously.11 Briefly, this was a single-blinded, two-arm, randomized controlled trial in which 46 older cancer survivors were evenly assigned to receive a one-year gardening intervention immediately or to a wait-list control arm where they received the gardening intervention after 1-year follow-up. The protocol and the consent form were approved by the Institutional Review Board of the University of Alabama at Birmingham (UAB). The trial was registered with ClinicalTrials.gov (NCT02150148).
Eligibility/Consent
Cancer survivors were recruited across Alabama from June 2014 – August 2014 largely by mailing letters of invitation to survivors identified through the UAB and the Alabama State Cancer Registries (making sure to obtain their primary oncologist’s permission for contact prior to the mailing). Community-based presentations at cancer support groups, fliers, television and radio announcements also drew self-referrals or referrals by community-based physicians. Eligibility criteria included: 1) 60 years of age or greater; 2) diagnosed with a loco-regionally staged cancer associated with at least a 60% five-year relative survival, i.e., in situ bladder; localized and regional: breast (female), Hodgkin Lymphoma, prostate, and thyroid; localized: cervix, colorectum, corpus and uterus, kidney/renal pelvis, Non-Hodgkin Lymphoma, oral cavity/pharynx, ovary, small intestine, and soft tissues; 3) completion of all cytotoxic and locoregional cancer therapy with the exception of ongoing adjuvant endocrine therapy; 4) ≥1 limitation on the SF-36 physical function subscale (at greater risk for functional decline);12 5) English speaking and writing; 6) community dwelling with residence able to accommodate one raised bed or four Earthboxes® in a location receiving at least six hours of daily sunlight and access to running water; 7) reporting <150 minutes of exercise/week on average; 8) reporting <5 servings of V&F/day on average; and 9) willingness to be randomized and adhere to study protocol. Potential participants were deemed ineligible if they already tended a vegetable garden or had counterindications to unsupervised PA, e.g., told by their physician not to exercise or had severe orthopedic, cardiovascular or pulmonary conditions. Written informed consent was obtained from all eligible participants.
Baseline Measures
Visits were scheduled at participants’ homes to collect the following: 1) accelerometers (Actical® Activity Monitor, Philips Respironics, Bend, OR) which were preprogrammed and sent 10 days prior to the home visit to collect objective data on PA; 2) anthropometric measures;13 3) physical performance data using the Senior Fitness Battery,14 plus hand grip strength, gait speed and balance; 4) finger- and toe-nail clippings, plus saliva to assess cortisol levels (stress hormone);15 and 5) blood to assess telomerase activity and interleukin (IL)-6 (biomarkers associated with aging, and inflammation and fatigue, respectively). Questionnaires comprised of scales/subscales that have been validated for use among cancer survivors and/or elderly populations with significant morbidity ascertained data on dietary intake,16 PA,17 quality-of-life,18 comorbidity,19 perceived stress,20 reassurance of worth,21 self-efficacy and social support to grow a vegetable garden, get more PA and eat more vegetables,22–24 and demographics (Table 1). Postage-paid, pre-addressed envelopes were provided to participants to mail-in questionnaires that were incomplete at the time of the home visit.
Table 1.
Description of intervention components and pre-post measures used in a pilot study of a home-based vegetable gardening intervention among older cancer survivors (n=46)
| Measure/Component | Description |
|---|---|
| Intervention Components | |
| Meet’n’Greet | Participants and Master Gardeners met each other at these initial 2-hour meetings convened at local county extension offices. Slide presentations were given about the gardening program and ample time provided for questions and answers. Most of the gardening supplies, with the exception of raised beds, Earthboxes® and soil (which were home-delivered) were provided at these meetings. |
| Master Gardener Mentoring | Master Gardeners were instructed to visit with the participant once a month and assess the home garden, during which time guidance would be given about pest control, harvesting, replanting, etc. Master Gardeners also were instructed to either email or telephone the participant in-between each of these visits to encourage engagement and provide ample mentorship on gardening techniques. |
| A private Facebook page was established for all intervention participants in which they could post their progress and discuss in an open forum any issues they were experiencing. | |
| Garden | Participants chose either a 4′x8′ raised bed or 4 Earthboxes® and were provided with appropriate soil mix; these supplies were delivered directly to their home. |
| Supplies | Standard supplies were: (1) garden and soaker hoses; (2) watering can; (3) spade; (4) cultivator; (5) hoe; (6) frost cover; (7) trellis; (8) seeds; (9) transplants; (10) fertilizer; (11) Neem oil/insect soap (to repel pests using “soft chemistry”); and (12) fertilizer; Deer fences were provided to those needing them. |
| Workbook | Pocket folders included contact information, as well as planting schedules and garden planning, sun safety, safe lifting (to avoid back injury and lymphedema), and trouble shooting. |
| Pre-post Evaluative Measures | |
| Vegetable and Fruit Intake [Eating at America’s Table Screener (EATS)]16 | 10-item questionnaire developed by the NCI that assesses both the frequency and portion size of F&V intake without imposing substantial participant burden. This instrument has proven reliability and validity in cancer populations and in the elderly, and has excellent concordance when compared to 24-hour recalls. |
| Physical Activity [Godin Leisure Time Exercise Questionnaire (GLTEQ)]17 | 3-item instrument that elicits self-reported frequency and average minutes of duration of strenuous, moderate and mild exercise/week was used based on excellent reliability and validity in cancer survivors. |
| Anthropometric Measures (Height, Weight and Waist Circumference)13 | Height and weight assessed in light clothing without shoes. Weight was measured using a calibrated portable scale (to nearest 0.1 kg) placed on non-carpeted flooring. Height was measured only at baseline upon inhale using a portable stadiometer (nearest 0.5 cm). Waist circumference was measured at the level of the umbilicus upon exhale using a non-stretch, tension-controlled tape measure (nearest 0.5 cm); two measures were taken and averaged. |
| Physical Performance (Senior Fitness Battery)14 | This battery includes assessments of lower and upper body strength (30-second chair stand, arm curl), endurance (2-minute step test), flexibility (chair sit-and-reach, back scratch), and agility/dynamic balance (8-foot Get Up & Go); the test battery provides an objective measure of physical function, is sensitive to change, and not associated with ceiling effects. Additional measures included (1) hand grip strength in kilograms measured using a dynamometer (Creative Products, Ann Arbor, MI); (2) gait speed as measured by the number of seconds needed to complete an 8-foot walk; and (3) balance measured by completion of side-by-side, tandem and semi-tandem stances for 10-seconds (range 0–6). |
| Perceived Stress20 [Perceived Stress Scale [PSS]) | The PSS is a 10-item scale used to measure the degree to which situations in an individual’s life are appraised with stress; it has been widely used and validated in multiple patient populations. |
| Reassurance of Worth21 | This 4-item subscale of the Social Provision Scale was used to assess the psychosocial benefits of gardening; it has been used previously in gardening interventions and provides information on enhanced self-esteem that results from increased independence and increased zest for life.79,91 Reliability estimates range from 0.60 to 0.70.223 |
| Quality of Life (QoL) [Short Form-36 Health Survey (SF-36)]12 | The SF-36 provides a global measure of health-related QoL, and component summaries (and specific subscales) for physical health (physical functioning, role limitations due to physical reasons, bodily pain, and general health perception subscales) and mental health (social functioning; vitality, energy and fatigue; role limitations due to emotional problems; and mental health subscales). The SF-36 is one of the most widely-used instruments to assess QoL across various patient populations and age groups. The intra-class correlations are high and range from 0.78 to 0.93. |
| Self-Efficacy22,24 | Self-efficacy is the organizing concept in SCT, and is defined as belief in the capability to organize and execute courses of action to deal with prospective situations. Self-efficacy beliefs are domain-specific; thus, participants were asked, “How confident (sure) they were that they could maintain a thriving vegetable garden, or exercise at least an additional 30 min/week or eat at least 1 more serving of V&F/day [anchors: “very sure (4),” “sure (3),” neither sure nor unsure (2),” “unsure (1),” or “very unsure (0)”]. |
| Social Support23 | Social support items for eating and exercise habits from Sallis et al.224 were employed given use in diverse samples and strong psychometric properties (α=.70). Frequency (anchors: “never,” “once a month or less,” “more than once a month, but less than once a week,” “more than once a week, but less than everyday,” or “everyday”) of friends and family either “listening to concerns,” “assisting with,” “encouraging choices favorable to,” and “agreeing with decisions to” eat more V&F, or exercise was assessed, and items were adapted to elicit similar information about tending a vegetable garden. |
Randomization/Intervention
Upon completion of the home visit, participants were randomly assigned with equal allocation to receive the gardening intervention either immediately or after a 1-year delay (wait-list control). Randomization was performed by an off-site statistician who block randomized (block size=4) by county and number of physical limitations (1 or >1). Concealed, coded envelopes were produced in sequence. Upon completion of baseline data collection, the participant was handed the sealed envelope and opened it to reveal their status.
In-depth information regarding the intervention is reported in the methods paper;11 components are summarized in Table 1. Briefly, each participant was provided with a 4′x8′ raised bed or four Earthboxes® (container gardens of equivalent square footage for use on balconies or patios) and other supplies needed to establish a successful spring, summer, and fall garden. Alabama Cooperative Extension Services MGs were to communicate with participants every two weeks over the course of a year, alternating home visits with contact by email or telephone, to establish a strong interactive relationship and build self-efficacy and behavioral capability to grow and maintain a successful vegetable garden using concepts integral to Social Cognitive Theory (SCT) and the Social Ecological Model.22, 25 As a retention strategy, holiday postcards were mailed throughout the year to both the intervention and control participants. Other than this, the wait-list control group was not contacted except for follow-up measures.
Follow-up Measures
At 1-year follow-up, home visits were repeated and all measures except demographics and height were reassessed. Evidence of active gardening also was assessed at the home visit among intervention arm participants and a debriefing telephone call assessed further information on fidelity, satisfaction, future gardening plans, and study suggestions (Table 4). Upon completion of follow-up, individuals in the wait-listed arm were provided with gardening supplies and access to a MG (study completion January 2016).
Table 4.
Debriefing survey of older cancer survivors (n=20) who completed the home vegetable gardening intervention
| Domain Item and Results - % (n) | Related Comments | Implications for Future Interventions/Trials | |
|---|---|---|---|
|
| |||
| Fidelity | When probed further about contact with MGs, only one participant voiced a need for more contact (one who was contacted by the MG every other month). All others stated that they were able to contact the MG as needed; 2 stated that once a month contact is the ideal frequency. One participant stated that an ankle break (unrelated to the intervention) interfered with her interactions with her MG and ability to work in the garden; this participant reported the lowest MG contact and one of those reporting the lowest gardening frequency. Another participant reported that her unexpected interstate move interfered with her participation, again a participant with low MG contact and gardening frequency. |
The frequency of contact may need to be further evaluated, though because many participant-MG dyads fell short of the twice-monthly contact goal, there also is a need for enhanced training of both the MG and the participant on the frequency of expected contact and more rigorous monthly tracking. While 85% of participants met expectations of working in their gardens at least several times/week, there is a need for enhanced communications between staff and participants regarding the goals of the program. Screen-out survivors who may be moving. |
|
| On average, how often did you communicate with your master gardener (MG)? | |||
| - More than once a month | 45% (9) | ||
| - Once a month | 25% (5) | ||
| - Every other month | 25% (5) | ||
| - Less than every other month | 5% (1) | ||
| On average, how often did you work in your garden? | |||
| - Several times a day | 5% (1) | ||
| - Once a day | 25% (5) | ||
| - Several times a week | 55% (11) | ||
| - Once a week | 15% (3) | ||
| On average, how many minutes per session did you spend? | |||
| - <15 minutes | 30% (6) | ||
| - 15–29 minutes | 65% (13) | ||
| - 30–44 minutes | 5% (1) | ||
|
| |||
| Satisfaction | Comments were overwhelmingly positive, “I thank God for this program,” “This program saved my life,” “Thank you so much,” “Great experience,” “Loved it,” and “After my health issues, it was extremely rewarding to work with the earth and grow healthy things to eat. The garden gave me happiness.” Those with lower satisfaction stated, “I wish I had enjoyed it more,” or comments related to a dislike of the outdoors. | Little action necessary. | |
| How would you rate your experience with this program (anchors: poor; fair; good; excellent)? | |||
| - Excellent | 70% (14) | ||
| - Good | 30% (6) | ||
| If given the chance, would you do it again? | |||
| - Yes, most definitely | 65% (13) | ||
| - Yes, probably | 20% (4) | ||
| - Maybe | 5% (1) | ||
| - No, probably | 5% (1) | ||
| - No, not at all | 5% (1) | ||
|
| |||
| Durability | Unsurprisingly, participants who reported higher levels of satisfaction voiced plans to continue gardening. One participant enrolled to become a MG. One participant planning not to continue stated, “I now know how wonderful fresh vegetables are… but I prefer to buy them.” Plans for expansion included a mixture of raised beds and container gardens. One participant stated a lack of room to expand. |
Little action necessary, though ensure that survivors who plan to continue gardening have appropriate support to do so. | |
| Do you plan to continue vegetable gardening? | |||
| - Yes, most definitely | 75% (15) | ||
| - Yes, probably | 10% (2) | ||
| - Maybe | 10% (2) | ||
| - No, not at all | 5% (1) | ||
| Do you plan to expand your vegetable garden? | |||
| - Yes, most definitely | 55% (11) | ||
| - Yes, probably | 15% (3) | ||
| - Maybe | 5% (1) | ||
| - No, probably | 5% (1) | ||
| - No, not at all | 20% (4) | ||
|
| |||
| Ratings on a scale of 1 (not at all) to 10 (tremendously) of how much the garden motivated them to (mean [SD])…. | Nine participants stated that they did more yardwork and nine reported more walking as a result of the intervention. Gym and weight training, as well as bicycling also were reported by a few. | Few fruits can be grown in sufficient quantities in a raised bed or container garden; therefore, assessing fruit intake may be unnecessary for future studies. | |
| - Eat a healthier diet | 8.1 (2.47) | ||
| - Eat more vegetables | 8.0 (2.36) | ||
| - Try new vegetables | 6.8 (2.84) | ||
| - Eat more fruit | 5.4 (3.10) | ||
| - Be more physically active | 7.5 (2.46) | ||
|
| |||
| Ratings on a scale of 1 (not at all) to 10 (tremendously) of the helpfulness of specific intervention supplies (mean [SD])…. | Participants expressed a need for workbooks to include more information on planting schedules (n=3), pest control (n=2) and maintaining soil quality (n=1). Four expressed a desire for recipes and more information on cancer fighting foods. | Notebooks should include ample information on planting schedules, pest control, soil quality, recipes, and the anticancer properties of specific vegetables. Omit soaker hoses from individuals receiving container gardens. Though watering cans received the lowest rating the variance was great and some participants found that they were extremely helpful. | |
| - Workbook | 6.4 (3.90) | ||
| - Spade | 8.1 (2.57) | ||
| - Hoe | 9.0 (1.73) | ||
| - Cultivator | 8.9 (1.81) | ||
| - Garden Hose | 8.9 (1.41) | ||
| - Soaker Hose | 5.7 (3.35) | ||
| - Watering Can | 4.5 (4.94) | ||
|
| |||
| Ratings of the helpfulness of MGs (anchors: very much, quite a bit, somewhat, a little bit, not at all) in… | A majority of comments of about MGs were positive (e.g., “I love my MG,” “My MG is awesome,” and “I knew I could always call or email my MG as needed”). However, the one participant reporting poorer ratings, stated a need for “better training of MGs.” | Clearly, most MGs were rated highly; however, competencies as related to vegetable gardening are needed since some MGs have expertise in other areas (e.g., ornamental plants). Also important is the need for back-ups or substitutes should personality conflicts arise, or if life circumstances affect the ability of MGs to follow through with mentorship. | |
| Garden design and planting | |||
| - Very much | 85% (17) | ||
| - Quite a bit | 5% (1) | ||
| - Somewhat | 5% (1) | ||
| - A Little bit | 5% (1) | ||
| Answering questions | |||
| - Very much | 75% (15) | ||
| - Quite a bit | 20% (4) | ||
| - A Little bit | 5% (1) | ||
|
| |||
| Estimated monetary value of program | While four participants placed values of $200–250 or stated, “y’all overpaid,” the clear majority valued the program above what it cost. Two participants valued the program at $1,000 and three stated that it was “priceless.” Additional participant comments included “It gave me a new focus on caring for the garden and sharing with others” and “What I gained in knowledge and encouragement was immeasurable. I am so thankful for the opportunity – I will garden now for years to come.” | ||
| The actual cost of the intervention was ~$500. Participants were informed of this amount and asked to place a monetary value on “How much the program meant to them” | |||
| - >$500 | 65% (13) | ||
| - =$500 | 15% (3) | ||
| - <$500 | 20% (4) | ||
Biospecimens were stored at −80°C until study completion, at which time they were batch-analyzed by blinded study staff in duplicate, with reruns conducted on samples providing discrepant readings >25%.11 Cortisol in saliva and nails was assessed using modified methods of Warnock et al.15 Plasma IL-6 was assessed via electrochemiluminescence. Telomerase activity was measured via enzyme-linked immunosorbent assay on peripheral blood mononuclear cells via methods of Saldanha et al.26
Statistical Analysis
The primary outcomes of this pilot study were feasibility-based, i.e., accrual, retention, acceptance, and absence of serious adverse events attributable to the intervention. Other analyses were secondary and descriptive, i.e., measures of central tendency and variance. Paired t tests and McNemars tests were used for within group comparisons over time for interval and dichotomous variables, respectively. Paired t tests and chi-square tests were used for between-group comparisons of baseline to 1-year follow-up change scores. All statistical analyses were conducted using SAS® version 9.4.27 Despite the focus on feasibility, apriori power calculations indicated 79% power to detect a between-arm difference of at least five points on the SF36 physical function subscale with the assumptions of 13% attrition, alpha <0.05, and a proportional between-arm difference of 15% vs. 55% using the Fisher’s exact test for proportions.
RESULTS
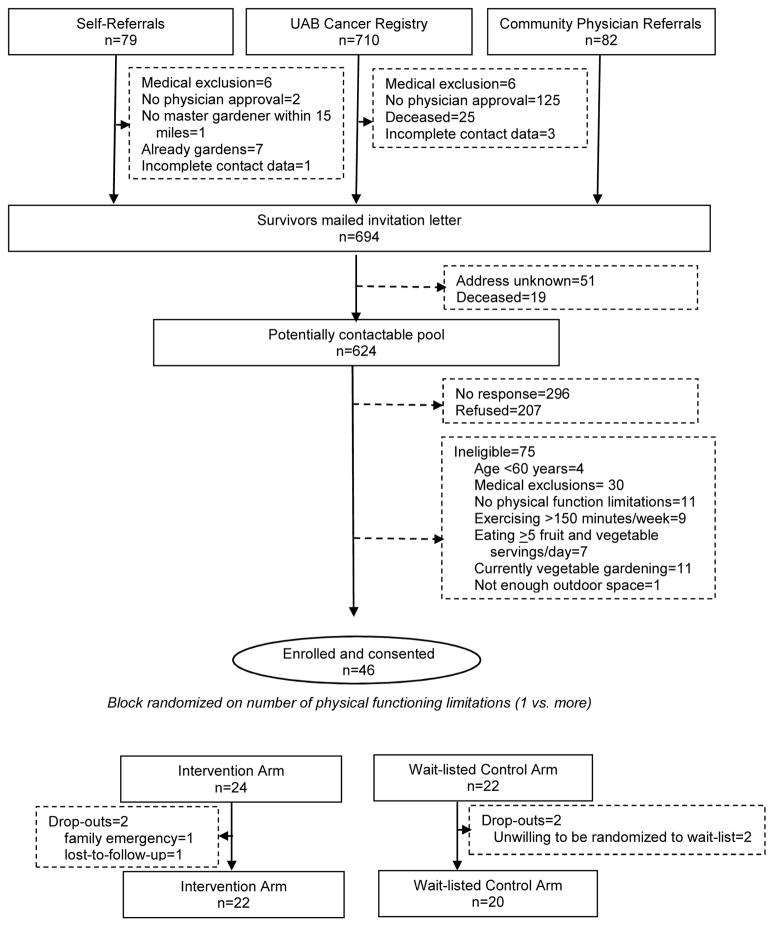
The trial CONSORT diagram is featured in Figure 1. Interest, as gauged by screener completion and a signed consent form, suggests that roughly 20% of older cancer survivors were interested in participating in this trial, though many screened-out due to medical exclusions, or because they lacked physical limitations, were already vegetable gardening or adhering to diet and exercise guidelines. There were only four drop-outs (8.7%) over the 1-year study, two in each study arm. The two drop-outs assigned to the wait-listed arm discontinued participation for the same reason - “they just couldn’t wait to garden.” This study experienced 91.3% retention over the 1-year study period and no attributable adverse events.
Figure 1.
CONSORT diagram of a vegetable gardening feasibility trial among 46 older cancer survivors
Baseline characteristics of the study sample are shown in Table 2. The sample was comprised primarily of non-Hispanic white, female breast cancer survivors who were married with a median educational level of “some college.” Only one participant currently smoked, but most were sedentary, overweight or obese, and had mean V&F intakes that were less than half of recommended levels.28 The sample reported multiple comorbidities and deficits in physical functioning. No between-arm differences were detected in any of the factors.
Table 2.
Characteristics of older cancer survivors (n=46) participating in a pilot study of a home-gardening intervention
| All (n=46) | Intervention (n=24) | Control (n=22) | |
|---|---|---|---|
|
| |||
| Age (years) | |||
| - Mean (SD) | 70.1 (8.1) | 70.4 (7.8) | 69.7 (8.5) |
| - Range | 60.0–91.8 | 60.0–85.6 | 60.0–91.8 |
|
| |||
| Type of Cancer - % (n) | |||
| - Breast | 58.7% (27) | 62.5% (15) | 54.5% (12) |
| - Prostate | 10.9% (5) | 4.2% (1) | 18.2% (4) |
| - Colorectal | 4.3% (2) | 8.3% (2) | 0.0% (0) |
| - Othera | 26.1% (12) | 25.0% (6) | 27.3% (6) |
|
| |||
| Cancer Treatment - % (n) | |||
| - Surgery | 84.8% (39) | 83.3% (20) | 86.4% (19) |
| - Radiation | 50.0% (23) | 58.3% (14) | 40.9% (9) |
| - Chemotherapy | 43.5% (20) | 50.0% (12) | 36.4% (8) |
| - Hormonal Therapy | 43.5% (20) | 45.8% (11) | 40.9% (9) |
| - None of the Above | 2.2% (1) | 4.2% (1) | 0% (0) |
|
| |||
| Years since Diagnosis | |||
| - Mean (SD) | 6.7 (7.7) | 6.7 (7.7) | 6.7 (7.9) |
| - Range | 0.8–30.3 | 0.8–28.7 | 0.9–30.3 |
|
| |||
| Female Sex - % (n) | 69.6% (32) | 75% (18) | 63.6% (14) |
|
| |||
| Race - % (n) | |||
| - Non-Hispanic White | 97.8% (45) | 95.8% (23) | 100% (22) |
| - African American | 2.2% (1) | 4.2% (1) | 0% (0) |
|
| |||
| Education - % (n) | |||
| - <High School | 8.7% (4) | 4.2% (1) | 13.6% (3) |
| - High School Graduate | 13.0% (6) | 8.3% (2) | 18.2% (4) |
| - Some College | 26.1% (12) | 33.3% (8) | 18.2% (4) |
| - College Graduate | 19.6% (9) | 16.7% (4) | 22.7% (5) |
| - Post-Graduate Degree | 22.7% (12) | 29.2% (7) | 22.7% (5) |
|
| |||
| Currently Employed - % (n) | 32.6% (15) | 25.0% (6) | 40.9% (9) |
|
| |||
| Marital Status - % (n) | |||
| - Married | 58.7% (27) | 58.3% (14) | 59.1% (13) |
| - Widowed | 28.3% (13) | 33.3% (8) | 22.7% (5) |
| - Other | 13.0% (6) | 8.3% (2) | 18.2% (4) |
|
| |||
| Number of Persons in Household - % (n) | |||
| - 1 | 28.9% (13) | 26.1% (6) | 31.8% (7) |
| - 2 | 53.3% (24) | 52.2% (12) | 54.5% (12) |
| - 3 or more | 17.8% (8) | 21.7% (5) | 13.6% (3) |
|
| |||
| Number of Co-morbidities - % (n) | |||
| - Mean (SD) | 4.0 (2.6) | 4.3 (2.7) | 3.7 (2.5) |
| - Range | 0–11 | 0–11 | 0–9 |
|
| |||
| Number of Functional Limitations12- % (n) | |||
| - 1 | 10.9% (5) | 12.5% (3) | 9.1% (2) |
| - 2 or more | 89.1% (41) | 87.5% (21) | 90.9% (20) |
| - Mean (SD) | 4.3 (2.4) | 4.3 (2.4) | 4.2 (2.5) |
| - Range | 1–9 | 1–9 | 1–9 |
|
| |||
| Social Readjustment Events | |||
| - Mean (SD) | 4.3 (4.7) | 4.5 (5.7) | 4.1 (3.3) |
| - Range | 0–26 | 0–26 | 1–13 |
|
| |||
| Current Smoker - % (n) | 2.3% (1) | 0% (0) | 4.5% (1) |
|
| |||
| Body Mass Index (BMI) | |||
| - Normal Weight (18.5–24.9 kg/m2) | 26.1% (12) | 20.8% (5) | 31.8% (7) |
| - Overweight (25.0–29.9 kg/m2) | 41.3% (19) | 45.8% (11) | 36.4% (8) |
| - Obese (>30 kg/m2) | 32.6% (15) | 33.3% (8) | 31.8% (7) |
|
| |||
| Moderate to Vigorous Physical Activity | |||
| - Mean (SD) Minutes/Week | 54.0 (84.5) | 72.1 (99.3) | 34.5 (59.1) |
|
| |||
| Vegetables & Fruit Intake | |||
| - Mean (SD) Servings/Day | 1.3 (1.1) | 1.3 (1.2) | 1.2 (1.1) |
Kidney, Lymphoma, Lung, Thyroid, Head & Neck, Multiple Myeloma, Pancreas
Participants entered the study with high levels of self-efficacy (or confidence) to garden, exercise, and increase V&F consumption, and no statistically significant differences in these potential mediators were observed over time; however, a significant increase was observed within the intervention arm in social support to garden (p=0.02). No within- or between-arm differences were observed regarding social support to eat more V&F or to exercise. Changes in behavioral, anthropometric, physical performance, biomarkers, and psychosocial outcomes are reported in Table 3. While directionality was of primary interest in this pilot study, within- and between-group differences were explored. Evidence of increased V&F intake was observed within the intervention arm (p=0.02), but not among controls; differences between arms also approached significance (p=.06). In contrast, mixed effects were seen for PA, and self- reported data did not track with accelerometer readings.
Table 3.
Baseline to 12-month changes in behavioral, psychosocial and clinical outcomes of 46 older cancer survivors enrolled in a vegetable gardening feasibility triala
| Mean (SE) | Gardening Intervention | Waitlist Control | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0M (n=24) | 12M (n=22) | Within-Arm Difference 0–12M p-value | 0M (n=22) | 12M (n=20) | Within-Arm Difference 0–12M p-value | |
|
| ||||||
| Health Behaviors | ||||||
| Vegetable & Fruit Intake (EATS)b | ||||||
| - Servings/day | 1.34(0.25) | 2.25(0.45) | 0.02 | 1.22(0.17) | 1.12(0.21) | 0.77 |
|
| ||||||
| Physical Activity (PA) | ||||||
| Moderate PA (GTLEQ) (min/week) | 59.77(20.47) | 105.00(32.76) | 0.26 | 22.50(7.18) | 52.14(30.64) | 0.47 |
| Accelerometer MET hours/weekc | 63.18(9.45) | 62.82(10.62) | 0.42 | 59.05(10.41) | 42.76(11.87) | 0.50 |
|
| ||||||
| Anthropometric Measures | ||||||
|
| ||||||
| Weight (kg) | 77.70(3.58) | 75.04(3.45) | 0.13 | 78.36(3.82) | 80.95(4.59) | 0.55 |
|
| ||||||
| BMI (kg/m2) | 28.53(1.04) | 27.82(1.19) | 0.17 | 27.99(0.97) | 28.49(1.18) | 0.57 |
|
| ||||||
| Waist Circumference (cm) | 92.92(2.86) | 95.22(2.67) | <0.01 | 91.89(3.08) | 99.85(3.79) | <0.01 |
|
| ||||||
| Physical Performance Measuresd | ||||||
|
| ||||||
| 2-Minute Step Test (steps) | 72.08(4.76) | 93.61(4.08) | <0.01 | 74.38(6.24) | 88.42(8.07) | 0.06 |
|
| ||||||
| Timed 8-foot Walk (seconds) | 3.55(0.28) | 2.54(0.16) | 0.04 | 3.68(0.30) | 3.07(0.37) | 0.01 |
|
| ||||||
| 8-foot Get-Up & Go (seconds) | 8.19(0.32) | 6.92(0.41) | 0.003 | 8.49(0.52) | 6.87(0.60) | 0.03 |
|
| ||||||
| 30-second Chair Stand (number of rises) | 12.46(0.57) | 13.74(0.77) | 0.19 | 14.14(1.19) | 13.94(1.23) | 0.66 |
|
| ||||||
| Chair Sit & Reach (inches) | 0.87(0.52) | 0.84(0.82) | 0.95 | −1.26(0.94) | −0.81(0.95) | 0.98 |
|
| ||||||
| Back Scratch (inches) | −1.49(1.21) | −2.80(0.97) | 0.37 | −3.28(1.25) | −3.40(1.36) | 0.89 |
|
| ||||||
| Arm Curl (number of curls) | 18.00(0.84) | 18.40(1.05) | 0.42 | 17.38(1.13) | 20.11(1.55) | 0.08 |
|
| ||||||
| Hand Grip Strength (kg) | 25.98(2.13) | 25.20(1.83) | 0.63 | 26.00(1.87) | 27.65(2.26) | 0.21 |
|
| ||||||
| Balance Testing (seconds) | 5.58(0.25) | 5.95(0.05) | 0.15 | 5.28(0.30) | 5.42(0.33) | 0.16 |
|
| ||||||
| Biomarkers | ||||||
|
| ||||||
| Salivary Cortisol (μg/dL) | 0.64(0.32) | 0.04(0.02) | 0.21 | 0.41(0.18) | 0.24(0.20) | 0.82 |
|
| ||||||
| Fingernail Cortisol (ng/g)f | 0.069(0.025) | 0.062(0.051) | 0.41 | 0.102(0.054) | 0.102(0.065) | 0.96 |
|
| ||||||
| Toenail Cortisol (ng/g)f | 0.022(0.073) | 0.015(0.007) | 0.39 | 0.040(0.060) | 0.149(0.065) | 0.64 |
|
| ||||||
| Telomerase | 0.014(0.007) | 0.001(0.001) | 0.05 | 0.014(0.006) | 0.012(0.007) | 0.81 |
|
| ||||||
| Interleukin (IL)-6 (pg/mL) | 1.77(0.52) | 1.12(0.10) | 0.24 | 1.59(0.31) | 1.46(0.23) | 0.52 |
|
| ||||||
| Psychosocial Measurese | ||||||
|
| ||||||
| Perceived Stress | 13.14(1.33) | 13.67(1.52) | 0.80 | 11.37(1.91) | 11.46(1.90) | 0.39 |
|
| ||||||
| Reassurance of Worth* | 12.95(0.36) | 13.44(0.40) | 0.18 | 13.53(0.48) | 13.08(0.59) | 0.07 |
|
| ||||||
| Quality of Life (SF-36)12 | ||||||
| Physical Summary Score * | 72.45(2.73) | 66.14(3.91) | 0.29 | 67.69(3.07) | 74.67(3.76) | 0.07 |
| - Physical functioning | 73.48(3.45) | 75.00(5.00) | 0.36 | 72.86(4.09) | 77.65(3.84) | 0.31 |
| - Physical role limitations ** | 71.74(4.34) | 63.99(6.91) | 0.22 | 64.58(5.07) | 79.41(5.56) | 0.01 |
| - Pain ** | 76.30(3.80) | 64.40(5.34) | 0.02 | 66.43(4.95) | 77.50(5.28) | 0.05 |
| - General Health | 68.26(3.15) | 66.14(3.92) | 0.89 | 66.90(4.02) | 64.12(4.91) | 0.19 |
| Mental Health Score* | 75.46(2.86) | 74.01(4.76) | 0.66 | 68.80(4.42) | 75.95(4.00) | 0.01 |
| - Emotional well-being | 76.74(3.09) | 80.00(3.61) | 0.53 | 74.52(4.85) | 76.47(5.40) | 0.35 |
| - Emotional role limitations | 81.88(4.37) | 79.76(5.99) | 0.84 | 76.59(5.65) | 87.25(4.35) | 0.03 |
| - Vitality* | 58.42(2.80) | 55.95(4.85) | 0.46 | 51.49(3.73) | 58.46(3.90) | 0.02 |
| - Social functioning | 84.78(3.24) | 80.36(6.49) | 0.44 | 72.62(6.08) | 81.62(5.04) | 0.07 |
Measures described in Methods and Table 1.
p<0.06;
MET-hours Metabolic Equivalent of Task (MET) is a physiological measure expressing the energy cost of physical activities, as compared to the reference metabolic rate of 3.5 ml O2·kg−1·min−1, (estimated oxygen consumption at rest). Higher levels indicate more physical activity.
For physical performance measures, higher values are favorable for the 2-minute step test, 30-second chair stand, arm curl, hand grip strength and balance testing, for all other measures lower values are considered more favorable.
For psychosocial measures, higher values are favorable for reassuance of worth (maximal score=16; minimal score=4) and the SF-36 (maximal score=100; minimal score=0), and lower values are favorable for perceived stress (maximal score=16; minimal score=0)
l nmol/g (0.3625 ng/g)
Between-arm difference of p<0.05;
Between-arm difference of p<0.01
Among these older cancer survivors, who were mostly overweight or obese, body weight and BMI did not change appreciably over the 1-year study period. However the enlargement of waist circumference, a parameter that generally increases with age-related changes in central body fat distribution,29 was significantly attenuated in the intervention arm as compared to controls (p=0.05).
Overall, physical performance assessments indicated that both arms experienced improvements over the study period in 8-of-9 measures. The intervention arm demonstrated significant improvements in the 2-minute step test, timed 8-foot walk, and the 8-foot up-and-go, whereas the control arm experienced statistically significant improvements in the latter two tasks. No statistically significant between-arm differences were noted.
No statistically significant changes were seen in levels of the stress hormone, cortisol, or in IL-6, a marker of inflammation. However, significant decreases in telomerase activity were seen in the gardening intervention arm. Self-reported assessments of psychosocial measures suggested that perceived stress was relatively stable within both arms over the 1-year study; however, reassurance of worth increased within the intervention arm and decreased among controls, resulting in significant between-group differences (p=.02). In contrast, more positive changes were seen for quality-of-life outcomes in the control versus intervention arms, i.e., improvements in 9-of-10 vs. 2-of-10 summary and subscale scores, respectively. These results were only partially corroborated by physical performance data.
Debriefing results show that while most participants (85%) reported working in their gardens several times/week, as was the goal, MG contact occurred less frequently than bimonthly in 55% of the dyads (Table 4). Injury and an unanticipated move accounted for 10% of participants reporting less frequent MG contact. Satisfaction was overwhelmingly positive; 100% of participants rated the experience “good-to-excellent,” and 85% stated that they would “do it again.” Moreover, 85% reported plans to continue gardening, with 70% stating plans for expansion. The vegetable garden was strongly attributed to eating a healthier diet, trying new vegetables, and obtaining more PA, but was less strongly ascribed to increasing fruit consumption. All supplies, except the soaker hose and watering can were considered helpful intervention components. More information on planting, pest control, soil quality, cancer-fighting attributes of specific vegetables and recipes was requested. Nearly all participants (90–95%) viewed their MGs as helpful in garden designing and planting, and in answering questions. Most participants estimated the monetary value of intervention at least as great as the actual cost.
DISCUSSION
This report is the first to describe the results of a vegetable gardening intervention on the health behaviors and outcomes of older cancer survivors. Data show that the intervention was safe, well received, and feasible. Moreover, the response rate of 20% among older cancer survivors, a population that has been repeatedly documented as “hard to reach,”30 is a clear victory and is roughly double the 10.8% reported by the Reach-out to ENhancE Wellness (RENEW) trial, the largest lifestyle intervention trial conducted among older cancer survivors (n=641).31 Participants assigned to the gardening intervention also manifested lesser gains in waist circumference, as well as increases in reported V&F consumption that not only were statistically significant, but more importantly for a pilot study, were of a clinically-significant effect size. The one serving increase in V&F intake observed in the intervention arm, and the differential between arms at 1-year follow-up, reportedly equates to a 5% reduction in all-cause mortality in a meta-analysis of 16 cohort studies across 833,234 adults of mean age 55.6 years;32 similar findings have been reported in older adults. Likewise, a 5 centimeter difference in waist circumference (magnitude of the between-arm difference in change scores), reportedly equates to an 8% reduction in all-cause mortality among adults of mean age 56 years,33 a finding that is supported by studies among older community-dwelling adults,34, 35 and specifically in colorectal cancer survivors.36 Moreover, like the nursing home study of Brown et al.7 that found a significant increase in reassurance of worth with a gardening intervention, the current study also observed significant increases in this same measure.21 This has obvious import for this specific study population that may feel disenfranchised not only because of their age, but also because of their illness.
Less clear were the potential effects on other outcomes. Increases in physical performance were noted in both groups and could be the result of a practice effect. Moreover, the lack of between-arm differences is likely attributed to the lack of power that is an overarching limitation of this pilot study. Indeed, between-arm differences in weight status, cortisol levels (particularly in saliva and toenails), IL-6, and telomerase activity may have emerged with an increased sample size.
An unexpected decrease in telomerase activity was noted within the intervention arm (p=0.05). Given reports of increased telomerase activity with 12-week mindfulness and lifestyle interventions in breast and prostate cancer survivors,37, 38 parallel findings were expected. However, the current study results are similar to the longer-term data reported by Ornish et al.39 of a lifestyle intervention in prostate cancer survivors in which telomerase activity was found to decrease over a 5-year period. Such data provide pause as to the utility of telomerase as a biomarker among cancer survivors; first, because of its variable activity over time and in response to lifestyle interventions and second, because while more activity might signal a longer lifespan, it also might serve as a potential threat to cancer control by prolonging the existence of abnormal cells.39
Also unexpected were the between-arm and within-arm changes in quality-of-life which suggested increased pain and reduced well-being with the gardening intervention. While pain is associated with increased PA among those who are sedentary, particularly with forward bending activities (such as gardening),40, 41 given prior research,7, 11 increases in quality-of-life were nonetheless expected with the intervention. However, subjective data collection was problematic in this pilot study, since many surveys were mailed-in after the home visits and after a considerable lag. This limitation potentially biases the results, since some participants may have already started the intervention by the time they completed the survey, thus attenuating or potentially changing the direction of effects. Therefore, timely collection of surveys in future research is imperative.
Other “lessons learned” came from the debriefing and suggested that while the gardening intervention was well received, some adaptations are necessary. First, while moves or injuries cannot be fully anticipated, efforts are needed to reduce this likelihood. Secondly, although the optimal frequency of MG-participant contact is unknown, increased communications regarding the goal of the biweekly contact and methods to enhance fidelity through increased tracking appear necessary. Third, while most participants perceived their MG as helpful and well trained, one participant was clearly dissatisfied. This suggests a need for further MG training or vegetable gardening competency attainment, and/or the ability to resolve personality conflicts amongst dyads. Fourth, the intervention could be strengthened by providing more information on planting, pest control, vegetable benefits and recipes, and streamlined by judiciously weighing needs for equipment like soaker hoses. The fact however that most participants planned to continue, and even expand their garden, shows the potential of this intervention to have long lasting benefits which need to be assessed in future studies.
Undoubtedly, this study was instrumental in beginning to address the research gap regarding the acceptability and potential benefits associated with a vegetable gardening intervention among older cancer survivors; hence, it contributes data in two unique areas: (1) holistic interventions that potentially affect several outcomes;10 and (2) older cancer survivors, an understudied population with several health-related needs or concerns.1, 8 Moreover, the randomized controlled design and reliance on registry-based recruitment were additional strengths. However, there are limitations that call for caution in interpreting the results, i.e., the lack of statistical power associated with the modest sample size, reliance on self-reported data, and the increased likelihood of Type I error associated with multiple comparisons.
CONCLUSIONS
This pilot vegetable gardening intervention in older cancer survivors was well accepted, safe, and feasible, and also significantly improved reassurance of worth and reduced gains in central adiposity. Data also suggest that it increased V&F consumption by approximately one serving/day. While other benefits are not as clear, results suggest that larger, future studies are warranted. A fully-powered, randomized controlled trial is currently underway and recruiting 426 older cancer survivors across Alabama (NCT02985411).
RESEARCH SNAPSHOT.
Research Question
Is a home vegetable gardening intervention feasible among older cancer survivors, and is it associated with improvements in diet and other health-related outcomes?
Key Findings
This feasibility trial among 46 older cancer survivors who were randomized to receive a yearlong vegetable gardening intervention immediately or to a wait-list control arm achieved 100% accrual, 91.3% retention and was safe; 100% of intervention participants rated their experience as “good-to-excellent,” and 85% would “do it again.” Data amongst intervention vs. control arm participants suggest improved reassurance of worth, attenuated increases in waist circumference, and trends toward improved vegetable and fruit consumption.
Acknowledgments
Funding Sources: National Institutes of Health (R21 CA182508, R25 CA047888, P30 DK079626, K07 AG043588) and the Diana Dyer Endowment of the American Institute for Cancer Research
The authors acknowledge the contributions of study staff (Carrie Howell, PhD and Teresa Martin), UAB Cancer Research Experiences for Students (CaRES) Interns (Hannah Brown Guthrie, Amber Hardeman, and Justine Goetzman), and work student students (Isabella Mak and Lora Roberson) for their contributions in collecting these data and in recruitment. We are grateful for the review by Jennifer Bail, PhD, RN. We also acknowledge Drs. Michael Meshad and Stephen Davidson, Amber Davis of East Alabama Medical Center, Cheryl Matrella of the UAB Cancer Care Network, and Bob Shepard for their contributions in recruiting the study samples. Further, the authors appreciate the efforts of Susan Rossman, Nancy Smith, Leonora Roberson, and Jennifer Hicks for their pioneering spirits and effort and we thank Alabama Cooperative Extension agents: Ellen Huckabay, James Miles, Mike McQueen, Lucy Edwards, Mallory Kelley, Danielle Carroll, Nelson Wynn, Bethany O’Rear, Charles Pinkston, Tim Crow, Danny Cain, Dan Porch, Renee Thompson, Ken Creel, and Eric Schavey. Most of all, we thank all of the survivors and Master Gardeners who make these studies possible. Funding was provided National Institutes of Health, National Cancer Institution R21 CA182508, National Institutes of Health, National Cancer Institute Cancer Prevention and Control Training Program Grant R25 CA047888, and National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases P30 DK079626, and the Diana Dyer Endowment of the American Institute for Cancer Research.
Footnotes
Conflicts: The authors have no conflicts to disclose
Author Contributions: WDW, ABC, JL, KPS and JFD designed the trial, methods, and obtained grant funding; HJC provided consul; WDW provided trial oversight and composed the initial draft of the manuscript; ABC performed randomization and statistical analysis; MGC, ADF and YT conducted accrual, data collection and cleaning; and MD and RK performed laboratory analysis. All authors reviewed and commented on subsequent drafts of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wendy Demark-Wahnefried, Professor of Nutrition Sciences, University of Alabama at Birmingham (UAB), 1824 6th Avenue, Wallace Tumor Institute, Room 102-M, Birmingham, AL 35294, T: 205-975-4022 F: 205-975-2592.
Mallory G. Cases, UAB Post-Doctoral Fellow, Other info as above.
Alan B. Cantor, Professor of Preventive Medicine, UAB, 1717 11th Avenue South, MT 516P, Birmingham, AL 35205, T: 205-934-2924 F: 205-996-2974.
Andrew D. Frugé, Assistant Professor of Nutrition, Dietetics & Hospitality Management, Auburn University, 101E PSB, 260 Lem Morrison Drive, Auburn, AL 36849, T: 334-844-3271 F: 334-844-3268.
Kerry P. Smith, State Master Gardener Program Coordinator, Alabama Cooperative Extension System, Auburn University. 101 Funchess Hall, Auburn University AL 36849, T: 334-844-3036 F: 334-844-5530.
Julie Locher, Professor of Medicine, Division of Gerontology, Geriatrics and Palliative Care, UAB, 933 19th Street South Birmingham, Alabama 35233, T: 205-934-7542 F: 205-975-5930.
Harvey J. Cohen, Professor of Medicine, Duke University Medical Center, Room 3502 Blue Zone Duke University Medical Center, Box 3003 Durham, NC 27710, T: 919-660-7502 F: 919-684-8569.
Yuko Tsuruta, Researcher V, UAB, 1824 6th Avenue, Wallace Tumor Institute, Room 102-L, Birmingham, AL 35294, T: 205-975-2418 F: 205-975-2592.
Michael Daniel, UAB Doctoral Candidate in Biology, 1300 University Blvd Birmingham, Alabama 35233, T: 205-934-4573 F: 205-975-6097.
Rishabh Kala, UAB Post Doctoral Fellow, 1300 University Blvd Birmingham, Alabama 35233, T: 205-934-4573 F: 205-975-6097.
Jennifer F. De Los Santos, Professor of Radiation Oncology, UAB, 1700 6th Avenue South, Birmingham AL 35233, T: 205-978-0257 F: 205-978-3928.
References
- 1.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park S, Shoemaker C, Haub M. Can older gardeners meet the physical activity recommendation through gardening? Horttechnol. 2008;18(4):639–643. [Google Scholar]
- 3.Blair CK, Madan-Swain A, Locher JL, et al. Harvest for health gardening intervention feasibility study in cancer survivors. Acta Oncol. 2013;52(6):1110–1118. doi: 10.3109/0284186X.2013.770165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waliczek TM, Sommerfeld AJ, McFarland AL, et al. Growing minds: Evaluating the relationship between gardening and fruit and vegetable consumption in older adults. Horttechnol. 2010;20(4):711–717. [Google Scholar]
- 5.Lombard KA, Forster-Cox S, Smeal D, et al. Diabetes on the Navajo nation: what role can gardening and agriculture extension play to reduce it? Rural Remote Health. 2006;6(4):640. [PubMed] [Google Scholar]
- 6.McCallum J, Simons LA, Simons J, et al. Delaying dementia and nursing home placement: the Dubbo study of elderly Australians over a 14-year follow-up. Ann N Y Acad Sci. 2007;1114:121–129. doi: 10.1196/annals.1396.049. [DOI] [PubMed] [Google Scholar]
- 7.Brown VM, Allen AC, Dwozan M, et al. Indoor gardening older adults: effects on socialization, activities of daily living, and loneliness. J Gerontol Nurs. 2004;30(10):34–42. doi: 10.3928/0098-9134-20041001-10. [DOI] [PubMed] [Google Scholar]
- 8.Henderson TO, Ness KK, Cohen HJ. Accelerated aging among cancer survivors: from pediatrics to geriatrics. American Society of Clinical Oncology educational book American Society of Clinical Oncology Meeting. 2014:e423–430. doi: 10.14694/EdBook_AM.2014.34.e423. [DOI] [PubMed] [Google Scholar]
- 9.Spees CK, Joseph A, Darragh A, et al. Health behaviors and perceptions of cancer survivors harvesting at an urban garden. Am J Health Behav. 2015;39(2):257–266. doi: 10.5993/AJHB.39.2.12. [DOI] [PubMed] [Google Scholar]
- 10.Stull VB, Snyder DC, Demark-Wahnefried W. Lifestyle interventions in cancer survivors: designing programs that meet the needs of this vulnerable and growing population. J Nutr. 2007;137(1 Suppl):243s–248s. doi: 10.1093/jn/137.1.243S. [DOI] [PubMed] [Google Scholar]
- 11.Cases MG, Fruge AD, De Los Santos JF, et al. Detailed methods of two home-based vegetable gardening intervention trials to improve diet, physical activity, and quality of life in two different populations of cancer survivors. Contemp Clin Trials. 2016;50:201–212. doi: 10.1016/j.cct.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware JEKM. Physical & Mental Health Summary Scales: A manual for users of Version 2. Lincoln, RI: QualityMetric, Inc; 2007. [Google Scholar]
- 13.Lohman TJ, Roache AF, Martorell R. Anthropometric Standardization Reference Manual. Med Sci Sports Exerc. 1992;24(8):952. [Google Scholar]
- 14.Rikli RE, Chodzko-Zajko W, Rikli RE, et al. The development and national norming of a functional fitness test for older adults. Med Sci Sports Exerc. 1999;31(Supplement):S399. [Google Scholar]
- 15.Warnock F, McElwee K, Seo RJ, et al. Measuring cortisol and DHEA in fingernails: a pilot study. Neuropsychiatr Dis Treat. 2010;6:1–7. [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute. [Accessed Oct 27, 2017];Fruit & Vegetable Screeners in the Eating at America’s Table Study (EATS) Available: http://appliedresearch.cancer.gov/diet/screeners/fruitveg/
- 17.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian J Appl Sport Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- 18.Anderson AS, Mackison D, Boath C, et al. Promoting changes in diet and physical activity in breast and colorectal cancer screening settings: an unexplored opportunity for endorsing healthy behaviors. Cancer Prev Res. 2013;6(3):165–172. doi: 10.1158/1940-6207.CAPR-12-0385. [DOI] [PubMed] [Google Scholar]
- 19.Fillenbaum C. Multidimensional Functional Assessment of Older Adults: The Duke Older Americans Resources and Services Procedures. PsycCRITIQUES. 1990;35(6) [Google Scholar]
- 20.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 21.Cutrona CERD. The provisions of social relationships and adaptation to stress. Greenwich, CT: JAI Press; 1987. [Google Scholar]
- 22.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 23.Sallis JF, Grossman RM, Pinski RB, et al. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16(6):825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 24.Sallis JF, Pinski RB, Grossman RM, et al. The development of self-efficacy scales for healthrelated diet and exercise behaviors. Health Educ Res. 1988;3(3):283–292. [Google Scholar]
- 25.Stokols D. Social ecology and behavioral medicine: implications for training, practice, and policy. Behav Med. 2000;26(3):129–138. doi: 10.1080/08964280009595760. [DOI] [PubMed] [Google Scholar]
- 26.Saldanha SN, Andrews LG, Tollefsbol TO. Analysis of telomerase activity and detection of its catalytic subunit, hTERT. Analyt Biochem. 2003;315(1):1–21. doi: 10.1016/s0003-2697(02)00663-2. [DOI] [PubMed] [Google Scholar]
- 27.SAS Institute Inc. SAS Version 9.4. Cary, NC: SAS Institute Inc; 2013. [Google Scholar]
- 28.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. [Accessed October 28, 2017];Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation. Available: http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf.
- 30.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 31.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301(18):1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berentzen TL, Jakobsen MU, Halkjaer J, et al. Changes in waist circumference and mortality in middle-aged men and women. PloS one. 2010;5(9) doi: 10.1371/journal.pone.0013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobs EJ, Newton CC, Wang Y, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170(15):1293–1301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- 35.Sui X, LaMonte MJ, Laditka JN, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298(21):2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haydon AM, Macinnis RJ, English DR, et al. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55(1):62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lengacher CA, Reich RR, Kip KE, et al. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer. Biol Res Nurs. 2014;16(4):438–447. doi: 10.1177/1099800413519495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 39.Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14(11):1112–1120. doi: 10.1016/S1470-2045(13)70366-8. [DOI] [PubMed] [Google Scholar]
- 40.Gibala MJ, McGee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev. 2008;36(2):58–63. doi: 10.1097/JES.0b013e318168ec1f. [DOI] [PubMed] [Google Scholar]
- 41.Miranda H, Viikari-Juntura E, Martikainen R, et al. Physical exercise and musculoskeletal pain among forest industry workers. Scand J Med Sci Sports. 2001;11(4):239–246. doi: 10.1034/j.1600-0838.2001.110408.x. [DOI] [PubMed] [Google Scholar]